Experimental and Molecular Dynamics Simulation Study on Sulfate Corrosion Resistance of Cellulose-Nanocrystal-Modified ECC
Abstract
1. Introduction
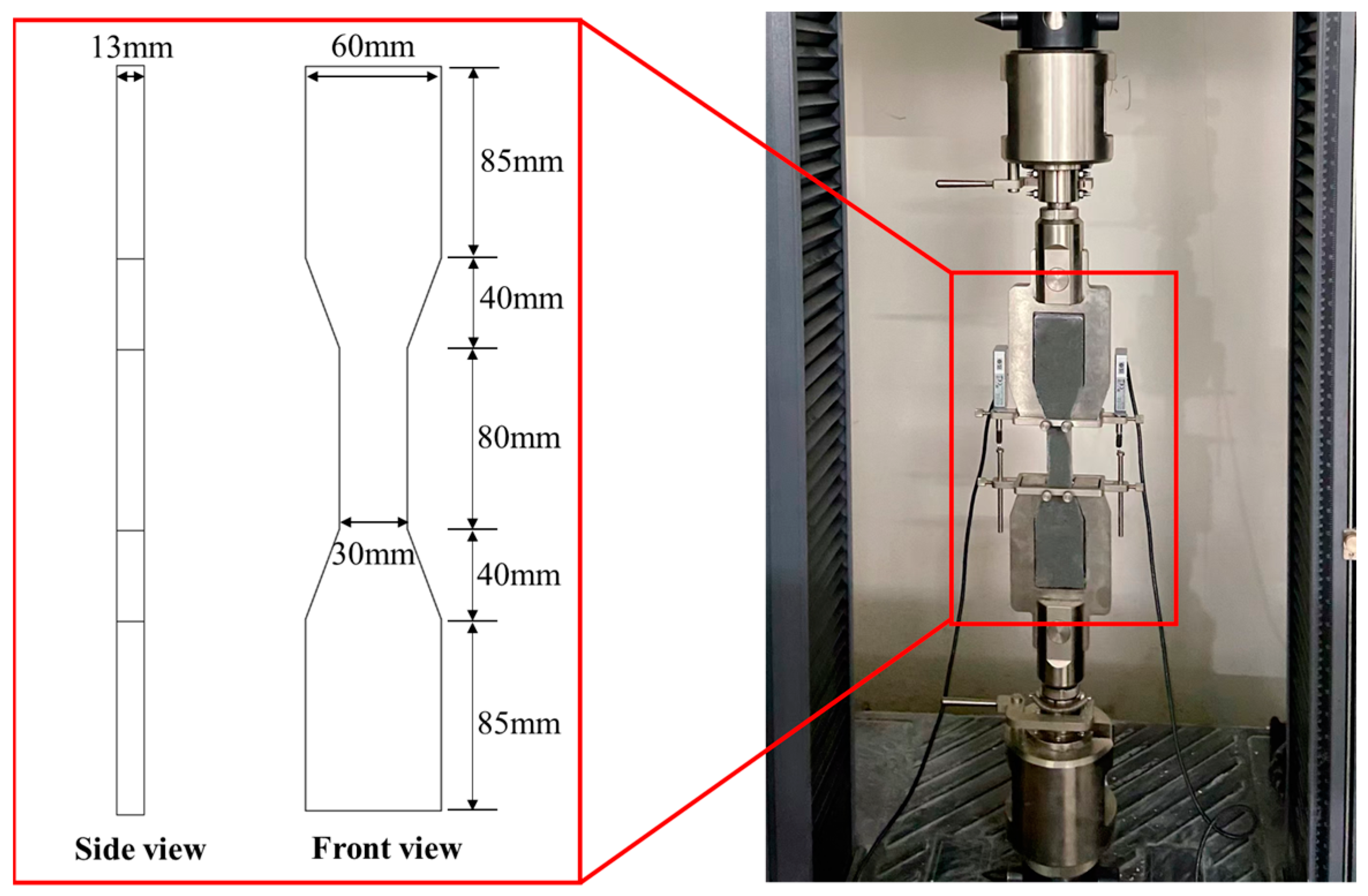
2. Materials and Methods
3. Test Results and Discussions
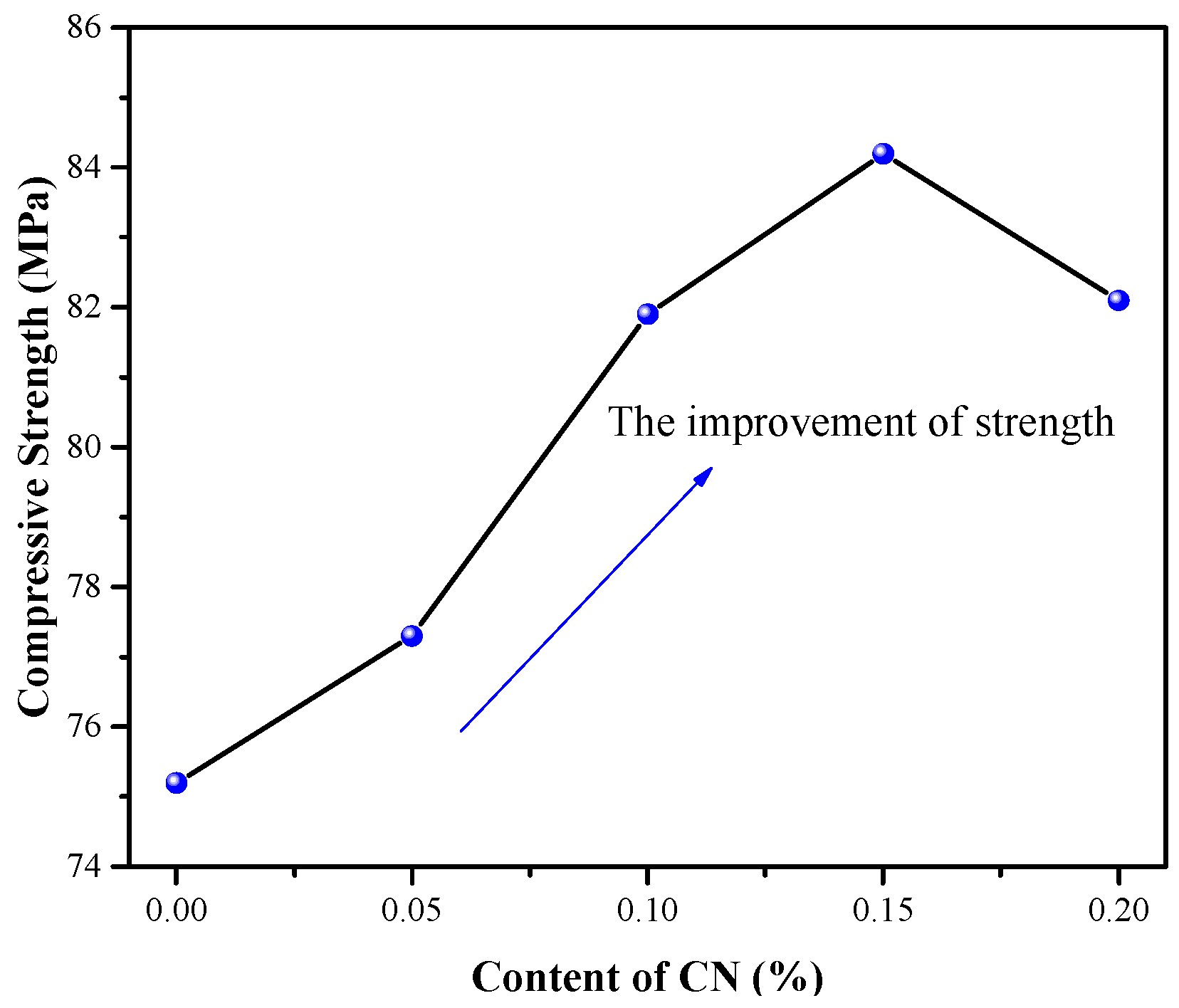
3.1. Effect of CN on Compressive Strength and Tensile Properties of ECC
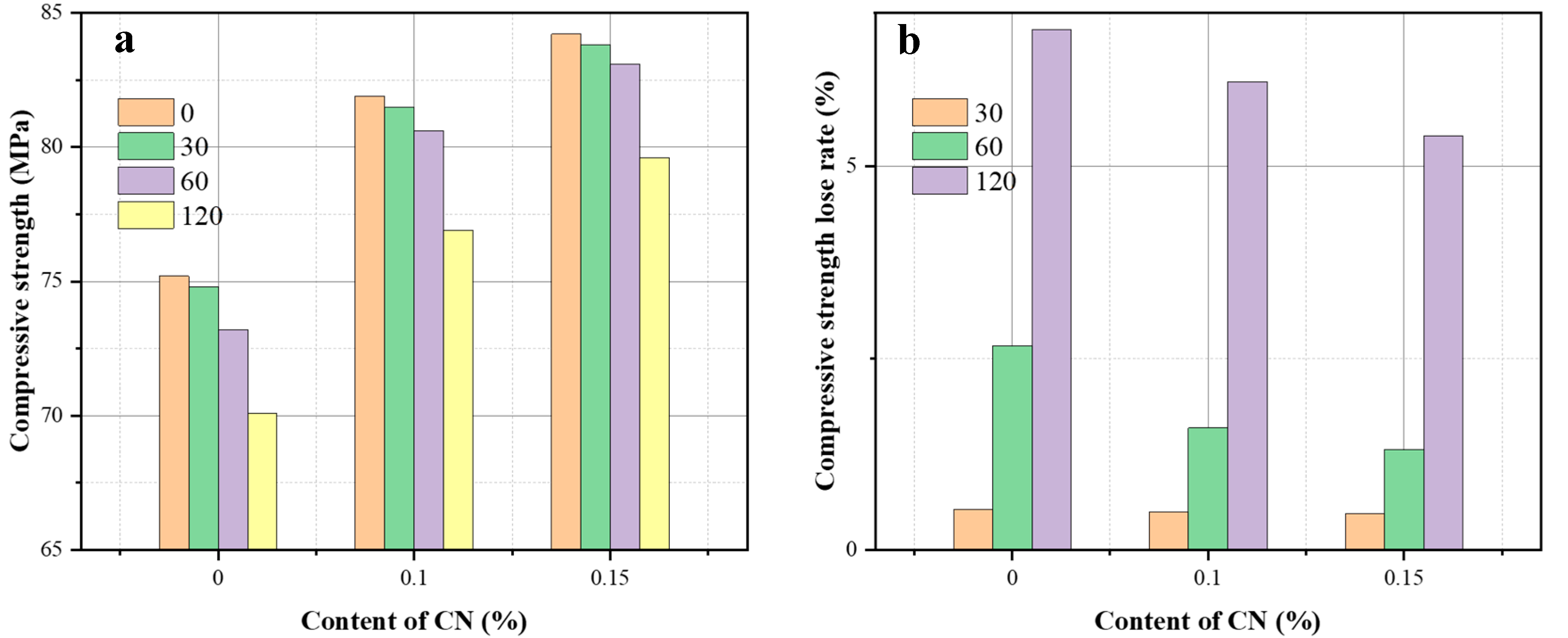
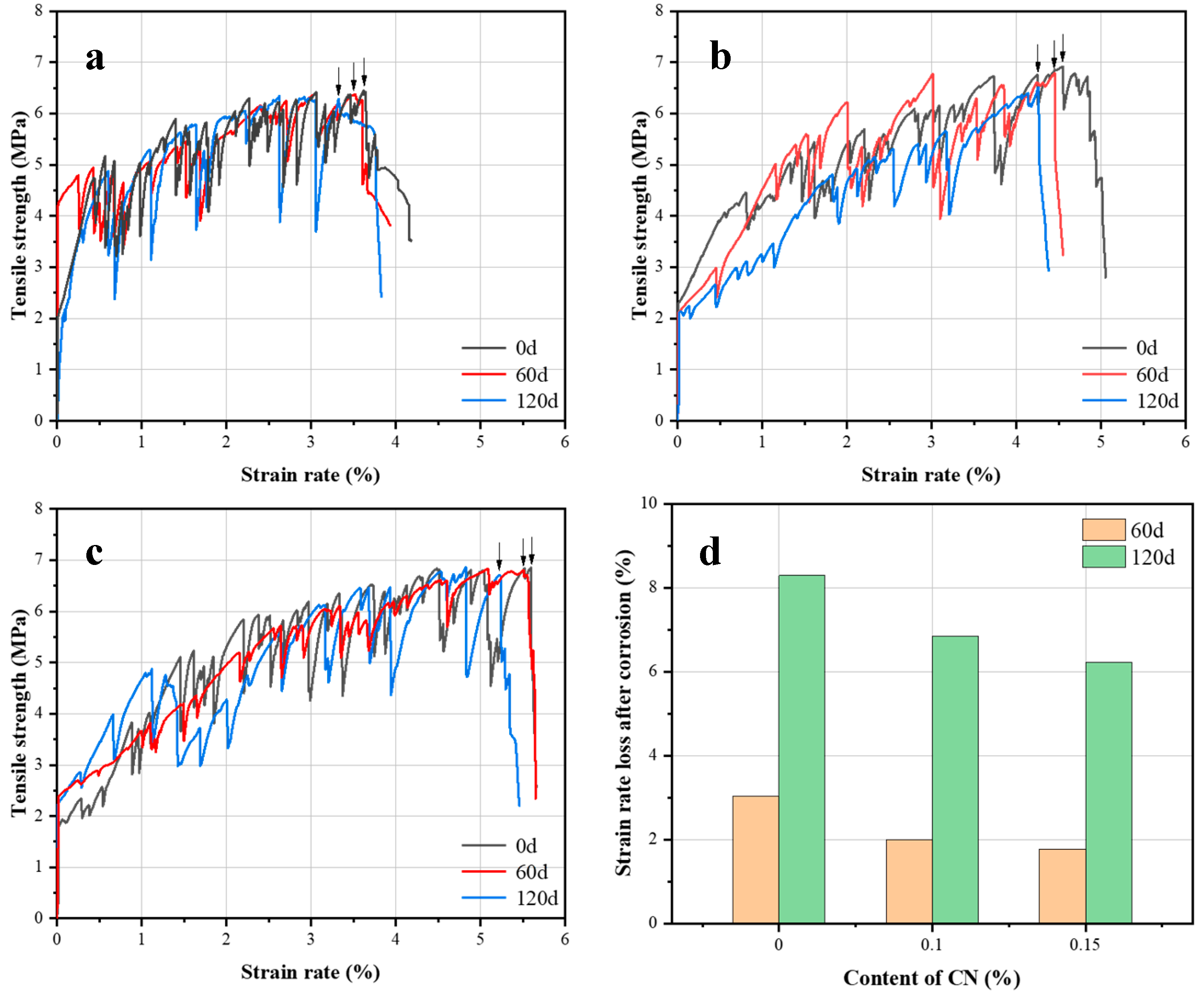
3.2. Effect of CN on Sulfate Corrosion Resistance of ECC
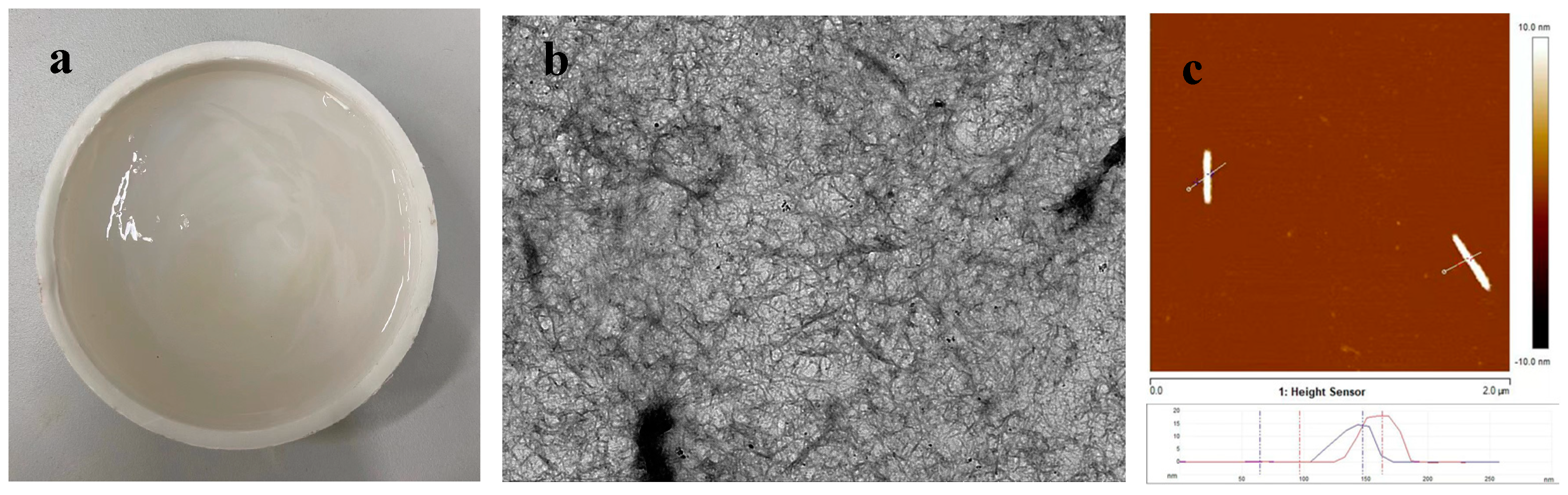
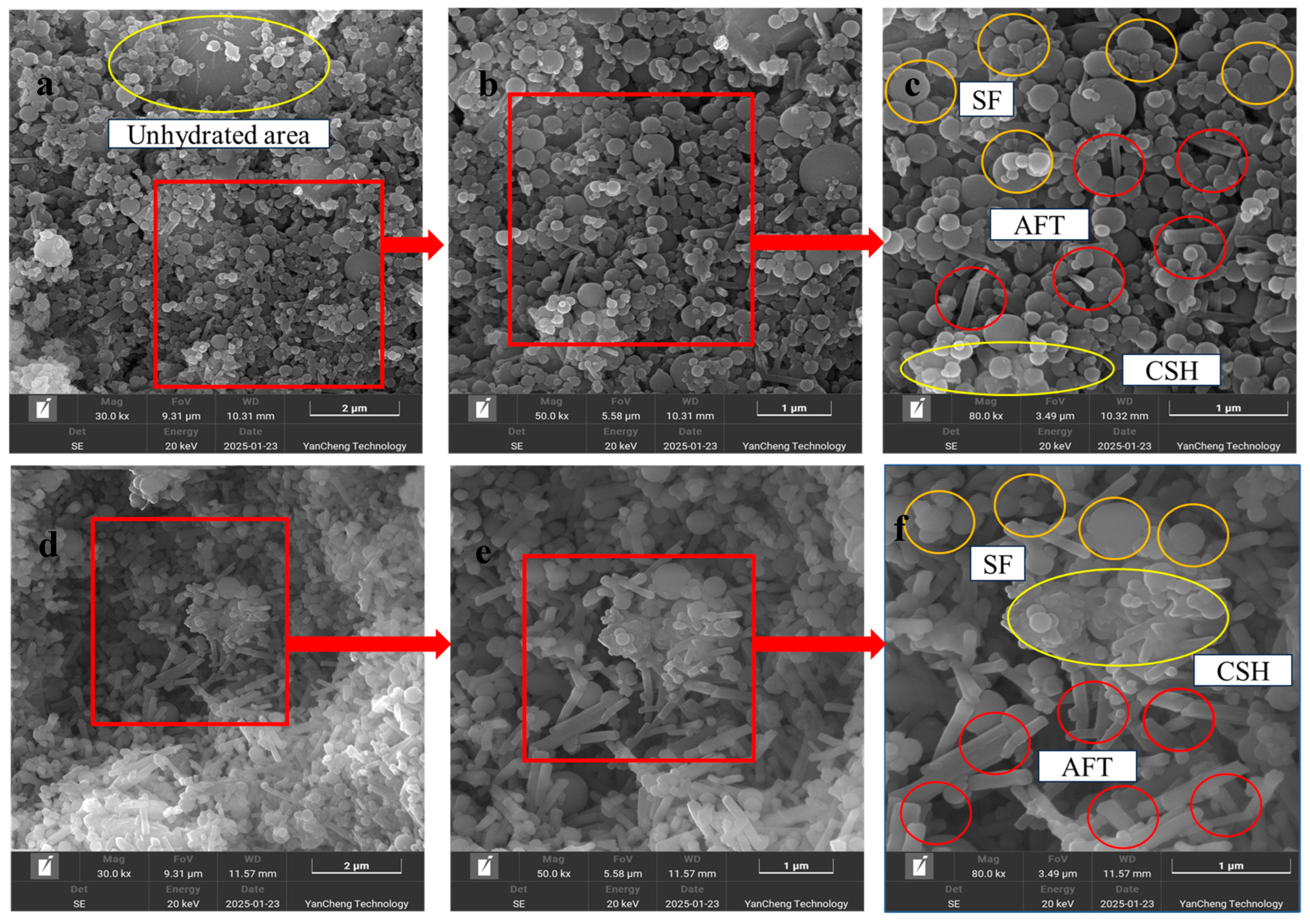
3.3. Micro-Morphology Inside the ECC Matrix
4. Molecular Dynamics Simulation
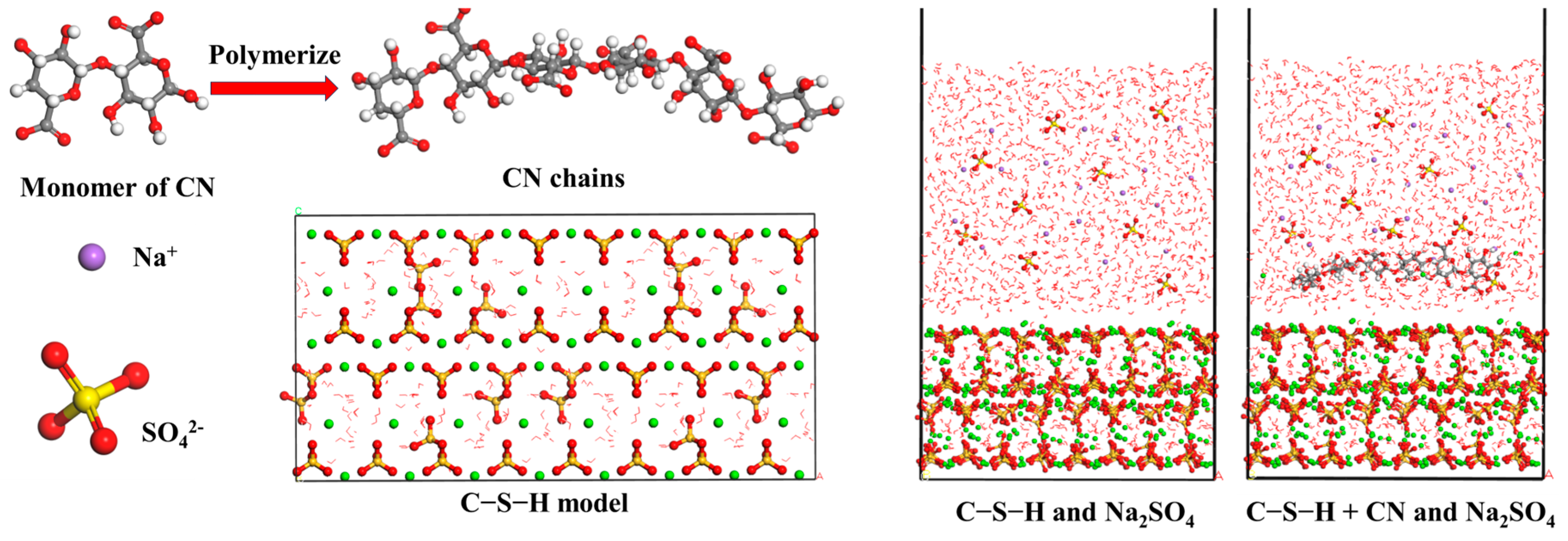
4.1. Modeling Details
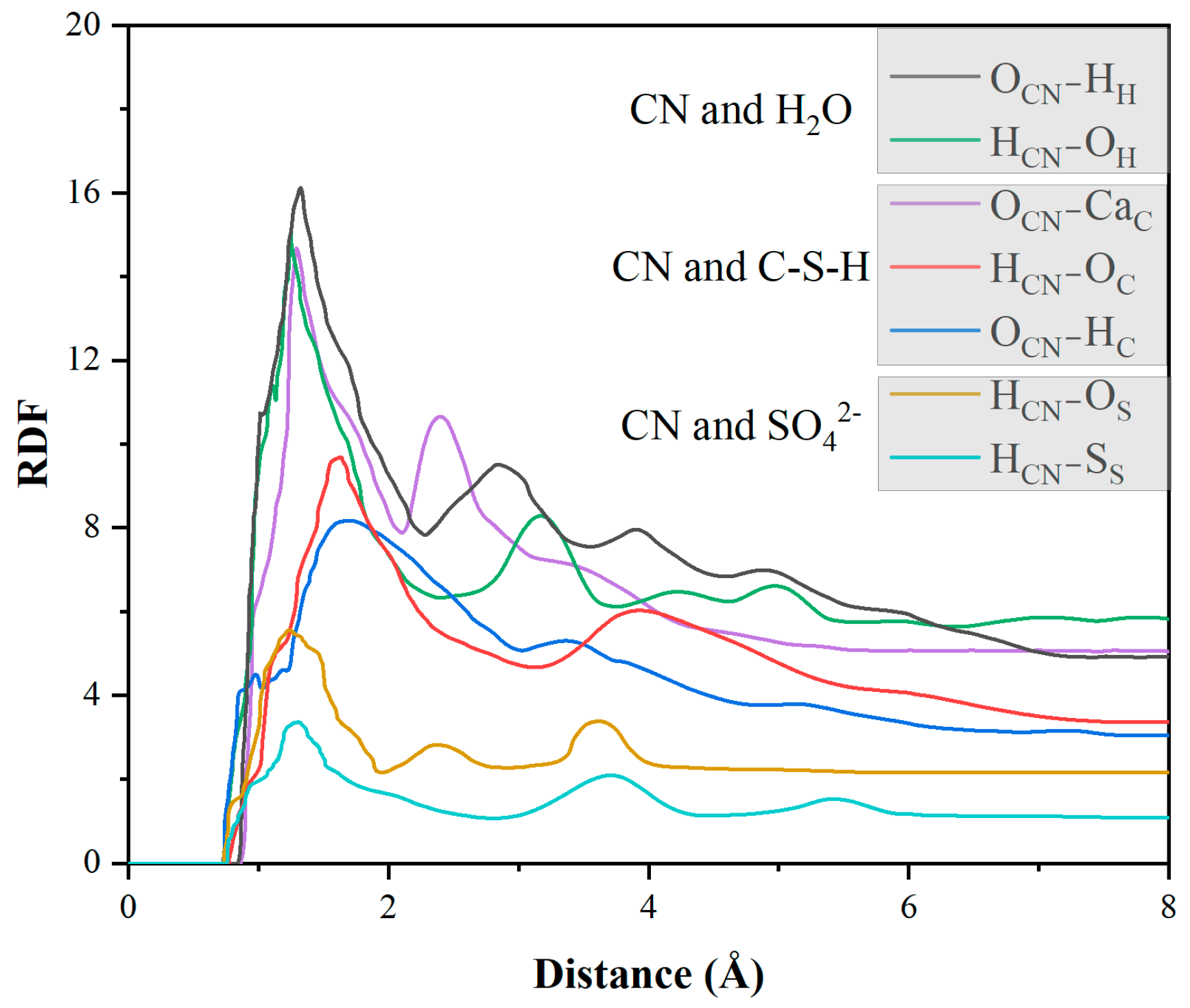
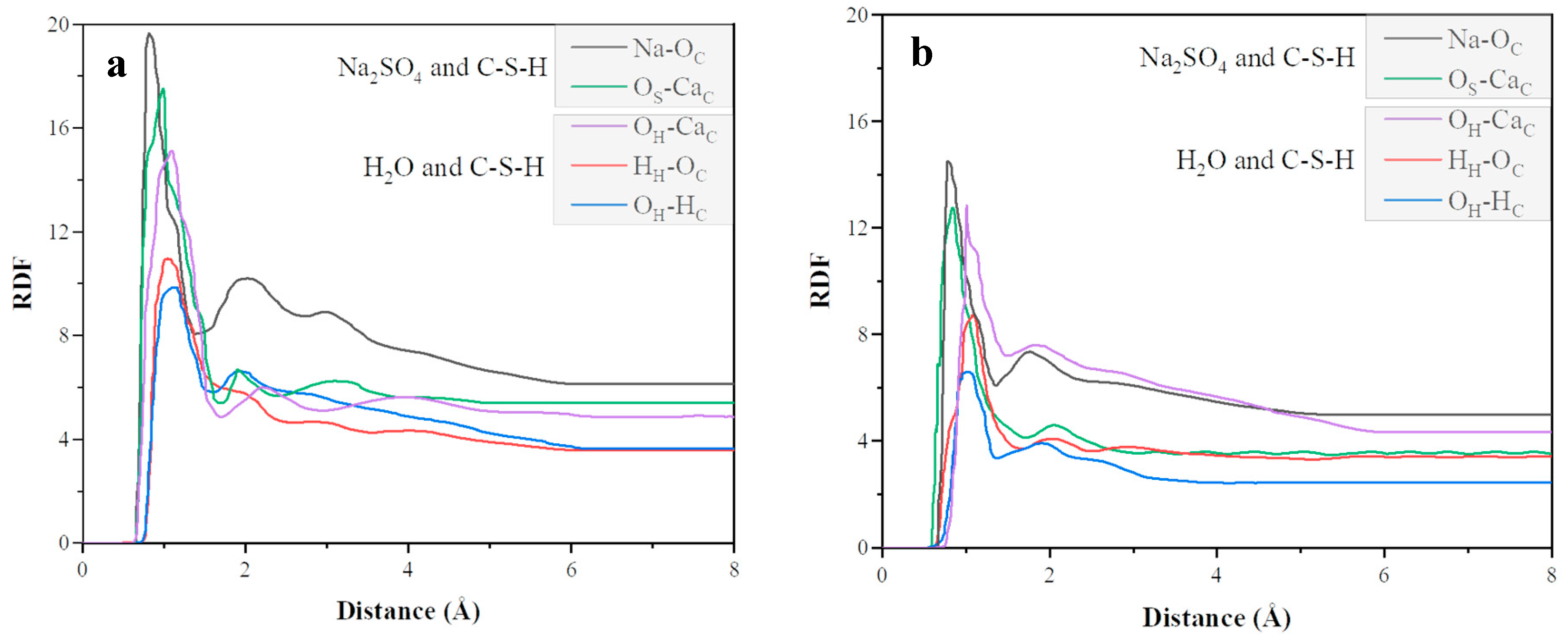
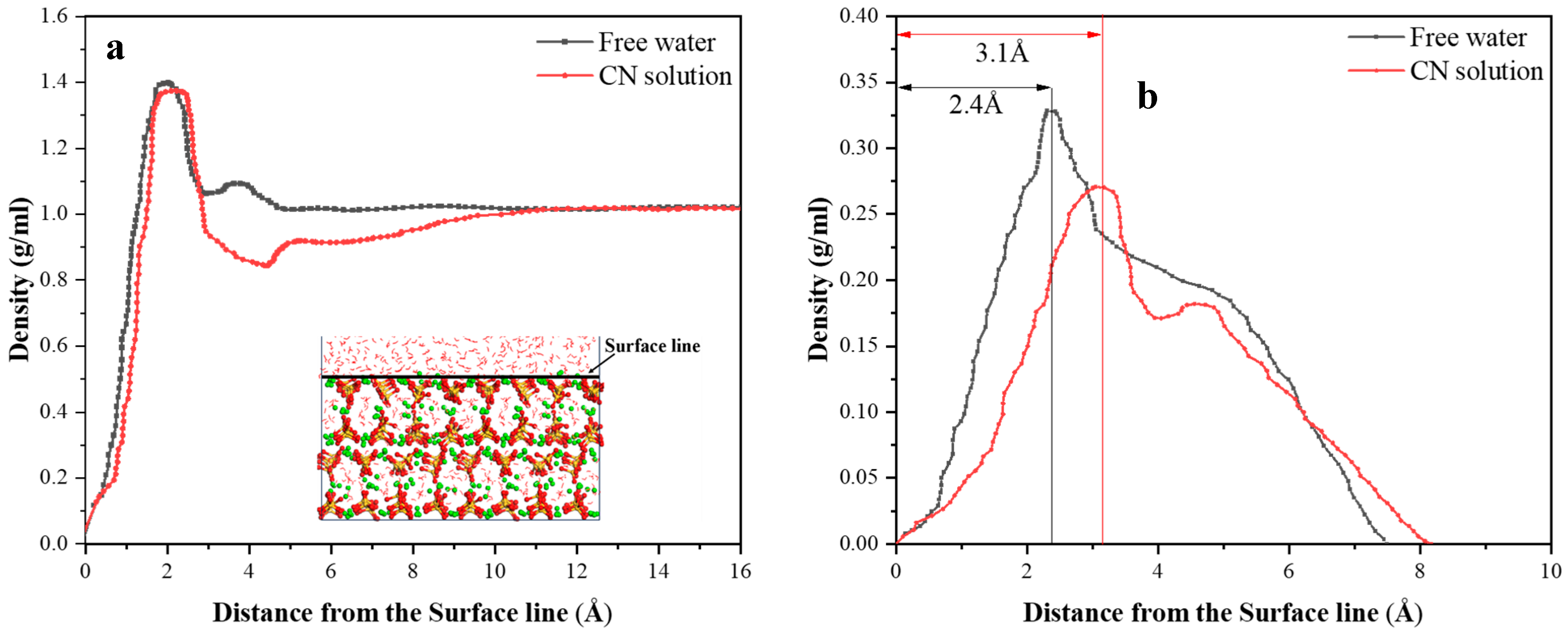
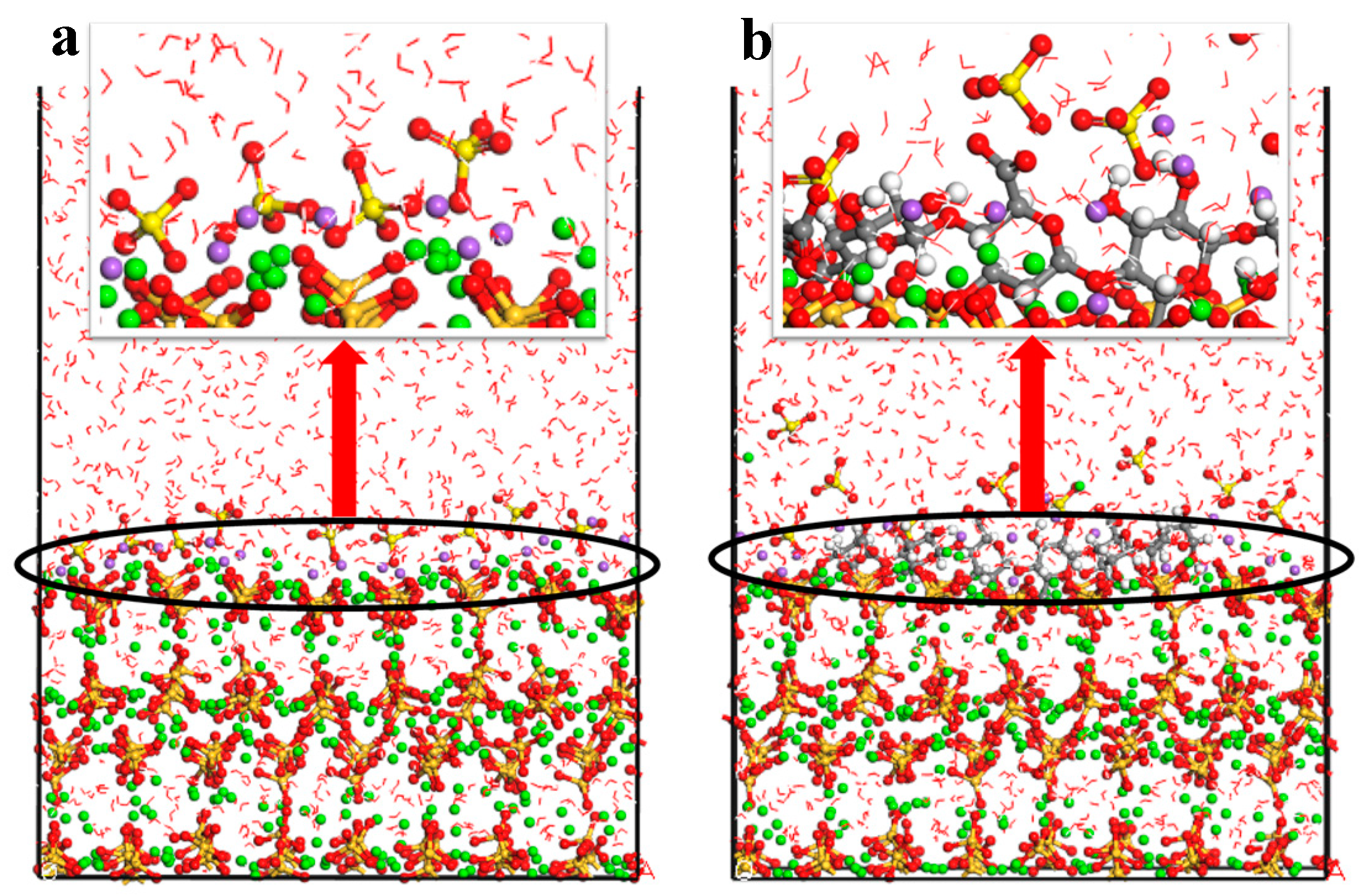
4.2. Adsorption Properties of CN and C-S-H Interfaces
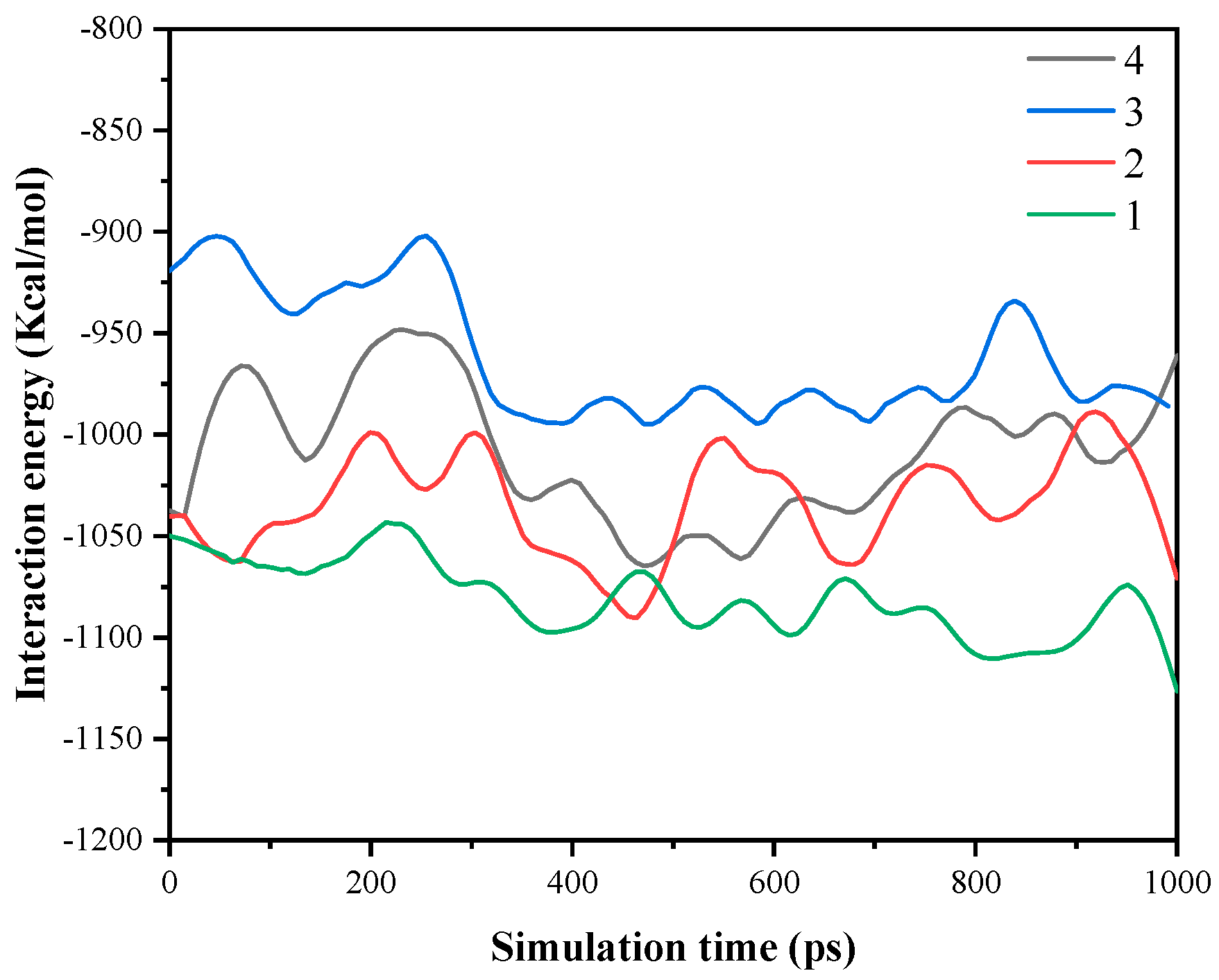
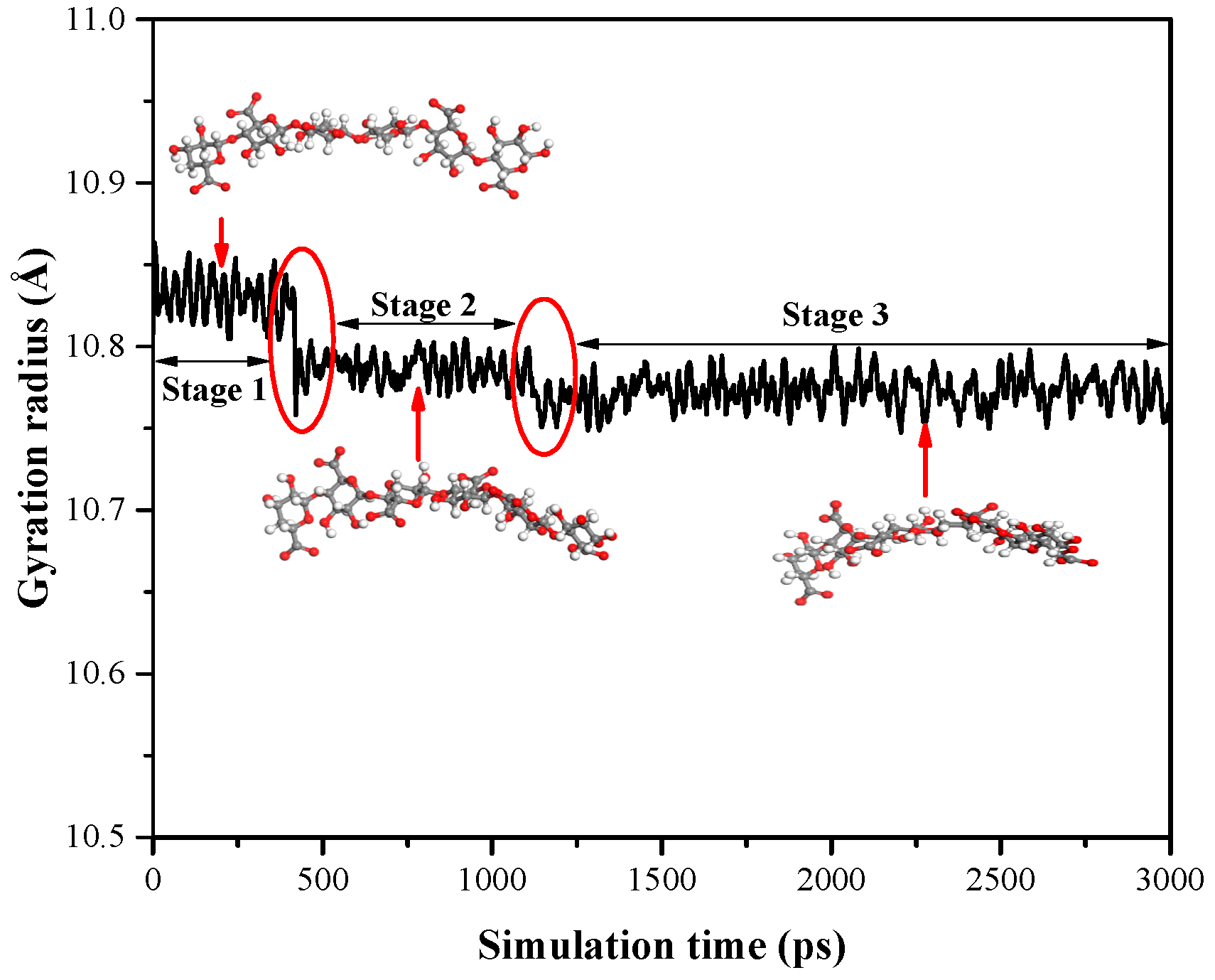
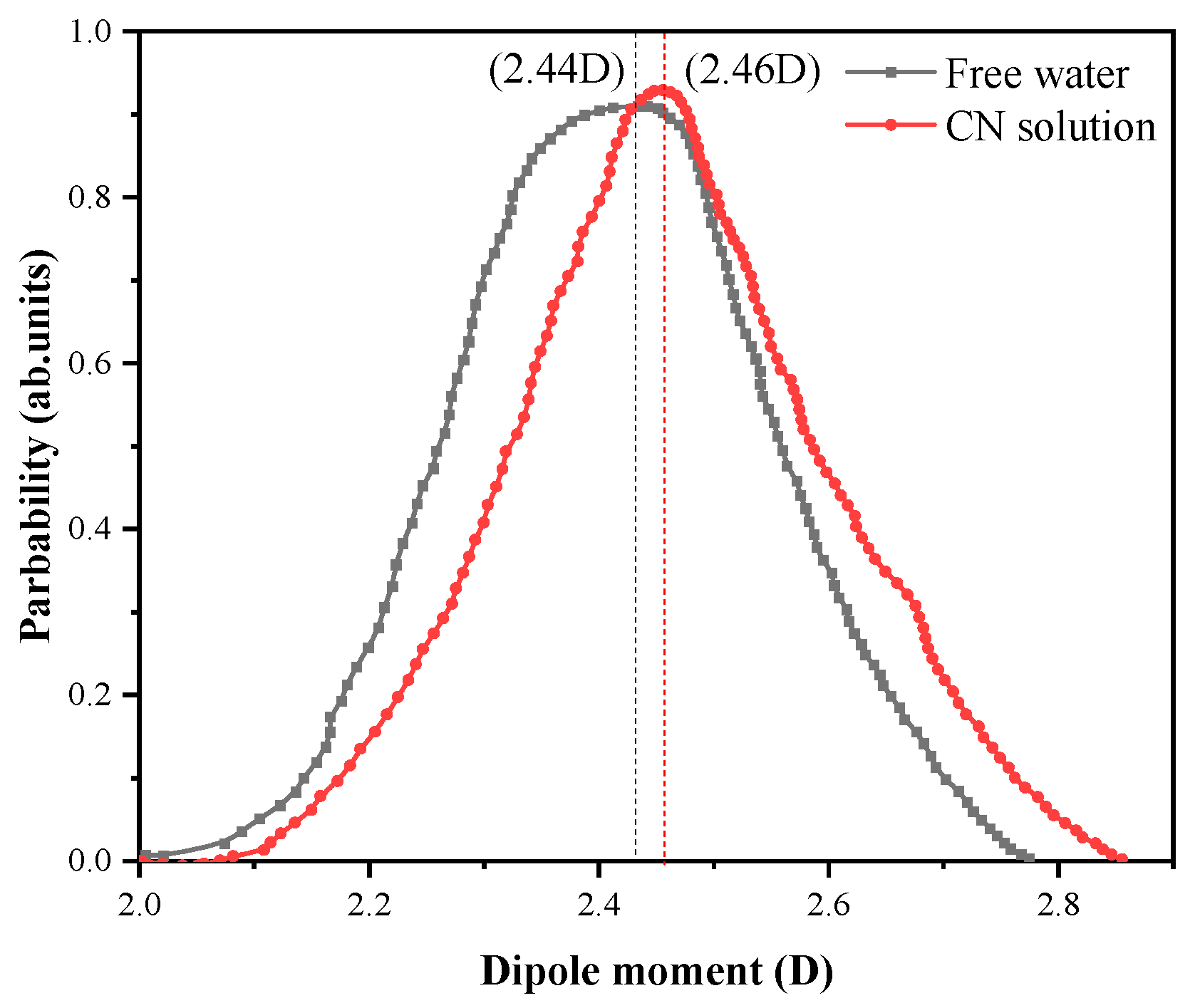
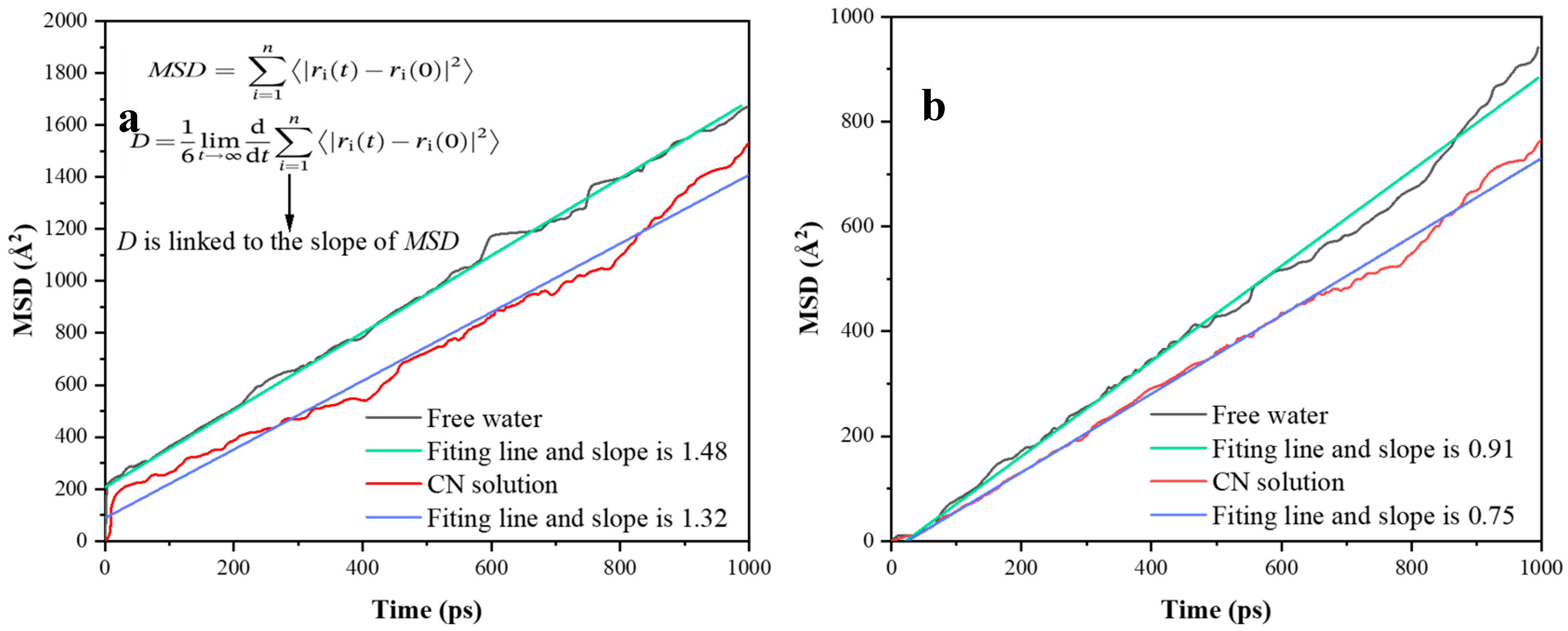
4.3. Kinetics at the Adsorption Interface
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, V.C.; Wang, S.; Wu, C. Tensile strain-hardening behavior of polyvinyl alcohol engineered cementitious composite (PVA-ECC). Mater. J. 2001, 98, 483–492. [Google Scholar]
- Li, V.C.; Bos, F.P.; Yu, K.Q.; McGee, W.; Ng, T.; Figueiredo, S. On the emergence of 3D printable engineered, strain hardening cementitious composites (ECC/SHCC). Cem. Concr. Res. 2020, 132, 106038. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, D.; Li, V.C. Centrifugally sprayed engineered cementitious composites: Rheology, mechanics, and structural retrofit for concrete pipes. Cem. Concr. Compos. 2022, 129, 104473. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, T.Y.; Wang, Y.C.; Li, V.C. Trenchless rehabilitation for concrete pipelines of water infrastructure: A review from the structural perspective. Cem. Concr. Compos. 2021, 123, 104193. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Y.X.; Leung, C.K.Y. Micromechanical modeling of crack-bridging relations of hybrid-fiber strain-hardening cementitious composites considering interaction between different fibers. Constr. Build. Mater. 2018, 182, 629–636. [Google Scholar] [CrossRef]
- Singh, S.B.; Munjal, P. Engineered cementitious composite and its applications. Mater. Today Proc. 2020, 32, 797–802. [Google Scholar] [CrossRef]
- Li, V.C. On engineered cementitious composites (ECC): A review of the material and its applications. J. Adv. Concr. Technol. 2003, 1, 215–230. [Google Scholar] [CrossRef]
- Zhu, J.X.; Weng, K.F.; Huang, B.T.; Xu, L.; Bai, J. Ultra-high-strength engineered cementitious composites (UHS-ECC) panel reinforced with FRP bar/grid: Development and flexural performance. Eng. Struct. 2024, 302, 117193. [Google Scholar] [CrossRef]
- Bonfim, W.B.; de Paula, H.M. Characterization of different biomass ashes as supplementary cementitious material to produce coating mortar. J. Clean. Prod. 2021, 291, 125869. [Google Scholar] [CrossRef]
- Zerihun, B.; Yehualaw, M.D.; Vo, D.H. Effect of agricultural crop wastes as partial replacement of cement in concrete production. Adv. Civ. Eng. 2022, 2022, 5648187. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, F.; Liu, J.C.; Wang, S. Eco-friendly high strength, high ductility engineered cementitious composites (ECC) with substitution of fly ash by rice husk ash. Cem. Concr. Res. 2020, 137, 106200. [Google Scholar] [CrossRef]
- Chen, H.; Yang, J. A new supplementary cementitious material: Walnut shell ash. Constr. Build. Mater. 2023, 408, 133852. [Google Scholar] [CrossRef]
- Camargo-Pérez, N.R.; Abellán-García, J.; Fuentes, L. Use of rice husk ash as a supplementary cementitious material in concrete mix for road pavements. J. Mater. Res. Technol. 2023, 25, 6167–6182. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.; Ji, Y.; She, W.; Yang, L.; Liu, G. A comparable study on the deterioration of limestone powder blended cement under sodium sulfate and magnesium sulfate attack at a low temperature. Constr. Build. Mater. 2020, 243, 118279. [Google Scholar] [CrossRef]
- O’Connell, M.; McNally, C.; Richardson, M.G. Performance of concrete incorporating GGBS in aggressive wastewater environments. Constr. Build. Mater. 2012, 27, 368–374. [Google Scholar] [CrossRef]
- Al-Dulaijan, S.U.; Maslehuddin, M.; Al-Zahrani, M.M.; Sharif, A.M.; Shameem, M.; Ibrahim, M. Sulfate resistance of plain and blended cements exposed to varying concentrations of sodium sulfate. Cem. Concr. Compos. 2003, 25, 429–437. [Google Scholar] [CrossRef]
- Sahmaran, M.; Li, V.C.; Andrade, C. Corrosion resistance performance of steel-reinforced engineered cementitious composite beams. ACI Mater. J. 2008, 105, 243. [Google Scholar]
- Abdulkadir, I.; Mohammed, B.S.; Liew, M.S.; Wahab, M.M.A. Modelling and optimization of the impact resistance of graphene oxide modified crumb rubber-ECC using response surface methodology. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; Volume 1197, p. 012043. [Google Scholar]
- Sabapathy, L.; Mohammed, B.S.; Al-Fakih, A.; Wahab, M.M.; Liew, M.S.; Amran, Y.H.M. Acid and sulphate attacks on a rubberized engineered cementitious composite containing graphene oxide. Materials 2020, 13, 3125. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, H.; Liu, J.; Xu, S.; Chen, Z. Experimental study on the salt freezing durability of multi-walled carbon nanotube ultra-high-performance concrete. Materials 2022, 15, 3188. [Google Scholar] [CrossRef]
- Mohseni, E.; Miyandehi, B.M.; Yang, J.; YangYazdi, M.A. Single and combined effects of nano-SiO2, nano-Al2O3 and nano-TiO2 on the mechanical, rheological and durability properties of self-compacting mor tar containing fly ash. Constr. Build. Mater. 2015, 84, 331–340. [Google Scholar] [CrossRef]
- Vanin, D.V.F.; Andrade, V.D.; Fiorentin, T.A.; Recouvreux, D.O.S.; Carminatti, C.A.; Al-Qureshi, H.A. Cement pastes modified by cellulose nanocrystals: A dynamic moduli evolution assessment by the impulse excitation technique. Mater. Chem. Phys. 2020, 239, 122038. [Google Scholar] [CrossRef]
- Zhou, S.; Qiu, Z.; Strømme, M.; Wang, Z. Highly crystalline PEDOT nanofiber templated by highly crystalline nanocellulose. Adv. Funct. Mater. 2020, 30, 2005757. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, S.K.; Kang, Y.; Kim, W. Fire resistance evaluation of fiber-reinforced cement composites using cellulose nanocrystals. Adv. Concr. Constr. 2019, 8, 311–320. [Google Scholar]
- Lee, H.J.; Kim, W. Long-term durability evaluation of fiber-reinforced ECC using wood-based cellulose nanocrystals. Constr. Build. Mater. 2020, 238, 117754. [Google Scholar] [CrossRef]
- Cao, Y.; Tian, N.; Bahr, D.; Zavattieri, P.D.; Youngblood, J.; Moon, R.J.; Weiss, J. The influence of cellulose nanocrystals on the microstructure of cement paste. Cem. Concr. Compos. 2016, 74, 164–173. [Google Scholar] [CrossRef]
- Lu, M.; Zheng, Y.Y.; Yin, Z.Y. Investigating the consolidation of kaolinite with Molecular Dynamics: The micro effective stress principle. Appl. Surf. Sci. 2025, 690, 162653. [Google Scholar] [CrossRef]
- Lu, M.; Zheng, Y.Y.; Yin, Z.Y. From sedimentation to consolidation of kaolinite: A molecular dynamic study. Comput. Geotech. 2024, 170, 106285. [Google Scholar] [CrossRef]
- Lu, M.; Zheng, Y.Y.; Yin, Z.Y. A molecular dynamics study on the softening of kaolinite in water: Weakening of tensile property during stretching and disintegration of structure during soaking. Comput. Geotech. 2024, 173, 106562. [Google Scholar] [CrossRef]
- Lu, M.; Diao, Q.F.; Zheng, Y.Y.; Yin, Z.Y.; Dai, Z. Influence of Water on Tensile Behavior of Illite through the Molecular Dynamics Method. Int. J. Geomech. 2024, 24, 04024024. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Yin, R.; Yao, J.; Zhao, B. Experimental and molecular dynamic study on the interfacial strengthening mechanism of PE fiber/cement mortar using advanced oxidation processes. Constr. Build. Mater. 2021, 309, 125144. [Google Scholar] [CrossRef]
- Wang, P.; Qiao, G.; Guo, Y.; Zhang, Y.; Jin, Z.; Zhang, J. Molecular dynamics simulation of the interfacial bonding properties between graphene oxide and calcium silicate hydrate. Constr. Build. Mater. 2020, 260, 119927. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Lin, H.; Huang, G.; Fan, Q.; Meng, D. Calculation of contact angle via Young-Dupré equation with Molecular Dynamic Simulation: Kaolinite as an example. Colloids Surf. A Physicochem. Eng. Asp. 2024, 697, 134469. [Google Scholar] [CrossRef]
- GB/T50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. Standerdization Administration of the People’s Republic of China: Beijing, China, 2009.
- Fan, Q.; Zheng, Y.; Meng, D.; Guo, Q.; Liu, Y.; Wu, H. Study on improving the performance of Engineered Cement-Based Composites by modifying binder system and polyethylene fiber/matrix interface. Colloids Surf. A Physicochem. Eng. Asp. 2025, 707, 135862. [Google Scholar] [CrossRef]
- Fan, Q.; Zheng, Y.; Liu, Y.; Liu, Y.; Meng, X.; Guo, H.; Wang, Z. Effect of modified cellulose nanocrystals on the structure of calcium silicate hydrate studied by molecular dynamics simulation and experiment. Langmuir 2023, 39, 16244–16260. [Google Scholar] [CrossRef]
- Cao, Y.; Zavaterri, P.; Youngblood, J.; Moon, R.; Weiss, J. The influence of cellulose nanocrystal additions on the performance of cement paste. Cem. Concr. Compos. 2015, 56, 73–83. [Google Scholar] [CrossRef]
- Fan, Q.C.; Meng, X.; Li, Z.D.; Ma, G.Y.; Wang, Z.P.; Zhang, K.; Meng, D. Experiment and molecular dynamics simulation of functionalized cellulose nanocrystals as reinforcement in cement composites. Constr. Build. Mater. 2022, 341, 127879. [Google Scholar] [CrossRef]
- Flores, J.; Kamali, M.; Ghahremaninezhad, A. An Investigation into the Properties and Microstructure of Cement Mixtures Modified with Cellulose Nanocrystal. Materials 2017, 10, 498. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Montes, F.; Suraneni, P.; Youngblood, J.; Weiss, J. The Influence of Cellulose Nanocrystals on the Hydration and Flexural Strength of Portland Cement Pastes. Polymers 2017, 9, 424. [Google Scholar] [CrossRef]
- Zheng, D.; Yang, H.; Feng, W.; Fang, Y.; Cui, H. Modification mechanism of cellulose nanocrystals in cement. Cem. Concr. Res. 2023, 165, 107089. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y.; Zhou, Y.; Zhang, W.; Li, W.; She, W.; Liu, J.; Miao, C. Multi-layered cement-hydrogel composite with high toughness, low thermal conductivity, and self-healing capability. Nat. Commun. 2023, 14, 3438. [Google Scholar] [CrossRef]
- Wang, P.; Duan, Y.; Zheng, H.; Chen, Z.; Wang, M.; Wang, X.; Li, H.; Hou, D. Molecular structure and dynamics of water on the surface of cement hydration products: Wetting behavior at nanoscale. Appl. Surf. Sci. 2023, 611, 155713. [Google Scholar] [CrossRef]
- Ramasamy, J.; Amanullah, M. Nanocellulose for oil and gas field drilling and cementing applications. J. Pet. Sci. Eng. 2020, 184, 106292. [Google Scholar] [CrossRef]
- Liu, K.; Cheng, X.; Ma, Y.; Gao, X.; Zhang, C.; Li, Z.; Zhuang, J. Analysis of interfacial nanostructure and interaction mechanisms between cellulose fibres and calcium silicate hydrates using experimental and molecular dynamics simulation data. Appl. Surf. Sci. 2020, 506, 144914. [Google Scholar] [CrossRef]
- Fan, Q.; Zheng, Y.; Yang, Y.; Liu, S.; Meng, D.; Guo, Q.; Liu, Y. Effect of interface properties between functionalized cellulose nanocrystals and tricalcium silicate on the early hydration mechanism of cement. Colloids Surf. A Physicochem. Eng. Asp. 2024, 698, 134552. [Google Scholar] [CrossRef]
- Lu, Z.; Yu, J.; Yao, J.; Hou, D. Experimental and molecular modeling of polyethylene fiber/cement interface strengthened by graphene oxide. Cem. Concr. Compos. 2020, 112, 103676. [Google Scholar] [CrossRef]
- Hou, D.; Lu, Z.; Li, X.; Ma, H.; Li, Z. Reactive molecular dynamics and experimental study of graphene-cement composites: Structure, dynamics and reinforcement mechanisms. Carbon 2017, 115, 188–208. [Google Scholar] [CrossRef]
- Lv, C.; Xue, Q.; Xia, D.; Ma, M.; Xie, J. Effect of chemisorption structure on the interfacial bonding characteristics of graphene-polymer composites. Appl. Surf. Sci. 2012, 258, 2077–2082. [Google Scholar] [CrossRef]
- Fan, Q.; Wang, Z.; Meng, X.; Zhang, K.; Ma, G.; Li, Z.; Meng, D. Multi-scale analysis of the strengthening mechanism of functionalized graphene as reinforcement in cement composites. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129729. [Google Scholar] [CrossRef]
- Fan, Q.; Liu, Y.; Zheng, Y.; Meng, D.; Guo, Q.; Zheng, H.; Guo, B.; Liao, W. The microscopic reinforcement mechanism of Zhuhai soft soil by cement-based stabilizer: From microscopic characterization to molecular dynamics simulation. Appl. Surf. Sci. 2025, 681, 161574. [Google Scholar] [CrossRef]
- Fan, Q.; Zheng, Y.; He, C.; Meng, D.; Guo, Q.; Liu, Y. Effect of interfacial properties between polyethylene and polyvinyl alcohol fiber/cement matrix on properties of mortar and ECC. Struct. Concr. 2024, 1–16. [Google Scholar] [CrossRef]

| Binder (100%) | PE (vol) | Water | PCE | Sand | ||
|---|---|---|---|---|---|---|
| Cement | Silica Fume | Fly Ash | ||||
| 70 | 20 | 10 | 2% | 22 | 2 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Xu, X.; Ni, S.; Meng, D.; Meng, X.; Xu, B. Experimental and Molecular Dynamics Simulation Study on Sulfate Corrosion Resistance of Cellulose-Nanocrystal-Modified ECC. Appl. Sci. 2025, 15, 3205. https://doi.org/10.3390/app15063205
Yu L, Xu X, Ni S, Meng D, Meng X, Xu B. Experimental and Molecular Dynamics Simulation Study on Sulfate Corrosion Resistance of Cellulose-Nanocrystal-Modified ECC. Applied Sciences. 2025; 15(6):3205. https://doi.org/10.3390/app15063205
Chicago/Turabian StyleYu, Lei, Xiaolong Xu, Songyuan Ni, Dan Meng, Xue Meng, and Binghua Xu. 2025. "Experimental and Molecular Dynamics Simulation Study on Sulfate Corrosion Resistance of Cellulose-Nanocrystal-Modified ECC" Applied Sciences 15, no. 6: 3205. https://doi.org/10.3390/app15063205
APA StyleYu, L., Xu, X., Ni, S., Meng, D., Meng, X., & Xu, B. (2025). Experimental and Molecular Dynamics Simulation Study on Sulfate Corrosion Resistance of Cellulose-Nanocrystal-Modified ECC. Applied Sciences, 15(6), 3205. https://doi.org/10.3390/app15063205




