Seasonal Pollution Levels and Heavy Metal Contamination in the Jukskei River, South Africa
Abstract
1. Introduction
2. Methodology
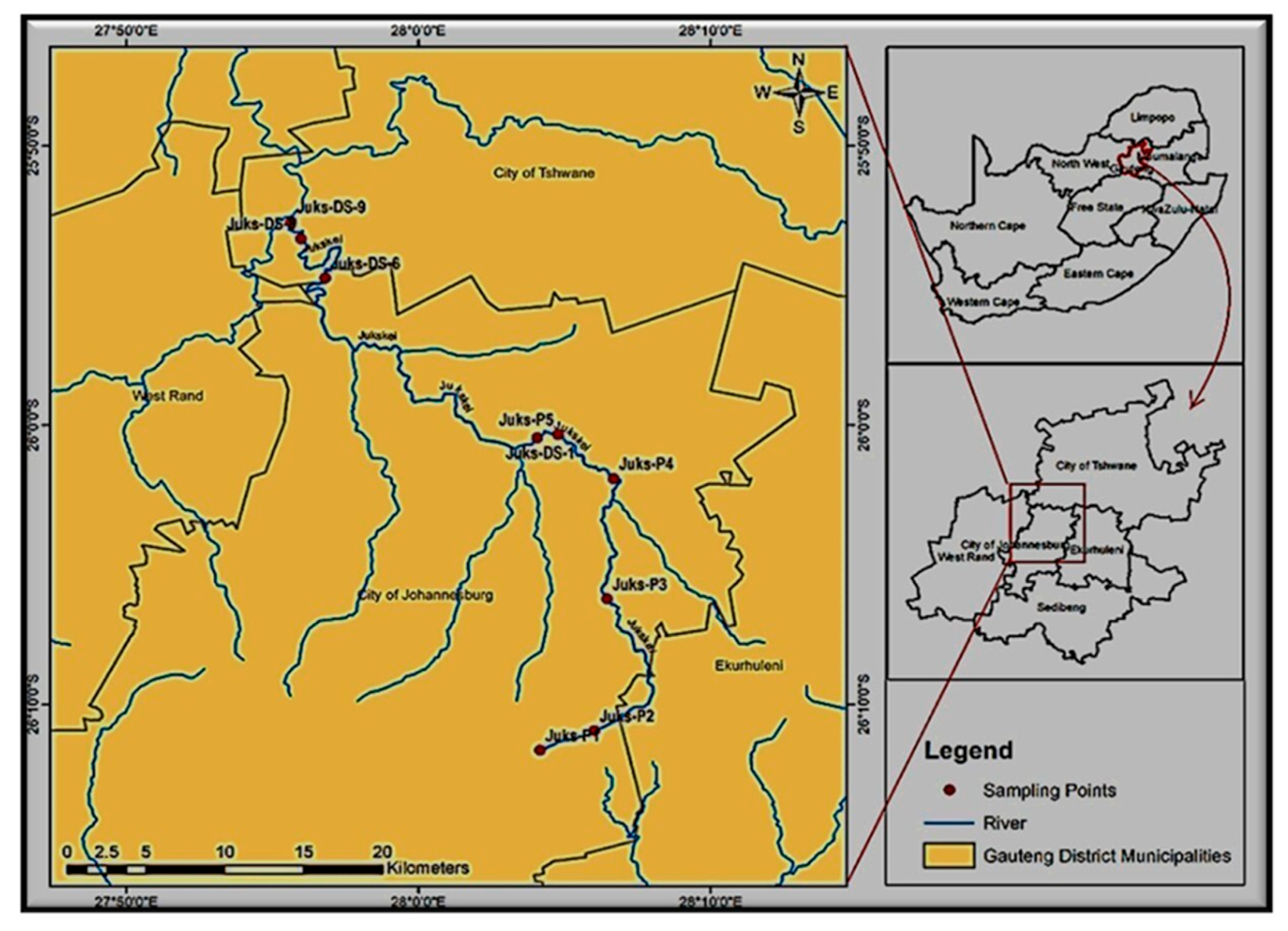
2.1. Study Area and Sampling Campaign
2.2. Reagents and Instrumentation
2.3. Sediments and Water Samples Preparation
2.4. Sediment Quality Assessment
2.5. Principal Component Analysis
3. Results and Discussion
3.1. Dry and Wet Season Concentrations of the Heavy Metals
3.2. Sediment and Water Quality Assessment Using Pollution Indices
3.3. Pearson Correlation Matrix
3.4. Source Apportionment Using of PCA
4. Recommendations
4.1. Proposed Regulations to Stem River Pollution
- Agricultural best management practices;
- Effluent discharge permits and inspection;
- Stormwater runoff regulations.
4.2. Recommendation for Pollution Control Strategies
- Pollution control education and awareness;
- Educating people on environmental issues and ways to lessen their effects. This may result in better-informed choices and environmental protection measures;
- Monitoring river pollution;
- Continuous river quality monitoring is crucial for identifying pollution hotspots and pollution origin;
- Compliance monitoring and legal responsibility;
- Government must enforce compliance and impose strict fines and imprisonment.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campos, C.J.A.; Alves, M.T.; Walker, D.I. Long term reductions of faecal indicator organisms in Chichester Harbour (England) following sewerage infrastructure improvements in the catchment. Sci. Total Environ. 2020, 733, 139061. [Google Scholar] [CrossRef] [PubMed]
- Karaouzas, I.; Smeti, E.; Vourka, A.; Vardakas, L.; Mentzafou, A.; Tornés, E.; Sabater, S.; Muñoz, I.; Skoulikidis, N.T.; Kalogianni, E. Assessing the ecological effects of water stress and pollution in a temporary river—Implications for water management. Sci. Total Environ. 2018, 618, 1591–1604. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K. Fresh Water availability and It’s Global challenge. J. Mar. Sci. Res. 2023, 2, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, S.; Tang, L.; Pan, X.; Pu, X.; Li, R.; Shen, C. Contribution of heavy metal in driving microbial distribution in a eutrophic river. Sci. Total Environ. 2020, 712, 136295. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.P.S. Water microbiology. Bacterial pathogens and water. Int. J. Environ. Res. Public Health 2010, 7, 3657–3703. [Google Scholar] [CrossRef]
- U.S. Department of the Navy. 3.2 Sediments and Water Quality; Training and Testing Draft EIS/OEIS; U.S. Department of the Navy: Hawaii, CA, USA, 2017. [Google Scholar]
- Bao, Q.; Liu, C.; Friese, K.; Dadi, T.; Yu, J.; Fan, C.; Shen, Q. Understanding the Heavy Metal Pollution Pattern in Sediments of a Typical Small- and Medium-Sized Reservoir in China. Int. J. Environ. Res. Public Health 2023, 20, 708. [Google Scholar] [CrossRef]
- Tundu, C.; Tumbare, M.J.; Onema, J.M.K. Sedimentation and its impacts/effects on river system and reservoir water quality: Case study of Mazowe catchment, Zimbabwe. Proc. Int. Assoc. Hydrol. Sci. 2018, 377, 57–66. [Google Scholar] [CrossRef]
- Buscaroli, A.; Zannoni, D.; Dinelli, E. Spatial distribution of elements in near surface sediments as a consequence of sediment origin and anthropogenic activities in a coastal area in northern Italy. Catena 2021, 196, 104842. [Google Scholar] [CrossRef]
- Osaro, I.L. Turbulent Suspension and Sediment Grains Transport in Natural Flows. Ph.D. Thesis, Royal Holloway, University of London, Egham, UK, August 2018. [Google Scholar]
- Letsoalo, M.R.; Mamo, M.A.; Ambushe, A.A. Synchronous Extraction and Quantitative Speciation of Arsenic and Chromium in Sediments by High-Performance Liquid Chromatography–Inductively Coupled Plasma–Mass Spectrometry (HPLC-ICP-MS). Anal. Lett. 2021, 54, 1943–1967. [Google Scholar] [CrossRef]
- Letsoalo, M.R.; Ambushe, A.A.; Mamo, M.A. Novel Chemoresistive Sensor for Sensitive Detection of Pb2+ Ions Using an Interdigital Gold Electrode Fabricated with a Reduced Graphene Oxide-Based Ion-Imprinted Polymer. ACS Omega 2021, 6, 31528–31538. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Mustafa, F.S.; Omer, K.M.; Hama, S.; Hamarawf, R.F.; Rahman, K.O. Heavy metal pollution in the aquatic environment: Efficient and low-cost removal approaches to eliminate their toxicity: A review. RSC Adv. 2023, 13, 17595–17610. [Google Scholar] [CrossRef] [PubMed]
- Biancacci, C.; Sanderson, J.C.; Evans, B.; Callahan, D.L.; Francis, D.S.; Skrzypczyk, V.M.; Cumming, E.E.; Bellgrove, A. Nutritional composition and heavy metal profiling of Australian kelps cultured in proximity to salmon and mussel farms. Algal Res. 2022, 64, 102672. [Google Scholar] [CrossRef]
- Oloruntoba, A.; Omoniyi, A.O.; Shittu, Z.A.; Ajala, R.O.; Kolawole, S.A. Heavy Metal Contamination in Soils, Water, and Food in Nigeria from 2000–2019: A Systematic Review on Methods, Pollution Level and Policy Implications. Water Air Soil Pollut. 2024, 235, 586. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Molecular, clinical and environmental toxicicology Volume 3: Environmental Toxicology. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud. Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Zainurin, S.N.; Wan Ismail, W.Z.; Mahamud, S.N.; Ismail, I.; Jamaludin, J.; Ariffin, K.N.; Wan Ahmad Kamil, W.M. Advancements in Monitoring Water Quality Based on Various Sensing Methods: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 14080. [Google Scholar] [CrossRef]
- Muñoz-Arcos, E.; Millward, G.E.; Clason, C.C.; Bravo-Linares, C.; Blake, W.H. Understanding the complexity of sediment residence time in rivers: Application of Fallout Radionuclides (FRNs). Earth Sci. Rev. 2022, 233, 104188. [Google Scholar] [CrossRef]
- Mukwevho, N.; Ntsasa, N.; Mkhohlakali, A.; Mabowa, M.H.; Chimuka, L.; Tshilongo, J.; Letsoalo, M.R. The Impact of Induced Industrial and Urban Toxic Elements on Sediment Quality. Water 2024, 16, 2485. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, R.; Satapathy, S.C.; Al-Ansari, N.; Singh, K.K.; Mahapatra, R.P.; Agarwal, A.K.; Le, H.V.; Pham, B.T. Analysis of Water Pollution Using Different Physicochemical Parameters: A Study of Yamuna River. Front. Environ. Sci. 2020, 8, 581591. [Google Scholar] [CrossRef]
- Jollife, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Varekar, V.; Karmakar, S.; Jha, R. Seasonal rationalization of river water quality sampling locations: A comparative study of the modified Sanders and multivariate statistical approaches. Environ. Sci. Pollut. Res. 2016, 23, 2308–2328. [Google Scholar] [CrossRef]
- Rimayi, C.; Chimuka, L.; Odusanya, D.; de Boer, J.; Weiss, J. Distribution of 2,3,7,8-substituted polychlorinated dibenzo-p-dioxin and polychlorinated dibenzofurans in the Jukskei and Klip/Vaal catchment areas in South Africa. Chemosphere 2016, 145, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Mukwevho, N.; Ntsasa, N.; Mkhohlakali, A.; Chimuka, L.; Tshilongo, J.; Mokgosi, D.; Mabowa, H.; Letsoalo, M.R. Examining the Effect of Induced Industrial and Urban Toxic Elements on Sediment Quality. In Recent Developments in Chemistry and Biochemistry Research Vol. 11; BP International: Tokyo, Japan, 2025; pp. 29–45. [Google Scholar] [CrossRef]
- Allion, K.; Kiemle, L.; Fuchs, S. Four Years of Sediment and Phosphorus Monitoring in the Kraichbach River Using Large-Volume Samplers. Water 2022, 14, 120. [Google Scholar] [CrossRef]
- Letsoalo, M.R.; Mamo, M.A.; Ambushe, A.A. Simultaneous quantitative speciation of selected toxic elements in water using high performance liquid chromatography coupled to inductively coupled plasma-mass spectrometry (HPLC-ICP-MS). Phys. Chem. Earth 2021, 124, 103011. [Google Scholar] [CrossRef]
- Letsoalo, M.R.; Godeto, T.W.; Magadzu, T.; Ambushe, A.A. Quantitative Speciation of Arsenic in Water and Sediment Samples from the Mokolo River in Limpopo Province, South Africa. Anal. Lett. 2018, 51, 2761–2775. [Google Scholar] [CrossRef]
- Huber, L. Understanding and Implementing ISO/IEC 17025 A Primer. p. 64, 2009. La démarche ISO 17025. Available online: https://www.agilent.com.cn/cs/library/primers/public/5990-4540CHCN_high.pdf (accessed on 11 March 2025).
- Rzetala, M.A.; Machowski, R.; Solarski, M.; Bakota, D.; Płomiński, A.; Rzetala, M. Toxic Metals, Non-Metals and Metalloids in Bottom Sediments as a Geoecological Indicator of a Water Body’s Suitability for Recreational Use. Int. J. Environ. Res. Public Health 2023, 20, 4334. [Google Scholar] [CrossRef]
- Cohen, D.; Rutherford, N. Technical Report on the Development of a Geochemical Atlas of Cyprus. Geol. Surv. Cyprus Lefkosia 2011, 1, 1–104. [Google Scholar]
- Cheng, H.; Huang, L.; Ma, P.; Shi, Y. Ecological risk and restoration measures relating to heavy metal pollution in industrial and mining wastelands. Int. J. Environ. Res. Public Health 2019, 16, 3985. [Google Scholar] [CrossRef]
- Rani, N.L.A.; Azid, A.; Khalit, S.I.; Gasim, M.B.; Juahir, H. Selected Malaysia air quality pollutants assessment using chemometrics techniques. J. Fundam. Appl. Sci. 2018, 9, 335. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, S.; Zhang, Z.; Aihemaiti, Y.; Yang, L.; Shao, Y.; Chen, Z.; Jiang, Y.; Jin, C.; Zheng, G. Dilution or enrichment: The effects of flood on pollutants in urban rivers. Environ. Sci. Eur. 2022, 34, 61. [Google Scholar] [CrossRef]
- Wittmann, G.T.W.; Forstnert, U. Metal enrichment in inland waters—The Jukskei and Hennops drainage. Water SA 1976, 2, 67–72. [Google Scholar]
- Lynch, S.F.L.; Batty, L.C.; Byrne, P. Environmental risk of severely Pb-contaminated riverbank sediment as a consequence of hydrometeorological perturbation. Sci. Total Environ. 2018, 636, 1428–1441. [Google Scholar] [CrossRef] [PubMed]
- Joe, D.J.; Choi, M.S.; Lee, J.H.; Kim, C.K.; Choi, M.S.; Shin, H.S. Discrimination of metal contaminant sources in river sediments influenced by mining and smelting activities using stable Pb and Zn isotopes. Environ. Sci. Pollut. Res. 2024, 31, 20521–20533. [Google Scholar] [CrossRef]
- Xie, S.; Liu, C.; He, B.; Chen, M.; Gao, T.; Wei, X.; Liu, Y.; Xia, Y.; Sun, Q. Geochemical Fractionation and Source Identification of Pb and Cd in Riparian Soils and River Sediments from Three Lower Reaches Located in the Pearl River Delta. Int. J. Environ. Res. Public Health 2022, 19, 13819. [Google Scholar] [CrossRef]
- Hossain, M.M.; Jahan, I.; Dar, M.A.; Dhanavade, M.J.; Mamtaz, A.F.B.; Maxwell, S.J.; Han, S.; Zhu, D. A Review of Potentially Toxic Elements in Sediment, Water, and Aquatic Species from the River Ecosystems. Toxics 2025, 13, 26. [Google Scholar] [CrossRef]
- Ntsasa, N.; Mkhohlakali, A.; Mogashane, T.; Tshilongo, J.; Letsoalo, M.R. Trends in Systematic Techniques for Pollutants Monitoring in the Environmental Water Systems. Available online: www.intechopen.com (accessed on 25 January 2025).
- Pascal, N.M.; Dieudonné, M.E.; Jean-Noël, M.K. Evaluation of the Level of Mercury Pollution in the Sediments of the Rivers Draining the Gold Panning Sites in the Territory of Fizi, Eastern Democratic Republic of Congo. J. Geosci. Environ. Prot. 2020, 8, 98277. [Google Scholar] [CrossRef]
- Niane, B.; Moritz, R.; Guédron, S.; Ngom, P.M.; Pfeifer, H.R.; Mall, I.; Poté, J. Effect of recent artisanal small-scale gold mining on the contamination of surface river sediment: Case of Gambia River, Kedougou region, southeastern Senegal. J. Geochem. Explor. 2014, 144, 517–527. [Google Scholar] [CrossRef]
- Phala, A.; Mistry, D.; Matlala, R.L.G. Implications of illegal mining in Gauteng Province. Int. J. Humanit. Soc. Sci. Invent. 2017, 6, 56–63. [Google Scholar]
- Asare-Donkor, N.K.; Adimado, A.A. Influence of mining related activities on levels of mercury in water, sediment and fish from the Ankobra and Tano River basins in South Western Ghana. Environ. Syst. Res. 2016, 5, 5. [Google Scholar] [CrossRef]
- Martinez, G.; McCord, S.A.; Driscoll, C.T.; Todorova, S.; Wu, S.; Araújo, J.F.; Vega, C.M.; Fernandez, L.E. Mercury contamination in riverine sediments and fish associated with artisanal and small-scale gold mining in Madre de Dios, Peru. Int. J. Environ. Res. Public Health 2018, 15, 1584. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, T.R.; Ferdous, J.; Haque, M.M.; Rahman, M.M.; Quraishi, S.B.; Rahman, M.S. Assessment of heavy metals and radionuclides in groundwater and associated human health risk appraisal in the vicinity of Rooppur nuclear power plant, Bangladesh. J. Contam. Hydrol. 2022, 251, 104072. [Google Scholar] [CrossRef]
- Lécrivain, N.; Clément, B.; Dabrin, A.; Seigle-Ferrand, J.; Bouffard, D.; Naffrechoux, E.; Frossard, V. Water-level fluctuation enhances sediment and trace metal mobility in lake littoral. Chemosphere 2021, 264, 128451. [Google Scholar] [CrossRef]
- Huffman, A.M.; Sikder, A.M. Assessment of Heavy Metal Pollution in the Sediments of the Roanoke River; The Geological Society of America: Boulder, CO, USA, 2017. [Google Scholar] [CrossRef]
- Geng, N.; Xia, Y.; Li, D.; Bai, F.; Xu, C. Migration and Transformation of Heavy Metal and Its Fate in Intertidal Sediments: A Review. Processes 2024, 12, 311. [Google Scholar] [CrossRef]
- Bartoszek, L.; Gruca-Rokosz, R.; Pękala, A.; Czarnota, J. Heavy Metal Accumulation in Sediments of Small Retention Reservoirs—Ecological Risk and the Impact of Humic Substances Distribution. Resources 2022, 11, 113. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Z.G.; Zeng, G.M.; Jiang, M.; Yang, Z.Z.; Cui, F.; Zhu, M.Y.; Shen, L.Q.; Hu, L. Effects of sediment geochemical properties on heavy metal bioavailability. Environ. Int. 2014, 73, 270–281. [Google Scholar] [CrossRef]
- Chen, C.F.; Ju, Y.R.; Lim, Y.C.; Chen, C.W.; Wu, C.H.; Lin, Y.L.; Dong, C.D. Dry and wet seasonal variation of total mercury, inorganic mercury, and methylmercury formation in estuary and harbor sediments. J. Environ. Manag. 2020, 253, 109683. [Google Scholar] [CrossRef]
- Duncan, A.E.; de Vries, N.; Nyarko, K.B. Assessment of heavy metal pollution in the main Pra River and its tributaries in the Pra Basin of Ghana. Environ. Nanotechnol. Monit. Manag. 2018, 10, 264–271. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Z.; Wu, Q.; Wang, W.; Peng, C.; Zeng, J.; Wang, Y. Urban geochemistry and human-impacted imprint of dissolved trace and rare earth elements in a high-tech industrial city, Suzhou. Elementa 2021, 9, 00151. [Google Scholar] [CrossRef]
- Addo-Bediako, A. Risk of Chemical Pollution in Olifants River Basin, South Africa: Human Health Implications. Limnol. Rev. 2025, 25, 1. [Google Scholar] [CrossRef]
- Abdullah, M.I.C.; Sah, A.S.R.M.; Haris, H. Geoaccumulation Index and Enrichment Factor of Arsenic in Surface Sediment of Bukit Merah Reservoir, Malaysia. Trop. Life Sci. Res. 2020, 31, 109–125. [Google Scholar] [CrossRef]
- Webster, J.; Iqani, M. Johannesburg’s shitty little river: Faecal discourse and discontent regarding the Jukskei. Soc. Dyn. 2024, 50, 109–127. [Google Scholar] [CrossRef]
- Fitchett, A. Suds for managing surface water in Diepsloot informal settlement, Johannesburg, South Africa. Water SA 2017, 43, 310–322. [Google Scholar] [CrossRef]
- Madzlan, N.A.H.; Suratman, S.; Mohamed, K.N.; Chuan, O.M. Seasonal Variation in Concentration of Heavy Metals in Tropical River Sediment. Malays. J. Anal. Sci. 2023, 27, 108–118. [Google Scholar]
- Habineza, E.; Makwinja, R.; Inagaki, Y. Contamination and health risks of trace metals in water and sediments of May Sieley stream, Ethiopia. Phys. Chem. Earth 2023, 129, 103315. [Google Scholar] [CrossRef]
- Lin, K.-N.; Lim, Y.-C.; Chen, C.-W.; Chen, C.-F.; Kao, C.-M.; Dong, C.-D. Spatiotemporal Variation and Ecological Risk Assessment of Heavy Metals in Industrialized Urban River Sediments: Fengshan River in Southern Taiwan as a Case Study. Appl. Sci. 2022, 12, 1013. [Google Scholar] [CrossRef]
- Akoto, O.; Ephraim, J.H.; Darko, G. Heavy metals pollution in surface soils in the vicinity of abundant railway servicing workshop in Kumasi, Ghana. Int. J. Environ. Res. 2008, 2, 359–364. [Google Scholar]
- Papu-Zamxaka, V.; Mathee, A.; Harpham, T.; Barnes, B.; Röllin, H.; Lyons, M.; Jordaan, W.; Cloete, M. Elevated mercury exposure in communities living alongside the Inanda Dam, South Africa. J. Environ. Monit. 2010, 12, 472–477. [Google Scholar] [CrossRef]
- Huizenga, J.M.; Harmse, J.T. Geological and anthropogenic influences on the inorganic water chemistry of the Jukskei River, Gauteng, South Africa. S. Afr. J. Geol. 2005, 108, 439–447. [Google Scholar] [CrossRef]
- Singh, S.; Tiwari, R.K.; Pandey, R.S. Water Pollution due to Discharge of Industrial Effluents. Int. Arch. Appl. Sci. Technol. 2018, 9, 111–121. [Google Scholar]
- Sampaio, N.A.S.; Mazza, F.C.; de Siqueira, S.S.S.; Miranda, J.E.; de Souza Moutinho, J.V.; de Oliveira Pacífico, L. Applications of Correlation Analysis in Environmental Problems. Rev. Gest. Soc. E Ambient. 2024, 18, 1–16. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar] [PubMed]
- Mishra, P.; Singh, U.; Pandey, C.; Mishra, P.; Pandey, G. Application of student’s t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 2019, 22, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Wijesiri, B.; Egodawatta, P.; McGree, J.; Goonetilleke, A. Influence of pollutant build-up on variability in wash-off from urban road surfaces. Sci. Total Environ. 2015, 527–528, 344–350. [Google Scholar] [CrossRef]
- Liu, X.; Sheng, Y.; Liu, Q.; Li, Z. Suspended particulate matter affects the distribution and migration of heavy metals in the Yellow River. Sci. Total Environ. 2024, 912, 169537. [Google Scholar] [CrossRef]
- Hassan, K.M.T.; Ferdoushi, Z.; Rana, M.M.; Alam, M.S. Assessing the Seasonal Variability of Water Quality and Heavy Metals Concentration in Sediment, Water, and Fish Muscles of Korotoa River in Bangladesh. Aquac. Res. 2024, 2024, 5343363. [Google Scholar] [CrossRef]
- Zhao, R.; Coles, N.A.; Wu, J. Status of heavy metals in soils following long-term river sediment application in plain river network region, southern China. J. Soils Sediments 2015, 15, 2285–2292. [Google Scholar] [CrossRef]
- UNEP. Final Review of Scientific Information on Cadmium; Chemicals Branch DTIE; United Nations Environment Programme: Nairobi, Kenya, 2010. [Google Scholar]
- Zhang, T.; Li, L.; Xu, F.; Chen, X.; Du, L.; Wang, X.; Li, Y. Assessing the remobilization and fraction of cadmium and lead in sediment of the Jialing River by sequential extraction and diffusive gradients in films (DGT) technique. Chemosphere 2020, 257, 127181. [Google Scholar] [CrossRef]
- Sulistyowati, L.; Nurhasanah, N.; Riani, E.; Cordova, M.R. Heavy metals concentration in the sediment of the aquaticenvironment caused by the leachate discharge from a landfill. Glob. J. Environ. Sci. Manag. 2023, 9, 323–336. [Google Scholar] [CrossRef]
- Lee, A.C.; Idrus, F.A.; Aziz, F. Cadmium and Lead Concentrations in Water, Sediment, Fish and Prawn as Indicators of Ecological and Human Health Risk in Santubong Estuary, Malaysia. Jordan J. Biol. Sci. 2021, 14, 317–325. [Google Scholar] [CrossRef]
- Hellar-Kihampa, H.; Mihale, M.J. Lead and Cadmium Levels in Water, Surficial Sediments, and Edible Biota of Urban Rivers in Dar es Salaam, Tanzania, During Two Seasons. Environ. Prot. Res. 2023, 3, 217–381. [Google Scholar] [CrossRef]
- Li, D.; Chang, F.; Zhang, Y.; Duan, L.; Liu, Q.; Li, H.; Hu, G.; Zhang, X.; Gao, Y.; Zhang, H. Arsenic migration at the sediment-water interface of anthropogenically polluted Lake Yangzong, Southwest China. Sci. Total Environ. 2023, 879, 163205. [Google Scholar] [CrossRef] [PubMed]
- Ambushe, A.A.; Letsoalo, M.R.; Lovia, D.; Matabane, C.P.; Molele, L.S.; Godeto, T.W.; Magadzu, T. Assessment of Potentially Toxic Elements and their Species in Selected Water Systems in Limpopo Province; Water Research Commission: Pretoria, South Africa, 2020. [Google Scholar]
- Van Eeden, E.S.; Liefferink, M.; Durand, J.V. Legal issues concerning mine closure and social responsibility on the West Rand. TD J. Transdiscipl. Res. S. Afr. 2009, 5, 51–71. [Google Scholar]
- Mccarthy, T.S.; Africa, S.; Africa, S. The impact of acid mine drainage in South Africa. S. Afr. J. Sci. 2011, 107, 1–7. [Google Scholar] [CrossRef]
- Abiye, T.A.; Ali, K.A. Potential role of acid mine drainage management towards achieving sustainable development in the Johannesburg region, South Africa. Groundw. Sustain. Dev. 2022, 19, 100839. [Google Scholar] [CrossRef]
- Nkinda, M.S.; Rwiza, M.J.; Ijumba, J.N.; Njau, K.N. Heavy metals risk assessment of water and sediments collected from selected river tributaries of the Mara River in Tanzania. Discov. Water 2021, 1, 3. [Google Scholar] [CrossRef]
- Ullrich, S.M.; Tanton, T.W.; Abdrashitova, S.A. Mercury in the aquatic environment: A review of factors affecting methylation. Crit. Rev. Environ. Sci. Technol. 2001, 31, 241–293. [Google Scholar] [CrossRef]
- Báez, A.; Belmont, R.; García, R.; Padilla, H.; Torres, M.C. Chemical composition of rainwater collected at a southwest site of Mexico City, Mexico. Atmos. Res. 2007, 86, 61–75. [Google Scholar] [CrossRef]
- Dube, R.A.; Maphosa, B.; Malan, A.; Fayemiwo, D.M.; Ramulondi, D.; Zuma, T.A. Response of Urban and Peri-Urban Aquatic Ecosystems to Riparian Zones Land Uses and Human Settlements: A Study of the Rivers, Jukskei, Kuils and Pienaars; Water Research Commission: Pretoria, South Africa, 2017. [Google Scholar]
- Yüksel, B.; Ustaoğlu, F.; Tokatli, C.; Islam, M.S. Ecotoxicological risk assessment for sediments of Çavuşlu stream in Giresun, Turkey: Association between garbage disposal facility and metallic accumulation. Environ. Sci. Pollut. Res. 2022, 29, 17223–17240. [Google Scholar] [CrossRef]
- CLiu, W.; Lin, K.H.; Kuo, Y.M. Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci. Total Environ. 2003, 313, 77–89. [Google Scholar] [CrossRef]
- Xing, M.; Yan, D.; Hai, M.; Zhang, Y.; Zhang, Z.; Li, F. Arsenic Contamination in Sludge and Sediment and Relationship with Microbial Resistance Genes: Interactions and Remediation. Water 2024, 16, 3633. [Google Scholar] [CrossRef]
- Shankar, S.; Shanker, U.; Shikha. Arsenic contamination of groundwater: A review of sources, prevalence, health risks, and strategies for mitigation. Sci. World J. 2014, 2014, 304524. [Google Scholar] [CrossRef] [PubMed]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Armah, F.A.; Boamah, S.A.; Quansah, R.; Obiri, S.; Luginaah, I. Unsafe occupational health behaviors: Understanding mercury-related environmental health risks to artisanal gold miners in ghana. Front. Environ. Sci. 2016, 4, 29. [Google Scholar] [CrossRef]
- Gibb, H.; Leary, K.G.O. WHO Comprehensive review of mercury in ASGM. Environ. Health Perspect. 2014, 122, 667–672. [Google Scholar] [CrossRef]
- Hoorzook, K.B.; Pieterse, A.; Heine, L.; Barnard, T.G.; van Rensburg, N.J. Soul of the jukskei river: The extent of bacterial contamination in the jukskei river in gauteng province, south africa. Int. J. Environ. Res. Public Health 2021, 18, 8537. [Google Scholar] [CrossRef]
- Mpanza, M.; Adam, E.; Moolla, R. Perceptions of external costs of dust fallout from gold mine tailings: West Wits Basin. Clean. Air J. 2020, 30, 1–12. [Google Scholar] [CrossRef]
| Scope of Ecological Risk Index (Eif) | Ecological Risk Level of Single-Factor Pollution | Scope of Toxicity Index (RI) | The Level of Potential Ecological Risk |
|---|---|---|---|
| Eif < 40 | low | RI < 150 | low-grade |
| 40 ≤ Eif < 80 | moderate | 150 ≤ RI < 300 | moderate |
| 80 ≤ Eif < 160 | higher | 300 ≤ RI < 600 | severe |
| 160 ≤ Eif < 320 | high | 600 ≤ RI | serious |
| Eif ≤ 320 | serious |
| Sampling Seasons | Sample ID | As (mg/kg) | Cd (mg/kg) | Hg (µg/kg) | Pb (mg/kg) | Th (mg/kg) | U (mg/kg) | Fe (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| Dry | Juks-P1 | 22.3 ± 0.90 | 0.85 ± 0.18 | 3650 ± 600 | 267 ± 13 | 5.93 ± 0.26 | 2.3 ± 0.5 | 47,250 ± 250 |
| Wet | 10.0 ± 0.06 | 0.16 ± 0.03 | 77.5 ± 3.54 | 38.8 ± 1.27 | 5.08 ± 0.02 | 2.02 ± 0.03 | 19,533 ± 896 | |
| Dry | Juks-P2 | 8.13 ± 0.60 | <0.01 | 8040 ± 770 | 35.5 ± 0 | 5.40 ± 0.33 | 2.45 ± 0.15 | 18,600 ± 0 |
| Wet | 8.6 ± 0.06 | 0.30 ± 0.03 | 20.7 ± 0.58 | 475 ± 4.04 | 4.59 ± 0.09 | 1.74 ± 0.06 | 36,600 ± 624 | |
| Dry | Juks-P3 | 7.38 ± 0.13 | 0.18 ± 0 | 7610 ± 350 | 25.7 ± 0.25 | 5.85 ± 0.08 | 2.60 ± 0.12 | 10,100 ± 0 |
| Wet | 2.35 ± 0.29 | <0.01 | 24.5 ± 2.12 | 12.6 ± 0.32 | 3.46 ± 0.08 | 1.26 ± 0.05 | 12,567 ± 231 | |
| Dry | Juks-P4 | 4.89 ± 0.16 | 0.10 ± 0 | 11,100 ± 230 | 15.9 ± 0.75 | 4.91 ± 0.42 | 1.88 ± 0.06 | 12,000 ± 0 |
| Wet | 5.77 ± 0.21 | <0.01 | 10.3 ± 0.99 | 13.0 ± 0.55 | 5.31 ± 0.02 | 2.86 ± 0.05 | 16,267 ± 1097 | |
| Dry | Juks-P5 | 3.89 ± 1.14 | 0.14 ± 0 | 11,700 ± 520 | 14.3 ± 1.41 | 5.15 ± 0.71 | 1.59 ± 0.18 | 6900 ± 282 |
| Wet | 2.1 ± 0.14 | 0.14 ± 0 | 15.0 ± 0 | 21.2 ± 0.14 | 5.84 ± 0.02 | 2.25 ± 0.06 | 13,233 ± 153 | |
| Dry | Juks-DS-1 | 7.18 ± 0.22 | <0.01 | 11,600 ± 500 | 47.5 ± 16.2 | 4.72 ± 0.11 | 1.73 ± 0.06 | 21,300 ± 0 |
| Wet | 1.6 ± 0.14 | 0.12 ± 0 | 16.0 ± 2.83 | 21.8 ± 0.42 | 4.14 ± 0.16 | 1.67 ± 0.01 | 10,267 ± 208 | |
| Dry | Juks-DS-6 | 6.05 ± 0.95 | 0.12 ± 0.0 | 12,100 ± 990 | 14.8 ± 2.85 | 3.89 ± 0.21 | 1.89 ± 0.26 | 8900 ± 424 |
| Wet | 1.09 ± 0.16 | <0.01 | 8.4 ± 0.85 | 15.8 ± 1.56 | 4.96 ± 0.62 | 1.34 ± 0.00 | 6900 ± 100 | |
| Dry | Juks-DS-8 | 3.55 ± 0.22 | 0.12 ± 0.09 | 11,900 ± 1150 | 16.7 ± 3.30 | 3.09 ± 0.17 | 1.37 ± 0.08 | 4750 ± 70 |
| Wet | 2.1 ± 0.14 | 0.26 ± 0.02 | 52.3 ± 4.2 | 28.2 ± 0.42 | 5.35 ± 0.29 | 2.81 ± 0.00 | 21,867 ± 513 | |
| Dry | Juks-DS-9 | 6.74 ± 1.42 | 0.16 ± 0.05 | 7540 ± 360 | 20.4 ± 0.06 | 8.16 ± 0.18 | 1.95 ± 0.01 | 27,250 ± 1484 |
| Wet | 1.3 ± 0.35 | 0.12 ± 0.01 | 22.0 ± 2.83 | 16.3 ± 0.40 | 4.72 ± 0.40 | 1.67 ± 0.08 | 11,033 ± 416 |
| Sampling Seasons | Sample ID | As (μg/L) | Cd (μg/L) | Hg (μg/L) | Pb (μg/L) | Th (μg/L) | U (μg/L) |
|---|---|---|---|---|---|---|---|
| Wet | Juks-P1 | <0.01 | <0.01 | 0.02 | <0.01 | <0.001 | 0.04 |
| Dry | <0.01 | <0.01 | 0.22 | <0.01 | 0.62 | 0.14 | |
| Wet | Juks-P2 | <0.01 | <0.01 | 0.02 | <0.01 | 0.01 | 0.05 |
| Dry | <0.01 | <0.01 | 0.1 | 0.03 | 0.42 | 0.44 | |
| Wet | Juks-P3 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | 0.16 |
| Dry | <0.01 | <0.01 | 0.1 | 0.05 | 0.36 | 0.78 | |
| Wet | Juks-P4 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | 0.27 |
| Dry | <0.01 | <0.01 | 0.07 | 0.08 | 0.34 | 0.91 | |
| Wet | Juks-P5 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | 0.34 |
| Dry | <0.01 | <0.01 | 0.06 | 0.03 | 0.23 | 0.95 | |
| Wet | Juks-DS-1 | <0.01 | <0.01 | 0.02 | <0.01 | 0.03 | 0.31 |
| Dry | <0.01 | <0.01 | 0.05 | 0.02 | 0.25 | 0.82 | |
| Wet | Juks-DS-6 | <0.01 | <0.01 | 0.02 | <0.01 | 0.09 | 0.32 |
| Dry | <0.01 | <0.01 | 0.06 | 0.11 | 0.22 | 0.55 | |
| Wet | Juks-DS-8 | <0.01 | <0.01 | 0.01 | <0.01 | 0.02 | 0.22 |
| Dry | <0.01 | <0.01 | 0.04 | 0.06 | 0.13 | 0.53 | |
| Wet | Juks-DS-9 | <0.01 | <0.01 | 0.01 | <0.01 | 0.01 | 0.22 |
| Dry | <0.01 | <0.01 | 0.03 | <0.01 | 0.09 | 0.49 |
| Sampling Seasons | Sample ID | As | Cd | Hg | Pb | Th | U |
|---|---|---|---|---|---|---|---|
| Dry | Juks-P1 | 3.91 | 0.188 | 6.93 | 4.16 | −1.34 | −2.71 |
| Wet | −0.51 | 1.75 | −3.64 | −4.24 | −1.18 | −2.51 | |
| Dry | Juks-P2 | −1.23 | 0.00 | 8.07 | 1.24 | −1.48 | −2.62 |
| Wet | −0.72 | −2.74 | −6.24 | 5.37 | −1.33 | −2.72 | |
| Dry | Juks-P3 | −1.37 | −2.06 | 7.99 | 0.77 | −1.36 | −2.53 |
| Wet | −2.59 | 0.00 | −6.25 | 0.13 | −1.73 | −3.19 | |
| Dry | Juks-P4 | −1.97 | −2.88 | 8.52 | 0.08 | −1.61 | −3.00 |
| Wet | −1.3 | 0.00 | −7.25 | 0.18 | −1.11 | −2.01 | |
| Dry | Juks-P5 | −2.29 | −2.43 | 8.60 | −0.09 | −1.54 | −3.24 |
| Wet | −2.76 | −3.84 | −6.25 | 0.88 | −0.98 | −2.35 | |
| Dry | Juks-DS−1 | −1.41 | 0.00 | 8.59 | 1.66 | −1.67 | −3.12 |
| Wet | −3.15 | −4.06 | −6.25 | 0.92 | −1.47 | −2.78 | |
| Dry | Juks-DS−6 | −1.65 | −2.59 | 8.65 | −0.02 | −1.94 | −2.99 |
| Wet | −3.7 | 0.00 | −7.24 | 0.45 | −1.21 | −3.10 | |
| Dry | Juks-DS−8 | −2.43 | −2.61 | 8.64 | 0.17 | −2.28 | −3.45 |
| Wet | −2.75 | −2.94 | −4.93 | 1.29 | −1.1 | −2.03 | |
| Dry | Juks-DS−9 | −1.50 | −2.21 | 7.97 | 0.44 | −0.88 | −2.95 |
| Wet | −3.44 | −4.05 | −6.24 | 0.5 | −1.28 | −2.78 |
| Sampling Seasons | Sample ID | As | Cd | Hg | Pb | Th | U |
|---|---|---|---|---|---|---|---|
| Dry | Juks-P1 | 1.76 | 0.06 | 183 | 26.7 | 0.593 | 0.23 |
| Wet | 0.79 | 0.32 | 3.88 | 3.88 | 0.51 | 0.2 | |
| Dry | Juks-P2 | 0.64 | 0.00 | 401 | 3.55 | 0.54 | 0.24 |
| Wet | 0.00 | 0.6 | 1.04 | 47.5 | 0.46 | 0.17 | |
| Dry | Juks-P3 | 0.58 | 0.36 | 380 | 2.57 | 0.59 | 0.6 |
| Wet | 0.00 | 0.00 | 1.23 | 1.26 | 0.35 | 0.13 | |
| Dry | Juks-P4 | 0.38 | 0.20 | 553 | 1.59 | 0.49 | 0.18 |
| Wet | 0.00 | 0.00 | 0.52 | 1.3 | 0.53 | 0.29 | |
| Dry | Juks-P5 | 0.31 | 0.20 | 584 | 1.43 | 0.52 | 0.19 |
| Wet | 0.00 | 0.28 | 0.75 | 2.13 | 0.58 | 0.23 | |
| Dry | Juks-DS-1 | 0.57 | 0.00 | 578 | 4.75 | 0.47 | 1.73 |
| Wet | 0.00 | 0.24 | 0.8 | 2.18 | 0.41 | 0.17 | |
| Dry | Juks-DS-6 | 0.48 | 0.25 | 602 | 1.48 | 0.39 | 0.19 |
| Wet | 0.00 | 0 | 0.42 | 1.58 | 0.5 | 0.13 | |
| Dry | Juks-DS-8 | 0.28 | 0.25 | 597 | 1.68 | 0.31 | 0.14 |
| Wet | 0.17 | 0.52 | 2.62 | 2.82 | 0.54 | 0.28 | |
| Dry | Juks-DS-9 | 0.53 | 0.32 | 377 | 2.04 | 0.82 | 0.19 |
| Wet | 0.10 | 0.24 | 1.1 | 1.63 | 0.47 | 0.17 |
| Sampling Seasons | Sample ID | As | Cd | Hg | Pb | Th | U |
|---|---|---|---|---|---|---|---|
| Dry | Juks-P1 | 0.37 | 0.36 | 38.6 | 5.65 | 0.13 | 0.05 |
| Wet | 0.4 | 0.16 | 1.98 | 1.99 | 0.26 | 0.16 | |
| Dry | Juks-P2 | 0.44 | 0 | 467 | 1.91 | 0.29 | 0.13 |
| Wet | 0.19 | 0.16 | 0.28 | 12.98 | 0.13 | 0.05 | |
| Dry | Juks-P3 | 0.54 | 0.34 | 355 | 2.41 | 5.47 | 0.24 |
| Wet | 0.14 | 0.3 | 0.28 | 1 | 0.13 | 0.05 | |
| Dry | Juks-P4 | 0.38 | 0.2 | 551 | 1.58 | 0.49 | 0.19 |
| Wet | 0.28 | 0 | 0.98 | 0.8 | 0.28 | 0.1 | |
| Dry | Juks-P5 | 0.44 | 0.4 | 840 | 1.63 | 0.74 | 0.23 |
| Wet | 0.13 | 0 | 0.32 | 1.09 | 0.33 | 0.32 | |
| Dry | Juks-DS-1 | 0.29 | 0.47 | 297 | 2.44 | 0.24 | 0.09 |
| Wet | 0.12 | 0.23 | 0.78 | 2.12 | 0.4 | 0.16 | |
| Dry | Juks-DS-6 | 0.54 | 0.28 | 677 | 1.66 | 0.44 | 0.21 |
| Wet | 0.12 | 0 | 0.61 | 2.29 | 0.72 | 0.19 | |
| Dry | Juks-DS-8 | 0.59 | 0.52 | 1257 | 3.53 | 0.65 | 0.29 |
| Wet | 0.08 | 0.24 | 1.19 | 1.29 | 0.24 | 0.13 | |
| Dry | Juks-DS-9 | 0.20 | 0.12 | 138 | 0.75 | 0.30 | 0.07 |
| Wet | 0.09 | 0.22 | 1.00 | 1.48 | 0.43 | 0.15 |
| Variables | As | Cd | Hg | Pb | Th | U | Fe |
|---|---|---|---|---|---|---|---|
| As | 1 | 0.369 | 0.52 | 0.558 | 0.113 | 0.204 | 0.71 |
| Cd | 0.369 | 1 | 0.434 | 0.633 | 0.242 | 0.269 | 0.777 |
| Hg | 0.52 | 0.434 | 1 | −0.053 | 0.118 | 0.231 | 0.306 |
| Pb | 0.558 | 0.633 | −0.053 | 1 | −0.105 | −0.118 | 0.872 |
| Th | 0.113 | 0.242 | 0.118 | −0.105 | 1 | 0.71 | 0.087 |
| U | 0.204 | 0.269 | 0.231 | −0.118 | 0.71 | 1 | 0.273 |
| Fe | 0.71 | 0.777 | 0.306 | 0.872 | 0.087 | 0.273 | 1 |
| Variables | As | Cd | Hg | Pb | Th | U | Fe |
|---|---|---|---|---|---|---|---|
| As | 1 | 0.903 | −0.846 | 0.978 | 0.306 | 0.505 | 0.915 |
| Cd | 0.903 | 1 | −0.749 | 0.934 | 0.238 | 0.288 | 0.779 |
| Hg | −0.846 | −0.749 | 1 | −0.762 | −0.652 | −0.734 | −0.853 |
| Pb | 0.978 | 0.934 | −0.762 | 1 | 0.193 | 0.339 | 0.875 |
| Th | 0.306 | 0.238 | −0.652 | 0.193 | 1 | 0.479 | 0.565 |
| U | 0.505 | 0.288 | −0.734 | 0.339 | 0.479 | 1 | 0.424 |
| Fe | 0.915 | 0.779 | −0.853 | 0.875 | 0.565 | 0.424 | 1 |
| Sampling Points | * RI Wet Season | * RI Dry Season |
|---|---|---|
| Juk-P1 | 193 | 7502 |
| Juk-P2 | 302 | 16,094 |
| Juk-P3 | 60.6 | 15,239 |
| Juk-P4 | 33.4 | 22,148 |
| Juk-P5 | 50.7 | 23,369 |
| Juk-DS-1 | 51.4 | 23,159 |
| Juk-DS-6 | 26.6 | 24,129 |
| Juk-DS-8 | 135 | 23,899 |
| Juk-DS-9 | 60.4 | 15,095 |
| Variables | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|
| As | 0.785 | −0.078 | 0.308 | −0.516 | 0.089 |
| Cd | 0.837 | −0.007 | −0.102 | 0.525 | 0.039 |
| Hg | 0.506 | 0.267 | 0.794 | 0.189 | 0.037 |
| Pb | 0.749 | −0.551 | −0.351 | −0.063 | 0.072 |
| Th | 0.278 | 0.825 | −0.315 | −0.052 | 0.372 |
| U | 0.385 | 0.819 | −0.186 | −0.104 | −0.367 |
| Fe | 0.949 | −0.211 | −0.143 | −0.03 | −0.146 |
| Eigenvalue | 3.260 | 1.779 | 1.013 | 0.596 | 0.310 |
| Variability (%) | 46.571 | 25.408 | 14.472 | 8.512 | 4.425 |
| Cumulative % | 46.571 | 71.978 | 86.450 | 94.962 | 99.387 |
| Variables | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|
| As | −0.963 | −0.232 | 0.092 | −0.092 | 0.046 |
| Cd | −0.875 | −0.383 | −0.04 | 0.288 | 0.049 |
| Hg | 0.949 | −0.246 | −0.055 | −0.083 | 0.17 |
| Pb | −0.912 | −0.4 | 0.025 | −0.049 | 0.015 |
| Th | −0.535 | 0.706 | −0.454 | 0.068 | 0.061 |
| U | −0.605 | 0.571 | 0.551 | −0.014 | 0.059 |
| Fe | −0.941 | −0.037 | −0.233 | −0.239 | −0.008 |
| Eigenvalue | 4.963 | 1.246 | 0.578 | 0.162 | 0.041 |
| Variability (%) | 70.897 | 17.806 | 8.260 | 2.318 | 0.584 |
| Cumulative % | 70.897 | 88.703 | 96.963 | 99.282 | 99.865 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukwevho, N.; Mabowa, M.H.; Ntsasa, N.; Mkhohlakali, A.; Chimuka, L.; Tshilongo, J.; Letsoalo, M.R. Seasonal Pollution Levels and Heavy Metal Contamination in the Jukskei River, South Africa. Appl. Sci. 2025, 15, 3117. https://doi.org/10.3390/app15063117
Mukwevho N, Mabowa MH, Ntsasa N, Mkhohlakali A, Chimuka L, Tshilongo J, Letsoalo MR. Seasonal Pollution Levels and Heavy Metal Contamination in the Jukskei River, South Africa. Applied Sciences. 2025; 15(6):3117. https://doi.org/10.3390/app15063117
Chicago/Turabian StyleMukwevho, Nehemiah, Mothepane H. Mabowa, Napo Ntsasa, Andile Mkhohlakali, Luke Chimuka, James Tshilongo, and Mokgehle R. Letsoalo. 2025. "Seasonal Pollution Levels and Heavy Metal Contamination in the Jukskei River, South Africa" Applied Sciences 15, no. 6: 3117. https://doi.org/10.3390/app15063117
APA StyleMukwevho, N., Mabowa, M. H., Ntsasa, N., Mkhohlakali, A., Chimuka, L., Tshilongo, J., & Letsoalo, M. R. (2025). Seasonal Pollution Levels and Heavy Metal Contamination in the Jukskei River, South Africa. Applied Sciences, 15(6), 3117. https://doi.org/10.3390/app15063117






