Development of Co-Axial Fibres Composed of CA (Mn 50,000) and PEGs (600 and 1000): Evaluation of the Influence of the Coagulation Bath
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Commercial cellulose acetate powder (CA, Sigma-Aldrich, St. Louis, MO, USA), with an acetyl content of 39.8 wt.%, average Mn = 50,000. It was selected because it is a non-toxic and natural material found in plants, and it is derived from cellulose; it is used to form the outer part of the co-axial fibres, generating a protective sheath [24].
- N,N-Dimethylformamide (DMF, 99.8%, Sigma-Aldrich, St. Louis, MO, USA). It was selected to dissolve CA because of its chemical compatibility (enhanced solubility) and moderate evaporation rate.
- Polyethylene glycol (600 and 1000) H(OCH2CH2)nOH, MP: ± 17–22 °C and MP: ± 37–40 °C (PEGs 600 and 1000, Thermo Fisher Scientific, Waltham, MA, USA). These are widely used PCMs. They were chosen for the PCF core due to their temperature range, unreactive nature towards other substances, phase change abilities, and heat storage capacity [25].
- Distilled water (dH2O) was used to dissolve PEGs;
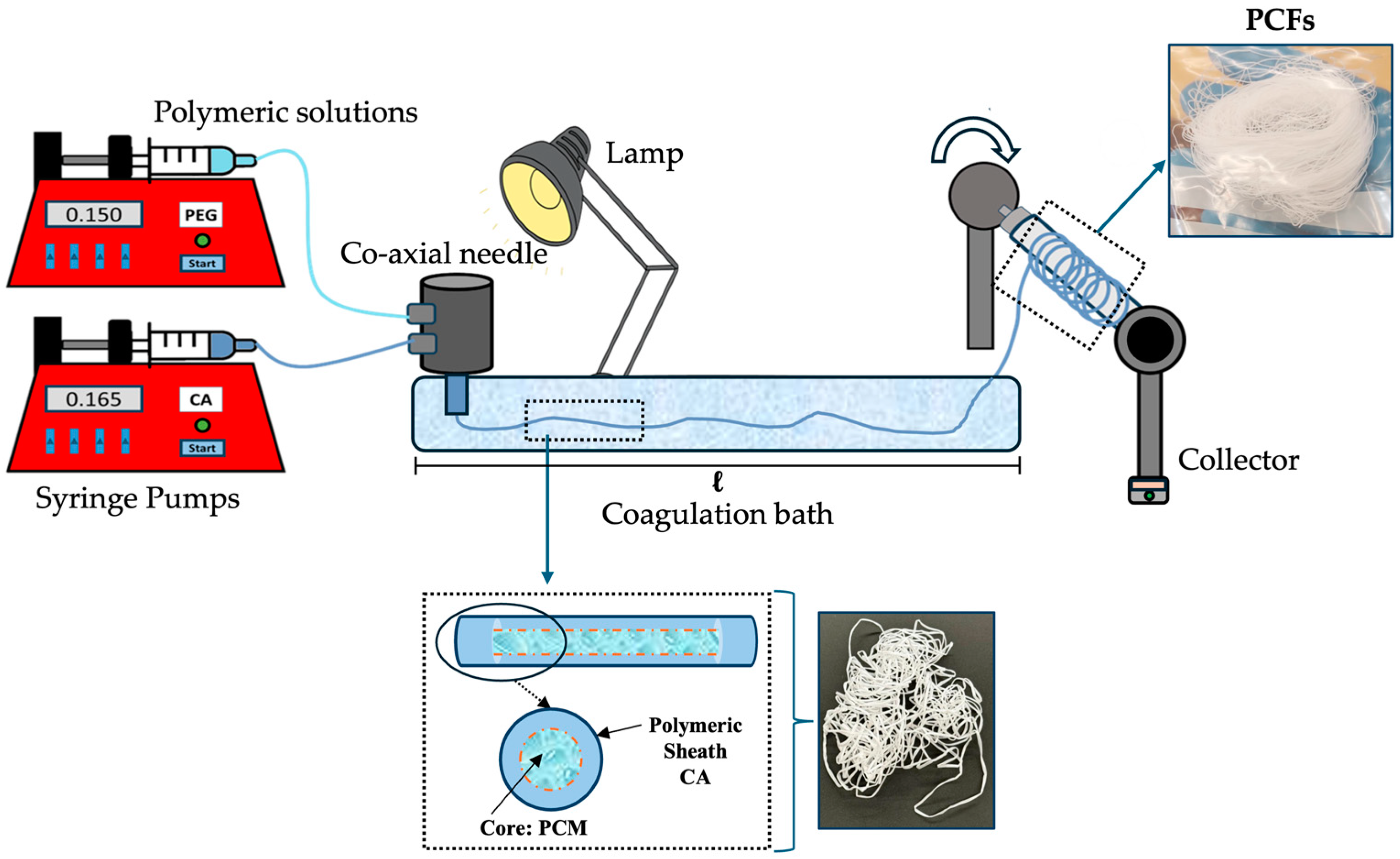
2.2. Wet-Spinning Production
2.3. Nomenclature of the Fibres
- 50 refers to the molecular weight of the CA (Mn 50,000);
- 600 indicates a molecular weight of the PEG;
- 90 indicates a concentration of 0.90 g/mol of the PEGs;
- 150 corresponds to a 0.150 mL/min ejection rate for the PEGs.
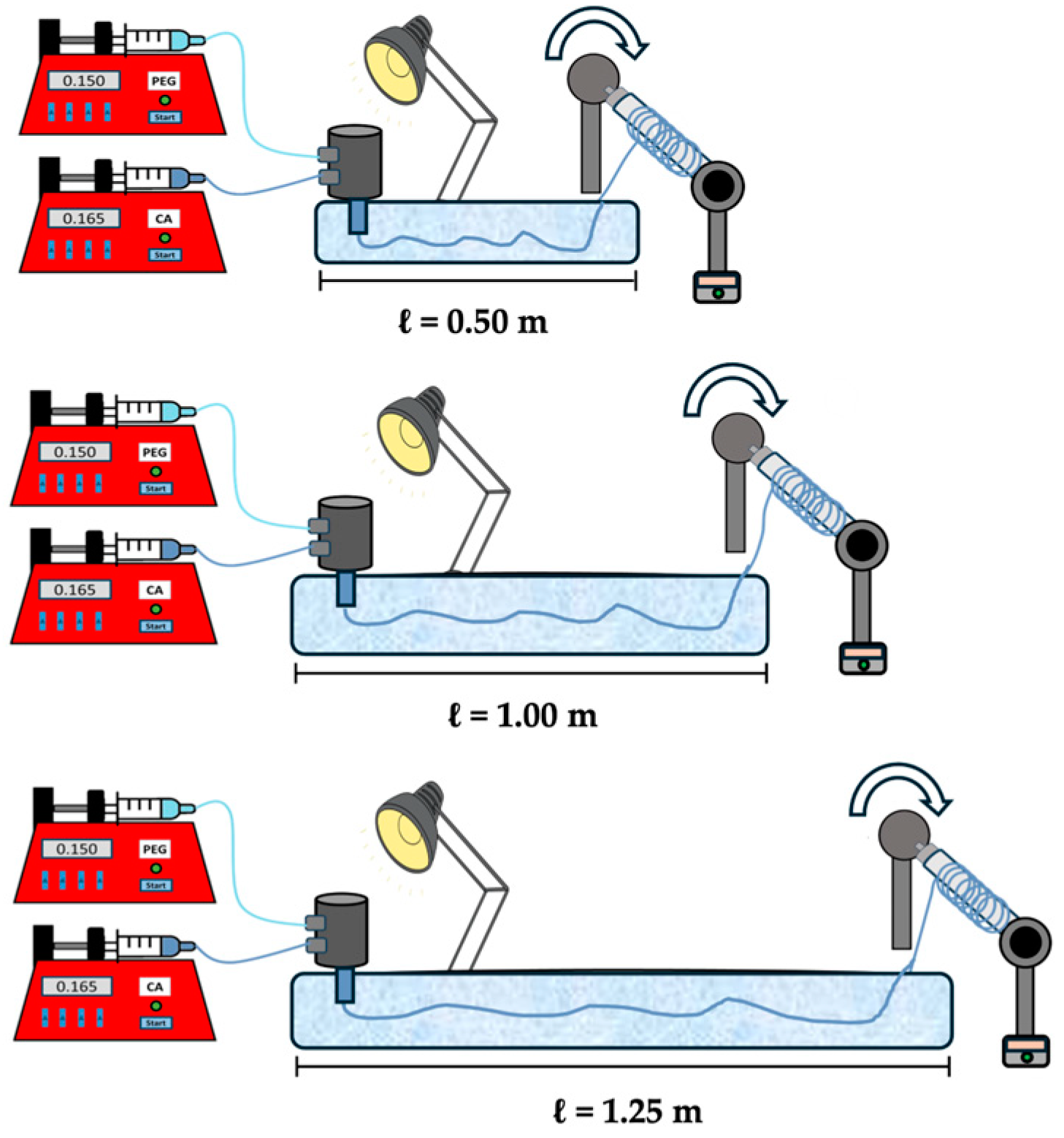
2.4. Coagulation Bath Optimisation
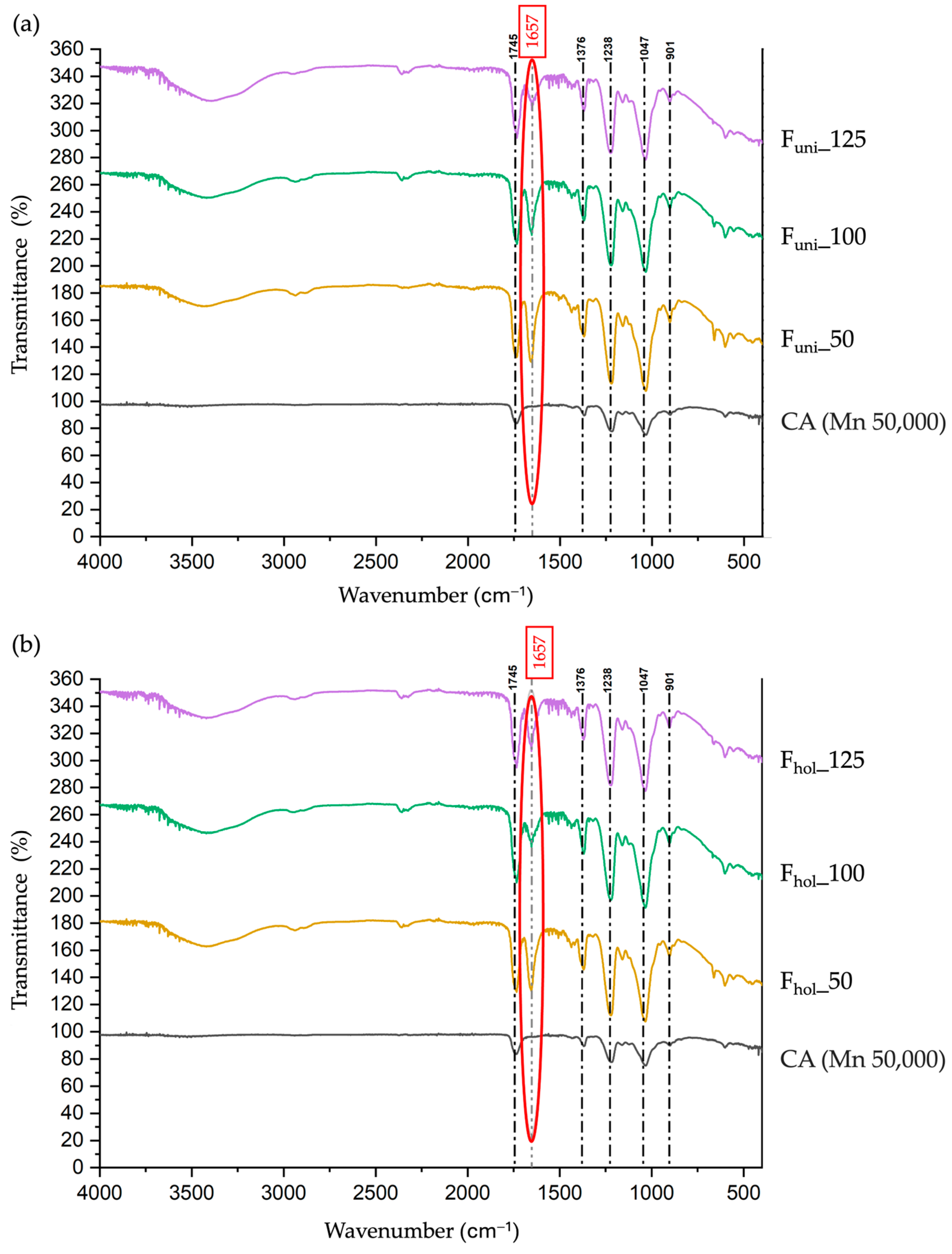
2.5. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR) and Index of Functional Groups
2.5.1. ATR-FTIR
2.5.2. Index of Functional Group
2.6. Bright-Field Microscopy
2.7. Thermal Analysis
2.7.1. Thermogravimetric Analysis (TGA)
2.7.2. Differential Scanning Calorimetry (DSC)
3. Results
3.1. Evaluation of the Coagulation Bath
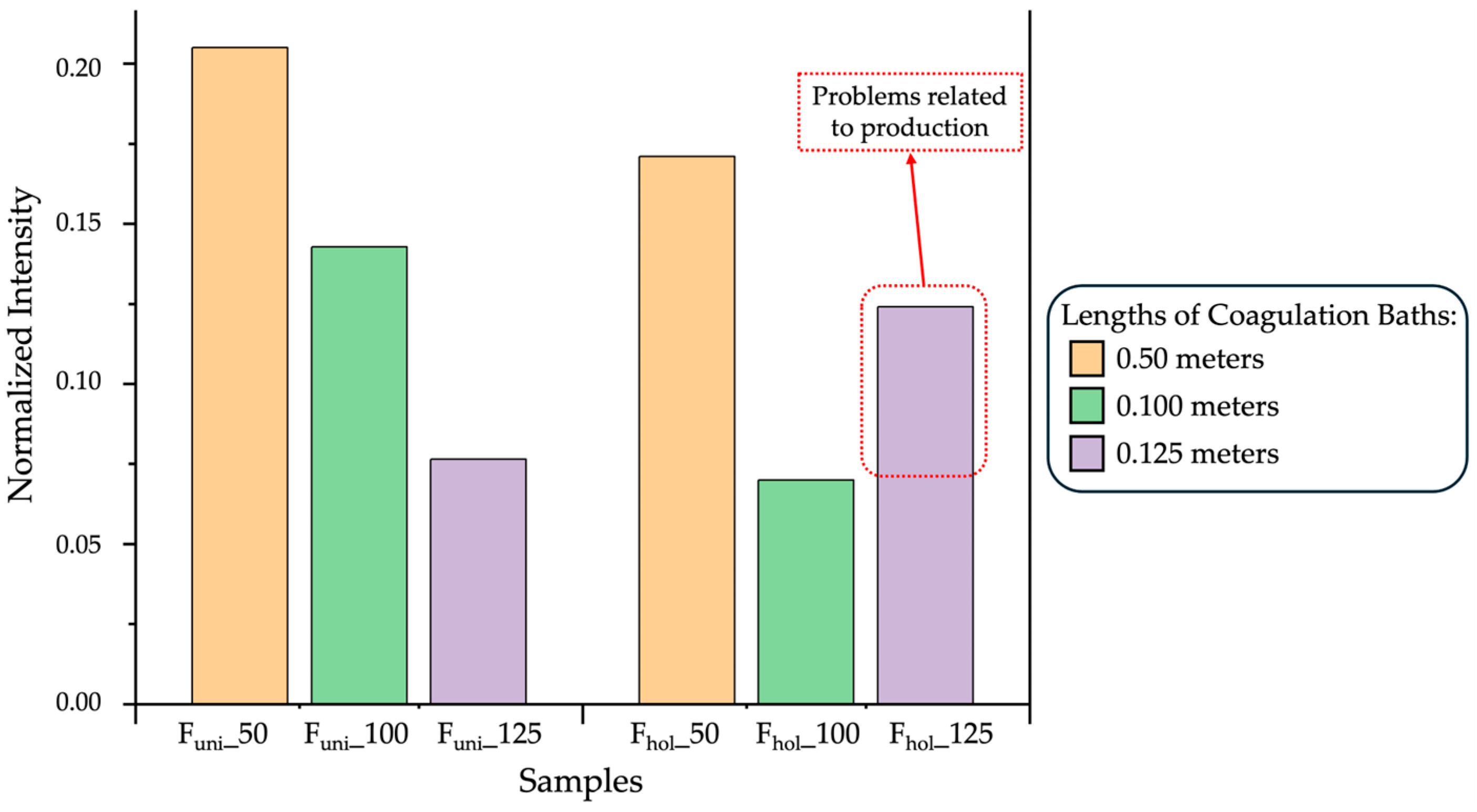
3.1.1. Influence of Different Lengths
3.1.2. Area Index of Functional Group
3.2. Morphology of PCFs
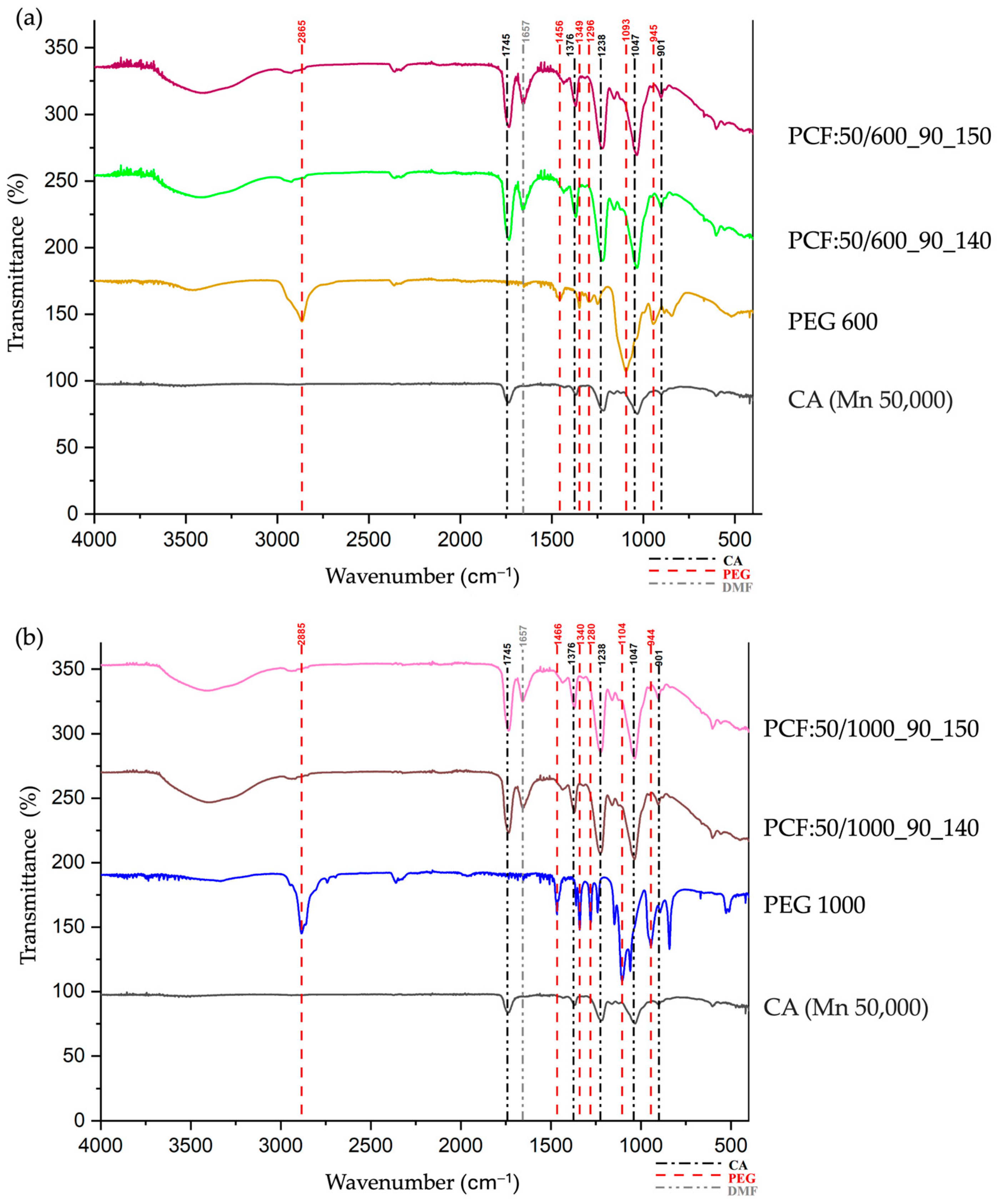
3.3. Chemical Analyses
3.4. Thermal Analyses
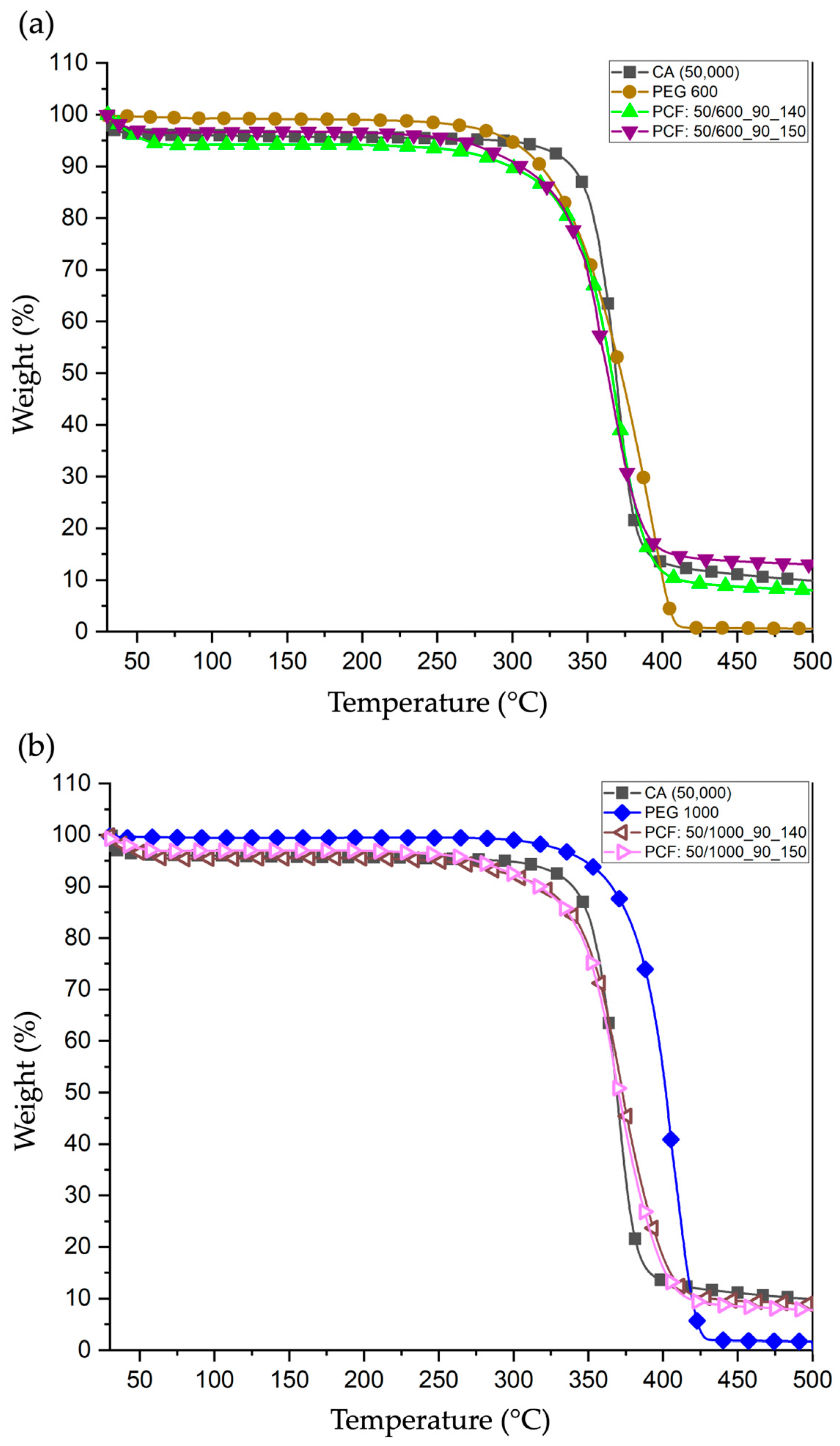
3.4.1. Thermogravimetric Analysis (TGA)
3.4.2. Differential Scanning Calorimetry (DSC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UHI | Urban Heat Island |
| PCM | Phase Change Materials |
| PCF | Phase Change Fibres |
| PEG | Polyethylene glycol |
| ATR-FTIR | Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy |
| TGA | Thermogravimetric Analysis |
| DSC | Differential Scanning Calorimetry |
| LHTS | Latent heat storage materials |
| DMF | Dimethylformamide |
| CA | Cellulose Acetate |
| dH2O | Distilled water |
| RT | Room temperature |
| Funi | Uniaxial fibres |
| Fhol | Hollow fibres |
| Mn | Molecular weight |
| Endo | Endothermic |
| Exo | Exothermic |
References
- Rajagopal, P.; Priya, R.S.; Senthil, R. A review of recent developments in the impact of environmental measures on urban heat island. Sustain. Cities Soc. 2023, 88, 104279. [Google Scholar] [CrossRef]
- Piracha, A.; Chaudhary, M.T. Urban Air Pollution, Urban Heat Island and Human Health: A Review of the Literature. Sustainability 2022, 14, 9234. [Google Scholar] [CrossRef]
- Segundo, I.R.; Freitas, E.; Branco, V.C.; Landi, S.; Costa, M.; Carneiro, J. Review and analysis of advances in functionalized, smart, and multifunctional asphalt mixtures. Renew. Sustain. Energy Rev. 2021, 151, 111552. [Google Scholar] [CrossRef]
- Kim, E.S.; Bae, C.; Ko, S.Y.; Won, J.E.; Lee, J.H.; Paio, Y.; Lee, D.K. Enhancing the effectiveness of heat adaptation strategies through citizen science-based outdoor thermal comfort. Heliyon 2024, 10, e39413. [Google Scholar] [CrossRef] [PubMed]
- Riahi, A.; Shafii, M.B. Experimental Evaluation of a Vapor Compression Cycle Integrated With a Phase Change Material Storage Tank for Peak Load Shaving. Eng. Sci. 2023, 23, 870. [Google Scholar] [CrossRef]
- Matos, A.M.; Delgado, J.M.P.Q.; Guimarães, A.S. Linking Energy Poverty with Thermal Building Regulations and Energy Efficiency Policies in Portugal. Energies 2022, 15, 329. [Google Scholar] [CrossRef]
- Gogoi, M.; Das, B.; Patowari, P.K. Characterization and performance analysis of modified phase change material with paraffin wax and waste exhaust carbon particles for thermal energy storage. J. Energy Storage 2025, 108, 115068. [Google Scholar] [CrossRef]
- Pinheiro, C.; Landi, S.; Lima, O.; Ribas, L.; Hammes, N.; Segundo, I.R.; Homem, N.C.; Branco, V.C.; Freitas, E.; Costa, M.F.; et al. Advancements in Phase Change Materials in Asphalt Pavements for Mitigation of Urban Heat Island Effect: Bibliometric Analysis and Systematic Review. Sensors 2023, 23, 7741. [Google Scholar] [CrossRef]
- Varadharajan, S.; Vasanthan, K.S.; Verma, S. Recent development in nano-phase change materials and their applications in enhancing thermal capacity of intelligent buildings: A state-of-the art review. J. Mater. Res. 2023, 38, 1463–1487. [Google Scholar] [CrossRef]
- Asadi, I.; Ji, G.; Steiner, G.; Baghban, M.H. Impact of microencapsulated phase change materials (PCMs) on the thermal and mechanical performance of cement mortar. Dev. Built Environ. 2024, 21, 100594. [Google Scholar] [CrossRef]
- Wang, X.; Ma, B.; Li, S.; Si, W.; Wei, K.; Zhang, H.; Zhou, X.; Fang, Y.; Kang, X.; Shi, W. Review on application of phase change materials in asphalt pavement. J. Traffic Transp. Eng. (Engl. Ed.) 2023, 10, 185–229. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, Y.; He, Y.; Jin, Z.; Zhang, Y.; Shi, L. Novel modular PCM wall board for building heating energy efficiency: Material preparation, manufacture and dynamic thermal testing. Appl. Therm. Eng. 2024, 256, 124168. [Google Scholar] [CrossRef]
- Drissi, S.; Ling, T.-C.; Mo, K.H. Development of leak-free phase change material aggregates. Constr. Build. Mater. 2020, 230, 117029. [Google Scholar] [CrossRef]
- Hammes, N.; Pinheiro, C.; Segundo, I.R.; Homem, N.C.; Silva, M.M.; Felgueiras, H.P.; Soares, G.M.B.; Freitas, E.; Costa, M.F.M.; Carneiro, J.A.O. Coaxial Fibres Incorporated with Phase Change Materials for Thermoregulation Applications. Appl. Sci. 2024, 14, 2473. [Google Scholar] [CrossRef]
- Miranda, C.S.; Marinho, E.; Seabra, C.L.; Evenou, C.; Lamartine, J.; Fromy, B.; Costa, S.P.; Homem, N.C.; Felgueiras, H.P.; Amorim, M. Antimicrobial, antioxidant and cytocompatible coaxial wet-spun fibers made of polycaprolactone and cellulose acetate loaded with essential oils for wound care. Int. J. Biol. Macromol. 2024, 277, 134565. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Q.; Hu, J.; Li, R.; Lin, J.; Liu, Y. Core-sheath phase change fibers via coaxial wet spinning for solar energy active storage. Compos. Part B Eng. 2022, 247, 110346. [Google Scholar] [CrossRef]
- Ahn, Y.-H.; DeWitt, S.J.A.; McGuire, S.; Lively, R.P. Incorporation of Phase Change Materials into Fibers for Sustainable Thermal Energy Storage. Ind. Eng. Chem. Res. 2021, 60, 3374–3384. [Google Scholar] [CrossRef]
- Niu, Z.; Qi, S.; Shuaib, S.S.A.; Züttel, A.; Yuan, W. Flexible core-sheath thermochromic phase change fibers for temperature management and electrical/solar energy harvesting. Compos. Sci. Technol. 2022, 226, 109538. [Google Scholar] [CrossRef]
- Radishevskii, M.B.; Serkov, A.T. Coagulation Mechanism in Wet Spinning of Fibres. Fibre Chem. 2005, 37, 266–271. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Im, J.; Lee, D.-M.; Kim, M.C.; Oh, D.; Son, J.; Park, S.; Hyun, K.; Jeong, B.; Lee, J. Coagulation engineering of surfactant-based wet spinning of carbon nanotube fibers. Carbon Lett. 2024, 34, 1803–1815. [Google Scholar] [CrossRef]
- Aneem, T.; Wong, S.; Afrin, H.; Nurunnabi, M.; Li, X.; Arafat, M. Investigation of coagulation process of wet-spun sodium alginate polymannuronate fibers with varied functionality using organic coagulants and cross-linkers. Mater. Today Chem. 2021, 22, 100580. [Google Scholar] [CrossRef]
- Pagliero, M.; Khayet, M.; García-Payo, C.; García-Fernández, L. Hollow fibre polymeric membranes for desalination by membrane distillation technology: A review of different morphological structures and key strategic improvements. Desalination 2021, 516, 115235. [Google Scholar] [CrossRef]
- Pereira, C.; Pinto, T.V.; Santos, R.M.; Correia, N. Sustainable and Naturally Derived Wet Spun Fibers: A Systematic Literature Review. Fibers 2024, 12, 75. [Google Scholar] [CrossRef]
- Pérez-Silva, I.; Canales-Feliciano, G.D.; Rodríguez, J.A.; Mendoza-Huizar, L.H.; Pérez-Estrada, S.; Ibarra, I.S.; Páez-Hernández, M.E. The Evaluation of Cellulose Acetate Capsules Functionalized for the Removal of Cd(II). Polymers 2023, 15, 3917. [Google Scholar] [CrossRef] [PubMed]
- Paberit, R.; Rilby, E.; Göhl, J.; Swenson, J.; Refaa, Z.; Johansson, P.; Jansson, H. Cycling Stability of Poly(ethylene glycol) of Six Molecular Weights: Influence of Thermal Conditions for Energy Applications. ACS Appl. Energy Mater. 2020, 3, 10578–10589. [Google Scholar] [CrossRef]
- Hammes, N.; Monteiro, J.; Pinheiro, C.; Felgueiras, H.P.; Soares, G.M.B.; Segundo, I.R.; Costa, M.F.M.; Carneiro, J. Exploring the Cutting Process of Coaxial Phase Change Fibers under Optical Characterization Tests. Appl. Sci. 2024, 14, 8050. [Google Scholar] [CrossRef]
- Segundo, I.R.; Landi, S.; Margaritis, A.; Pipintakos, G.; Freitas, E.; Vuye, C.; Blom, J.; Tytgat, T.; Denys, S.; Carneiro, J. Physicochemical and Rheological Properties of a Transparent Asphalt Binder Modified with Nano-TiO2. Nanomaterials 2020, 10, 2152. [Google Scholar] [CrossRef]
- Ratri, C.R.; Aguta, T.B.; Arundati, A.H.; Rohib, R.; Chalid, M.; Astutiningsih, S.; Nugraha, A.F. Effect of Coagulation Bath Composition on Cellulose-Based Polymer Electrolyte Fabricated via Non-Solvent-Induced Phase Separation Method. Int. J. Technol. 2023, 14, 1605–1614. [Google Scholar] [CrossRef]
- Lin, L.; Liu, C.; Sacci, R.L.; Chen, X.C.; Doughty, B. Molecular structures of residual solvent in polyacrylonitrile based electrolytes: Implications for conductivity and stability. J. Chem. Phys. 2024, 161, 233578. [Google Scholar] [CrossRef]
- Nguyen, K.D. Temperature Effect of Water Coagulation Bath on Chitin Fiber Prepared through Wet-Spinning Process. Polymers 2021, 13, 1909. [Google Scholar] [CrossRef]
- Kulichikhin, V.; Makarov, I.; Mironova, M.; Golova, L.; Vinogradov, M.; Shandryuk, G.; Levin, I.; Arkharova, N. A Role of Coagulant in Structure Formation of Fibers and Films Spun from Cellulose Solutions. Materials 2020, 13, 3495. [Google Scholar] [CrossRef] [PubMed]
- Sanahuja-Embuena, V.; Khensir, G.; Yusuf, M.; Andersen, M.F.; Nguyen, X.T.; Trzaskus, K.; Pinelo, M.; Helix-Nielsen, C. Role of Operating Conditions in a Pilot Scale Investigation of Hollow Fiber Forward Osmosis Membrane Modules. Membranes 2019, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Huang, J.; Guo, Z.; Bin Xu, B.; Cao, Y.; Ren, J.; Hou, H.; Xiao, Y.; Elashiry, M.; El-Bahy, Z.M.; et al. Hydrophobic Multilayered PEG@PAN/MXene/PVDF@SiO2 Composite Film with Excellent Thermal Management and Electromagnetic Interference Shielding for Electronic Devices. Small 2024, 20, e2402938. [Google Scholar] [CrossRef]
- Cao, H.; Xu, Z.; Zhang, T.; Zhao, Y. Highly stretchable phase change fibers from coaxial wet spinning of nonaqueous emulsions for temperature regulation. Chem. Eng. J. 2023, 478, 147389. [Google Scholar] [CrossRef]
- Coelho, G.; Champion, D.; Heintz, O.; Krystianiak, A.; Debon, S.; Deleris, I.; Wallecan, J.; Roudaut, G. Impact of processing and storage on citrus fiber functionality: Insights from spectroscopic techniques. Int. J. Biol. Macromol. 2024, 282, 137281. [Google Scholar] [CrossRef]
- Sukmawan, R.; Kusmono; Wildan, M.W. Study of alkali and acetylation treatments on sisal fibers compatibility with low-amine/epoxy stoichiometric ratio. Results Eng. 2024, 24, 103127. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yoo, C.G. Observation of Potential Contaminants in Processed Biomass Using Fourier Transform Infrared Spectroscopy. Appl. Sci. 2020, 10, 4345. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Magazù, S. Ethylene Glycol—Polyethylene Glycol (EG-PEG) Mixtures: Infrared Spectra Wavelet Cross-Correlation Analysis. Appl. Spectrosc. 2017, 71, 401–409. [Google Scholar] [CrossRef]
- Yacob, N.; Yusof, M.R.; Mahmud, M.; Mohamed, A.Z.; Badri, K.H. Effect of Different Molecular Weight and Concentration of Polyethylene Glycol (PEG) on Tensile and Morphology of Sago Starch Film. ASM Sci. J. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Teixeira, S.C.; de Oliveira, T.V.; Fortes-Da-Silva, P.; Raymundo-Pereira, P.A.; Ribeiro, A.R.C.; Batista, L.F.; Gomes, N.O.; Stringheta, P.C.; Soares, N.d.F.F. Investigation of the influence of plasticizers on the biodegradability of cellulose acetate. J. Appl. Polym. Sci. 2023, 140, 54316. [Google Scholar] [CrossRef]
- Sadeghpour, M.; Yusoff, R.; Aroua, M.K.; Tabandeh, M. Modification of polyethylene glycol with choline chloride and evaluation of the CO2 absorption capacity of their aqueous solutions. Greenh. Gases Sci. Technol. 2018, 8, 324–334. [Google Scholar] [CrossRef]
- Lascurain, P.G.G.; Frigione, M.; Sarcinella, A.; Linares, F.; Schröer, L.; Cnudde, V.; Toniolo, L.; Goidanich, S. Improving PEG confinement in smart aggregates for lime-based mortars. Constr. Build. Mater. 2024, 454, 138996. [Google Scholar] [CrossRef]
- Prasad, N.S.; Babarao, R.; Madapusi, S.; Sridhar, S.; Choudhury, N.R.; Bhargava, S.K. Residual solvent induced physical morphology and gas permeation in polyamide-imide membrane: Experimental investigation and molecular simulations. Eur. Polym. J. 2022, 165, 111012. [Google Scholar] [CrossRef]
- Minea, A.A. State of the Art in PEG-Based Heat Transfer Fluids and Their Suspensions with Nanoparticles. Nanomaterials 2021, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, H.; Zhang, N.; Sun, Q.; Cao, X. Effect of water content on the phase transition temperature, latent heat and water uptake of PEG polymers acting as endothermal-hydroscopic materials. J. Therm. Anal. Calorim. 2016, 126, 699–708. [Google Scholar] [CrossRef]
- Noel, J.; Jannot, Y.; Métivier, C.; Sgreva, N.R. Thermal characterization of polyethylene glycol 600 in liquid and solid phase and across the phase transition. Thermochim. Acta 2022, 716, 179326. [Google Scholar] [CrossRef]
- Hu, Y.; Liao, Y.; Zheng, Y.; Ikeda, K.; Okabe, R.; Wu, R.; Ozaki, R.; Xu, J.; Xu, Q. Influence of Cooling Rate on Crystallization Behavior of Semi-Crystalline Polypropylene: Experiments and Mathematical Modeling. Polymers 2022, 14, 3646. [Google Scholar] [CrossRef]
- Yan, Y.; Li, W.; Zhu, R.; Lin, C.; Hufenus, R. Flexible Phase Change Material Fiber: A Simple Route to Thermal Energy Control Textiles. Materials 2021, 14, 401. [Google Scholar] [CrossRef]


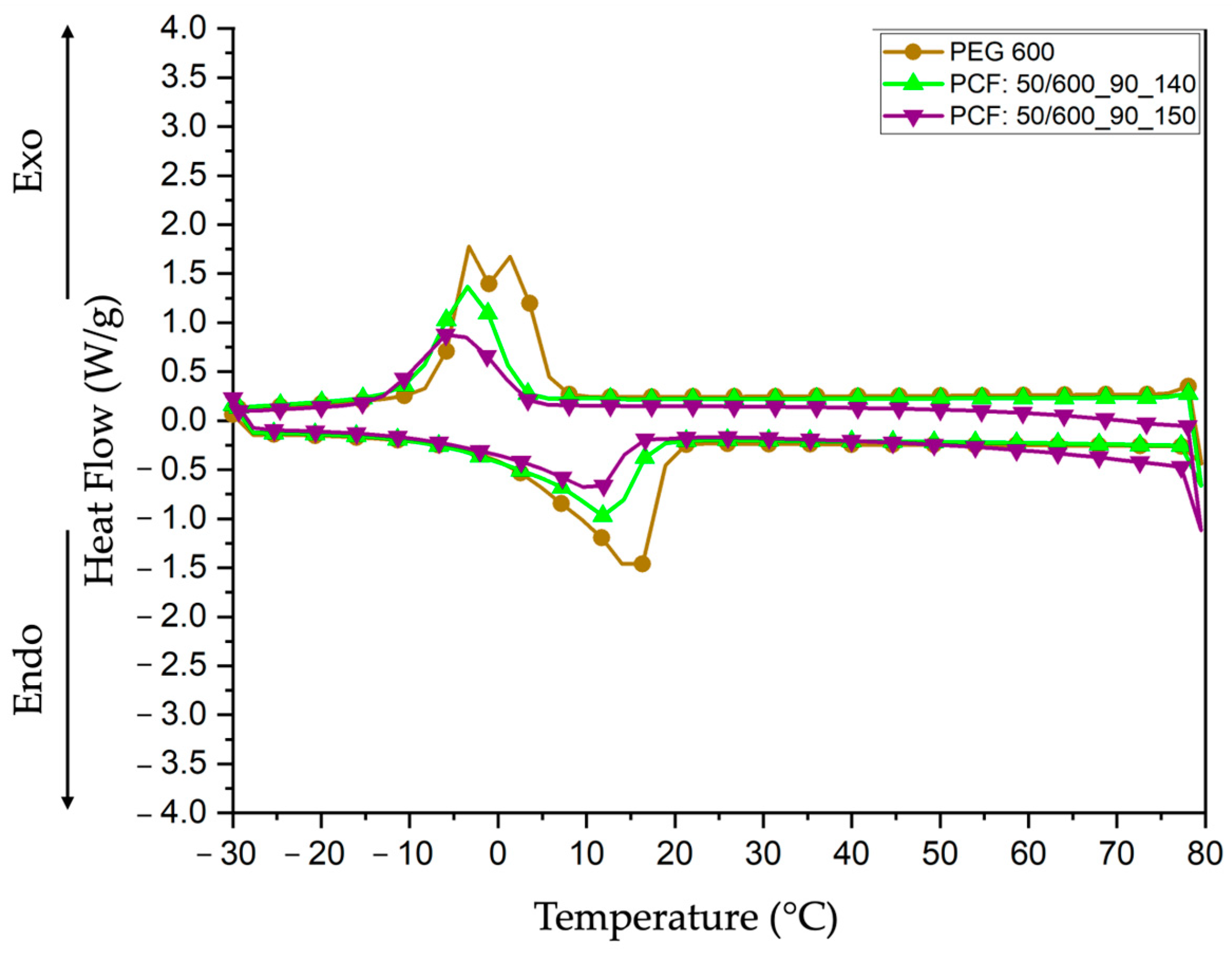
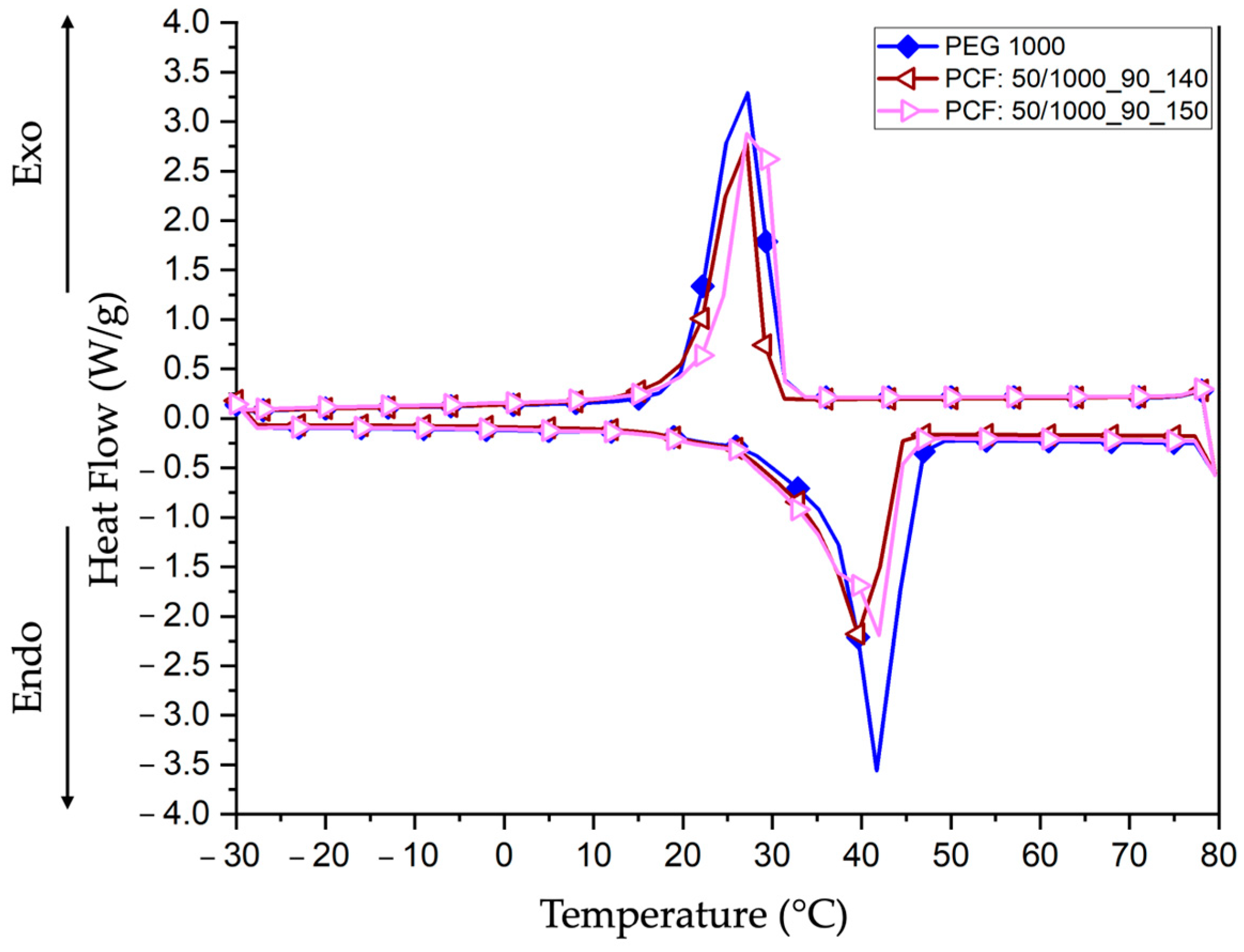
| Samples | Peak Temperature Onset (°C) | Enthalpy Endo (J/g) | Peak Temperature Endset (°C) | Enthalpy Exo (J/g) |
|---|---|---|---|---|
| PEG 600 | 15.76 | 68.21 | −1.21 | 72.74 |
| PCF:50/600_90_140 | 12.64 | 39.24 | −3.78 | 50.17 |
| PCF:50/600_90_150 | 11.04 | 30.59 | −5.30 | 40.93 |
| Samples | Peak Temperature Onset (°C) | Enthalpy Endo (J/g) | Peak Temperature Endset (°C) | Enthalpy Exo (J/g) |
|---|---|---|---|---|
| PEG 1000 | 41.24 | 93.62 | 26.59 | 112.96 |
| PCF:50/1000_90_140 | 40.32 | 83.47 | 26.95 | 84.66 |
| PCF:50/1000_90_150 | 41.13 | 98.88 | 28.35 | 88.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammes, N.; Monteiro, J.; Rocha Segundo, I.; Felgueiras, H.P.; Silva, M.M.; Costa, M.F.M.; Carneiro, J. Development of Co-Axial Fibres Composed of CA (Mn 50,000) and PEGs (600 and 1000): Evaluation of the Influence of the Coagulation Bath. Appl. Sci. 2025, 15, 3028. https://doi.org/10.3390/app15063028
Hammes N, Monteiro J, Rocha Segundo I, Felgueiras HP, Silva MM, Costa MFM, Carneiro J. Development of Co-Axial Fibres Composed of CA (Mn 50,000) and PEGs (600 and 1000): Evaluation of the Influence of the Coagulation Bath. Applied Sciences. 2025; 15(6):3028. https://doi.org/10.3390/app15063028
Chicago/Turabian StyleHammes, Nathalia, José Monteiro, Iran Rocha Segundo, Helena P. Felgueiras, M. Manuela Silva, Manuel F. M. Costa, and Joaquim Carneiro. 2025. "Development of Co-Axial Fibres Composed of CA (Mn 50,000) and PEGs (600 and 1000): Evaluation of the Influence of the Coagulation Bath" Applied Sciences 15, no. 6: 3028. https://doi.org/10.3390/app15063028
APA StyleHammes, N., Monteiro, J., Rocha Segundo, I., Felgueiras, H. P., Silva, M. M., Costa, M. F. M., & Carneiro, J. (2025). Development of Co-Axial Fibres Composed of CA (Mn 50,000) and PEGs (600 and 1000): Evaluation of the Influence of the Coagulation Bath. Applied Sciences, 15(6), 3028. https://doi.org/10.3390/app15063028











