Abstract
This study aimed to assess the effect of real-time vertical ground reaction force (VGRF) biofeedback on sagittal plane hip and knee joint biomechanics and extensor muscle activities in older adults. Fifteen healthy older adults (71 ± 5.8 years) walked on a treadmill while instructed to increase their first peak of VGRF via biofeedback. Whole-body kinetic and kinematic data and electromyography data for the vastus lateralis and gluteus maximus muscles were recorded. A one-way repeated measure ANOVA followed by post hoc analysis was conducted. Results showed increases in peak VGRF (20.95%), knee extension torque (73.7%), knee flexion angle (53.8%), and vastus lateralis muscle activity (72.1%) during the loading response, with percentage changes calculated as the mean of acquisition and recall trials relative to baseline walking. In contrast, no significant effect on peak hip extension torque and hip flexion angle over time was observed. These findings suggest that biofeedback can induce greater vertical support forces production with increased knee extension torque and extensor muscle activity. In addition, older adults adopted higher vertical support without increasing hip joint torque, potentially aiding in mitigating age-related distal-to-proximal joint torque redistribution. These findings suggest that VGRF biofeedback could potentially be an effective intervention to enhance knee extensor activation and mobility in older adults without increasing hip load.
1. Introduction
As people age, the generation of vertical support forces during walking declines [1,2,3,4,5]. Vertical support forces are the forces acting on the body in the vertical direction, playing a crucial role in maintaining stability and supporting body weight during weight-bearing activities. The magnitude of vertical ground reaction forces (VGRF) is influenced by factors such as muscle strength, gait mechanics, and overall body mass. In older adults, a decline in VGRF is often associated with lower-limb muscle weaknesses and slower walking speed in older adults [6,7] and may increase the risk of falls. VGRF during the stance phase relies on the combined output of ankle, knee, and hip extension torques [8]. Compared to younger adults, older adults show a redistribution of joint torques, relying more on hip extensors and less on ankle plantar flexors and knee extensors [1,5,9,10,11,12,13,14,15,16]. This distal-to-proximal joint torque redistribution appears to be consistent regardless of walking speed, level of physical activity, or gender [10,17] and is associated with an increased energy cost of walking in the elderly population [18].
With aging, there is a well-documented reduction in knee extensor function [19,20,21,22], which has been associated with slower walking speed and shorter step length in healthy older adults [23]. After heel strike, knee joint flexion provides shock absorption during the loading response phase when the body weight is transferred onto the leading limb. At this moment, knee extensor muscles contract eccentrically to control the knee flexion motion [24,25]. Older adults show a moderate degree of atrophy in their quadriceps muscles [20], which may compromise knee extensor function during gait. The higher reliance on hip extensor torque in older adults suggests a potential compensatory mechanism for knee extensor muscle weakness [15,26]. Consequently, task-oriented walking training targeting both vertical support forces and knee extensor torque in older adults is needed.
Previous research has shown that real-time force biofeedback effectively increases VGRF peak and knee extension torque in individuals with knee pathologies. For instance, in post-ACL reconstruction patients, a 5% increase in the first peak of VGRF led to a 13.22% increase in knee extension torque [27,28]. However, no studies have specifically investigated the effect of VGRF biofeedback on older adults, who typically exhibit lower VGRF during walking and rely more on hip extensors due to knee extensor weakness. This study addresses this gap by investigating whether VGRF biofeedback can enhance knee extension torque and reduce compensatory hip torque, thereby mitigating age-related distal-to-proximal joint torque redistribution. Therefore, the purpose of this study was to determine the effect of VGRF real-time biofeedback on knee and hip joint kinetics, kinematics, and extensor muscle activities in older adults. We hypothesized that older adults would show increased peak VGRF, peak knee extension torque, peak knee flexion angle, knee flexion excursion, and knee extensor muscle activities during biofeedback, immediately following the biofeedback, and after a 5 min break, compared to baseline walking. We further hypothesize that with increasing VGRF, older adults will show greater knee extension torque contribution relative to hip extension torque during biofeedback and recall trials compared to baseline walking.
2. Materials and Methods
2.1. Participants
Fifteen healthy older adults (65–83 years, 6 female) were recruited from university community and senior activity centers. Participants were included if they were able to walk on a treadmill for at least 6 min without any assistive devices. Exclusion criteria included having any lower extremity musculoskeletal injury in the six months prior to data collection and any neurological and cardiovascular restrictions limiting participation in daily physical activities. A sample size of 12 was estimated with an α-error of 0.05 and power of 0.80, based on effect sizes from a previous study [27] (G*power Statistical Power Analysis Software v3.1). Fifteen participants were included to account for dropouts. This study was approved by the Institutional Review Board of University of Texas at Austin (HRP-UT 901), and all participants provided written informed consent.
2.2. Experimental Protocol and Visual Biofeedback
A total of 42 reflective markers were attached to anatomical landmarks on the trunk, pelvis, and lower limbs (acromioclavicular joints, jugular notch of the clavicle, the xiphoid process of the sternum, spinous process of C7 and T10, anterior superior iliac spine, posterior superior iliac spine, lateral femur epicondyle, lateral malleolus, the first, second, and fifth metatarsal heads, and calcaneus), 4-marker rigid clusters were used for tracking the thigh and shank, and additional medial knee and ankle markers were added during static standing calibration trial (Figure 1).
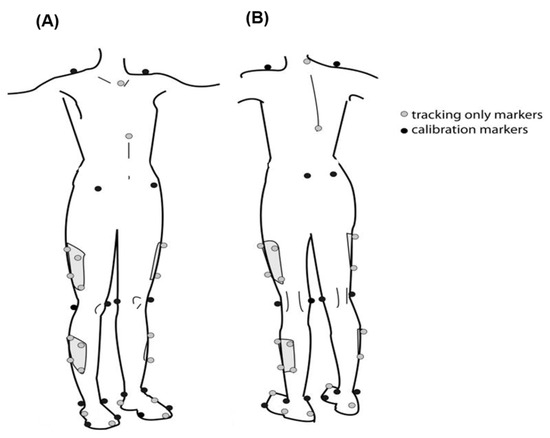
Figure 1.
Front (A) and rear (B) views of marker placement, showing tracking-only markers (gray) and calibration markers (black) on key anatomical landmarks, including the shoulders, pelvis, thighs, knees, shins, ankles, and feet.
Participants wore a harness solely for safety purposes during treadmill walking. To determine their comfortable walking speed (CWS), participants gradually increased the treadmill speed in 0.1 m/s increments every 15 s until they reported their preferred speed. Following this, participants walked at their CWS for 3 min as a warm-up. A 3 min baseline walking trial was then conducted at a fixed speed of 1.2 m/s to minimize the influence of speed variations on gait parameters such as joint kinematics and kinetics [29].
The biofeedback training phase consisted of three 6 min bouts of walking at a fixed speed of 1.2 m/s. The first bout served as familiarization with the VGRF biofeedback system. The second bout was designated as the acquisition phase, during which the last minute of walking with visual biofeedback active was used to define the acquisition trial. The third bout reinforced the training and was immediately followed by a one-minute walking trial without visual feedback, defined as recall 1. Participants were provided with a 5 min seated rest period between each biofeedback bout (Figure 2). After completing the third bout and the recall 1 trial, participants rested for another 5 min in a seated position. This was followed by a 3 min walking trial without visual feedback, defined as recall 2. During both recall trials, the visual biofeedback was removed, and participants were instructed to attempt to maintain the same increased VGRF as during the biofeedback trials.”
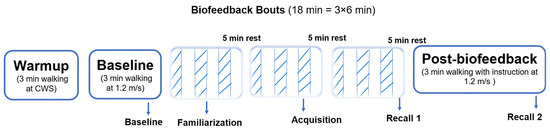
Figure 2.
The VGRF biofeedback training protocol. The alternating stripes and blanks indicate that biofeedback was on and off, respectively, during that minute.
Real-time VGRF biofeedback was displayed on a screen using D-flow software (version 3.20.0), with VGRF trajectories for the right and left limbs shown as blue and red graphs, respectively. A horizontal target line (yellow line), set at 10% above baseline during familiarization and 15% above baseline during training, guided participants to increase their VGRF (Figure 3). To reduce reliance on visual feedback, intermittent biofeedback (1 min on, 1 min off) was employed during the training bouts (Figure 2).

Figure 3.
The experimental setup involved projecting real-time vertical ground reaction force (VGRF) biofeedback in front of the participants with a horizontal yellow targeted line set at 10 to 15% higher than their baseline first peak of VGRF.
2.3. Data Collection and Processing
The kinetic and kinematic data were collected during the last minute of baseline, the first minute of the third bout of biofeedback adaptation (acquisition), immediately after biofeedback (recall 1), and after a 5 min break (recall 2). GRFs were captured at 1000 Hz using a force platform embedded in the instrumented split-belt treadmill (Motek Medical, Amsterdam, The Netherlands). Three-dimensional kinematic data were captured at 100 Hz using a 10-camera Vicon motion capture analysis system (Oxford Metrics, Oxford, UK). Surface electromyography (EMG) electrodes with wireless preamplifiers (Trigno, Delsys, Inc., Boston, MA, USA) were placed on the right leg’s gluteus maximus (GMAX) and vastus lateralis (VL) muscles. EMG signals were sampled at 2000 Hz and visually checked for quality when participants contracted each muscle. Each participant’s comfortable and fastest overground walking speed was measured twice along a 10 m walkway before and after the training session to evaluate the effect of VGRF biofeedback on overground walking speed.
GRFs and marker trajectories were low-pass filtered using a fourth-order Butterworth filter at 20 Hz and 6 Hz, respectively. Kinetic and kinematic variables for the last 30 s of each trial were calculated in Visual3D (C-motion Inc., Germantown, MD, USA). Net joint torques were calculated via inverse dynamics. EMG signals were processed in MATLAB (23.2.0.2391609 (R2023b)) (MathWorks, Inc., Natick, MA, USA) using a Butterworth bandpass filter (16–500 Hz), full-wave rectification, and a Butterworth low-pass filter at 20 Hz. EMG signals during biofeedback and recall trials were normalized to the mean EMG signal during the stance phase of baseline walking.
All the kinetic and kinematic variables were recorded during the last minute of each trial of the baseline, acquisition, and recall trials and analyzed during the last 30 s of each trial.
Primary outcome variables included peak VGRF, knee extension torque, and knee flexion angle during the first half of the stance phase. Secondary outcome measures included peak hip extension torque, hip flexion angle, knee flexion excursion, knee extension torque contribution, and peak VL and GMAX normalized EMG during the first half of the stance phase. Knee flexion excursion was calculated as the difference between peak knee flexion angle during loading response and knee flexion angle at heel strike [27]. To evaluate how biofeedback affects the knee extension torque changes relative to the hip extension torque, the knee extension torque relative contribution was calculated as
Knee Extension Torque Contribution Percentage = (Knee extension torque/ (Knee extension torque + Hip extension torque)) ×100.
A higher percentage indicates a greater reliance on knee extension torque relative to hip extension torque. All variables were calculated for each stride and averaged across strides on the right leg for each trial.
2.4. Statistical Analysis
Shapiro–Wilk’s test was used to evaluate the distribution of all variables. A one-way repeated measure analysis of variance (rmANOVA) was used to test for a significant main effect of time point (i.e., baseline, acquisition, recall 1, and recall 2) for each variable. If significance was detected, post hoc pairwise comparisons using the Bonferroni adjustment were performed to compare differences between each time point. A paired-t test was used to compare the overground walking speed before and after training. An alpha level of 0.05 was used for all analyses.
3. Results
Two participants were excluded due to technical issues, as we were unable to measure force data from the force plates embedded in the treadmill. Data from the remaining thirteen participants (age: 71 ± 5.82 years, height: 172.61 ± 8.14 cm, weight: 76.63 ± 17.49 kg, body mass index (BMI): 25.50 ± 4.67 kg/m2) were analyzed.
All variables were normally distributed. No significant differences in step length across time points were found (F3,36 = 0.43, p = 0.73; Table 1). A significant main effect of time point on cadence was detected (F3,36 = 4.50, p = 0.02; Table 1), with higher cadence during acquisition, recall 1, and recall 2 compared to baseline (p < 0.05 for all comparisons) (Table 1).

Table 1.
Gait kinematics and percentage of knee extension torque contribution at a controlled speed of 1.2 m/s across walking conditions (N = 13).
Figure 4 presents VGRF, knee and hip joints angles, and torque trajectories during the stance phase of walking from a representative participant across different time points. A significant main effect of time point on the peak VGRF was detected (F3,36 = 24.68, p < 0.001; Table 1, Figure 5). The first peak of VGRF was greater during acquisition and recall trials compared to baseline (p < 0.05 for all comparisons), with no significant differences between acquisition and recall trials. These data suggest that older adults were able to increase the first peak of VGRF by an average of 20.95% with visual biofeedback during acquisition and recall trials compared to baseline walking.
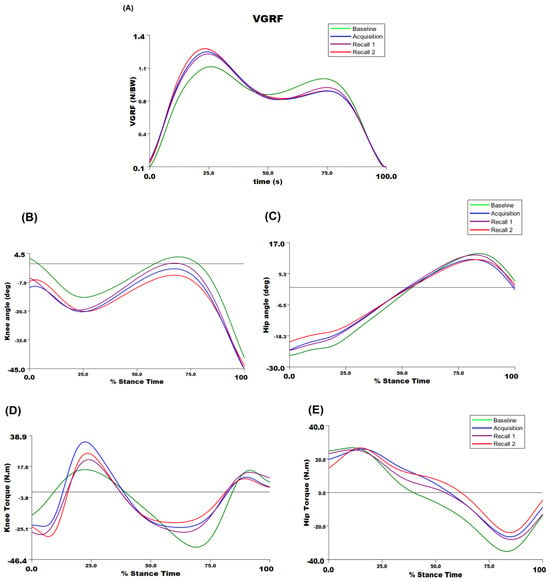
Figure 4.
The biomechanical outcome trajectories for a representative participant for (A) vertical ground reaction force; (B) knee joint angle; (C) hip joint angle; (D) knee joint torque; and (E) hip joint torque during the stance phase of walking across time points. For joint angles and torques, the positive sign is extension, and the negative sign is flexion.
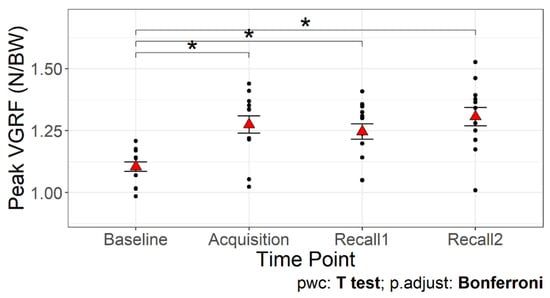
Figure 5.
The first peak of the vertical ground reaction force across time points. The red triangles are the mean values with error bars showing standard error. Asterisks (*) indicate significant differences compared to baseline walking, p < 0.05.
There was a significant main effect of time point on the peak knee flexion angle during the first half of the stance phase (F3,36 = 24.66, p < 0.001). The peak knee flexion angle was greater during the acquisition and recall trials than baseline (p < 0.001 for all comparisons; Figure 6A), with no difference between acquisition and recall trials. Additionally, knee flexion excursion showed a significant main effect of time point (F3,36 = 4.44, p < 0.05; Table 1). Knee flexion excursion was smaller during acquisition and recall 2 trials compared to baseline (p < 0.05). In addition, the peak knee flexion angle during the swing phase showed a significant main effect of time point (F3,36 = 7.97, p < 0.001; Table 1). The peak knee flexion angle during the swing phase was greater during acquisition and recall 2 compared to baseline (p < 0.05), but not during recall 1. No significant main effect was observed for the peak hip flexion angle (p = 0.11; Figure 6B). These results suggest that while older adults responded to VGRF biofeedback by increasing knee flexion during swing and loading response, their knee position remained relatively unchanged during the first half of the stance phase. Additionally, the lack of change in hip flexion angle indicates that they maintained their usual hip mechanics, suggesting a localized effect of the biofeedback on knee mechanics rather than a broader alteration in gait strategy.
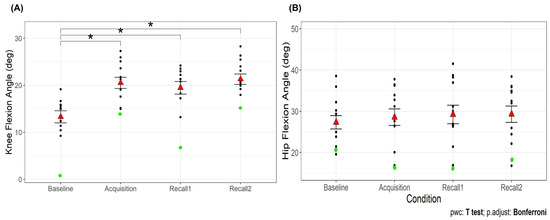
Figure 6.
Peak knee flexion angle (A) and peak hip flexion angle (B) during the first half of the stance phase of walking across time points. The red triangles are the mean values with error bars showing standard error. Asterisks (*) indicate significant difference from baseline walking, p < 0.05. The green dots are the participant (#15) who has been identified as an outlier during baseline and recall 1 for knee flexion angle.
There was a significant main effect of time point on peak knee extension torque (F3,36 = 9.79, p < 0.001). The peak knee extension torque was greater during the acquisition and recall 1 trials than baseline (p < 0.05 for all comparisons; Figure 7A). No significant main effect was observed for hip extension torque (p = 0.44; Figure 7B). Additionally, there was a significant main effect of time point on the percentage of knee extension torque contribution (F3,36 = 10.67, p < 0.001; Table 1), with higher percentages during acquisition and recall trials compared to baseline (p < 0.05). This suggests that VGRF biofeedback effectively increased knee extension torque during walking, with sustained effects observed in recall trials. The improvement persisted even after a short break, although the statistical significance in recall 2 was dependent on the exclusion of an outlier. In contrast, hip extension torque remained unchanged, indicating that participants did not compensate by increasing hip involvement. Furthermore, the increased percentage of knee extension torque contribution suggests a shift in joint-loading strategy, with a greater reliance on knee extensors rather than hip extensors, potentially mitigating age-related distal-to-proximal torque redistribution.
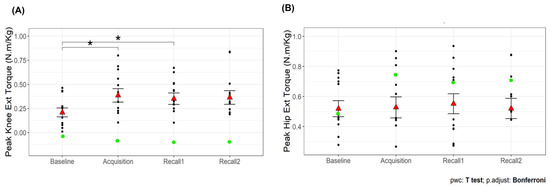
Figure 7.
Peak knee extension torque (A) and peak hip extension torque (B) during the first half of the stance phase of walking across time points. The red triangles are the mean values with error bars showing standard error. Asterisks (*) indicate significant difference from baseline walking, p < 0.05. The green dots are the participant (#15) who has been identified as an outlier during baseline and recall 1 for knee flexion angle.
Our analysis identified an outlier in knee flexion angle during the baseline and recall 1 trial, represented by a green dot in Figure 6A. The inclusion or exclusion of this outlier did not affect the overall results for most variables. However, for knee extension torque during recall 2, the significance of the difference from baseline was shown only after excluding the outlier.
There was a significant main effect of time point on maximum EMG of VL (F3,36 = 4.02, p < 0.05; Figure 8A) and GMAX (F3,36 = 6.26, p < 0.05; Figure 8B). Post hoc comparisons show that older adults increased their VL and GMAX muscle activities during acquisition and recall trials compared to baseline walking (p < 0.05), with no difference between acquisition and recall trials. These results showing that increasing the first peak of VGRF led to a greater activation of both knee and hip extensor muscles suggest an adaptive response to generate higher vertical support forces during walking.
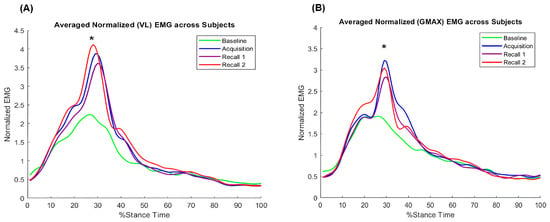
Figure 8.
Group average of normalized electromyography during the stance phase of walking across time points: (A) vastus lateralis (VL), (B) gluteus maximus (GMAX). Asterisks (*) indicate significant difference from baseline walking, p < 0.05.
A significant difference was found in overground comfortable walking speed before (1.35 ± 0.31) m/s or 4.86 ± 1.12 km/h) and after (1.46 ± 0.24 m/s or 5.26 ± 0.86 km/h) a single session of biofeedback walking (t = −2.52, df = 12, p = 0.02), but no difference was observed for overground faster walking speed.
4. Discussion
This study determined the short-term effects of real-time force feedback on hip and knee joint kinetics, kinematics, and extensor muscle activities. Our findings support our primary hypothesis that older adults increased their peak VGRF during loading response, with greater knee extension torque and knee extensor muscle activity during biofeedback and recall trials compared to baseline walking. In contrast, compared to baseline walking, older adults did not demonstrate an increase in their hip extension torque during biofeedback or recall trials. These results suggest that older adults can use biofeedback to increase the knee extension torque and generate vertical forces without increasing the contribution from the hip extension torque. In the clinic, wearable shoe sensors could be used to provide real-time feedback, encouraging older adults with reduced VGRF to improve force generation and enhance knee extensor engagement, potentially improving mobility.
In our study, older adults walked at a constant speed of 1.2 m/s across all conditions. This speed falls within the range of preferred walking speeds for older adults [12,30]. We observed that they walked with a higher cadence during biofeedback and recall trials without changing step length. We report the group average results for cadence and step length, rather than individual steps. The statistical significance of each variable was influenced by the mean differences and standard deviation (variability). It is possible that, because the variability in cadence is lower compared to step length, ANOVA was able to detect significance in cadence but not in step length.
Participants increased their first peak of VGRF by an average of 15.45% from baseline to acquisition, 12.27% in recall 1, and 19% in recall 2 trials. Consistent with previous studies [27,31], we showed that the biofeedback was effective in increasing the first peak of VGRF not only during the acquisition phase but also during recall trials, reflecting the short-term retention of the learned response. In addition, overground comfortable walking speed was increased after the single session biofeedback exercise, suggesting that limb loading could affect forward progression and that VGRF biofeedback has the potential to increase walking speed. Only one participant showed an increased VGRF, by 2.9%, during acquisition and did not show an increase during recall 2, possibly due to fatigue or recall difficulty. This highlights the need to consider individual variability and suggests that some participants may require additional rest or different strategies to maintain the learned behavior.
Analysis of knee and hip joint angles and torques demonstrated patterns consistent with previous research [9,32]. Hip and knee flexion were observed after foot contact, with peak hip and knee extension torque to stabilize the leg and support the body’s weight. Knee extension torque increased during biofeedback and recall trials by an average of 73.7% relative to baseline walking. A previous study on knee extension torque biofeedback demonstrated that targeting a 40% increase in peak knee extension torque during early stance resulted in a 9% increase in vertical ground reaction force (VGRF) [33]. There are pros and cons to using knee extension torque biofeedback compared to VGRF biofeedback. One key challenge of knee extension torque biofeedback is the complexity of real-time computation, as it requires a precise marker-based model and intricate biomechanical calculations. Additionally, participants may struggle to interpret knee extension torque feedback effectively. On the other hand, VGRF biofeedback offers several practical advantages: it is easier to implement, relies on readily available force sensors, and is generally more intuitive for real-time interpretation.
In the present study, participant #15 (the ‘green dot participant’) was identified as an outlier for knee flexion angle during baseline and recall 1 walking (Figure 6A). Including or excluding this participant did not affect knee flexion angle, so their data were included in all other analyses. Despite this deviation, statistical outcomes remained consistent across all variables except for knee extension torque during recall 2, where results varied depending on the inclusion or exclusion of this participant. When the outlier was excluded, a significant difference in knee extension torque was observed during recall 2 compared to baseline. This participant exhibited a distinct walking pattern compared to the rest of the group, characterized by walking with more extended (straighter) knees. As a result, they generated the lowest knee extension torque during walking.
In a healthy gait, the support moment is achieved by coordinated contributions from the ankle, knee, and hip joints [8]. With aging, older adults may increase their hip joint torque to compensate for lower ankle and knee torque output [9]. While dominant contribution from any individual joint is likely not desirable, an approach that allows individuals to modulate relative joint contributions may offer an opportunity for improving gait efficiency or safety. In the present study, biofeedback was used to promote increases in the first peak of VGRF. Our participants did not increase the hip flexion angle or hip extension torque. Rather, they increased the maximum knee flexion angle and produced higher knee extension torques, consistent with previous findings in patients with anterior cruciate ligament reconstruction [27,28]. These results suggest that VGRF biofeedback can increase vertical support through enhanced knee extensor torque, without changes in hip extension torque, making it a potential therapeutic tool for older adults with low VGRF and knee extension torque.
With aging, there is a general reduction in the lower extremity’s range of motion. Older adults often exhibit a reduced knee range of motion and lower peak knee flexion angle during loading response compared to younger adults [34]. Typically, a decrease in the peak knee flexion angle might suggest that the GRF torque arm was closer to the knee joint center, resulting in a lower knee extension torque. Interestingly, in this study we found that older adults increased knee flexion angle and knee extension torque during acquisition and recall trials. Additionally, the knee flexion angle during the swing phase increased, indicating that older adults bent their knees more during the swing phase with increased VGRF, leading to more flexed knees during the stance phase. Despite this, they exhibited reduced knee flexion excursion during biofeedback trials. This finding contrasts with a previous study in younger adults, where an increase in VGRF via biofeedback resulted in greater knee flexion excursions during biofeedback compared to baseline walking [27]. A potential explanation is that older adults may have stiffened up their legs to manage the increased loading force during biofeedback. Exercise programs using biofeedback to increase VGRF should consider the potential implications of reduced knee flexion excursion and evaluate its relevance to their specific target population.
Our study found that the VL and GMAX muscle EMG profiles during the first half of the stance phase were similar to previous studies, showing peaks in activities for both muscles during the loading response [35,36]. Both muscles’ activities increased during biofeedback and recall trials compared to baseline walking (Figure 8), indicating enhanced neuromuscular recruitment of hip and knee extensor muscles in response to the increase in the first peak of VGRF. The increased EMG of VL is consistent with higher knee extension torque during the loading response. However, the increased EMG of GMAX did not directly support the non-significant change in hip extension torque. This discrepancy might be due to the inverse dynamic approach not accounting for co-contraction of agonist and antagonist muscles. Older adults demonstrate higher antagonist coactivation during level walking compared to young adults [37,38]. Increased GMAX muscle activation could be accompanied by co-contraction of hip flexor muscles, which was not measured in this study. Future studies are needed to determine if VGRF biofeedback affects co-contraction of lower extremities in older adults.
There are some limitations in this study. First, we included healthy older adults who walked without any assistive devices in the community. Thus, the results may or may not be generalizable to frail older adults. Future research should consider including clinical populations with limb loading deficiencies during early stance to determine the applicability of VGRF biofeedback for individuals with specific mobility challenges. Second, we used a fixed walking speed of 1.2 m/s, which may not reflect the comfortable speed of all participants. On average, participants’ comfortable walking speed (CWS) overground was 1.35 ± 0.31 m/s, slightly higher than the fixed speed, while their treadmill CWS was 0.9 ± 0.17 m/s, which was lower than the fixed speed. This discrepancy suggests that 1.2 m/s may have been slightly above their natural treadmill walking speed, potentially causing discomfort or subtle adjustments in gait mechanics. Future studies should examine how deviations from a fixed speed influence biofeedback outcomes. Third, the study had a relatively small sample size of 13 older adults. While previous studies have used similar sample sizes to examine the effects of aging on walking [9,14], future research should include larger cohorts to enhance the robustness of the findings. Additionally, this study only involved a single session of biofeedback training, limiting our ability to assess the long-term effects of this intervention. We also did not measure muscle fatigue and cognitive load, both of which could impact the feasibility of force biofeedback. While biofeedback may help increase knee extension torque, it could also elevate fatigue risk, necessitating careful rest period management. Future studies should incorporate measures of muscle fatigue and energy expenditure to better understand the trade-offs between biofeedback benefits and the risks of fatigue in older adults. Finally, this study did not examine muscle co-contractions in relation to increasing the first peak of VGRF. Future research should explore these aspects to provide a more comprehensive understanding of the biomechanical changes induced by VGRF biofeedback during gait.
5. Conclusions
Our study showed the adaptability of older adults’ gait mechanics in response to real-time VGRF biofeedback during the loading response phase of walking. They achieved increased VGRF along with significant rises in knee extension torque and knee extensor muscle activity. However, no corresponding increase in hip extension torque was observed. This indicates that older adults can enhance vertical support through knee biomechanics adjustments rather than relying on increased hip extension torque.
Author Contributions
Conceptualization, F.F., S.S. and H.-Y.H.; methodology, F.F. and S.S.; software, F.F. and H.-Y.H.; validation, F.F. and H.-Y.H., formal analysis, F.F.; investigation, F.F.; resources F.F. and H.-Y.H.; data curation, F.F. and H.-Y.H.; writing—original draft preparation, F.F.; writing—review and editing, F.F., S.S. and H.-Y.H.; visualization, F.F.; supervision, H.-Y.H.; project administration. F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the University of Texas at Austin (protocol code HRP-UT901 [07/05/2021]).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Boyer, K.A.; Johnson, R.T.; Banks, J.J.; Jewell, C.; Hafer, J.F. Systematic review and meta-analysis of gait mechanics in young and older adults. Exp. Gerontol. 2017, 95, 63–70. [Google Scholar] [CrossRef]
- Larish, D.D.; Martin, P.E.; Mungiole, M. Characteristic Patterns of Gait in the Healthy Old. Ann. N. Y. Acad. Sci. 1988, 515, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Maie, K.; Kondo, S. The characteristics of walking in old men analysed from the ground reaction force. J. Anthropol. Soc. Nippon. 1988, 96, 7–15. [Google Scholar] [CrossRef][Green Version]
- Lim, J.Y.; Yi, Y.; Jung, S.W.; Park, D.-S. Comparison of vertical ground reaction forces between female elderly and young adults during sit-to-stand and gait using the Nintendo Wii Balance Board. Phys. Ther. Rehabil. Sci. 2018, 7, 179–185. [Google Scholar] [CrossRef]
- Toda, H.; Nagano, A.; Luo, Z. Age and gender differences in the control of vertical ground reaction force by the hip, knee and ankle joints. J. Phys. Ther. Sci. 2015, 27, 1833–1838. [Google Scholar] [CrossRef]
- Takahashi, T.; Ishida, K.; Hirose, D.; Nagano, Y.; Okumiya, K.; Nishinaga, M.; Doi, Y.; Yamamoto, H. Vertical ground reaction force shape is associated with gait parameters, timed up and go, and functional reach in elderly females. J. Rehabil. Med. 2004, 36, 42–45. [Google Scholar] [CrossRef]
- LaRoche, D.P.; Millett, E.D.; Kralian, R.J. Low strength is related to diminished ground reaction forces and walking performance in older women. Gait Posture 2011, 33, 668–672. [Google Scholar] [CrossRef]
- Winter, D.A. Overall principle of lower limb support during stance phase of gait. J. Biomech. 1980, 13, 923–927. [Google Scholar] [CrossRef] [PubMed]
- DeVita, P.; Hortobagyi, T. Age causes a redistribution of joint torques and powers during gait. J. Appl. Physiol. 2000, 88, 1804–1811. [Google Scholar] [CrossRef]
- Savelberg, H.H.C.M.; Verdijk, L.B.; Willems, P.J.B.; Meijer, K. The robustness of age-related gait adaptations: Can running counterbalance the consequences of ageing? Gait Posture 2007, 25, 259–266. [Google Scholar] [CrossRef]
- Monaco, V.; Rinaldi, L.A.; Macrì, G.; Micera, S. During walking elders increase efforts at proximal joints and keep low kinetics at the ankle. Clin. Biomech. 2009, 24, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Silder, A.; Heiderscheit, B.; Thelen, D.G. Active and passive contributions to joint kinetics during walking in older adults. J. Biomech. 2008, 41, 1520–1527. [Google Scholar] [CrossRef]
- Lim, Y.P.; Lin, Y.-C.; Pandy, M.G. Lower-limb muscle function in healthy young and older adults across a range of walking speeds. Gait Posture 2022, 94, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Cofré, L.E.; Lythgo, N.; Morgan, D.; Galea, M.P. Aging modifies joint power and work when gait speeds are matched. Gait Posture 2011, 33, 484–489. [Google Scholar] [CrossRef] [PubMed]
- McGibbon, C.A. Toward a Better Understanding of Gait Changes with Age and Disablement: Neuromuscular Adaptation. Exerc. Sport Sci. Rev. 2003, 31, 102–108. [Google Scholar] [CrossRef]
- Kirkwood, R.N.; Gomes, H.D.A.; Sampaio, R.F.; Culham, E.; Costigan, P. Biomechanical analysis of hip and knee joints during gait in elderly subjects. Acta Ortop. Bras. 2007, 15, 267–271. [Google Scholar] [CrossRef]
- Buddhadev, H.H.; Martin, P.E. Effects of age and physical activity status on redistribution of joint work during walking. Gait Posture 2016, 50, 131–136. [Google Scholar] [CrossRef]
- Delabastita, T.; Hollville, E.; Catteau, A.; Cortvriendt, P.; De Groote, F.; Vanwanseele, B. Distal-to-proximal joint mechanics redistribution is a main contributor to reduced walking economy in older adults. Scand. J. Med. Sci. Sports 2021, 31, 1036–1047. [Google Scholar] [CrossRef]
- Lindle, R.S.; Metter, E.J.; Lynch, N.A.; Fleg, J.L.; Fozard, J.L.; Tobin, J.; Roy, T.A.; Hurley, B.F. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J. Appl. Physiol. 1997, 83, 1581–1587. [Google Scholar] [CrossRef]
- Lanza, I.R.; Towse, T.F.; Caldwell, G.E.; Wigmore, D.M.; Kent-Braun, J.A. Effects of age on human muscle torque, velocity, and power in two muscle groups. J. Appl. Physiol. 2003, 95, 2361–2369. [Google Scholar] [CrossRef]
- Ikezoe, T.; Mori, N.; Nakamura, M.; Ichihashi, N. Atrophy of the lower limbs in elderly women: Is it related to walking ability? Eur. J. Appl. Physiol. 2011, 111, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Savelberg, H.H.C.M.; Meijer, K. The Effect of Age and Joint Angle on the Proportionality of Extensor and Flexor Strength at the Knee Joint. J. Gerontol. Ser. A 2004, 59, 1120–1128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Demura, T.; Demura, S.; Uchiyama, M.; Sugiura, H. Examination of Factors Affecting Gait Properties in Healthy Older Adults: Focusing on Knee Extension Strength, Visual Acuity, and Knee Joint Pain. J. Geriatr. Phys. Ther. 2014, 37, 52. [Google Scholar] [CrossRef]
- Sadeghi, H.; Prince, F.; Zabjek, K.F.; Sadeghi, S.; Labelle, H. Knee flexors/extensors in gait of elderly and young able-bodied men (II). Knee 2002, 9, 55–63. [Google Scholar] [CrossRef]
- Messier, S.P.; DeVita, P.; Cowan, R.E.; Seay, J.; Young, H.C.; Marsh, A.P. Do older adults with knee osteoarthritis place greater loads on the knee during gait? A preliminary study. Arch. Phys. Med. Rehabil. 2005, 86, 703–709. [Google Scholar] [CrossRef]
- McGibbon, C.A.; Krebs, D.E. Discriminating age and disability effects in locomotion: Neuromuscular adaptations in musculoskeletal pathology. J. Appl. Physiol. 2004, 96, 149–160. [Google Scholar] [CrossRef]
- Luc-Harkey, B.A.; Franz, J.R.; Blackburn, J.T.; Padua, D.A.; Hackney, A.C.; Pietrosimone, B. Real-time biofeedback can increase and decrease vertical ground reaction force, knee flexion excursion, and knee extension moment during walking in individuals with anterior cruciate ligament reconstruction. J. Biomech. 2018, 76, 94–102. [Google Scholar] [CrossRef]
- Evans-Pickett, A.; Davis-Wilson, H.C.; Luc-Harkey, B.A.; Blackburn, J.T.; Franz, J.R.; Padua, D.A.; Seeley, M.K.; Pietrosimone, B. Biomechanical effects of manipulating peak vertical ground reaction force throughout gait in individuals 6–12 months after anterior cruciate ligament reconstruction. Clin. Biomech. 2020, 76, 105014. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, C.A.; Fukuchi, R.K.; Duarte, M. Effects of walking speed on gait biomechanics in healthy participants: A systematic review and meta-analysis. Syst. Rev. 2019, 8, 153. [Google Scholar] [CrossRef]
- Franz, J.R.; Kram, R. Advanced age affects the individual leg mechanics of level, uphill, and downhill walking. J. Biomech. 2013, 46, 535–540. [Google Scholar] [CrossRef]
- Armitano-Lago, C.; Pietrosimone, B.; Evans-Pickett, A.; Davis-Wilson, H.; Franz, J.R.; Blackburn, T.; Kiefer, A.W. Cueing Changes in Peak Vertical Ground Reaction Force to Improve Coordination Dynamics in Walking. J. Mot. Behav. 2022, 54, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kuhman, D.; Willson, J.; Mizelle, J.C.; DeVita, P. The relationships between physical capacity and biomechanical plasticity in old adults during level and incline walking. J. Biomech. 2018, 69, 90–96. [Google Scholar] [CrossRef]
- Munsch, A.E.; Pietrosimone, B.; Franz, J.R. The effects of knee extensor moment biofeedback on gait biomechanics and quadriceps contractile behavior. PeerJ 2020, 8, e9509. [Google Scholar] [CrossRef]
- Begg, R.K.; Sparrow, W.A. Ageing effects on knee and ankle joint angles at key events and phases of the gait cycle. J. Med. Eng. Technol. 2006, 30, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Van Criekinge, T.; Saeys, W.; Hallemans, A.; Van de Walle, P.; Vereeck, L.; De Hertogh, W.; Truijen, S. Age-related differences in muscle activity patterns during walking in healthy individuals. J. Electromyogr. Kinesiol. 2018, 41, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.P.; Lin, Y.-C.; Pandy, M.G. Muscle function during gait is invariant to age when walking speed is controlled. Gait Posture 2013, 38, 253–259. [Google Scholar] [CrossRef]
- Lee, H.-J.; Chang, W.H.; Choi, B.-O.; Ryu, G.-H.; Kim, Y.-H. Age-related differences in muscle co-activation during locomotion and their relationship with gait speed: A pilot study. BMC Geriatr. 2017, 17, 44. [Google Scholar] [CrossRef]
- Hortobágyi, T.; Solnik, S.; Gruber, A.; Rider, P.; Steinweg, K.; Helseth, J.; DeVita, P. Interaction between age and gait velocity in the amplitude and timing of antagonist muscle coactivation. Gait Posture 2009, 29, 558–564. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).