The Osteoinductive Effect of Water-Soluble Matrix from Nano-Nacre Particles of Haliotis diversicolor (H. diversicolor) Abalone on MC3T3-E1 Osteoblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Nano-Nacre Particles Preparation
2.2. Extraction of WSM from Nano-Nacre Particles
2.3. Western Blotting for WSM Protein Profiling
2.4. Cell Culture and WSM Treatment
2.5. Cell Viability
2.6. Detection of Osteogenic Gene Markers
2.7. Quantitative Calcium Deposition
2.8. Statistics Analyses
3. Results
3.1. Characterization of Nano-Nacre Particles
3.2. Western Blotting
3.3. The WSM from Nano-Nacre Particles Is Non-Cytotoxic to MC3T3-E1 Cells
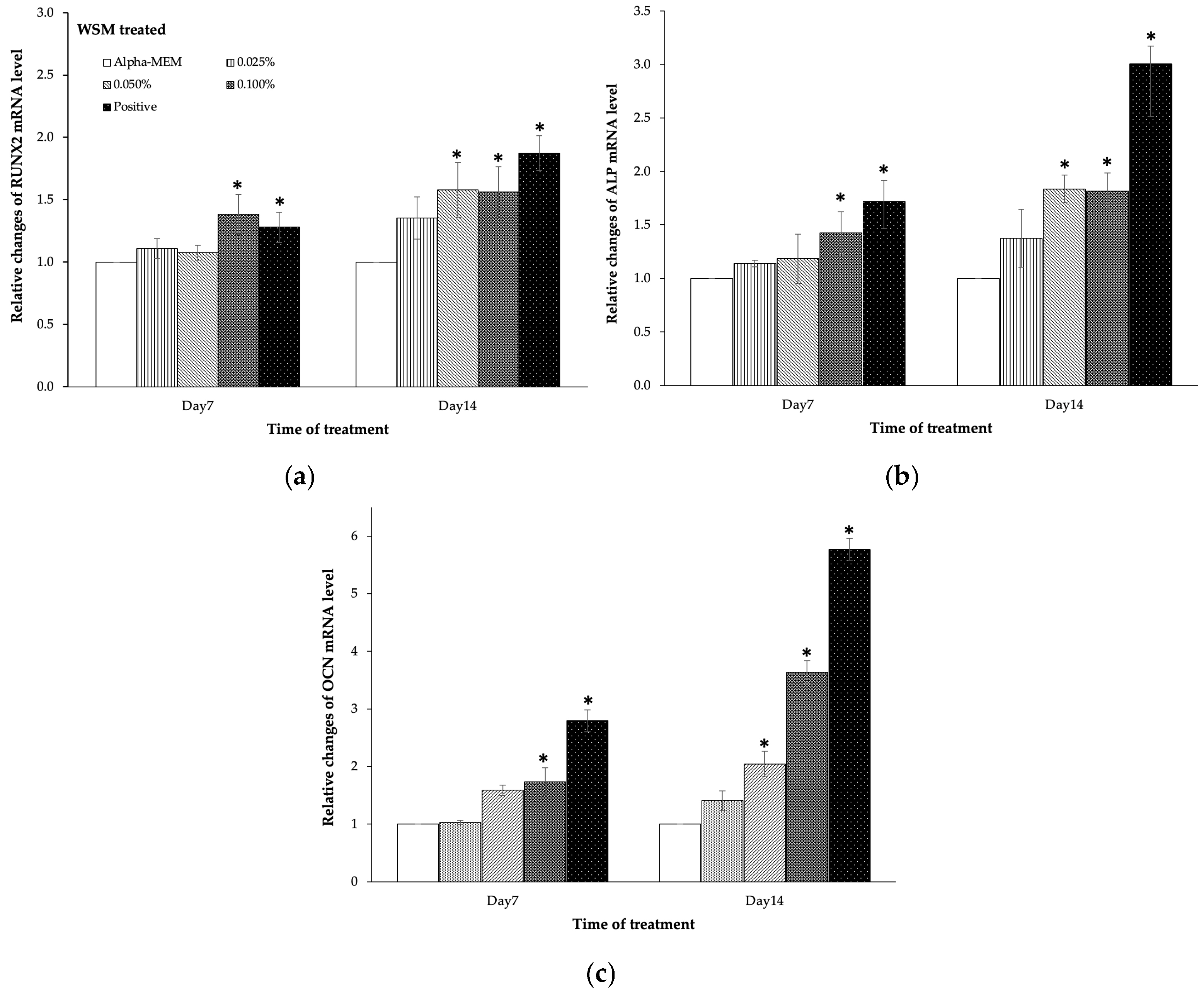
3.4. The WSM from Nano-Nacre Particles Promoted MC3T3-E1 Cells Differentiation
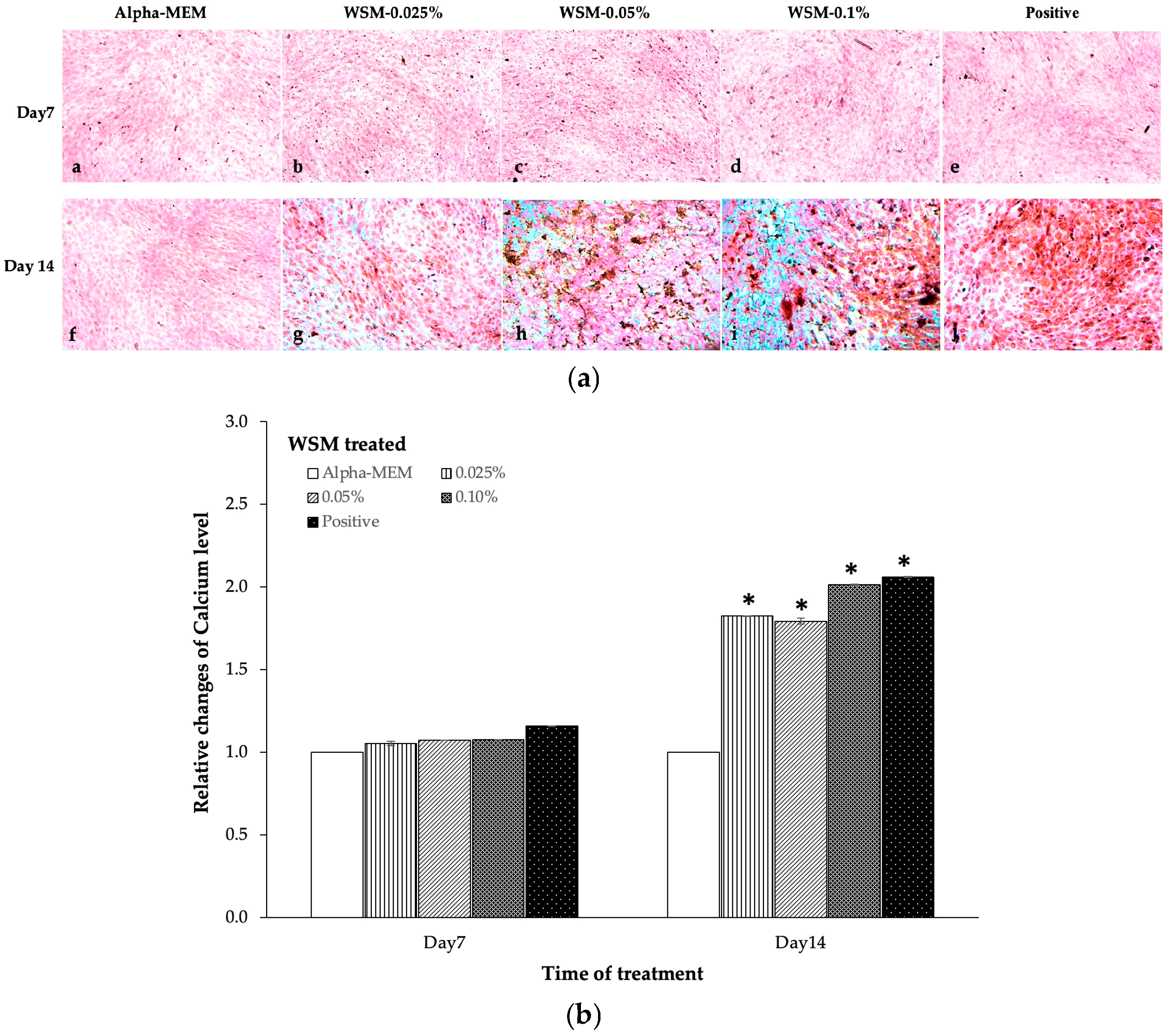
3.5. The WSM from Nano-Nacre Particles Accelerated Mineralization of MC3T3-E1 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Cooper, C.; Rizzoli, R.; Reginster, J.-Y.; on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2013, 24, 23–57. [Google Scholar] [CrossRef] [PubMed]
- Popp, A.W.; Varathan, N.; Buffat, H.; Senn, C.; Perrelet, R.; Lippuner, K. Bone Mineral Density Changes After 1 Year of Denosumab Discontinuation in Postmenopausal Women with Long-Term Denosumab Treatment for Osteoporosis. Calcif. Tissue Int. 2018, 103, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Guise, T.A.; Yin, J.J.; Mohammad, K.S. Role of Endothelin-1 in Osteoblastic Bone Metastases. Cancer 2003, 97, 779–784. [Google Scholar] [CrossRef]
- McClung, M.R. Romosozumab for the treatment of osteoporosis. Osteoporos. Sarcopenia 2018, 4, 11–15. [Google Scholar] [CrossRef]
- Farkas, S.; Szabó, A.; Hegyi, A.E.; Török, B.; Fazekas, C.L.; Ernszt, D.; Kovács, T.; Zelena, D. Estradiol and Estrogen-like Alternative Therapies in Use: The Importance of the Selective and Non-Classical Actions. Biomedicines 2022, 10, 861. [Google Scholar] [CrossRef]
- Vecchio, K.S.; Zhang, X.; Massie, J.B.; Wang, M.; Kim, C.W. Conversion of bulk seashells to biocompatible hydroxyapatite for bone implants. Acta Biomater. 2007, 3, 910–918. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Glimcher, M.J. Bone mineral: Update on chemical composition and structure. Osteoporos. Int. 2009, 20, 1013–1021. [Google Scholar] [CrossRef]
- Camprasse, S.; Camprasse, G.; Pouzol, M.; Lopez, E. Artificial dental root made of natural calcium carbonate (Bioracine). Clin. Mater. 1990, 5, 235–250. [Google Scholar] [CrossRef]
- Camprasse, G.; Camprasse, S.; Gill, G.A. Substitution of the dental root by aquatic invertebrate skeletons in animals and man. C. R. Acad.Sci. 1988, 307, 485–491. [Google Scholar]
- Berland, S.; Delattre, O.; Borzeix, S.; Catonne, Y.; Lopez, E. Nacre/bone interface changes in durable nacre endosseous implants in sheep. Biomaterials 2005, 26, 2767–2773. [Google Scholar] [CrossRef]
- Zhang, G.; Brion, A.; Willemin, A.-S.; Piet, M.-H.; Moby, V.; Bianchi, A.; Mainard, D.; Galois, L.; Gillet, P.; Rousseau, M. Nacre, a natural, multi-use, and timely biomaterial for bone graft substitution. J. Biomed. Mater. Res. Part A 2017, 105, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Iandolo, D.; Laroche, N.; Nguyen, D.K.; Normand, M.; Met, C.; Zhang, G.; Vico, L.; Mainard, D.; Rousseau, M. Preclinical safety study of nacre powder in an intraosseous sheep model. BMJ Open Sci. 2022, 6, e100231. [Google Scholar] [CrossRef] [PubMed]
- Green, D.W.; Kwon, H.-J.; Jung, H.-S. Osteogenic Potency of Nacre on Human Mesenchymal Stem Cells. Mol. Cells 2015, 38, 267–272. [Google Scholar] [CrossRef]
- Xu, J.; Rao, Y.; Wu, X.; Jiang, J.; Yu, M.; Chen, X.; Wang, H. The osteoinductive effect of nano-nacre particles on MC-3T3 E1 preosteoblast through controlled release of water-soluble matrix and calciumions. Dent. Mater. J. 2019, 38, 981–986. [Google Scholar] [CrossRef]
- Miyamoto, H.; Miyashita, T.; Okushima, M.; Nakano, S.; Morita, T.; Matsushiro, A. A carbonic anhydrase from the nacreous layer in oyster pearls. Proc. Natl. Acad. Sci. USA 1996, 93, 9657–9660. [Google Scholar] [CrossRef] [PubMed]
- Sudo, S.; Fujikawa, T.; Nagakura, T.; Ohkubo, T.; Sakaguchi, K.; Tanaka, M.; Nakashima, K.; Takahashi, T. Structures of mollusc shell framework proteins. Nature 1997, 387, 563–564. [Google Scholar] [CrossRef]
- Samata, T.; Hayashi, N.; Kono, M.; Hasegawa, K.; Horita, C.; Akera, S. A new matrix protein family related to the nacreous layer formation of Pinctada fucata. FEBS Lett. 1999, 462, 225–229. [Google Scholar] [CrossRef]
- Miyashita, T.; Takagi, R.; Okushima, M.; Nakano, S.; Miyamoto, H.; Nishikawa, E.; Matsushiro, A. Complementary DNA Cloning and Characterization of Pearlin, a New Class of Matrix Protein in the Nacreous Layer of Oyster Pearls. Mar. Biotechnol. 2000, 2, 409–418. [Google Scholar] [CrossRef]
- Weiss, I.; Kaufmann, S.; Mann, K.; Fritz, M. Purification and Characterization of Perlucin and Perlustrin, Two New Proteins from the Shell of the Mollusc Haliotis laevigata. BBRC Biochem. Biophys. Res. Commun. 2000, 267, 17–21. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Ma, Z.; Xie, L.; Zhang, R. A Novel Matrix Protein p10 from the Nacre of Pearl Oyster (Pinctada fucata) and Its Effects on Both CaCO3 Crystal Formation and Mineralogenic Cells. Mar. Biotechnol. 2006, 8, 624–633. [Google Scholar] [CrossRef]
- Yano, M.; Nagai, K.; Morimoto, K.; Miyamoto, H. A novel nacre protein N19 in the pearl oyster Pinctada fucata. BBRC Biochem. Biophys. Res. Commun. 2007, 362, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Wong, K.L.; Xu, Z.Y.; Au, K.Y.; Lee, N.L.; Su, C.; Su, W.-W.; Li, P.-B.; Shaw, P.-C. N16, a Nacreous Protein, Inhibits Osteoclast Differentiation and Enhances Osteogenesis. J. Nat. Prod. 2016, 79, 204–212. [Google Scholar] [CrossRef]
- Mann, K.; Cerveau, N.; Gummich, M.; Fritz, M.; Mann, M.; Jackson, D.J. In-depth proteomic analyses of Haliotis laevigata (greenlip abalone) nacre and prismatic organic shell matrix. Proteome Sci. 2018, 16, 11. [Google Scholar] [CrossRef]
- Suwannasing, C.; Buddawong, A.; Khumpune, S.; Habuddha, V.; Weerachatyanukul, W.; Asuvapongpatana, S. Bone Morphogenetic Protein 2/4 in Mollusk, Haliotis diversicolor: Its Expression and Osteoinductive Function In Vitro. Mar. Biotechnol. 2021, 23, 836–846. [Google Scholar] [CrossRef]
- Pereira-Mouriès, L.; Almeida, M.J.; Ribeiro, C.; Peduzzi, J.; Barthélemy, M.; Milet, C.; Lopez, E. Soluble silk-like organic matrix in the nacreous layer of the bivalve Pinctada maxima. Eur. J. Biochem. 2002, 269, 4994–5003. [Google Scholar] [CrossRef]
- Shi, Y.; Zheng, X.; Zhan, X.; Wang, A.; Gu, Z. cDNA microarray analysis revealing candidate biomineralization genes of the pearl oyster, Pinctada fucata martensii. Mar. Biotechnol. 2016, 18, 336–348. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Singha, P.K.; Dey, S. Water Soluble Bioactives of Nacre Mediate Antioxidant Activity and Osteoblast Differentiation. PLoS ONE 2013, 8, e84584. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, W.; Fan, H.; Xu, P. Water-soluble nano-pearl powder promotes MC3T3-E1 cell differentiation by enhancing autophagy via the MEK/ERK signaling pathway. Mol. Med. Rep. 2018, 18, 993–1000. [Google Scholar] [CrossRef]
- Wang, X.; Harimoto, K.; Fuji, R.; Liu, J.; Li, L.; Wang, P.; Akaike, T.; Wang, Z. Pinctada fucata mantle gene 4 (PFMG4) from pearl oyster mantle enhances osteoblast differentiation. Biosci. Biotechnol. Biochem. 2015, 79, 558–565. [Google Scholar] [CrossRef]
- Peverali, F.A.; Basdra, E.K.; Papavassiliou, A.G. Stretch-mediated activation of selective MAPK subtypes and potentiation of AP-1 binding in human osteoblastic cells. J. Mol. Med. 2001, 7, 68–78. [Google Scholar] [CrossRef]
- Lian, J.B.; Stein, G.S.; Stein, J.L.; van Wijnen, A.J. Osteocalcin gene promoter: Unlocking the secrets for the regulation of osteoblast growth and differentiation. J. Cell. Biochem. 1998, 72, 62–72. [Google Scholar] [CrossRef]
- Rousseau, M.; Pereira-Mouriès, L.; Almeida, M.J.; Milet, C.; Lopez, E. The water-soluble matrix fraction from the nacre of Pinctada maxima produces earlier mineralization of MC3T3-E1 mouse pre-osteoblasts. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 135, 1–7. [Google Scholar] [CrossRef]
- Tanasawet, S.; Withyachumnarnkul, B.; Changsangfar, C.; Cummins, S.F.; Sroyraya, M.; Sangsuwan, P.; Kitiyanant, Y.; Asuvapongpatana, S.; Weerachatyanukul, W. Isolation of Organic Matrix Nacreous Proteins from Haliotis diversicolor and Their Effect On In Vitro Osteoinduction. Malacologia 2013, 56, 107–119. [Google Scholar] [CrossRef]
- Jackson, D.J.; McDougall, C.; Green, K.; Simpson, F.; Wörheide, G.; Degnan, B.M. A rapidly evolving secretome builds and patterns a sea shell. BMC Biol. 2006, 4, 40. [Google Scholar] [CrossRef]
- Marie, B.; Marie, A.; Jackson, D.J.; Dubost, L.; Degnan, B.M.; Milet, C.; Marin, F. Proteomic analysis of the organic matrix of the abalone Haliotis asinina calcified shell. Proteome Sci. 2010, 8, 54. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, L.; Meng, Q.; Jing, T.; Pu, R.; Chen, L.; Zhang, R. A novel matrix protein participating in the nacre framework formation of pearl oyster, Pinctada fucata. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 135, 565–573. [Google Scholar] [CrossRef]
- Checa, A. A new model for periostracum and shell formation in Unionidae (Bivalvia, Mollusca). Tissue Cell 2000, 32, 405–416. [Google Scholar] [CrossRef]
- Marin, F.; Roy, N.L.; Marie, B. The formation and mineralization of the mollusk shell. Front. Biosci. Schol. Ed. 2012, 4, 1099–1125. [Google Scholar] [CrossRef]
- Marin, F.; Amons, R.; Guichard, N.; Stigter, M.; Hecker, A.; Luquet, G.; Layrolle, P.; Alcaraz, G.; Riondet, C.; Westbroek, P. Caspartin and calprismin, two proteins of the shell calcitic prisms of the Mediterranean fan mussel Pinna nobilis. J. Biol. Chem. 2005, 280, 33895–33908. [Google Scholar] [CrossRef]
- Kong, Y.; Jing, G.; Yan, Z.; Li, C.; Gong, N.; Zhu, F.; Li, D.; Zhang, Y.; Zheng, G.; Wang, H.; et al. Cloning and Characterization of Prisilkin-39, a Novel Matrix Protein Serving a Dual Role in the Prismatic Layer Formation from the Oyster Pinctada fucata. J. Biol. Chem. 2009, 284, 10841–10854. [Google Scholar] [CrossRef]
- Hayashi, M.; Maeda, S.; Aburatani, H.; Kitamura, K.; Miyoshi, H.; Miyazono, K.; Imamura, T. Pitx2 prevents osteoblastic transdifferentiation of myoblasts by bone morphogenetic proteins. J. Biol. Chem. 2008, 283, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef]
- Ge, C.; Xiao, G.; Jiang, D.; Yang, Q.; Hatch, N.E.; Roca, H.; Franceschi, R.T. Identification and functional characterization of ERK/MAPK phosphorylation sites in the Runx2 transcription factor. J. Biol. Chem. 2009, 284, 32533–32543. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwannasing, C.; Prapan, A.; Surinlert, P.; Sombutkayasith, C.; Weerachatyanukul, W. The Osteoinductive Effect of Water-Soluble Matrix from Nano-Nacre Particles of Haliotis diversicolor (H. diversicolor) Abalone on MC3T3-E1 Osteoblasts. Appl. Sci. 2025, 15, 2907. https://doi.org/10.3390/app15062907
Suwannasing C, Prapan A, Surinlert P, Sombutkayasith C, Weerachatyanukul W. The Osteoinductive Effect of Water-Soluble Matrix from Nano-Nacre Particles of Haliotis diversicolor (H. diversicolor) Abalone on MC3T3-E1 Osteoblasts. Applied Sciences. 2025; 15(6):2907. https://doi.org/10.3390/app15062907
Chicago/Turabian StyleSuwannasing, Chanyatip, Ausanai Prapan, Piyaporn Surinlert, Chanyarak Sombutkayasith, and Wattana Weerachatyanukul. 2025. "The Osteoinductive Effect of Water-Soluble Matrix from Nano-Nacre Particles of Haliotis diversicolor (H. diversicolor) Abalone on MC3T3-E1 Osteoblasts" Applied Sciences 15, no. 6: 2907. https://doi.org/10.3390/app15062907
APA StyleSuwannasing, C., Prapan, A., Surinlert, P., Sombutkayasith, C., & Weerachatyanukul, W. (2025). The Osteoinductive Effect of Water-Soluble Matrix from Nano-Nacre Particles of Haliotis diversicolor (H. diversicolor) Abalone on MC3T3-E1 Osteoblasts. Applied Sciences, 15(6), 2907. https://doi.org/10.3390/app15062907






