Unraveling Mass Transfer and Reaction Processes in CVD-Grown MoS2 Films: A Multiphysical Field Coupling Study
Abstract
1. Introduction
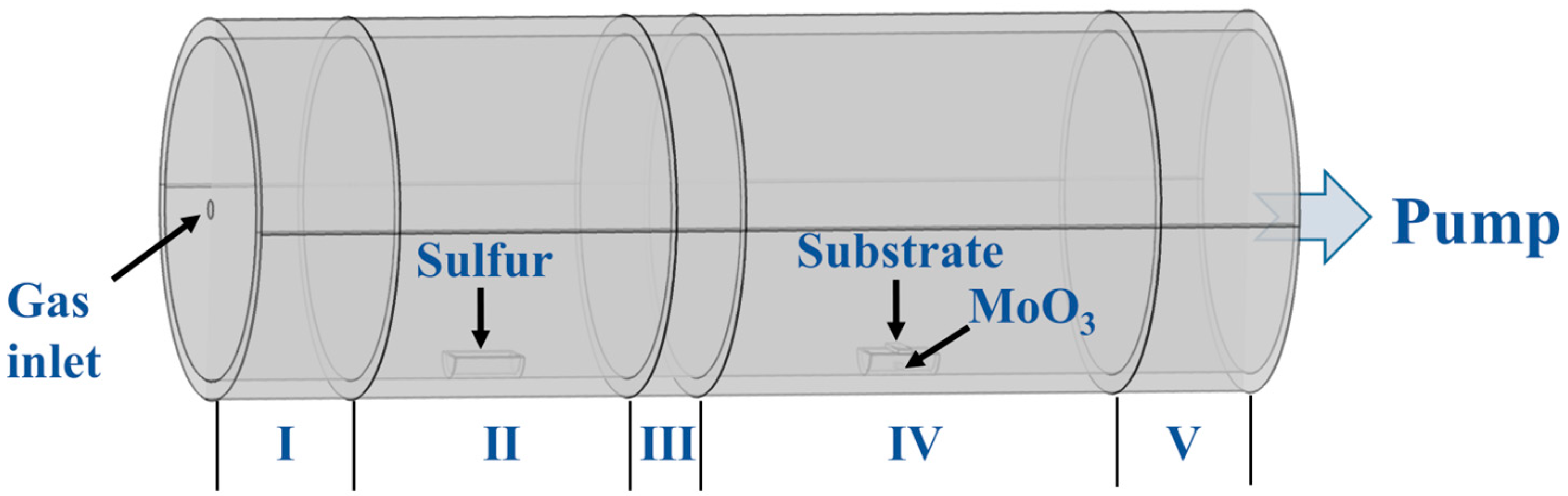
2. Experimental Methods
3. Construction of Multiphysics Coupling Model
4. Results and Discussion
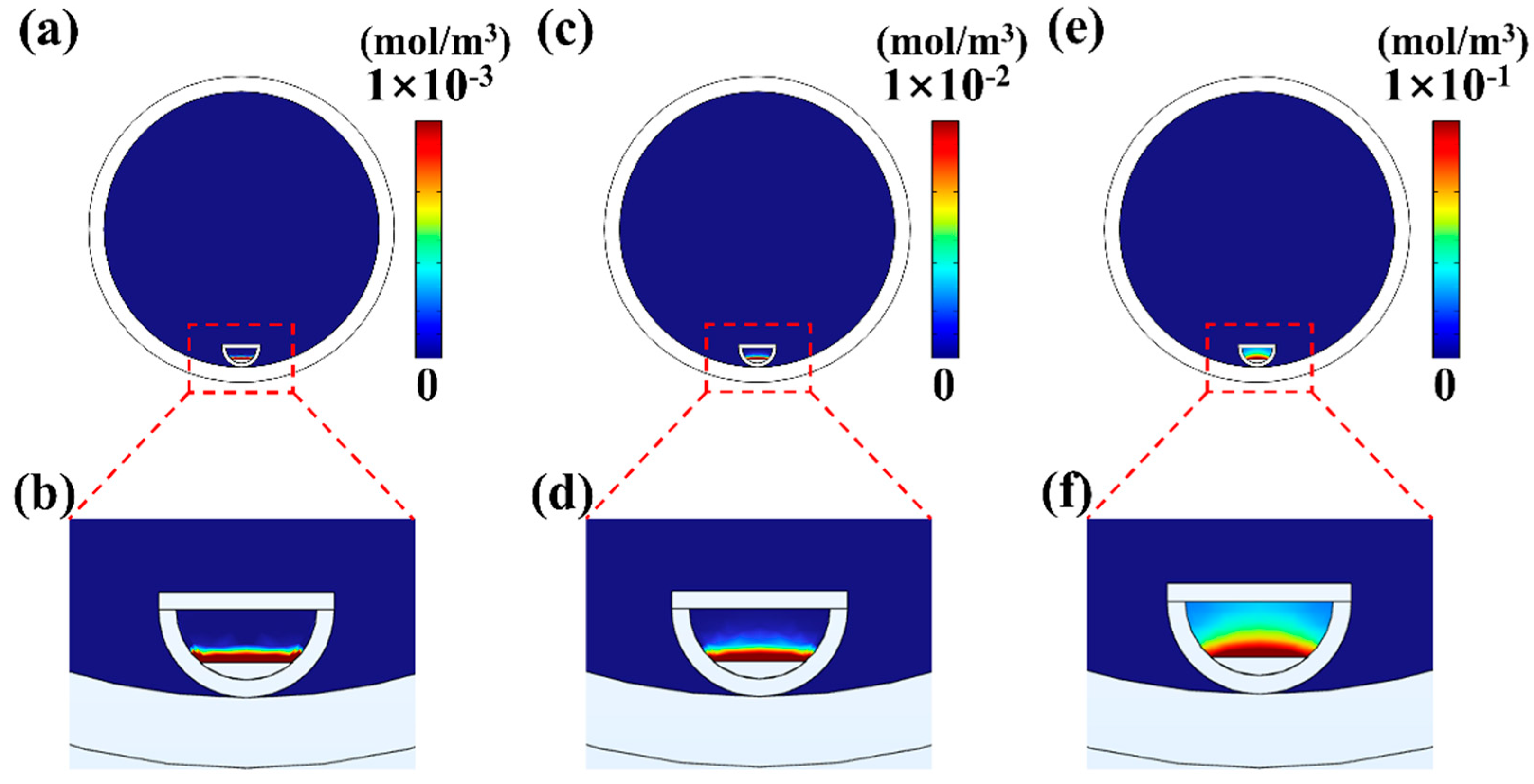
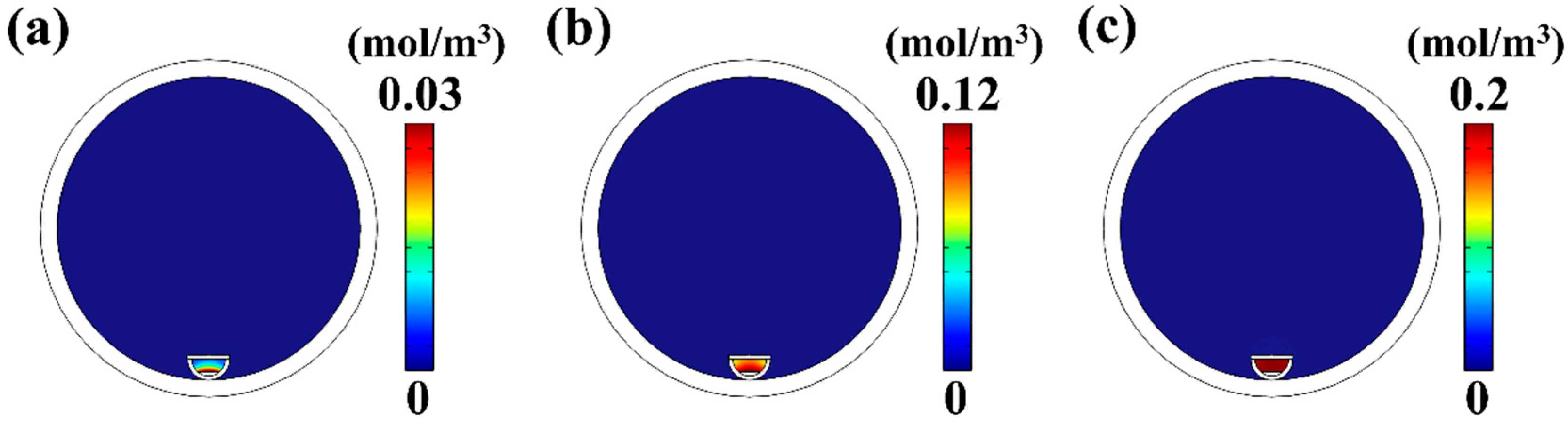
4.1. The Influence of Temperature on MoS2 Growth
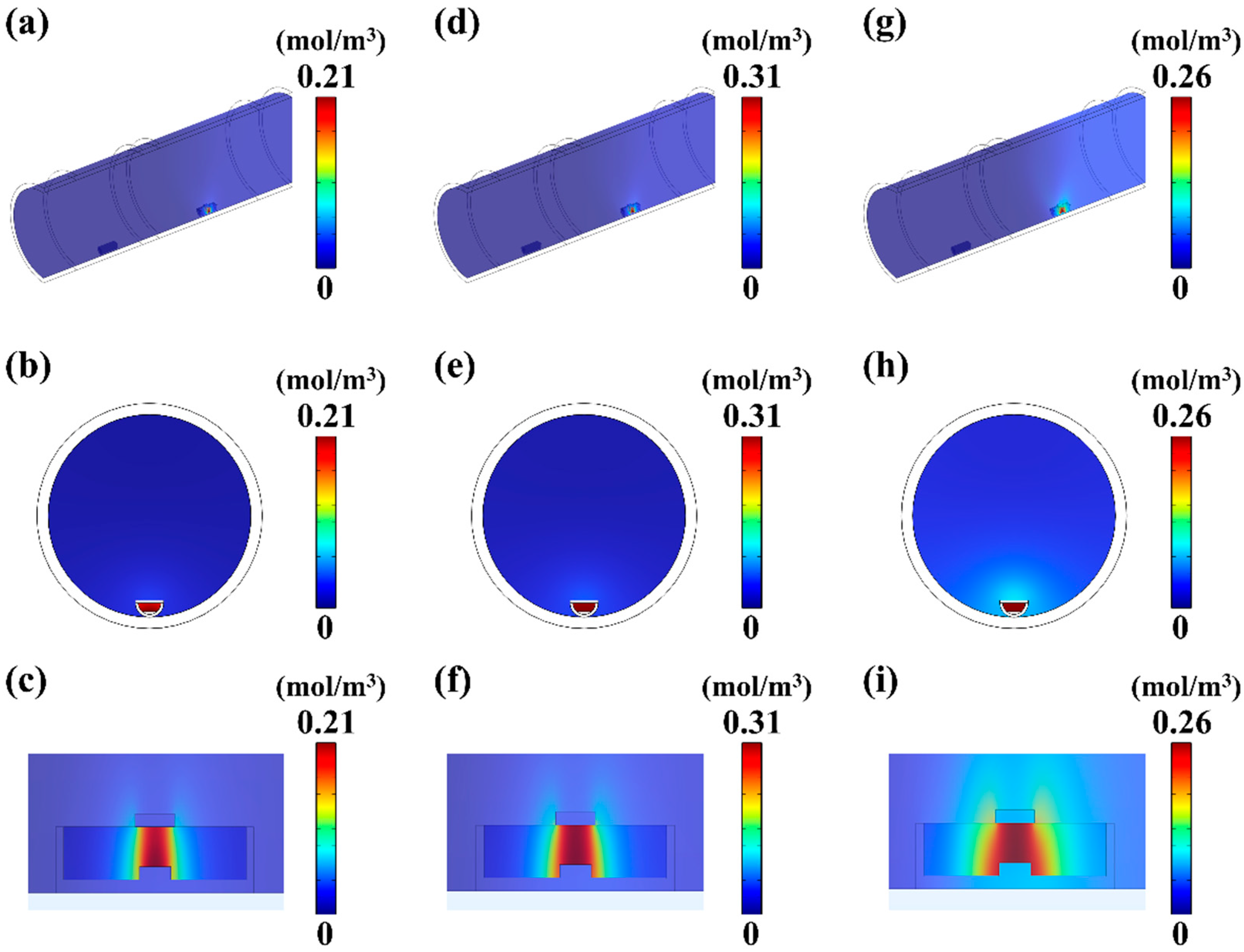
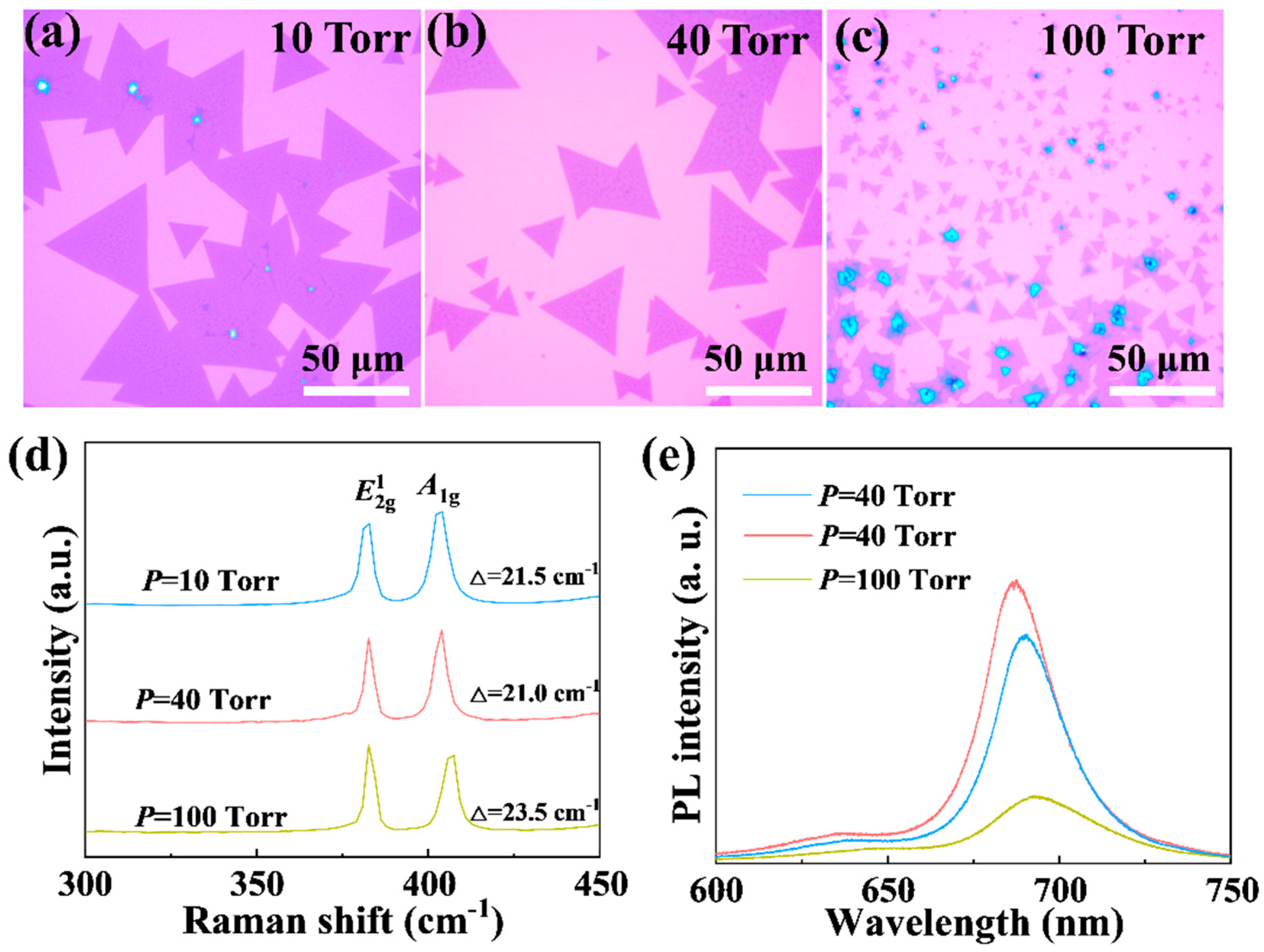
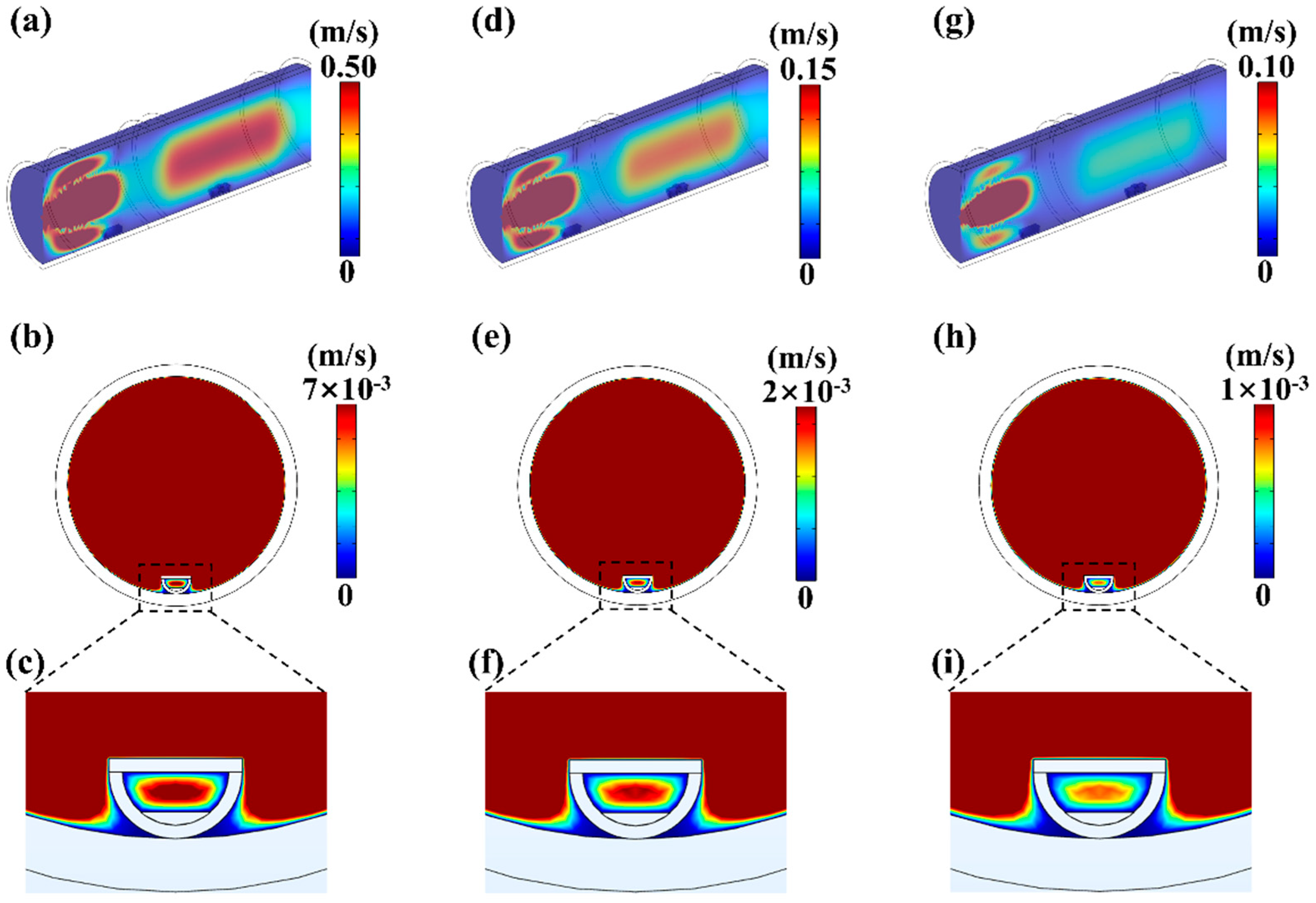
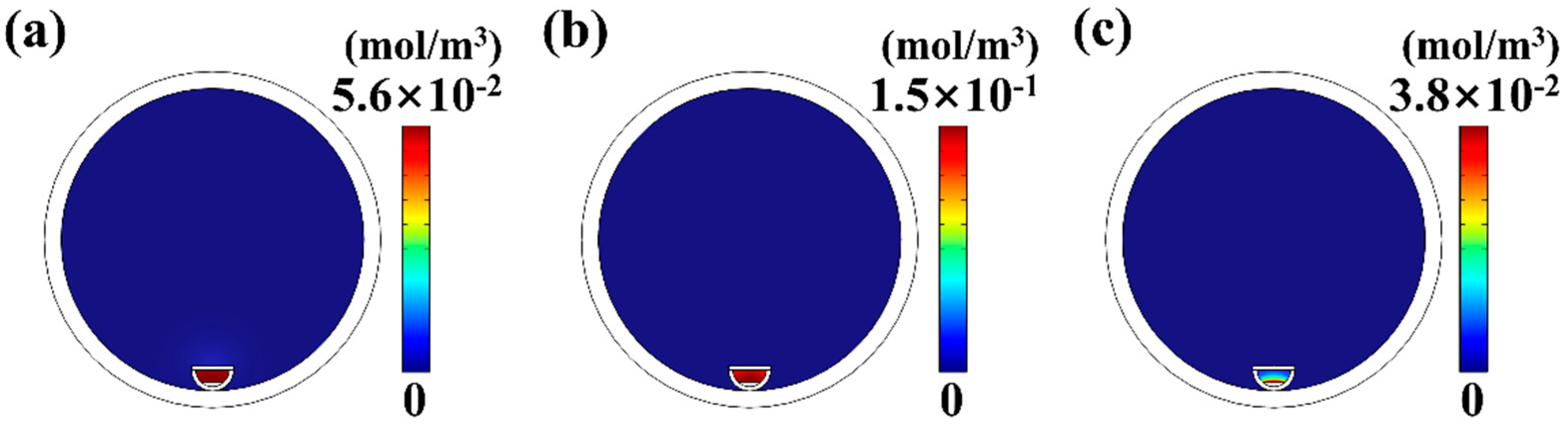
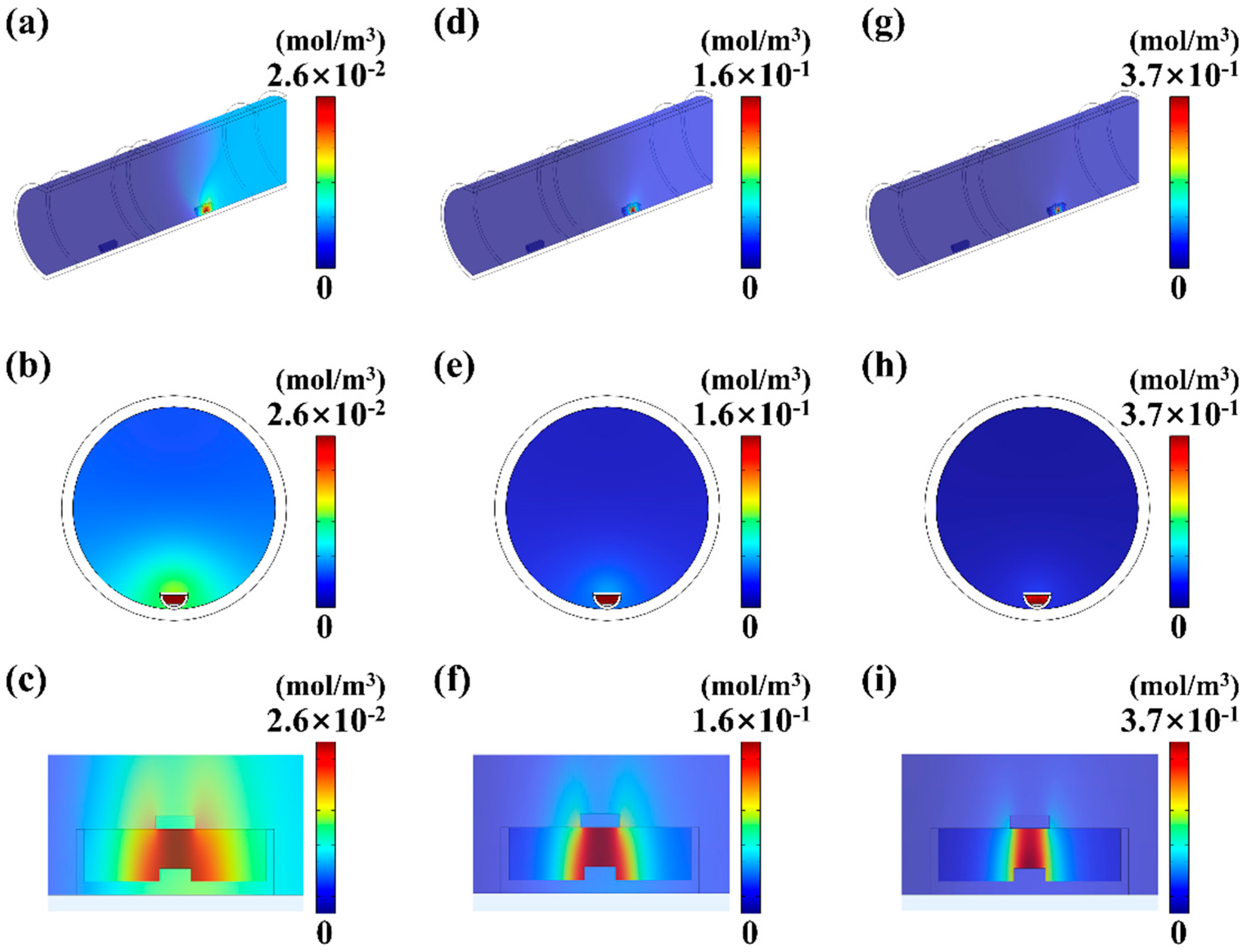
4.2. The Influence of Pressure on MoS2 Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waldrop, M.M. The chips are down for Moore’s law. Nature 2016, 530, 144. [Google Scholar] [CrossRef] [PubMed]
- Vashishtha, P.; Abidi, I.H.; Giridhar, S.P.; Verma, A.K.; Prajapat, P.; Bhoriya, A.; Murdoch, B.J.; Tollerud, J.O.; Xu, C.; Davis, J.A.; et al. CVD-grown monolayer MoS2 and GaN thin film heterostructure for a self-powered and bidirectional photodetector with an extended active spectrum. ACS Appl. Mater. Interfaces 2024, 16, 31294. [Google Scholar] [CrossRef]
- Li, M.Y.; Su, S.K.; Wong, H.P.; Li, L.J. How 2D semiconductors could extend Moore's law. Nature 2019, 567, 169. [Google Scholar] [CrossRef] [PubMed]
- Migliato Marega, G.; Zhao, Y.; Avsar, A.; Wang, Z.; Tripathi, M.; Radenovic, A.; Kis, A. Logic-in-memory based on an atomically thin semiconductor. Nature 2020, 587, 72. [Google Scholar] [CrossRef]
- Kwak, D.; Polyushkin, D.K.; Mueller, T. In-sensor computing using a MoS2 photodetector with programmable spectral responsivity. Nat. Commun. 2023, 14, 4264. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.-C.; Su, C.; Lin, Y.; Chou, A.-S.; Cheng, C.-C.; Park, J.-H.; Chiu, M.-H.; Lu, A.-Y.; Tang, H.-L.; Tavakoli, M.M.; et al. Ultralow contact resistance between semimetal and monolayer semiconductors. Nature 2021, 593, 211. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, X.; Shin, H.-J.; Park, S.; Huang, Y.; Duan, X. Promises and prospects of two-dimensional transistors. Nature 2021, 591, 43. [Google Scholar] [CrossRef]
- Das, S.; Sebastian, A.; Pop, E.; McClellan, C.J.; Franklin, A.D.; Grasser, T.; Knobloch, T.; Illarionov, Y.; Penumatcha, A.V.; Appenzeller, J.; et al. Transistors based on two-dimensional materials for future integrated circuits. Nat. Electron. 2021, 4, 786. [Google Scholar] [CrossRef]
- Zhang, X.; Grajal, J.; Vazquez-Roy, J.L.; Radhakrishna, U.; Wang, X.; Chern, W.; Zhou, L.; Lin, Y.; Shen, P.-C.; Ji, X.; et al. Two-dimensional MoS2-enabled flexible rectenna for Wi-Fi-band wireless energy harvesting. Nature 2019, 566, 368. [Google Scholar] [CrossRef]
- Vashishtha, P.; Dash, A.; Prajapat, P.; Goswami, P.; Walia, S.; Gupta, G. Self-powered broadband photodetection of MoS2/Sb2Se3 heterostructure. ACS Appl. Opt. Mater. 2023, 1, 1952. [Google Scholar] [CrossRef]
- Vashishtha, P.; Dash, A.; Walia, S.; Gupta, G. Self-bias Mo–Sb–Ga multilayer photodetector encompassing ultra-broad spectral response from UV–C to IR–B. Opt. Laser Technol. 2025, 181, 111705. [Google Scholar] [CrossRef]
- Duan, X.; Wang, C.; Shaw, J.C.; Cheng, R.; Chen, Y.; Li, H.; Wu, X.; Tang, Y.; Zhang, Q.; Pan, A.; et al. Lateral epitaxial growth of two-dimensional layered semiconductor heterojunctions. Nat. Nanotechnol. 2014, 9, 1024. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, P.; Duan, X.; Zang, K.; Luo, J.; Duan, X. Robust epitaxial growth of two-dimensional heterostructures, multiheterostructures, and superlattices. Science 2017, 357, 788. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Memaran, S.; Xin, Y.; Balicas, L.; Gutierrez, H.R. One-pot growth of two-dimensional lateral heterostructures via sequential edge-epitaxy. Nature 2018, 553, 63. [Google Scholar] [CrossRef]
- Zheng, B.; Ma, C.; Li, D.; Lan, J.; Zhang, Z.; Sun, X.; Zheng, W.; Yang, T.; Zhu, C.; Ouyang, G.; et al. Band alignment engineering in two-dimensional lateral heterostructures. J. Am. Chem. Soc. 2018, 140, 11193. [Google Scholar] [CrossRef] [PubMed]
- Cun, H.; Macha, M.; Kim, H.; Liu, K.; Zhao, Y.; LaGrange, T.; Kis, A.; Radenovic, A. Wafer-scale MOCVD growth of monolayer MoS2 on sapphire and SiO2. Nano Res. 2019, 12, 2646. [Google Scholar] [CrossRef]
- Zhu, H.; Nayir, N.; Choudhury, T.H.; Bansal, A.; Huet, B.; Zhang, K.; Puretzky, A.A.; Bachu, S.; York, K.; Mc Knight, T.V.; et al. Step engineering for nucleation and domain orientation control in WSe2 epitaxy on c-plane sapphire. Nat. Nanotechnol. 2023, 18, 1295. [Google Scholar] [CrossRef]
- Olding, J.N.; Henning, A.; Dong, J.T.; Zhou, Q.; Moody, M.J.; Smeets, P.J.M.; Darancet, P.; Weiss, E.A.; Lauhon, L.J. Charge separation in epitaxial SnS/MoS2 vertical heterojunctions grown by low-temperature pulsed MOCVD. ACS Appl. Mater. Interfaces 2019, 11, 40543. [Google Scholar] [CrossRef]
- Yu, H.; Huang, L.; Zhou, L.; Peng, Y.; Li, X.; Yin, P.; Zhao, J.; Zhu, M.; Wang, S.; Liu, J.; et al. Eight In. wafer-scale epitaxial monolayer MoS2. Adv. Mater. 2024, 36, 2402855. [Google Scholar] [CrossRef]
- Li, L.; Wang, Q.; Wu, F.; Xu, Q.; Tian, J.; Huang, Z.; Wang, Q.; Zhao, X.; Zhang, Q.; Fan, Q.; et al. Epitaxy of wafer-scale single-crystal MoS2 monolayer via buffer layer control. Nat. Commun. 2024, 15, 1825. [Google Scholar] [CrossRef]
- Liu, L.; Li, T.; Ma, L.; Li, W.; Gao, S.; Sun, W.; Dong, R.; Zou, X.; Fan, D.; Shao, L.; et al. Uniform nucleation and epitaxy of bilayer molybdenum disulfide on sapphire. Nature 2022, 605, 69. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liao, M.; Zhao, W.; Liu, G.; Zhou, X.J.; Wei, Z.; Xu, X.; Liu, K.; Hu, Z.; Deng, K.; et al. Wafer-scale growth and transfer of highly-oriented monolayer MoS2 continuous films. ACS Nano 2017, 11, 12001. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, N.; Tang, J.; Zhu, J.; Zhang, Q.; Jia, Q.; Lu, Y.; Wei, Z.; Yu, H.; Zhao, Y.; et al. Wafer-scale highly oriented monolayer MoS2 with large domain sizes. Nano Lett. 2020, 20, 7193. [Google Scholar] [CrossRef]
- Xu, X.; Guo, T.; Kim, H.; Hota, M.K.; Alsaadi, R.S.; Lanza, M.; Zhang, X.; Alshareef, H.N. Growth of 2D materials at the wafer scale. Adv. Mater. 2022, 34, 2018258. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Ge, M.; Ma, Y.; Abbas, A.N.; Zhou, C. Step-edge-guided nucleation and growth of aligned WSe2 on sapphire via a layer-over-layer growth mode. ACS Nano 2015, 9, 8368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, J.; Ding, F. Strategies, status, and challenges in wafer scale single crystalline two-dimensional materials synthesis. Chem. Rev. 2021, 121, 6321. [Google Scholar] [CrossRef]
- Xu, J.; Ho, D. Modulation of the reaction mechanism via S/Mo: A rational strategy for large-area MoS2 growth. Chem. Mater. 2021, 33, 3249. [Google Scholar] [CrossRef]
- Rajan, A.G.; Warner, J.H.; Blankschtein, D.; Strano, M.S. Generalized mechanistic model for the chemical vapor deposition of 2D transition metal dichalcogenide monolayers. ACS Nano 2016, 10, 4330. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, X.; Wei, J.; Wang, S.; Chen, S.; Wu, S.; Ji, M.; Sun, Z.; Xu, Z.; Bao, W.; et al. 12-inch growth of uniform MoS2 monolayer for integrated circuit manufacture. Nat. Mater. 2023, 22, 1324. [Google Scholar] [CrossRef]
- Lei, J.; Xie, Y.; Yakobson, B.I. Gas-phase “prehistory” and molecular precursors in monolayer metal dichalcogenides synthesis: The case of MoS2. ACS Nano 2021, 15, 10525. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Zheng, J.; Gu, J.; Huang, Z.; Lu, L.; Li, H.; Chen, Z.; Yang, S. High-efficiency purification of CH4 and H2 energy sources enabled by a phosphotungstic acid-supported Os single-atom catalyst. J. Mater. Chem. A 2023, 11, 24698. [Google Scholar] [CrossRef]
- Ren, L.-K.; Zhu, L.-F.; Qi, T.; Tang, J.-Q.; Yang, H.-Q.; Hu, C.-W. Performance of dimethyl sulfoxide and brønsted acid catalysts in fructose conversion to 5-hydroxymethylfurfural. ACS Catal. 2017, 7, 2199. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From Bulk to Monolayer MoS2: Evolution of raman scattering. Adv. Funct. Mater. 2012, 22, 1385. [Google Scholar] [CrossRef]
- Verma, A.K.; Rahman, M.A.; Vashishtha, P.; Guo, X.; Sehrawat, M.; Mitra, R.; Giridhar, S.P.; Waqar, M.; Bhoriya, A.; Murdoch, B.J.; et al. Oxygen-passivated sulfur vacancies in monolayer MoS2 for enhanced piezoelectricity. ACS Nano 2025, 19, 3478. [Google Scholar] [CrossRef]


| Source | Pressure | Temperature | Mo3O9 Flow |
|---|---|---|---|
| MoO3 powder | 70 Torr | 1033 K | 1.9 × 10−4 mol min−1 cm−2 |
| 1053 K | 3.9 × 10−4 mol min−1 cm−2 | ||
| 1073 K | 6.6 × 10−4 mol min−1 cm−2 |
| Source | Pressure | Temperature | Mo3O9 Flow |
|---|---|---|---|
| MoO3 powder | 10 Torr | 1053 K | 9.4 × 10−4 mol min−1 cm−2 |
| 40 Torr | 5.5 × 10−4 mol min−1 cm−2 | ||
| 70 Torr | 3.9 × 10−4 mol min−1 cm−2 | ||
| 100 Torr | 2.4 × 10−4 mol min−1 cm−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Lin, J.; Zhang, Q.; Liu, Y.; Han, S.; Zhou, Y.; Chen, S.; Zhong, S.; Su, X.; Zhang, Q.; et al. Unraveling Mass Transfer and Reaction Processes in CVD-Grown MoS2 Films: A Multiphysical Field Coupling Study. Appl. Sci. 2025, 15, 2627. https://doi.org/10.3390/app15052627
Yang Z, Lin J, Zhang Q, Liu Y, Han S, Zhou Y, Chen S, Zhong S, Su X, Zhang Q, et al. Unraveling Mass Transfer and Reaction Processes in CVD-Grown MoS2 Films: A Multiphysical Field Coupling Study. Applied Sciences. 2025; 15(5):2627. https://doi.org/10.3390/app15052627
Chicago/Turabian StyleYang, Zhen, Jinwei Lin, Qing Zhang, Yutian Liu, Shujun Han, Yanbin Zhou, Shuo Chen, Shenlong Zhong, Xianli Su, Qingjie Zhang, and et al. 2025. "Unraveling Mass Transfer and Reaction Processes in CVD-Grown MoS2 Films: A Multiphysical Field Coupling Study" Applied Sciences 15, no. 5: 2627. https://doi.org/10.3390/app15052627
APA StyleYang, Z., Lin, J., Zhang, Q., Liu, Y., Han, S., Zhou, Y., Chen, S., Zhong, S., Su, X., Zhang, Q., & Tang, X. (2025). Unraveling Mass Transfer and Reaction Processes in CVD-Grown MoS2 Films: A Multiphysical Field Coupling Study. Applied Sciences, 15(5), 2627. https://doi.org/10.3390/app15052627





