Abstract
Prosthetics, a rapidly advancing field in dentistry, aims to improve patient comfort and aesthetics by addressing the challenge of replacing missing teeth. A critical obstacle in dental implantation is the condition of the jawbone, which often necessitates reconstruction prior to implant placement. Guided bone regeneration (GBR) and guided tissue regeneration (GTR) techniques utilize membranes that act as scaffolds for bone and tissue growth while serving as barriers against rapidly proliferating cells and pathogens. Commonly used membranes, such as poly(tetrafluoroethylene) (PTFE) and collagen, have significant limitations—PTFE is non-bioresorbable and requires secondary removal, while collagen lacks adequate mechanical strength and exhibits unpredictable degradation rates. To overcome these challenges, nanofiber membranes produced via electrospinning using polylactic acid (PLA) were developed. The novel composites were functionalized with bioactive additives, including periclase (MgO) nanoparticles and polydopamine (PDA), to enhance osteoblast adhesion, antibacterial properties, and tissue regeneration. This study comprehensively evaluated the biological, mechanical, and physicochemical properties of the prepared nanofibrous scaffolds. Experimental results revealed controlled degradation rates and improved hydrophilicity due to surface modifications with PDA and MgO. Moreover, the nanofibers exhibited enhanced swelling behavior, which promoted nutrient exchange while maintaining structural integrity over prolonged periods. The incorporation of bioactive additives contributed to superior osteoblast proliferation, antibacterial activity, and growth factor immobilization, supporting bone tissue regeneration. These findings suggest that the developed nanofibrous composites are a promising candidate for GBR and GTR applications, offering a balanced combination of biological activity, mechanical performance, and degradation behavior tailored for clinical use.
1. Introduction
The field of prosthodontics, driven by an aging population, the desire for improved quality of life, and aesthetic considerations, represents one of the most rapidly advancing areas in dentistry today. Prosthodontics focuses on the replacement of missing teeth to restore both function and aesthetics. A primary limitation of dental implantation, however, lies in the condition of the jawbone, which significantly influences the success of the procedure. In many cases, bone reconstruction is required prior to implant placement to ensure optimal outcomes.
A modern approach to address this challenge involves the application of guided bone regeneration (GBR) and guided tissue regeneration (GTR) techniques. These methods utilize a membrane that acts as a scaffold for bone tissue regeneration, providing both mechanical support and a conducive environment for tissue growth. The procedure typically involves exposing the targeted area, thoroughly cleansing it, and applying the membrane. This membrane functions as a barrier, preventing the infiltration of rapidly proliferating epithelial and connective tissue cells, as well as pathogens, thereby creating favorable conditions for bone growth to fill the defect [1,2,3,4,5,6,7].
Currently, the most commonly used membranes for guided bone regeneration (GBR) and guided tissue regeneration (GTR) are composed of poly(tetrafluoroethylene) (PTFE) or collagen. However, these materials possess certain limitations that necessitate the development of superior alternatives. PTFE membranes are biocompatible, stable, and mechanically durable. However, they are non-bioresorbable, requiring additional procedures for removal, which can cause patient discomfort and may potentially damage newly formed tissue. On the other hand, collagen-based membranes, while bioresorbable and biocompatible, exhibit low mechanical strength and unpredictable resorption rates, which can compromise their performance [8,9,10,11,12].
Synthetic biodegradable polymer membranes, such as those made from poly(lactic acid) (PLA), are also available. However, PLA membranes often exhibit low hydrophilicity, which limits tissue adhesion and osteoconductivity. The incorporation of functional additives into PLA membranes could mitigate these drawbacks and enhance their performance. Additionally, nanofiber membranes, owing to their high porosity, increased surface area, and improved mechanical strength and elasticity, offer enhanced placement stability and superior barrier properties [13,14,15,16,17,18,19,20].
Electrospinning is a widely utilized technique for producing nano- and microscale polymer and composite fibers under high voltage. This technique involves two primary methods: using a polymer or composite dissolved in a highly volatile solvent or employing a thermoplastic polymer in a liquefied state. Fiber characteristics are significantly influenced by process parameters, such as applied voltage, needle-to-collector distance, solvent type, flow rate, and temperature. Moreover, because electrospinning can be performed under ambient conditions, it preserves the biological activity of thermolabile compounds. Post-treatment techniques can further enhance material functionality, and the process offers the advantage of reducing reliance on toxic solvents [21,22,23,24,25,26,27,28].
Periclase, the highly crystalline form of magnesium oxide, is notable for its chemical stability and inertness, distinguishing it from its amorphous counterpart. Periclase nanoparticles possess antibacterial properties and, when incorporated into polymer matrices, enhance mechanical strength. Magnesium itself plays a critical role in influencing bone density and structure. Studies have demonstrated that magnesium oxide (MgO) nanoparticles significantly improve osteoblast adhesion and proliferation.
PLA has gained significant attention in bone tissue engineering due to its favorable characteristics, including biocompatibility, biodegradability, and customizable mechanical properties. It is also known to support osteoblast attachment and proliferation, which makes it suitable for GBR and GTR applications. As PLA degrades, it releases lactic acid, a metabolite that is naturally processed by the body, reducing the risk of adverse tissue reactions. Nevertheless, it may cause a local pH decrease, leading to inflammation. Thus, it is important to acknowledge while developing a novel biomaterial for this purpose that its degradation rate is affected by various factors such as molecular weight, crystallinity, and environmental conditions. An appropriate choice of PLA type may help to have an impact on its performance over time. PLA’s chemical structure allows for modifications and the addition of bioactive materials. These may include ceramics, nanoparticles, or other polymers to improve their mechanical properties, bioactivity, and ability to support bone regeneration. Although PLA has many advantages, it still has some challenges that must be overcome, including its hydrophobic nature and slow degradation under physiological conditions. Advanced fabrication methods like electrospinning enable PLA processing, followed by the integration of functional nanomaterials with its matrix, which have shown promise in addressing these limitations. Recent research highlights the potential of PLA-based nanofibrous scaffolds to deliver the mechanical strength, porosity, and bioactivity needed for effective bone regeneration. Particularly promising ones are those enhanced with bioactive additives [29,30,31,32].
Polydopamine (PDA), a polymer derived from natural sources, is biocompatible, hydrophilic, and mucoadhesive. It also exhibits antioxidant, metal-ion chelating, and antibacterial properties due to its catecholamine and hydroxyl functional groups. PDA enables bioconjugation with a wide range of biomedical materials and facilitates cellular interactions. It is synthesized through the auto-oxidation and polymerization of dopamine. Growth factors are soluble proteins secreted by cells that regulate proliferation, migration, and differentiation by binding to specific transmembrane receptors, thus promoting wound healing. Despite their considerable therapeutic potential, growth factors are limited by their short half-lives, low stability, and rapid enzymatic degradation at body temperature. Encapsulation within a polymer matrix can address these challenges by providing protection and enabling controlled release. The nanofibrous composite presented in this study represents an innovative biomaterial for GBR and GTR applications. It offers a combination of advantageous biological and mechanical properties, making it a promising candidate for use in tissue regeneration thanks to the use of potentially bioactive components, which may affect osseointegration as well as new bone formation [33,34,35,36].
2. Materials and Methods
2.1. Materials
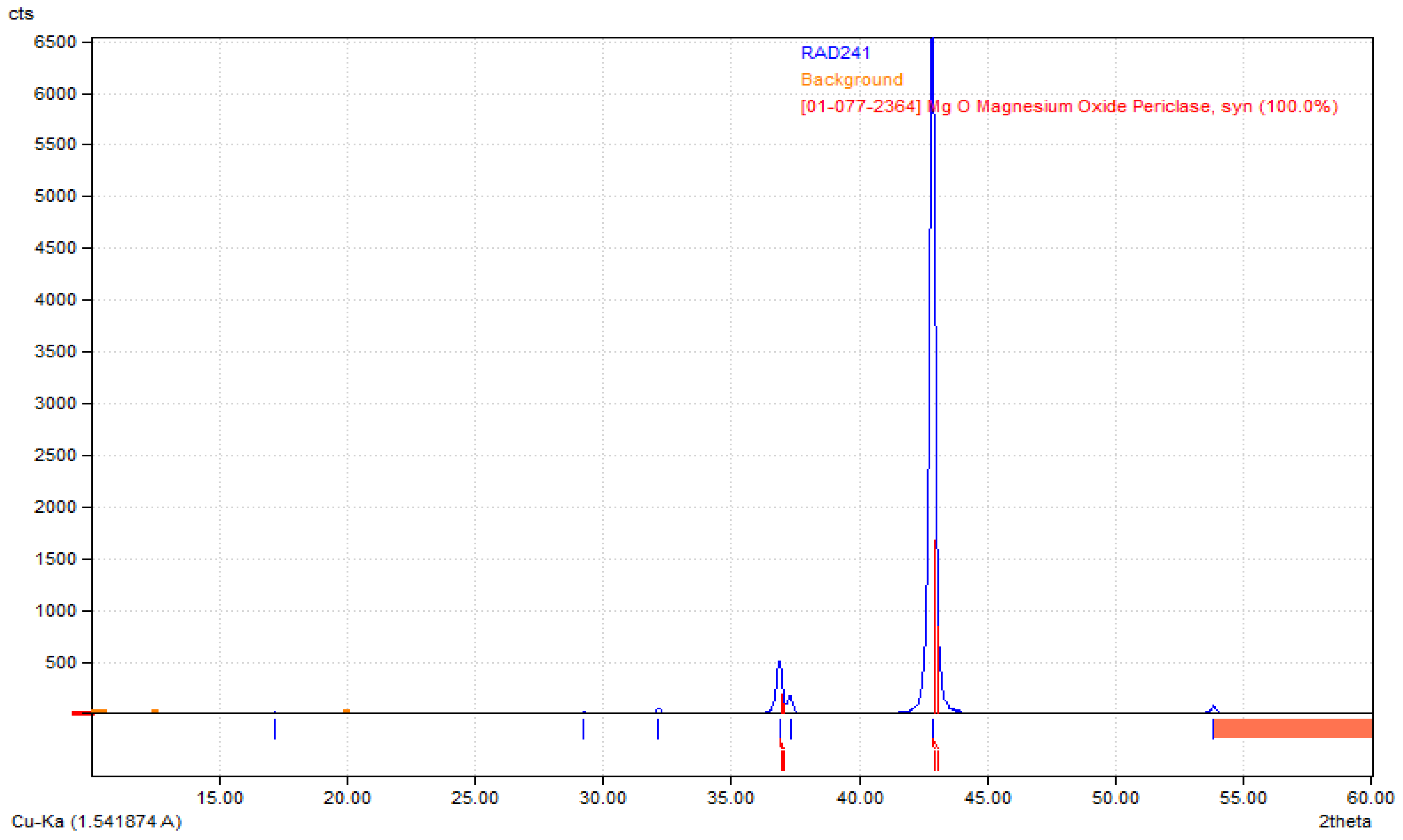
Poly(L-lactic acid) (PLA) of molecular weight 193.3 kg/mol was purchased from Good Fellow (UK), acetone (CAS: 67-64-1), dopamine chloride (CAS: 62-31-7), MgSO4 (CAS: 7487-88-9), Dulbecco’s Modified Eagle Medium (DMEM) with glucose and phenol red content cell culture medium, fetal bovine serum (FBS), phosphate buffer solution (PBS sterile, pH = 7.4), CaCl2·2H2O (10035-04-8), KCl (CAS: 7447-40-7), KOH (1310-58-3), KH2PO4 (CAS: 7778-77-0), Na2HPO4 (CAS: 7558-79-4), NaHCO3 (CAS: 144-55-8), NaCl (CAS: 7647-14-5), HCl (7647-01-0), NaOH (CAS: 1310-73-2), osteosarcoma MG-63 cell line (The European Collection of Authenticated Cell Cultures (ECACC)), trypsin with EDTA, antibiotics (streptomycin/penicillin), cell proliferation kit XTT (Roche, Basel, Switzerland) were purchased from Sigma Aldrich, Poznań, Poland. All solvents were of analytic-grade purity. Highly crystalline magnesium oxide, i.e., periclase, the so-called dead MgO, was obtained by roasting magnesium hydroxide in an argon atmosphere at temperatures of 1100 °C and 1300 °C, with a temperature step of 100 °C, was used to modify the membranes. Its structure has been confirmed by XRD analysis (Figure 1).
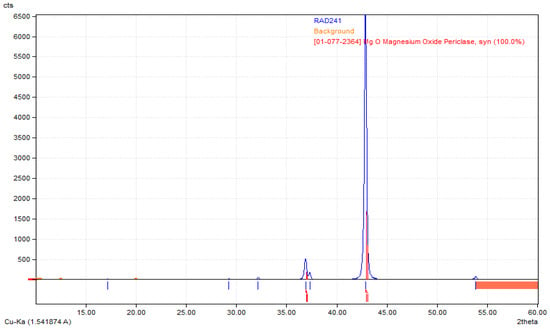
Figure 1.
XRD diffractogram confirming preparation of highly crystalline periclase.
2.2. Methods
2.2.1. Biomaterials Preparation
The nanofibrous biomaterial was obtained by electrospinning of 10% solution of PLA in acetone of periclase, polydopamine NPs (0.5% ethanol solution), and BMP-2 growth factor. The mixture thus obtained was subjected to ultrasound with a power of 150 W and a frequency of 50 kHz for 30 min. Then, electrospinning was carried out at variable parameters. For the experiments, samples with the most favorable structural integrity and homogeneity were used, which were obtained using a voltage of 25 kV, the mixture feed rate using a syringe pump was 10 mL/h, and the distance of the needle from the rotating collector was 10 cm. After electrospinning, the fibrous material was dried at a temperature of 25 °C and a pressure of 1 atm until the solvent was completely removed and a dry mass was obtained. The chemical composition of the samples is given in Table 1.

Table 1.
Chemical composition of prepared composites.
2.2.2. Ansys Analysis
To simulate the electrospinning phenomenon, the ANSYS Maxwell software was used (version 2020 R2). The Electric Transient simulation was performed for three different supplied voltage values (25 kV, 30 kV, and 35 kV). The needle-to-collector distance was stated as 50 and 100 mm. To present the field distribution results, the Authors selected the exemplary cases. The generated was related to grid densities in regions of electric field concentration. The simulation time was 30 s.
The simulation conditions were as follows:
- Needle top to collector distance: 50 and 100 mm, representing the working distance in the electrospinning setup;
- Grounded collector (the collector was set to ground potential to establish a stable electric field gradient between the needle and the collector);
- Number of mesh elements: 1,233,843 (a high-resolution mesh consisting of 1,233,843 elements was generated to ensure accurate field distribution analysis and capture detailed changes in the electric field);
- Needle material: stainless steel (stainless steel was selected as the needle material due to its high electrical conductivity and compatibility with the electrospinning process);
- Collector material: aluminum (aluminum was chosen as the collector material, leveraging its excellent conductivity and lightweight properties);
- Needle filling: acetone (the needle was modeled as containing acetone, reflecting the solvent used during the electrospinning process);
- Syringe material: plastic (plastic (PS) was used to model the syringe, consistent with the experimental apparatus).
The analysis type was set as Electric Transient with a simulation time of 30 s. The simulation area was restricted to a 570 × 600 × 90 mm box. The voltage excitation applied to the needle was set as 25 kV, 30 kV, and 35 kV, with zero potential assumed for the collector. The crated model allows for changing the needle position and dimension without the new mesh settings. The vector and magnitude field intensity distribution display were chosen for the presentation of the results.
2.2.3. Chemical Structure Study
Chemical structure was evaluated using Fourier-transform infrared spectroscopy (FT-IR) equipped with ATR adapter (diamond crystal). For the experiments, Thermo Nicolet Nexus 470 FT-IR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) was used. The spectra were collected in range from 4000 to 500 nm, resolution 4 cm. All samples were dried before the analysis.
2.2.4. Morphology Study
To investigate morphology of the semi-products and ready materials, TEM and SEM analyses were carried out.
Nanomaterials were investigated by Transmission Electron Microscope purchased from Jeol (Peabody, MA, USA). For this reason, nanoparticle suspensions were instilled on copper meshes and left to dry. Next, Ready products were investigated by FEI Quanta 650 FEG Scanning Electron Micro-scope purchased from FEI (ThermoFisher Scientific, Hillsboro, OR, USA). Samples before analysis were sputtered with copper. Images were taken under various magnifications, SEM HV = 20 kV using BSE/SE detector, WD = 14.98 mm.
2.2.5. Determination of Swelling Degree in Water, SBF and PBS
To determine swelling abilities of the prepared nanofibers, 10 mg of each sample was placed in distilled water, SBF (pH = 6.5 to imitate artificial saliva), and PBS and left for 24 h. After fixed period intervals, the sample was weighed again. The swelling degree was calculated using Equation (1):
where
SD = (Wt/W0)
SD—swelling degree (g/g);
Wt—sample weight after 24 h (g);
W0—initial weight of the sample (g).
2.2.6. Antibacterial Properties Study
The study was carried out on gram-positive (+) S. aureus and negative E. coli (−) bacteria strains (PolAura, Dywity, Poland) via dilution test. For this reason, bacteria suspensions were added to the samples, placed in the cell culture medium, and incubated at 37 C. After fixed time intervals (2, 4, 6, 24 h), 200 μL of the liquid from each well was collected and placed on agar plates, followed by further incubation. After 24 h, colony counting was carried out, and CFU was determined for each sample.
2.2.7. Cytotoxicity Study
The human osteosarcoma MG-63 cell line, which is commonly used in cytotoxicity tests of biomaterials in accordance with the ISO 10993 standard [37], was used for the experiments. The culture was conducted for 168 h under standard conditions (5% CO2 concentration, high humidity, 37 °C) using DMEM (Gibco, Waltham, MA, USA) as a medium which was changed every 48 h. The observation of cell morphology under inverted microscope was performed using 40× magnification (Delta Optical IB-100 microscope, Planeta Oczu, Zielona Góra, Poland). The XTT assay was used to measure cell viability by measuring absorbances at 450 nm according to producer’s protocol (Roche).
3. Results and Discussion
3.1. Electric Field Intensity Study
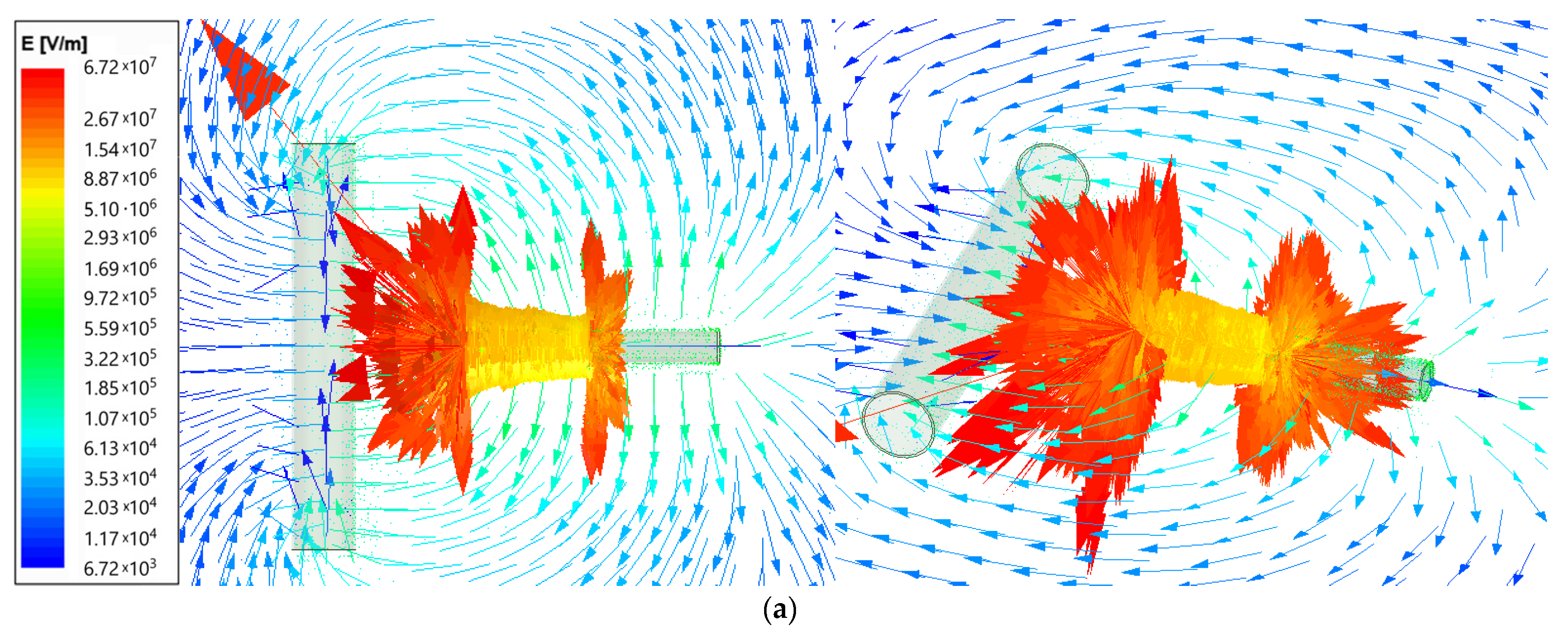
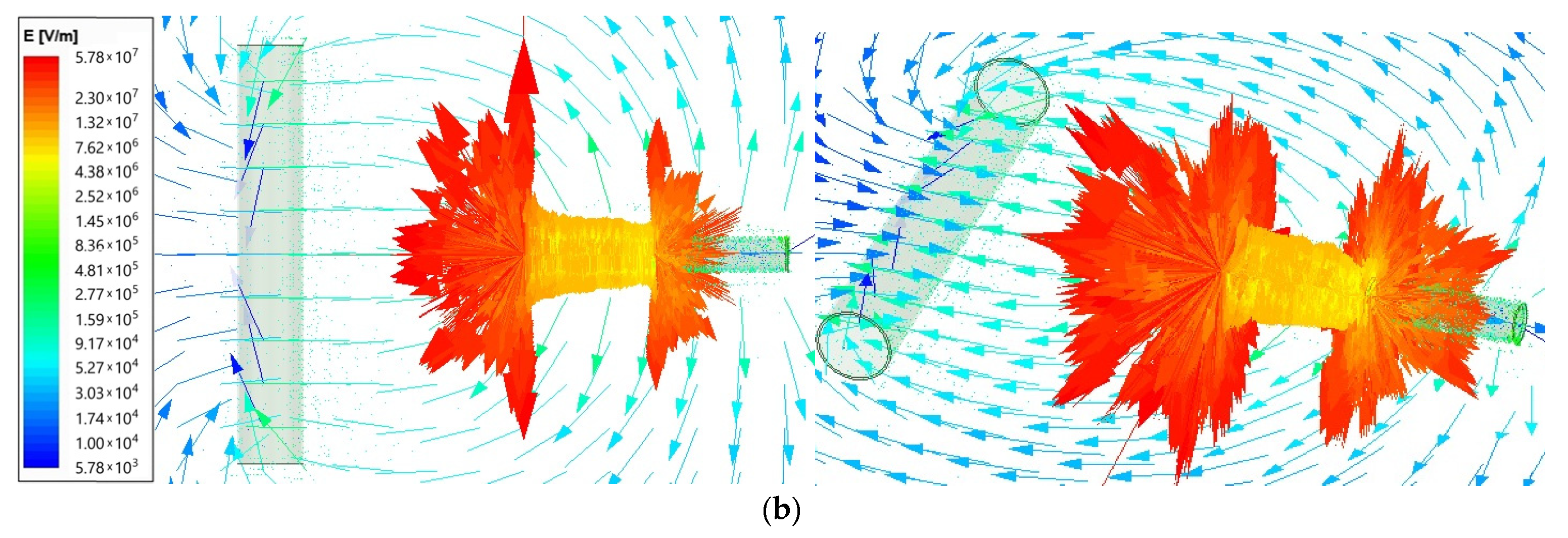
Using the ANSYS Maxwell software, the field analysis was performed (type Electric Transient). The electric field intensity (E) for the electrospinning machine is presented as vector distribution on plots from Figure 2. The needle length was set as 6 cm and was supplied by voltage 25 kV for two needle-to-collector distance cases: 50 and 100 mm. The arrows show the electric field direction; their length is proportional to the field intensity. The results are given using the logarithmic scale.
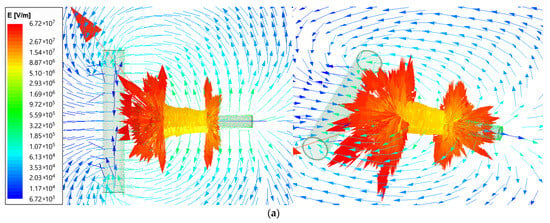
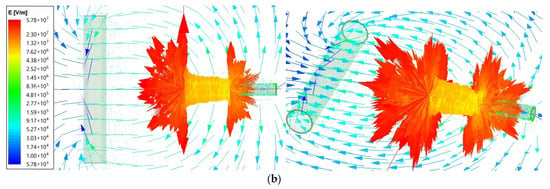
Figure 2.
Vector plot of electric field intensity for electrospinning machine for (a)—50 mm; (b)—100 mm needle-to-collector distance.
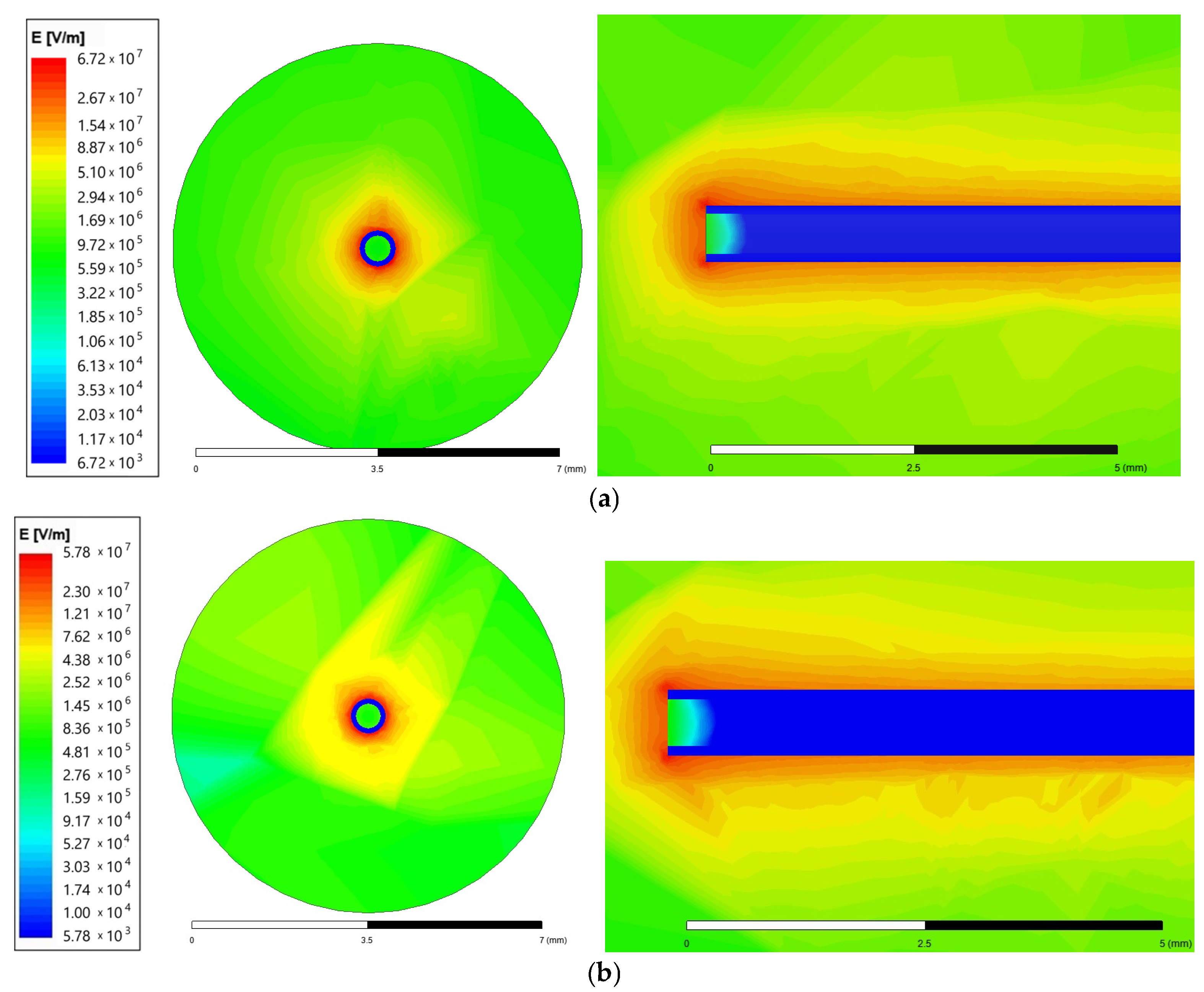
Figure 3 contains the magnitude field distribution on the surface placed at the needle top and on the surface crossing the needle. A 6 cm length needle was supplied with a voltage of 25 kV for two needle-to-collector distance cases: 50 and 100 mm. The plots also use the logarithmic scale.
Figure 3.
Magnitude of the electric field intensity for the needle end and surroundings (front view and top view) for (a)—50 mm; (b)—100 mm needle-to-collector distance.
The maximum electrical field intensity value is 6.73 × 107 for the 50 mm needle-to-collector distance and 5.78 × 107 for the 100 mm needle-to-collector distance. The highest values are concentrated close to the corners of the needle end, as presented in Figure 3 on the top view. Therefore, the next comparison was performed for the smaller distance value of 50 mm.
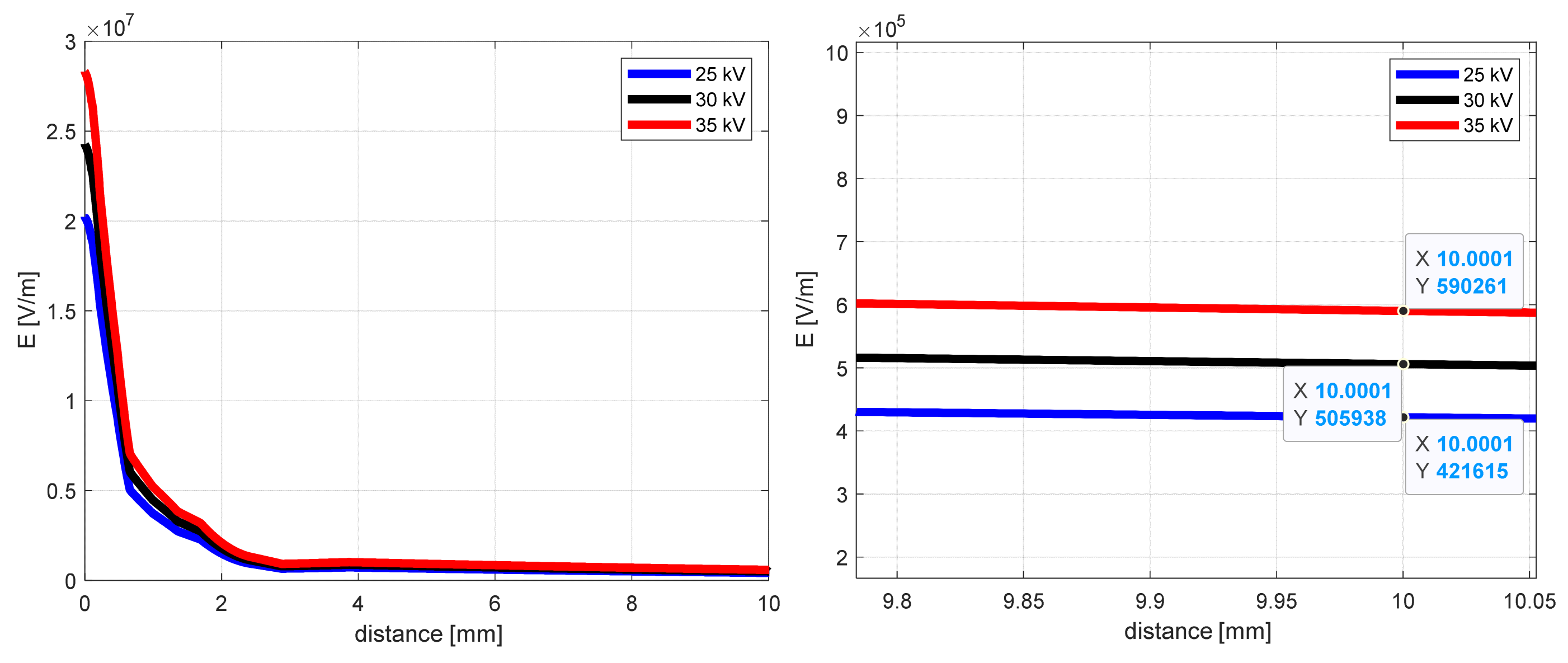
The influence of the supplied voltage for the electric field intensity as a distance function, from the middle of the needle end to the collector (y-coordinate), is shown in Figure 4 for the case of 50 mm distance. The other conditions of the simulation remained unchanged.
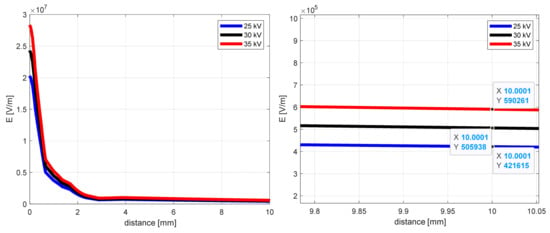
Figure 4.
The electric field intensity as a distance function from the needle end (y-coordinate) for 50 mm needle-to-collector distance.
Analyzing the curves from Figure 4, the notable differences in electric field intensity one can see close to the needle end (0–2 mm). As the distance increases, these values approach each other and, after 10 mm, are about 590 kV/m, 506 kV/m, and 422 kV/m for 25 kV, 30 kV, and 35 kV, respectively. Close to the collector (after 50 mm), the electric field intensities for selected supply voltage values are as follows: 267 kV/m, 320 kV/m, and 373 kV/m.
The maximum electric field intensity values for selected supplied voltages are presented in Table 1. As shown in Figure 3, the maximum field value concentrates close to the needle end corners.
While changing the voltage value from 25 to 30 kV, the maximum field intensity value increases by 20.0%. Raising the voltage from 30 to 35 kV, this change is about 16.6%. As mentioned in [6], the knowledge of external electric field value may be used to calculate the electrospun fiber diameter using the Scaling law [38,39,40,41,42,43,44].
3.2. Chemical Structure Study
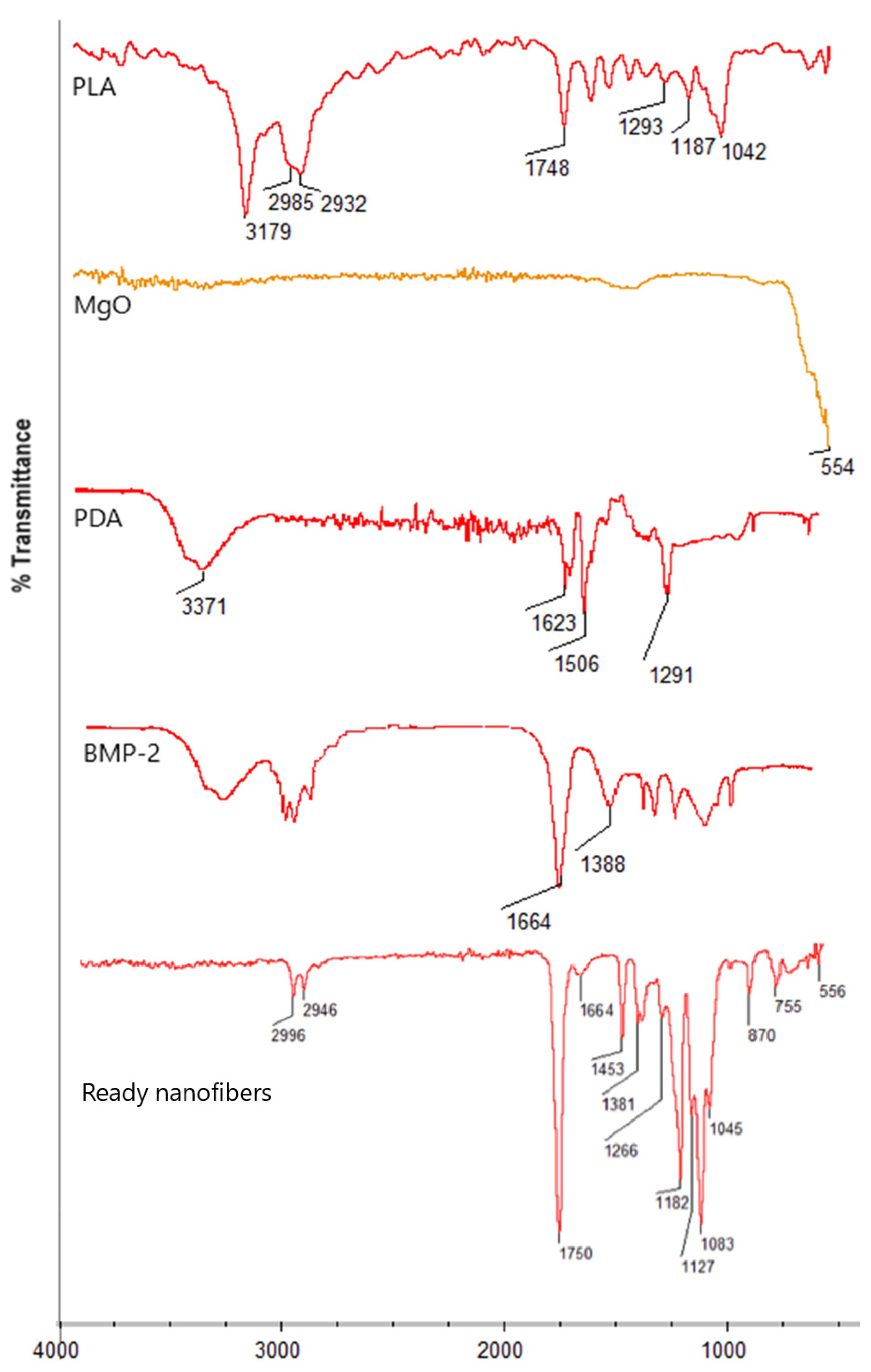
Figure 5 presents general scheme for biomaterials preparation and future possible use. Figure 6 reveals the results of the FT-IR study of the raw materials (a) and ready nanofibers (b) Pure PLA spectrum reveals bands coming from aliphatic moieties at 3179, 2985, and 2932 cm−1 as well as bands representing ester bonds between mers at 1748 cm−1. Periclase nanoparticles exhibit a sharp band at 554 cm−1, typical for MgO. Poly(dopamine) spectrum shows typical bands at 3371 cm−1 coming from o stretching vibration of NH and OH groups, while bands at 1623 and 1506 cm−1 correspond to C=C stretching vibrations present in an aromatic ring. The band at 1291 cm−1 comes from hydroxyl moiety from the aromatic ring. BMP-2 exhibits typical bands at 1664 cm−1 coming from peptide (amide) bonds and 1388 cm−1, which can be assigned to amide bond moieties. In the case of ready fibers, it can be noted that typical for ester bonds, bands at 1750 cm−1 are visible together with bands at 2996 cm−1 and 2946 cm−1 coming from -CH3 and -CH2- moieties confirming lack of biodegradation of the PLA during processing. Moreover, bands corresponding to poly(dopamine) nanoparticles’ presence at 1453 cm−1 and 1266 cm−1 can be distinguished, as well as bands typical to inorganic MgO nanoparticles (556 cm−1), which proves their incorporation. Finally, the presence of BMP-2 in the polymeric matrix is confirmed by the band corresponding to peptide bonds with a maximum of 1664 cm−1. At the same time, no bands coming from the solvent can be spotted, thus confirming nanofibrous composites purity.
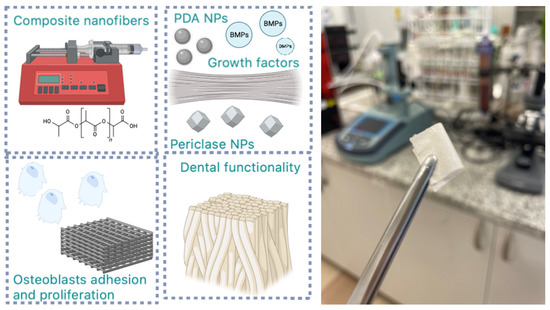
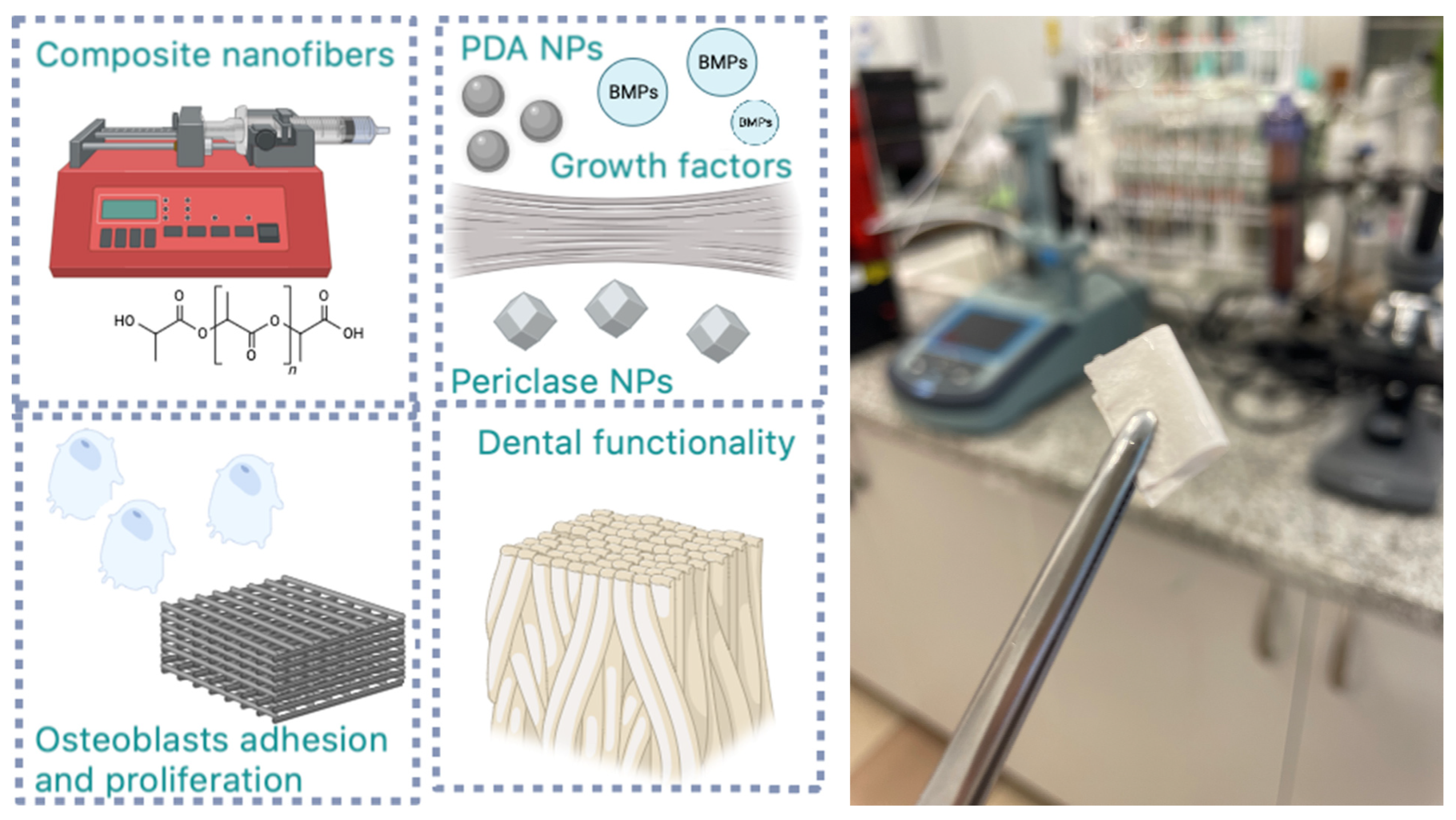
Figure 5.
General scheme for biomaterials preparation and application.
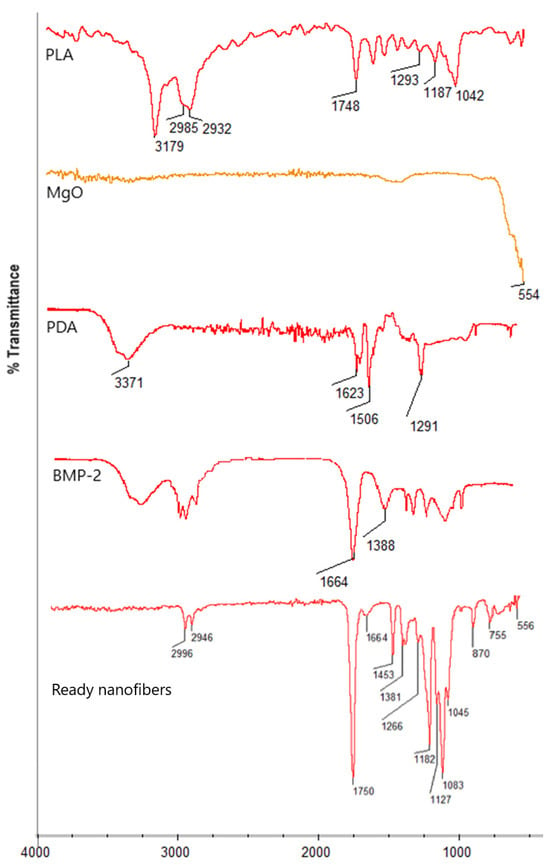
Figure 6.
FT-IR spectrum of raw materials and ready nanofibers.
3.3. Morphology Study
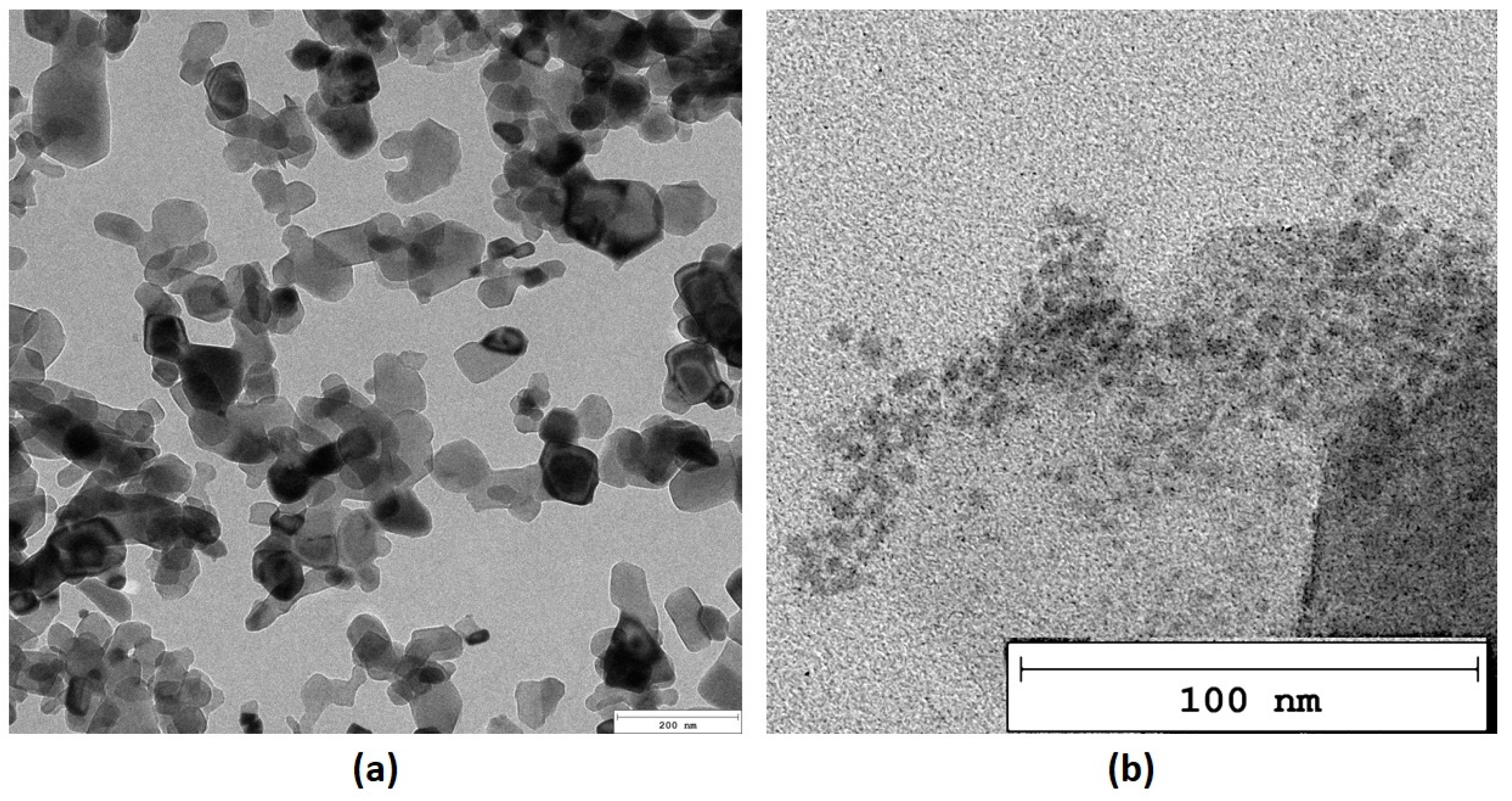
Nanoparticles, due to their size, may exhibit some unique features strictly correlated with their size and morphology. Thus, both inorganic and organic NPs (periclase/PDA) were investigated over a TEM microscope. As it can be observed in Figure 7a MgO nanoparticles are predominantly rectangular in shape, exhibiting well-defined edges and corners. This geometric form is indicative of their crystalline structure. The size of the nanoparticles varies from 20 to 100 nm in length. On the contrary, polymeric NPs prepared from dopamine chloride are characterized by a more regular, oval morphology. Moreover, periclase NPs appear darker compared to the surrounding media in the TEM microphotograph, which indicates higher electron density due to their heavy metal composition. This contrast allows for easy identification against other materials in the sample. As shown in Figure 7b, PDA NPs are generally smaller than the MgO nanoparticles and may exhibit a more irregular or spherical shape. These particles are typically distributed in the vicinity of the MgO nanoparticles, appearing a little bit clustered or aggregated. The surface of the poly(dopamine) nanoparticles shows a rougher texture compared to the smooth, crystalline surface of the MgO particles. This roughness can be attributed to the polymeric nature of poly(dopamine), which is composed of various functional groups that enhance its interaction with other materials. Poly(dopamine) nanoparticles appear less dense than the MgO nanoparticles, resulting in lighter shades in the TEM images.
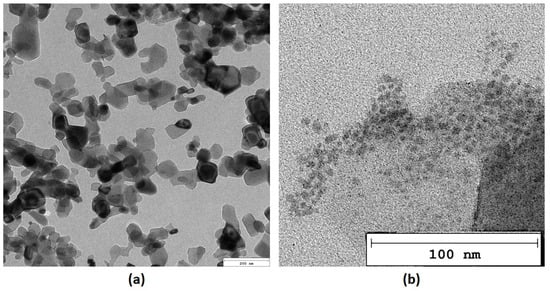
Figure 7.
TEM microphotographs (a)—periclase (b)—poly(dopamine).
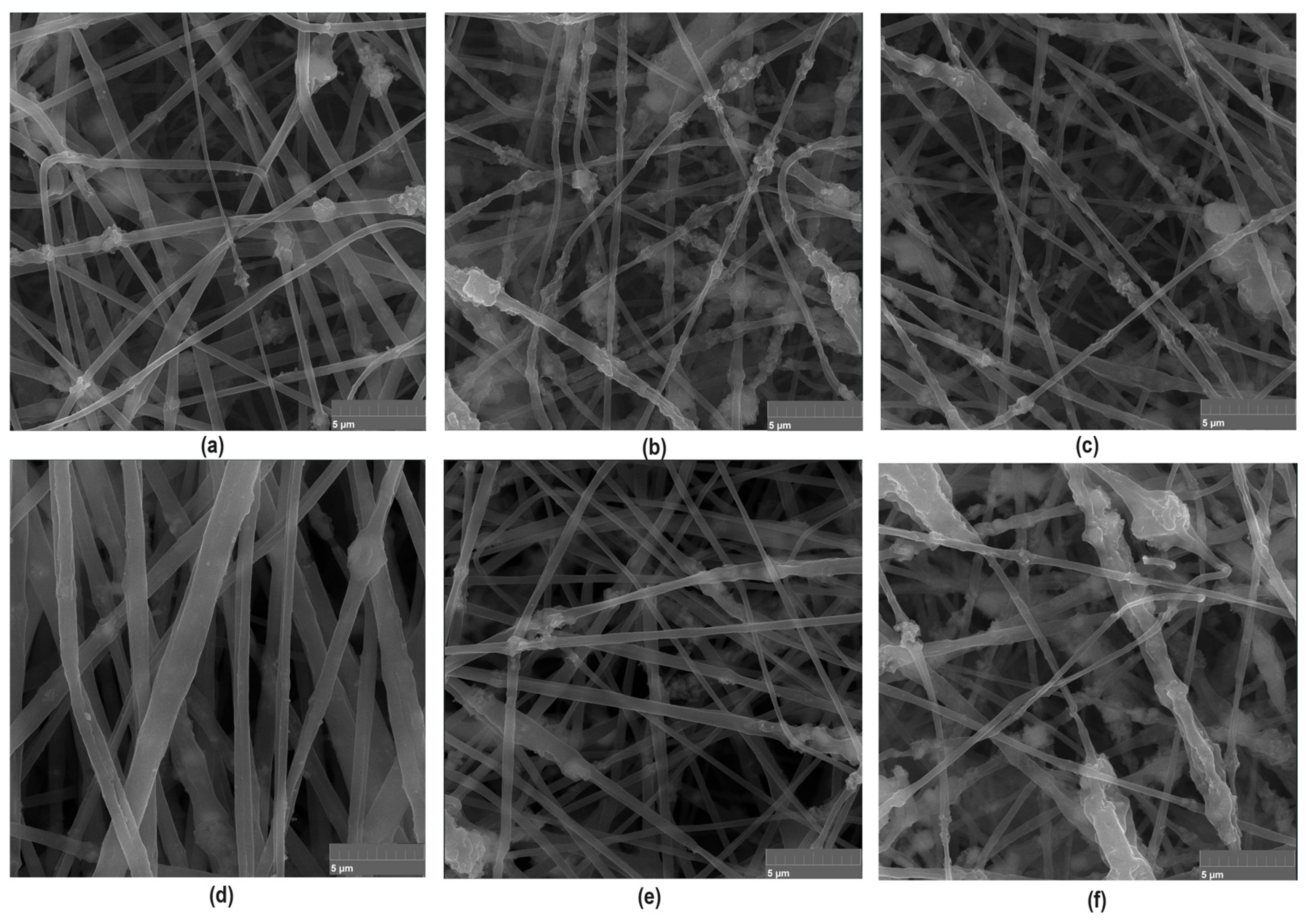
To further investigate newly prepared biomaterials and confirm their fibrous nature, SEM microscope imaging has been carried out. As shown in Figure 8, all prepared samples are characterized with nanofibrous structure. The PLA nanofibers are characterized by uniform, elongated shapes with diameters in the micro- and nanometer range. The fibers may appear as continuous strands, forming a web-like structure that is highly porous and provides a large surface area, which is beneficial for applications in tissue engineering and guided bone regeneration process. Moreover, such morphology prevents biofilm formation and bacteria adhesion. Importantly, the presence of MgO nanoparticles on the surface of the PLA nanofibers is visible in the SEM images. These nanoparticles appear as discrete particles scattered inside the fiber matrix. The crystalline MgO in the form of periclase nanoparticle contributes to the mechanical strength and may enhance the bioactivity of the fibers due to prolonged Mg2+ ions release, which take part in the osteogenesis process. The poly(dopamine) nanoparticles can be observed as a secondary layer on the MgO-coated PLA nanofibers. The poly(dopamine) appears as darker, irregularly shaped particles, which may vary in size. This coating can improve the adhesion of biological molecules and cells due to the presence of functional groups introduced by poly(dopamine), enhancing the overall bioactivity of the scaffold. As shown in Figure 8, the combination of MgO and poly(dopamine) nanoadditives may introduce additional surface roughness to the nanofibers. The SEM images reveal a textured surface, promoting cell adhesion and proliferation, which is essential for tissue engineering applications. What is important, the addition of PDA and periclase NPs did not negatively affect the morphology and spatial micro-architecture of the biomaterials. The porosity allows for nutrient and waste exchange, making the scaffolds suitable for cell culture environments. Thus, performed PLA-based nanofibers preparation by means of electrospinning resulted in the formation of a complex and bioactive surface that is beneficial for various biomedical applications. The SEM images show a well-distributed layer of nanoparticles on the fibers without significant aggregation, which is crucial for maintaining the functional properties of the nanofibers.
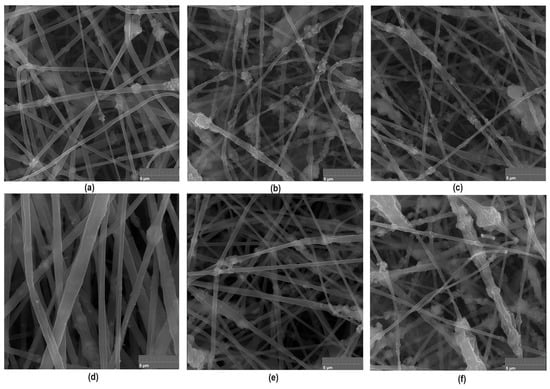
Figure 8.
Nanofibers morphology containing periclase (×20k magnification): (a)—sample 2; (b)—sample 3; (c)—sample 4 (d)—sample 4 with extra magnification (×50k); (e)—sample 5; (f)—sample 6.
SEM images confirmed that there is a relationship between electric field intensity and fiber diameter (scaling law), which allowed for precise control of fiber dimension. Thus, it was possible that they would remain in the nanometer range for increased surface area and bioactivity, even when modified with additional nanoscale additives. In performed theoretical studies regarding the voltage applied during the process, as shown in Table 2, it was found that this voltage directly affects the maximum field intensity, which influences the polymer jet’s stretching force. The application of higher voltages increases field intensity, leading to thinner fibers obtainment but, on the other hand, may also introduce instability if not carefully controlled. Our research showed that electrical field simulations lead to the preparation of the material, which is characterized by uniform morphology and high porosity, which is crucial for biomedical applications, especially in terms of tissue recovery. Successful integration of periclase and PDA nanoparticles enabled potential bioactivity enhancement via promoting osteogenesis and cell adhesion.

Table 2.
The maximum value of the electric field intensity for electrospinning machine.
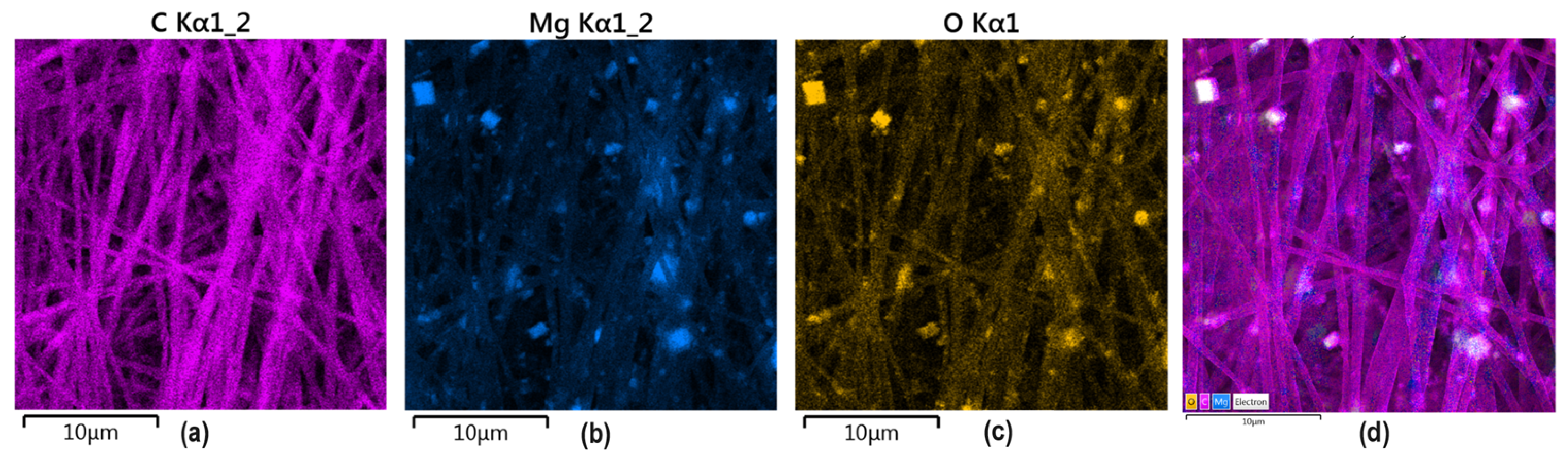
To confirm MgO and PDA presence inside the nanofibers, EDX analysis has been carried out. As shown in Figure 9, MgO crystalline particles are clearly visible inside the polymeric matrix composed mainly of carbon and oxygen. No additional impurities are present. It can be noticed that nanoparticles are uniformly distributed inside the material and exhibit a homogenous shape. Such distribution is very important from the future application point of view since it will provide good mechanical durability and bioactivity, which results from MgO presence. Moreover, it shows that electrospinning parameters were well-adjusted since ready material is characterized by repeatable architecture. Such NPs distribution will help to provide good coverage of new bone formation areas and will be able to interact with cells and biomolecules. Superficial placement of MgO particles will also help to prevent infections due to bacteriostatic effects due to direct interaction with pathogens. Such elemental distribution is especially important in the case of highly porous, nanofibrous membranes, which are characterized by low thickness, and their primary mode of action strictly depends on the fibers’ alignment and nanoadditives presence at the superficial layers.
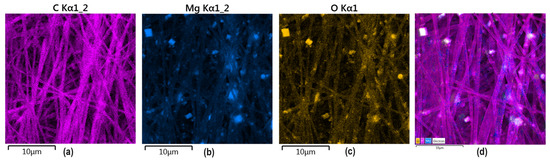
Figure 9.
EDS analysis of the GBR membrane sample 6: (a) pink—carbon; (b) blue magnesium; (c) yellow oxygen; (d) all elements marked (C, Mg, O).
3.4. Swelling Abilities Study
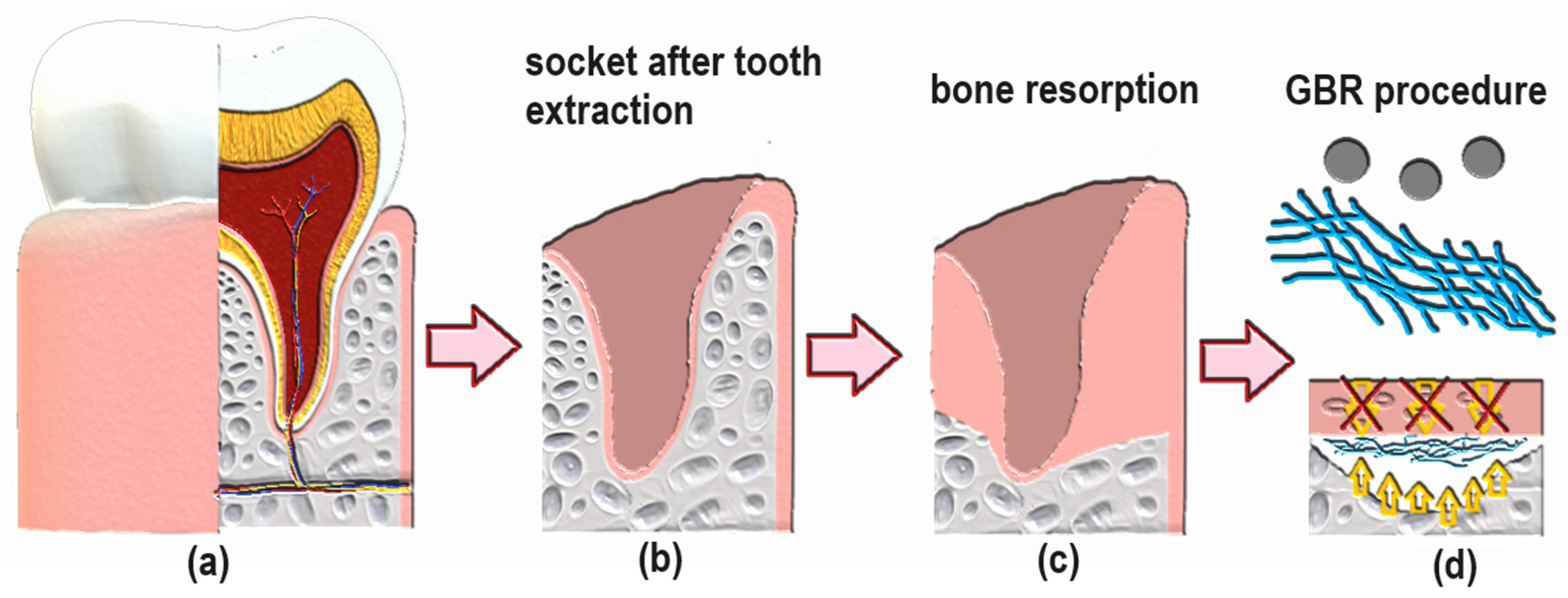
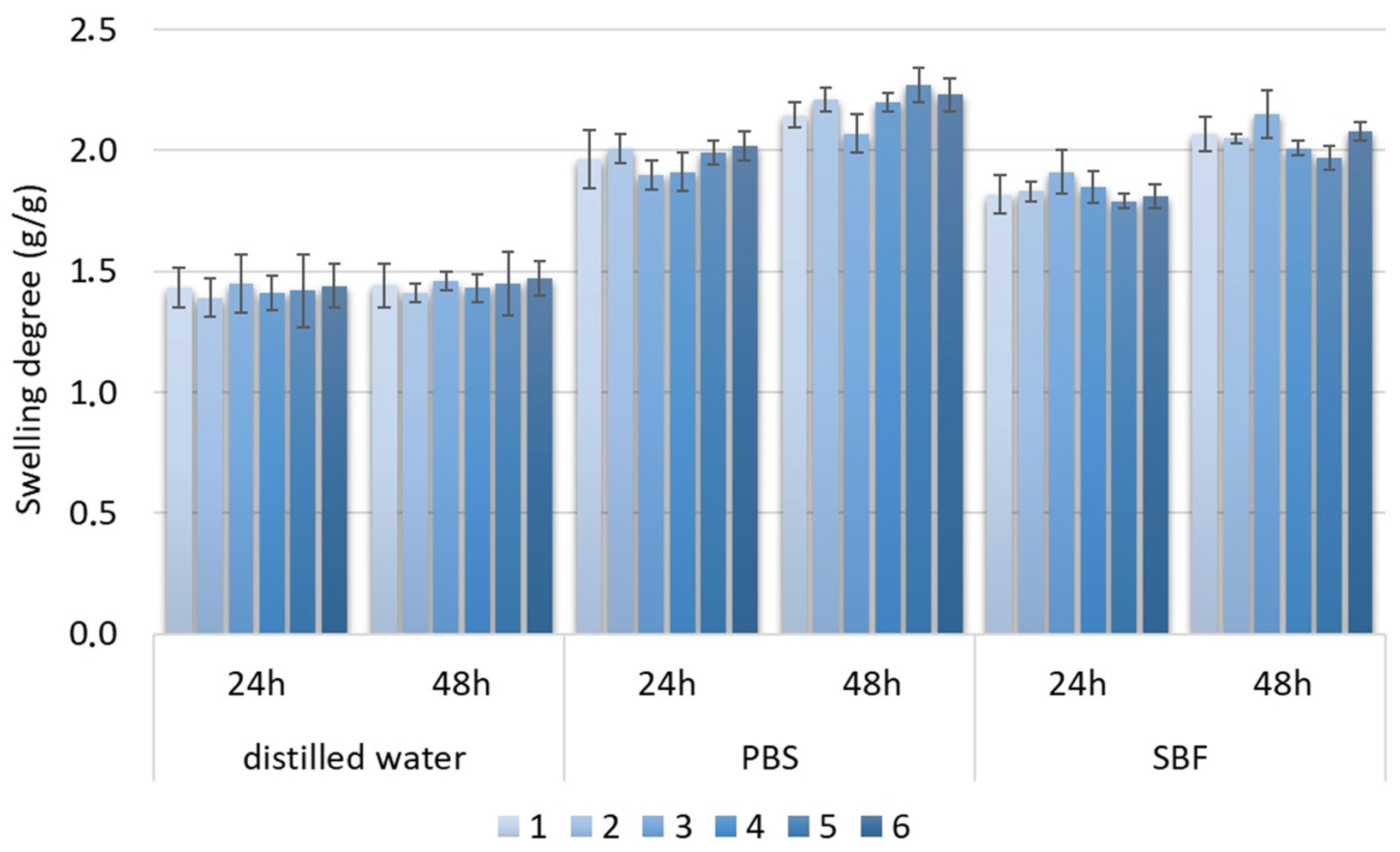
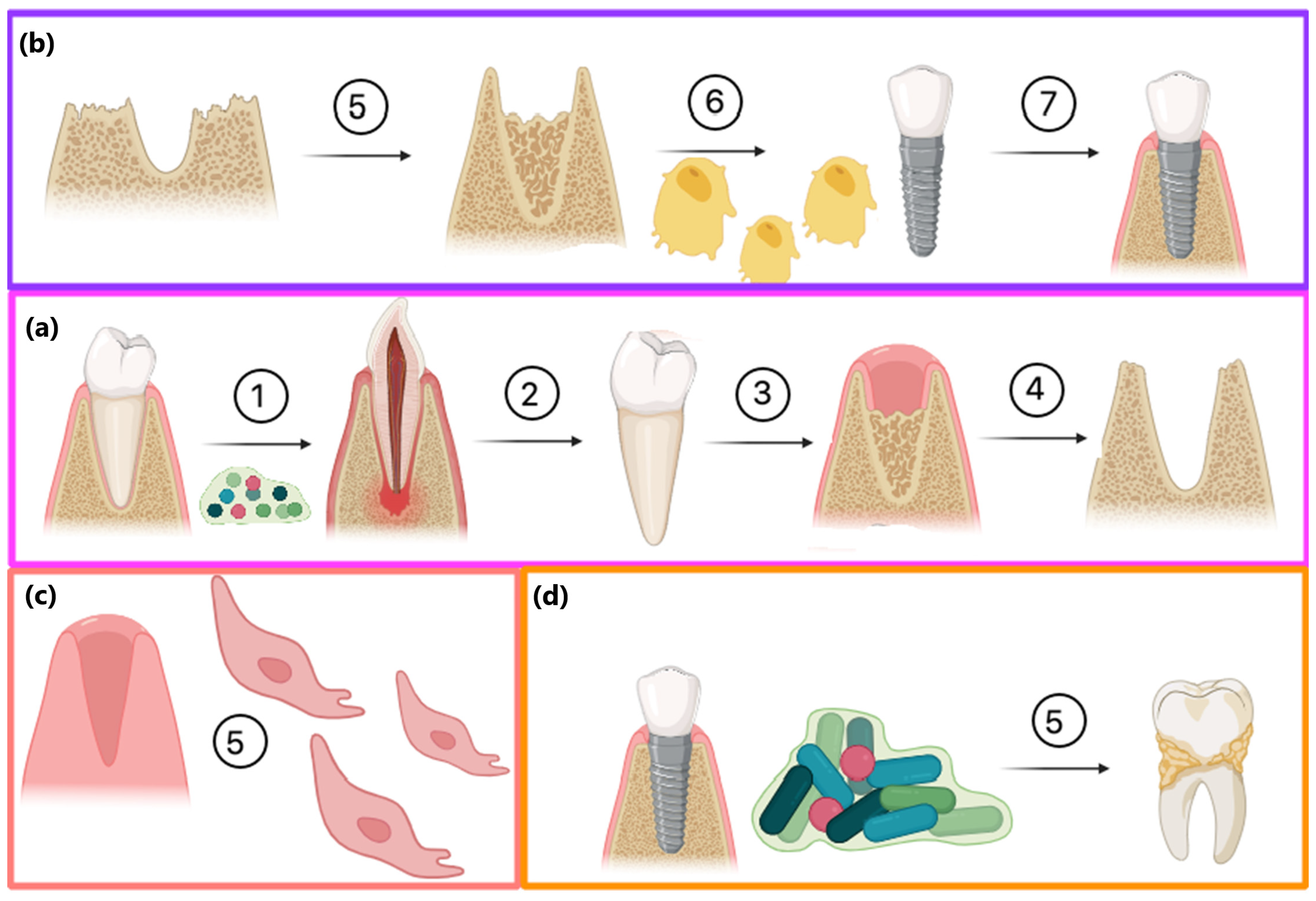
The swelling degree describes the possibility of the evaluated material absorbing water and other different solvents. Biomaterials that are dedicated to medical applications, especially in the field of tissue regeneration, should be able to provide an aqueous environment for proliferating cells. Nevertheless, under certain circumstances, the material should not increase its volume too much and create overly wide spaces. Guided bone tissue regeneration relying on the application of bioactive membranes can be included. As shown in Figure 9, after tooth extraction occurs and an empty socket is generated (b), bone resorption occurs (c). Thus, to perform implantation, this hard tissue must be restored for proper implant fixation followed by osseointegration. To make this happen, it is crucial to provide appropriate conditions for bone recovery while simultaneously creating a barrier that would prevent invasion of attachment gingival epithelium cells, preventing cell competition that would be detrimental to bone mineralization since osteogenic cells are known for slower proliferation. Application of GBR membrane (d) provides space for osteoblasts proliferation, bone mineralization, ECM deposition and angiogenesis due to mechanical separation from epithelium cells. Moreover, appropriate porosity and antibacterial activity enable the prevention of biofilm formation (Figure 8). The creation of such biomaterial leads to full bone regeneration (Figure 10). Figure 11 presents results from the swelling abilities study. It can be noticed that all samples had a certain ability to absorb various media, including distilled water, simulated body fluid, and phosphate buffer solution. Due to mediocre wettability, the lowest swelling degrees were determined for distilled water and the highest for SBF, which can be assigned to the presence of various ions that may interact with nanoparticles and functional groups of PLA. After 48 h, a clear difference in value changes was observed depending on the medium. PBS showed the largest increase (0.20–0.27), suggesting that this medium had the most noticeable effect on the samples, potentially stimulating their activity or properties over time. SBF also showed an increase but to a lesser extent (0.15–0.23), indicating a more moderate response compared to PBS. In contrast, water exhibited the smallest increase (0.02), suggesting minimal changes in the samples in this medium. These findings imply that PBS and SBF had a more pronounced effect on the samples than water, and these differences may indicate various mechanisms of interaction between the media and samples at different time points. Water molecule migration inside biomaterials can be explained by the presence of periclase nanoparticles as well as three-dimensional, fibrous microarchitecture, which promotes the sorption process. It must be acknowledged that biomaterials incubated in ions containing fluids—SBF and PBS—undergo constant swelling for 48 h, which shows that before application to the patient, they should undergo a conditioning process. Nevertheless, all samples exhibited similar sorption capabilities, which can be assigned to comparable preparation pathways and chemical composition. Such results show that newly developed biomaterials are characterized by high repeatability, which is crucial for future clinical applications. Taking into consideration the fact that after 48 h, investigated materials stopped absorbing the swelling medium, it can be assumed that it should not cause any additional effects of prolonged exposure on mechanical integrity. Volumetric and dimensional stability is crucial for future applications in physiological environments, especially craniofacial areas, where dynamic stresses, including compression, tension, and shear forces, are present. Thus, the continuous swelling may alter the biomaterial’s structural and mechanical properties. The choice of fairly stable in aqueous media PLA as a main raw material should potentially avoid this problem after in vivo application.
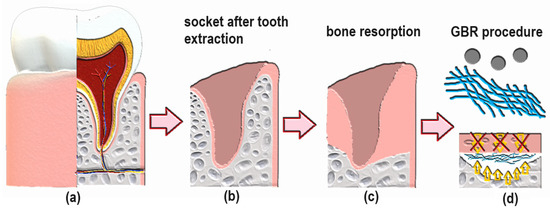
Figure 10.
General scheme of GBR procedure after tooth extraction: (a) healthy socket with tooth; (b) empty socket after tooth extraction; (c) natural bone resorption process after tooth removal; (d) guided bone regeneration due to separation of cavity from epithelial cells.
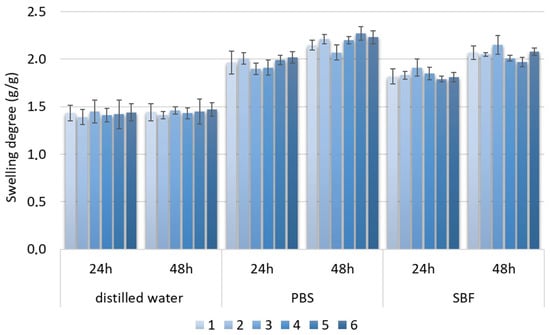
Figure 11.
Swelling degree of samples 1–6.
A great threat to successful bone regeneration is followed by implantation, fixation, and osseointegration in microbial infection, which may lead to various dental diseases and inflammation. Figure 12 shows possible pathways of the GBR procedure and the role of biofilm formation. Firstly, microbes present in the oral cavity may lead to caries and tooth loss (a). After tooth extraction, two possible modes of action may be undertaken. The most promising method is to perform the GBR procedure using bioactive membranes with dual modes of action, namely creating proper space for bone formation with osteoblast stimulation as well as antibacterial activity and biofilm formation prevention. Such attitude may lead to successful implant fixation and osseointegration. Without any additional activities, the socket can be filled with epithelial cells with complete bone loss, enabling implant fixation (c). Another option is to perform GBR, rebuild resorbed tissue, and perform implantation using standard, e.g., collagen-based membranes. However, without proper prevention during the recovery process and insufficient hygiene, biofilm may be formed, leading to dental diseases (d).
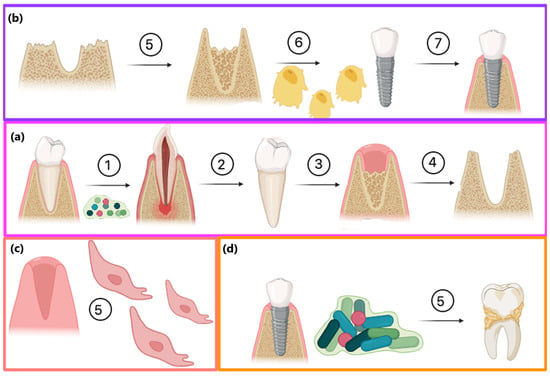
Figure 12.
Possible consequences of tooth extraction: (a) tooth loss due to caries: 1—dental biofilm formation, 2—caries leading to tooth loss, 3—empty socket after extraction, 4—bone resorption; (b) the most promising pathway including GBR procedure: 5—bone augmentation, 6—proliferating osteoblasts, 7—successful implantation and osseofixation without biofilm formation (c) alternative scenario without GBR procedure—socket filling with epithelial cells (d) issues with osseofixation and caries of adjacent teeth due to lack of biofilm formation prevention.
Table 3 and Table 4 show the results of the antibacterial properties studied against two bacteria strains, namely e. coli and s. aureus. As can be seen, in contrast to standard physiochemical properties, the chemical composition of membranes had a crucial effect on bacteria division and the number of colonies forming units (CFU). In both cases (bacteria gram (−) and (+)), samples 1–4 did not exhibit sufficient antibacterial activity to reduce the number of CFU. On the contrary, samples 5 and 6, which contained higher amounts of periclase nanoparticles, exhibited a hampering effect on bacteria multiplication. Importantly, in both cases, a bacteriostatic effect was observed after 2 h of incubation; after this period, it slightly weakened, and after 6 h or longer, an antibacterial effect was observed. Of note, higher antibacterial efficiency was observed for gram-negative bacteria, which can be attributed to the thinner cell walls and lower content of murein. Although antibacterial activity increased along with MgO content, samples containing a higher amount of periclase were characterized by low homogeneity and some agglomerates, resulting in overall material quality. Moreover, an MgO content of around 10% caused a significant culture medium increase (up to 8.4), which had a negative impact on cell viability.

Table 3.
The results of the antibacterial properties study of the membranes against Escherichia coli, CFU; *—impossible CFU count.

Table 4.
The results of the antibacterial properties study of the membranes against Staphylococcus aureus, CFU; *—impossible CFU count.
3.5. Cytotoxicity Assessment
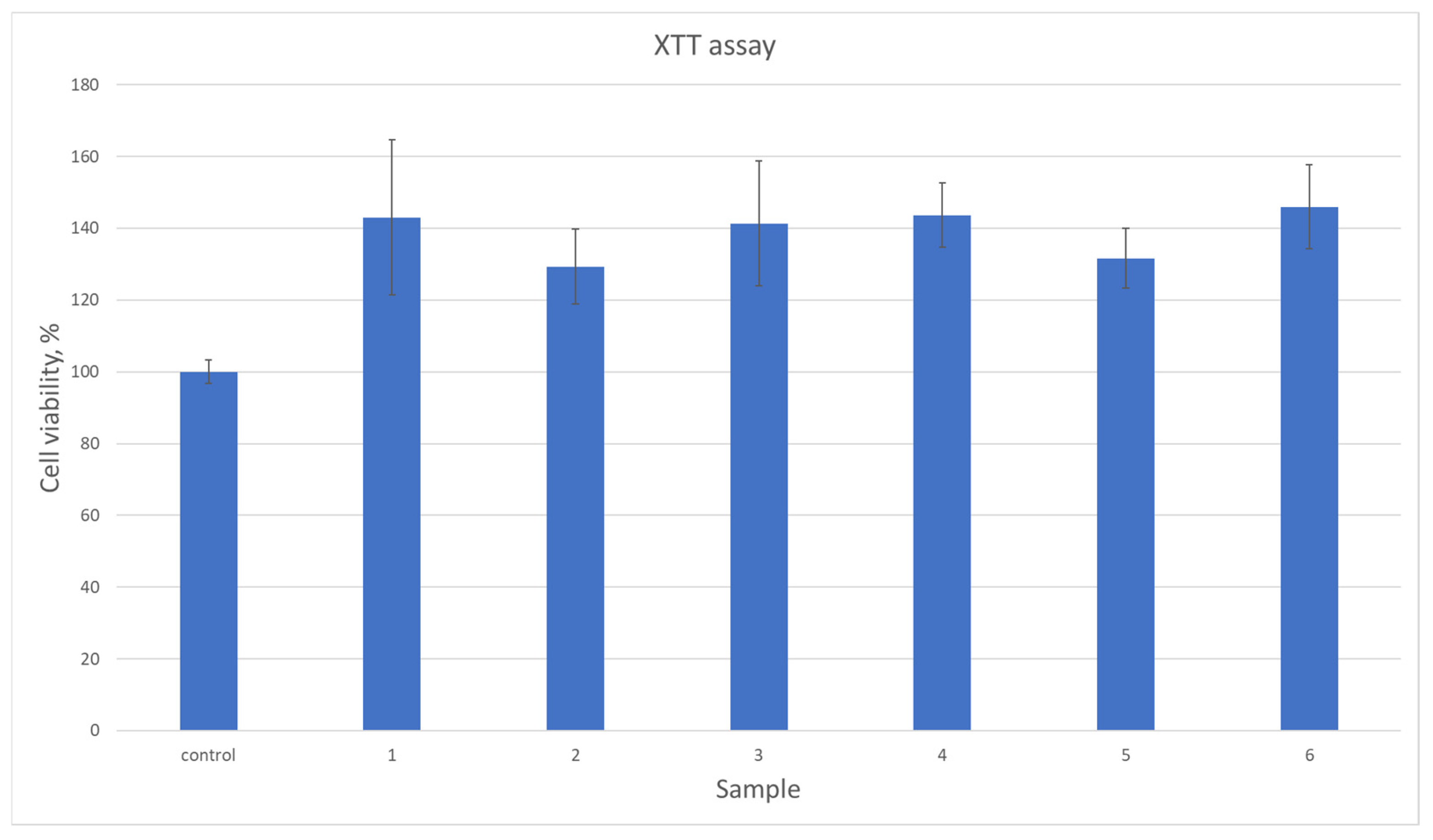
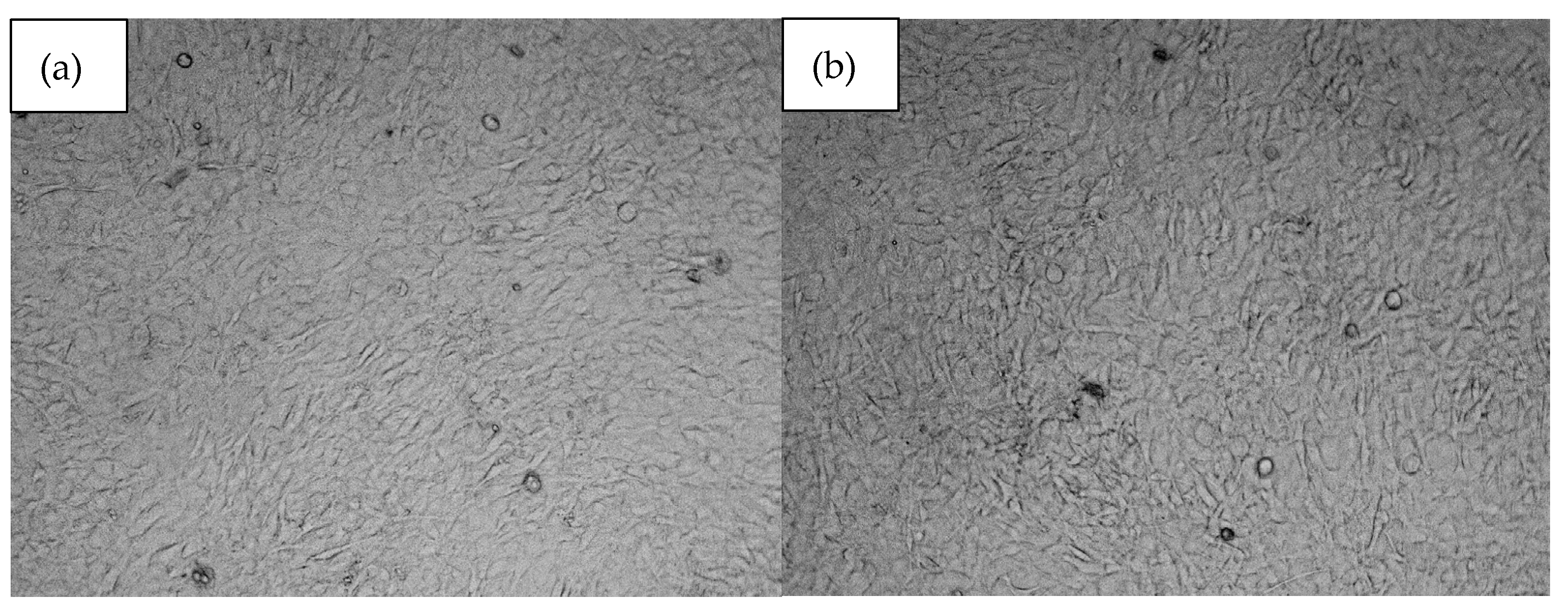
All biomaterials, despite numerous favorable physicochemical characteristics, must meet the most important criteria, namely biocompatibility. This property is evaluated both quantitatively and qualitatively. Figure 13 reveals results of cytotoxicity study carried out using XTT assay which determines the amount of metabolically active cells. For the experiment, osteosarcoma cell line MG-63 has been used which is typically chosen for bone-dedicated medical devices. According to ISO-10993 [37] norm for medical device biological evaluation, the material can be described as non-toxic if compared to control cell culture cells viability, its viability is at least 70%. As shown in Figure 13, all samples did not exhibit any negative impact. Moreover, in all cases the number of viable cells was higher than for standard culture. Such results can be assigned to the BMP-2 incorporation as well as the use of raw materials known for their biocompatibility. This property was further investigated during a direct contact test [32,33,34,35]. Based on the cell viability data (Figure 13), the samples exhibit a significant increase in cell viability compared to the control group. The average viability across all samples was approximately 138.83%, with individual sample values ranging from 128% to 144%. The highest increase was observed in samples at 144%, suggesting a robust effect on cell survival, while the lowest increase of 128% still demonstrates a clear improvement over the control. The moderate standard deviation (7.56%) indicates relatively consistent results across samples. These findings suggest that the tested samples have a positive impact on cell viability, which could be of interest for further research into their potential applications. As shown in Figure 14, photographs of cell cultures carried out in the presence of samples 5 and 6 created a complete monolayer. All cells are attached to the surface. They are flattened and connected. The cells had elongated morphology, typical for osteoblast-like cells. Also, the ECM formation process could be noticed. A positive impact on cell proliferation may also be assigned to the presence of MgO in the form of periclase nanoparticles, which acted as a filler. It is well known that Mg2+ ions play a crucial role during bone regeneration. Firstly, magnesium hampers the activity of osteoclasts, cells responsible for bone resorption. At the same time, Mg2+ stimulates osteoblasts differentiation and positively affects new tissue formation, helping to create extracellular matrix followed by its mineralization. Importantly, magnesium also affects neovascularization; thus, it enables the delivery of oxygen and nutrients to maturating bone. Secondary activities include modulation of various signaling pathways, especially modulation of calcium ions homeostasis and a reduction in inflammatory responses. The choice of magnesium incorporated inside the matrix in the form of highly crystalline periclase enables a reduction in rapid MgO reactions with water and SBF ions and provides the possibility of the presence of Mg2+ ions being prolonged through a timed release in the regeneration site.
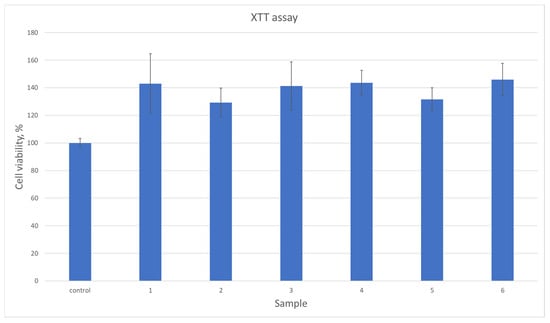
Figure 13.
Quantitative cytotoxicity study on MG-63 osteosarcoma cells.
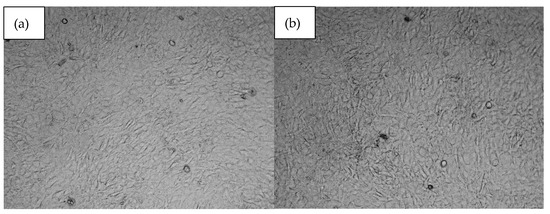
Figure 14.
Microscopic images of (a)—sample 5 and (b)—sample 6.
4. Conclusions
Most patients with tooth loss opt for dental implants to restore full dentition. A key step before implant placement is the recovery of resorbed bone and filling the empty socket, which has led to increased interest in guided bone regeneration (GBR) procedures. Both biodegradable and permanent membranes are used for this purpose, with fourth-generation biomaterials standing out due to their dual role in providing mechanical barriers and stimulating biological processes like osteoblast proliferation and biomineralization. The goal of this study was to develop GBR membranes through electrospinning that not only meet the basic requirements for dental materials but also surpass current resorbable and non-resorbable options. Theoretical studies were conducted to determine optimal process parameters for nanofibrous PLA-based materials containing periclase nanoparticles and BMP-2 growth factors. Our study revealed that the most favorable samples in terms of nanofibers alignment, additives distribution, and membrane integrity are obtained using 25 kV and a distance between the needle and collector of 10 cm. FT-IR spectroscopy confirmed their composition, and swelling tests in various media, along with antibacterial testing against two bacterial strains, were successful. Furthermore, cytotoxicity studies on the MG-63 cell line demonstrated good biocompatibility and enhanced proliferation activity. These promising results suggest that the developed biomaterials exhibit reproducible, desirable properties and warrant further investigation for GBR applications.
Author Contributions
Conceptualization, A.S.-B. and J.R.-P.; methodology A.S.-B., J.R.-P., N.R.-P. and Ł.J.; validation, A.S.-B., J.R.-P. and Ł.J.; investigation, A.S.-B., J.R.-P., Ł.J., N.R.-P., K.Ł., T.G. and P.R.; data curation, A.S.-B. and J.R.-P.; writing—original draft preparation, A.S.-B. and J.R.-P.; resources: Ł.J., J.R.-P., M.T. and N.R.-P. supervision, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by This research was funded by Ministry of Education and Science, TRL 4.0 Grant number MNiSW/2020/344/DIR.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided Bone Regeneration: Materials and Biological Mechanisms Revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Halbritter, S.; Harnisch, H.; Weber, H.P.; Buser, D. A Retrospective Analysis of Patients Referred for Implant Placement to a Specialty Clinic: Indications, Surgical Procedures, and Early Failures. Int. J. Oral Maxillofac. Implants 2008, 23, 1109–1116. [Google Scholar] [PubMed]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological Principle and Therapeutic Applications. Clin. Oral Implants Res. 2010, 21, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Oller, I.; Padial-Molina, M.; Galindo-Moreno, P.; O’Valle, F.; Jódar-Reyes, A.B.; Peula-García, J.M. Bone Regeneration from PLGA Micro-Nanoparticles. Biomed. Res. Int. 2015, 2015, 415289. [Google Scholar] [CrossRef]
- Padial-Molina, M.; O’Valle, F.; Lanis, A.; Mesa, F.; Dohan Ehrenfest, D.M.; Wang, H.L.; Galindo-Moreno, P. Clinical Application of Mesenchymal Stem Cells and Novel Supportive Therapies for Oral Bone Regeneration. Biomed. Res. Int. 2015, 2015, 341327. [Google Scholar] [CrossRef]
- Gallo, S.; Pascadopoli, M.; Pellegrini, M.; Pulicari, F.; Manfredini, M.; Zampetti, P.; Spadari, F.; Maiorana, C.; Scribante, A. Latest Findings of the Regenerative Materials Application in Periodontal and Peri-Implant Surgery: A Scoping Review. Bioengineering 2022, 9, 594. [Google Scholar] [CrossRef]
- Kormas, I.; Pedercini, A.; Alassy, H.; Wolff, L.F. The Use of Biocompatible Membranes in Oral Surgery: The Past, Present & Future Directions. A Narrative Review. Membranes 2022, 12, 841. [Google Scholar] [CrossRef]
- Deng, Y.; Liang, Y.; Liu, X. Biomaterials for Periodontal Regeneration. Dent. Clin. N. Am. 2022, 66, 659–672. [Google Scholar] [CrossRef]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold Design for Bone Regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef]
- Sarkari, S.; Khajehmohammadi, M.; Davari, N.; Li, D.; Yu, B. The Effects of Process Parameters on Polydopamine Coatings Employed in Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2022, 10, 1005413. [Google Scholar] [CrossRef]
- Batul, R.; Tamanna, T.; Khaliq, A.; Yu, A. Recent Progress in the Biomedical Applications of Polydopamine Nanostructures. Biomater. Sci. 2017, 5, 1204–1229. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, H.; Hanks, T.W. Polydopamine: A Bioinspired Adhesive and Surface Modification Platform. Polym. Int. 2022, 71, 578–582. [Google Scholar] [CrossRef]
- Kwon, I.S.; Bettinger, C.J. Polydopamine Nanostructures as Biomaterials for Medical Applications. J. Mater. Chem. B 2018, 6, 6895–6903. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi Yazdi, M.; Zare, M.; Khodadadi, A.; Seidi, F.; Sajadi, S.M.; Zarrintaj, P.; Arefi, A.; Saeb, M.R.; Mozafari, M. Polydopamine Biomaterials for Skin Regeneration. ACS Biomater. Sci. Eng. 2022, 8, 2196–2219. [Google Scholar] [CrossRef]
- Jia, L.; Han, F.; Wang, H.; Zhu, C.; Guo, Q.; Li, J. Polydopamine-Assisted Surface Modification for Orthopaedic Implants. J. Orthop. Transl. 2019, 17, 82–95. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, B.; Xu, Y. Surface Characteristics of a Self-Polymerized Dopamine Coating Deposited on Hydrophobic Polymer Films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef]
- Abbas, I.K.; Adim, K.A. Synthesis and Characterization of Magnesium Oxide Nanoparticles by Atmospheric Non-Thermal Plasma Jet. Kuwait J. Sci. 2023, 50, 223–230. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Z.; Tang, T.; Li, T.; Xu, H.; Sun, H.; Lin, Y.; Tonin, B.S.H.; Ye, Z.; Fu, J. Bioactive Dental Resin Composites with MgO Nanoparticles. ACS Biomater. Sci. Eng. 2023, 9, 4632–4645. [Google Scholar] [CrossRef]
- Sahmani, S.; Saber-Samandari, S.; Khandan, A.; Mohammadi Aghdam, M. Influence of MgO Nanoparticles on the Mechanical Properties of Coated Hydroxyapatite Nanocomposite Scaffolds Produced via Space Holder Technique: Fabrication, Characterization and Simulation. J. Mech. Behav. Biomed. Mater. 2019, 95, 76–88. [Google Scholar] [CrossRef]
- Singh, A.K.; Pramanik, K.; Biswas, A. MgO Enables Enhanced Bioactivity and Antimicrobial Activity of Nano Bioglass for Bone Tissue Engineering Application. Mater. Technol. Adv. Perform. Mater. 2019, 34, 13. [Google Scholar] [CrossRef]
- Hickey, D.J.; Ercan, B.; Sun, L.; Webster, T.J. Adding MgO Nanoparticles to Hydroxyapatite-PLLA Nanocomposites for Improved Bone Tissue Engineering Applications. Acta Biomater. 2015, 14, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel Biomaterial Strategies for Controlled Growth Factor Delivery for Biomedical Applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Terranova, L.; Louvrier, A.; Hébraud, A.; Meyer, C.; Rolin, G.; Schlatter, G.; Meyer, F. Highly Structured 3D Electrospun Conical Scaffold: A Tool for Dental Pulp Regeneration. ACS Biomater. Sci. Eng. 2021, 7, 5775–5787. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Yin, J.; Xu, L.; Ahmed, A. Batch Preparation and Characterization of Electrospun Porous Polylactic Acid-Based Nanofiber Membranes for Antibacterial Wound Dressing. Adv. Fiber Mater. 2022, 4, 832–844. [Google Scholar] [CrossRef]
- Alqahtani, A.M. Guided Tissue and Bone Regeneration Membranes: A Review of Biomaterials and Techniques for Periodontal Treatments. Polymers 2023, 15, 3355. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.M.G.; Kowoli, M.J.; Janowski, G.M. Recent Advances in the Development of GTR/GBR Membranes for Periodontal Regeneration—A Materials Perspective. Dent. Mater. 2012, 28, 703–712. [Google Scholar] [CrossRef]
- Jiang, J.; Duan, H.W.; He, T.H.; Li, B. Electric Field Simulation and Experimentation of Needle-Plate Type Electrospinning Machine. J. Comput. Theor. Nanosci. 2015, 12, 2016–2022. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, D.; Montagne, K.; Ushida, T.; Furukawa, K.S. Differentiation-Inducing Effect of Osteoclast Microgrooves for the Purpose of Three-Dimensional Design of Regenerated Bone. Acta Biomater. 2023, 168, 174–184. [Google Scholar] [CrossRef]
- Wang, G.; Liu, C.; Bu, W.; Yang, Y.; Guo, F.; Li, J.; Wang, E.; Mao, Y.; Mai, H.; You, H.; et al. 3D Printing of Polylactic Acid/Boron Nitride Bone Scaffolds: Mechanical Properties, Biomineralization Ability and Cell Responses. Ceram. Int. 2023, 49, 25886–25898. [Google Scholar] [CrossRef]
- Guo, F.; Wang, E.; Yang, Y.; Mao, Y.; Liu, C.; Bu, W.; Li, P.; Zhao, L.; Jin, Q.; Liu, B.; et al. A Natural Biomineral for Enhancing the Biomineralization and Cell Response of 3D Printed Polylactic Acid Bone Scaffolds. Int. J. Biol. Macromol. 2023, 242, 124728. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wang, E.; Huang, Y.; Yang, Y.; Zhang, T.; You, H.; Long, Y.; Guo, W.; Liu, B.; Wang, S. Biomolecule-Grafted GO Enhanced the Mechanical and Biological Properties of 3D Printed PLA Scaffolds with TPMS Porous Structure. J. Mech. Behav. Biomed. Mater. 2024, 157, 106646. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.-J.; Seok, H. Silk Protein-Based Membrane for Guided Bone Regeneration. Appl. Sci. 2018, 8, 1214. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, W.; Wu, X.; Gao, X.; Song, F.; Duan, B.; Lu, A.; Yang, H.; Huang, C. Janus Membrane with Intrafibrillarly Strontium-Apatite-Mineralized Collagen for Guided Bone Regeneration. ACS Nano 2024, 18, 7204–7222. [Google Scholar] [CrossRef]
- Sheng, R.; Mu, J.; Chernozem, R.V.; Mukhortova, Y.R.; Surmeneva, M.A.; Pariy, I.O.; Ludwig, T.; Mathur, S.; Xu, C.; Surmenev, R.A.; et al. Fabrication and Characterization of Piezoelectric Polymer Composites and Cytocompatibility with Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2023, 15, 2009–2020. [Google Scholar] [CrossRef]
- Malaiappan, S.; Harris, J. Osteogenic Potential of Magnesium Oxide Nanoparticles in Bone Regeneration: A Systematic Review. Cureus 2024, 16, e55502. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Jiang, J.-G.; Wang, Z.; Duan, H.-W.; Liu, J.-Q.; Guo, X.-W. Electric Field Simulation and Effect of Different Solvent Ratios on the Performance of Single Electrospun PVDF/PEI Composite Film. Adv. Mater. Sci. Eng. 2016, 2016, 4329283. [Google Scholar] [CrossRef]
- Duan, H.; Jiang, J. Experimentation and Finite Element Analysis of Electric Field Structure of Electrospinning Machine. In Advances in Computer Science, Intelligent System and Environment; Jin, D., Lin, S., Eds.; Advances in Intelligent and Soft Computing; Springer: Berlin/Heidelberg, Germany, 2011; Volume 105, pp. 283–289. [Google Scholar]
- Hekmati, A.H.; Rashidi, A.; Ghazisaeidi, R.; Drean, J.-Y. Effect of Needle Length, Electrospinning Distance, and Solution Concentration on Morphological Properties of Polyamide-6 Electrospun Nanowebs. Text. Res. J. 2013, 83, 1452–1466. [Google Scholar] [CrossRef]
- Gupta, A.; Ayithapu, P.; Singhal, R. Study of the Electric Field Distribution of Various Electrospinning Geometries and Its Effect on the Resultant Nanofibers Using Finite Element Simulation. Chem. Eng. Sci. 2021, 235, 116463. [Google Scholar] [CrossRef]
- Wei, L.; Liu, C.; Dong, J.; Fan, X.; Zhi, C.; Sun, R. Process Investigation of Nanofiber Diameter Based on Linear Needleless Spinneret by Response Surface Methodology. Polym. Test. 2022, 110, 107577. [Google Scholar] [CrossRef]
- Mamtha, V.; Murthy, H.N.N.; Authade, P.; Sridhar, R. Study of Electrospun Fiber Diameter Using ANSOFT and ANSYS. Mater. Today Proc. 2018, 5, 21529–21537. [Google Scholar] [CrossRef]
- Cramariuc, B.; Cramariuc, R.; Scarlet, R.; Maneac, L.R.; Lupu, I.G.; Cramariuc, O. Fiber diameter in electrospinning process. J. Electrost. 2013, 71, 189–198. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).