Abstract
The aim of this study was to evaluate the effect of a 4-week respiratory muscle training (RMT) intervention versus continuous positive airway pressure (CPAP) on cardiorespiratory parameters and cognitive function in patients with OSA. Twenty-eight male patients with OSA were divided into two groups (RMTgroup n = 14 and CPAPgroup n = 14) and we recorded cardiopulmonary parameters and completed the questionnaires for cognitive impairment (MoCA) and sleep quality (PSQI) before and after 4 weeks. We observed differences before and after the intervention in cardiopulmonary parameters: RMTgroup: breathing reserve (p < 0.001) and oxygen breathing (p = 0.002), at the end of the exercise test and pulse-respiratory quotient at rest (p < 0.001), end of exercise (p = 0.020), and recovery (p < 0.001), mean arterial pressure (RMTgroup p = 0.035, CPAPgroup p = 0.032); cognitive function assessment: RMTgroup: 7% improvement in MoCAscore (p < 0. 001). and in the attention–working memory domain by 13% (p = 0.001), CPAPgroup: improvement in the short-term memory recall domain by 28% (p = 0.001) and in the visuospatial domain by 18% (p = 0.027); sleep quality scores: RMTgroup: 22% improvement in PSQIscore (p = 0.013), and 50% reduction in ‘cannot breathe comfortably’ domain (p = 0.013), CPAPgroup: 31% increase in ‘waking in the middle of the night or early in the morning’ domain (p = 0.044). Our findings support the implementation of RMT programs in patients with OSA as an effective and beneficial practice to promote exercise and reduce symptoms of sleep disturbance.
1. Introduction
Obstructive sleep apnea (OSA) is a sleep disorder characterized by repeated episodes of partial or complete obstruction of the upper airway during sleep. This obstruction causes breathing to stop and start intermittently, leading to fragmented sleep and reduced oxygen levels in the blood [1]. OSA is estimated to affect 2–9% of adults in the general population and is more common in men than women (approximately 25% of men and 10% of women), although the prevalence may be higher as many cases go undiagnosed [2]. Body mass index, age, gender, and neck circumference are the risk factors which are associated with the presence of sleep-disordered breathing (SDB), while the severity of SDB is associated with the risk of developing diabetes, hypertension, metabolic syndrome, and depression [3]. Obesity is a significant risk factor for OSA, and people with a higher body mass index are at increased risk, with over 60% of obese people estimated to have OSA [2]. Other risk factors include conditions such as high blood pressure, type 2 diabetes, and cardiovascular disease, which are associated with higher rates of OSA. In addition, certain anatomical features, such as a large neck circumference or a small airway, can increase the risk [4].
In recent years, there has been growing literature exploring the bilateral relationship between SBD and cognitive functions. SBD is a factor in cognitive impairment in patients with and/or without COVID-19 disease [5], with executive function, attention, and memory and learning most affected [6]. A structured exercise program (tele-exercise or hybrid exercise) can improve fitness indicators and cognitive domains, interpreting a multidimensional, non-pharmacological relationship between physical activity parameters and cognitive function [7] with beneficial effects on brain health and cognitive function by improving neurovascular and cerebral oxygenation, reducing sympathetic overactivity and improving vascular function both at rest and during exercise [8]. Previous studies have shown that low-intensity exercise and/or no exercise contributes to the development of OSA, while high-intensity exercise protects patients from developing moderate to severe OSA [9], providing a wide range of benefits to the general population: improved cardiorespiratory and metabolic profiles, beneficial biological and psychological effects on the brain, improved cognitive function and sleep, inducing potent neuroplastic phenomena, partly mediated by epigenetic mechanisms [10]. In our previous study [11], patients with apnea hypopnea index (AHI) ≥ 30 events/hour showed higher values of maximal inspiratory pressure compared to those <30 events/hour due to intermittent breath-holding during hypoxia-reoxygenation, which probably increases intrathoracic pressure with successive changes in transmural pressure of the cardiac cavities (inspiratory and expiratory pressures provide key insights into respiratory mechanics, e.g., stiff lungs require higher inspiratory pressures, airway obstruction increases expiratory pressures, increased pressures indicate greater work of breathing, and both help to assess gas exchange and alveolar stability), while inspiratory muscle training alleviates accompanying SDB hypertension [11].
Despite efforts to develop interventions and exercise protocols for patients with OSA, there are currently no studies investigating the efficacy of respiratory muscle training and its impact on cardiopulmonary parameters in combination with cognitive function. The effectiveness of exercise programs for patients with OSA is well documented [12], but there are no studies that suggest alternative training methods for patients with OSA who are unable to exercise due to limitations (e.g., musculoskeletal disability). The types of exercise programs in patients with OSA contribute to counteracting the disease-related syndromes due to the low adherence to continuous positive airway pressure (CPAP) treatment in a percentage that in adults reaches the 46 to 83% of patients with OSA [13]. This randomized controlled trial aims to evaluate the impact of a 4-week respiratory muscle training (RMT) intervention on cardiorespiratory parameters and cognitive function in patients with obstructive sleep apnea (OSA). Additionally, it seeks to compare outcomes between patients undergoing RMT and those receiving standard CPAP treatment.
2. Materials and Methods
2.1. Participants
Our randomized controlled trial included male patients (Figure 1) newly diagnosed with obstructive sleep apnea [14]. All patients were prescribed treatment with CPAP and after 4 weeks their adherence was evaluated. Patients who received CPAP treatment for 4 weeks but had low adherence for any reason, e.g., usage discomfort despite trying different masks, noise annoyance, and airflow despite trying different CPAP devices, <4 h nightly for consecutive days >10 days, etc. [15], were consecutively recruited into our study between June 2023 and September 2024, discontinued any treatment for OSA for 8 weeks, and were enrolled in the respiratory (inspiratory and expiratory) muscle training (RMT) group for a 4-week intervention program. Patients with high adherence to CPAP (>4 h nightly use) and physical inactivity [16,17] served as the control group (CPAPgroup). The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board/Ethics Committee of the Medical School of the University of Thessaly (2229/13.04.2022), by the National Bioethics Committee of Cyprus (EEBK/EP 2023/60), the State Health Services Organization of Cyprus (05.34.001.002/44/23) and ClinicalTrials.gov (NCT06467682). All participants gave written informed consent in accordance with the Helsinki Declaration and personal data in accordance with the European Parliament and Council of the European Union [18].
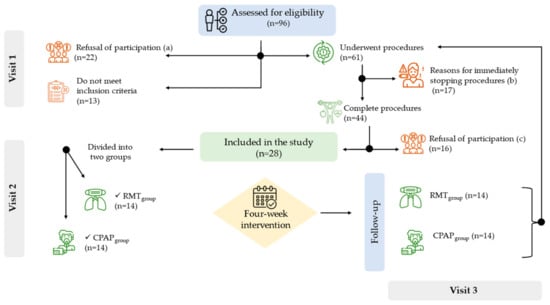
Figure 1.
Flow chart. Visit 1 = data collected, visit 2 = instructions for respiratory muscle training and CPAP treatment adherence; visit 3 = follow-up after 4-week intervention. a = reasons for refusal to participate: 45% said they were not interested, 36% said they were afraid they would not be able to do it, and 18% said they were not familiar with the technology. b = reasons for interrupting the procedures: 47% do not cooperate with physical fitness tests, 24% report dizziness and chest pain during spirometry, 18% report knee pain during physical fitness tests, and 12% show hypostasis during 30 s Sit-to-Stand test. c = reasons for refusal to participate after completed procedures: 50% said “I don’t want to be subjected to these tests again”, 31% said “I wanted to take the tests as a check-up”, and 19% said “I don’t find it interesting to take part”. The procedures are described in the Section 2.3.
2.2. Inclusion Criteria
Inclusion criteria were age ≥25 to ≤65 years, AHI ≥15 to ≤30 events/h (to exclude the possibility of the development of comorbidity, e.g., cardiovascular and/or metabolic diseases, etc.) [19], body mass index <40 kg/m2, physical inactivity [16,17], without known neurodegenerative disease, musculoskeletal disability, active self-reported symptoms and comorbidity [20], and access to new technologies and the internet [21,22].
2.3. Measurements
2.3.1. Anthropometric Characteristics and Body Composition
Anthropometric characteristics and body mass were recorded for all participants (Seca 700, Hamburg, Germany). Body surface area (BSA, m2) [23] and body mass index (BMI) were calculated. For body composition, the Biody XPERTTM (Amino Gram, La Ciotat, France) was used according to the manufacturer’s recommendations and following all necessary guidelines for accurate measurements [24].
2.3.2. Pulmonary Function Test and Respiratory Muscle Strength
Pulmonary function testing was performed using the MasterScreen-CPX pneumotachograph (VIASYS HealthCare, Hoechberg, Germany) with standard spirometry to measure lung volumes and carbon monoxide diffusing capacity from a seated position. All tests were performed by the same operator in the morning according to American Thoracic Society and European Respiratory Society guidelines [25]. Maximum inspiratory pressure (MIP) and maximum expiratory pressure (MEP) were recorded using a portable device, AirOFit PRO™ (AirOFit, Copenhagen, Denmark). The AirOFit PRO™ e-unit contains pressure sensors and a Bluetooth transmitter and allows breathing patterns to be measured and visualized on a phone or tablet via an app [11,21]. The MIP and MEP were calculated using the following equations [26]:
and respiratory muscle strength (RMS) was calculated using the equation [21].
MIP(cmH2O) = 63.27 − 0.55 × age(years) + 17.96 × 0 + 0.58 × body mass(kg)
MEP(cmH2O) = −61.41 + 2.29 × age(years) − 0.03 × age(years)2 + 33.72 × 0 +1.40 × waist circumference(cm)
2.3.3. Physical Fitness Tests
The 30 s Sit-to-Stand (30 s StS) test was used to assess physical fitness. All participants sat on a stable chair of standard height, approximately 46 cm. During the 30 s StS test, all participants performed as many full stands as possible without the use of hands and without encouragement. Cardiopulmonary parameters (Cosmed Quark CPET, Rome, Italy) [11,17,27], blood pressure (Mac, Osaka, Japan), and heart rate using chest belt Bluetooth and ANT+ technologies were recorded before, immediately after, and during the first minute of the recovery from the 30 s StS test. Mean arterial blood pressure (MAP) was calculated using the equation [28] MAP(mmHg) = , the maximum heart rate (HRmax) was calculated according to equation [HRmax (bpm) = 207 − 0.7 × age (years)] [29], the oxygen breath was calculated according to equation [30] and the pulse respiration quotient was calculated according to equation [31]. Baseline values were the average of one minute of continuous recording before the start of the test, end of test values was the highest value recorded, and recovery values were the average of one minute of continuous recording after the test. Prior to the 30 s Sit-to-Stand test, all participants performed a handgrip strength test using the electronic dynamometer (Camry, EH 101, South El Monte, CA, USA) from a seated position on a chair of standard height, approximately 46 cm, with the participant’s elbow at the side of the body and flexed at 90 degrees; the forearm and wrist remained in a neutral position and the handle of the dynamometer rested on the middle of the four fingers [32]. All participants performed a maximum isometric effort with both hands for 5 s and the mean values of each trial were calculated.
2.3.4. Cognitive and Sleep Quality Assessment
Prior to the physical fitness tests, all subjects completed the Montreal Cognitive Assessment (MoCA) [33] and Pittsburgh Sleep Quality Index (PSQI) questionnaires [34]. The MoCA questionnaire was used to detect cognitive impairment caused by sleep disturbance [35] and was administered by a certified examiner (CYSTAVA710719592-01). PSQI was used to measure sleep quality and patterns using self-report questionnaires over a one-month period. The PSQI consists of 19 individual items, creating 7 components that produce a global score. All the above assessments were made in baseline and in the 4-week follow-up.
2.3.5. Interventions Program
The intervention respiratory muscle training program lasted 4 weeks. Each patient participated in a daily training session lasting approximately 35 min. In the RMTgroup, a small, portable, lightweight mouth pressure manometer was provided for respiratory muscle training (AirOFit PRO™ company https://www.airofit.com/, accessed on 10 July 2023). All patients performed 3 sets of 12 repetitions daily, with 40 s rest between repetitions and 2 min between sets, for MIP and MEP, respectively. There is a rest period of 6 min between the MIP and the MEP workout. For the first 2 weeks, the intensity was 80% of MIP and MEP, and for the remaining 2 weeks, the intensity was 90% of MIP and MEP. Intensity was calculated according to maximal inspiratory pressure at baseline. During training, participants viewed real-time measurements and breathing patterns via a mobile app (software version 2.5.8). Program adherence was assessed by one video call per week for both groups (RMTgroup and CPAPgroup). Each video call focused on whether participants were adhering to the program daily and troubleshooting.
2.3.6. Statistical Analysis
Normality of data was assessed using the Kolmogorov–Smirnov one-sample test. Data are presented as percentages for qualitative variables, mean ± standard deviation (SD) for parametric variables, and median and 25th and 75th percentiles for non-parametric variables. Independent samples t-tests or Mann–Whitney U-tests were used to assess differences between groups (RMTgroup versus CPAPgroup). Paired t-test or Wilcoxon signed-rank test were used to assess within groups differences before and after the 4-week intervention period (RMTgroup and CPAPgroup). Correlations between continuous variables were assessed using Spearman’s Rho and Pearson’s R correlation coefficients for non-parametric and parametric variables, respectively. A Bonferroni post hoc test was used to detect differences between means. Two-way repeated measures analysis of variance (2 measurements × 2 groups) was used to test for differences in dependent variables and interactions. Cohen’s d was calculated from the mean difference between groups (M1 and M2) and by the pooled SD: Cohen’s d = and SDpooled = . For all tests, a p-value of <0.05 was considered statistically significant. The IBM SPSS 21 statistical package (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
3. Results
Adherence to the program was 82% for the RMTgroup and 86% for the CPAPgroup. For the RMTgroup, adherence was recorded by connection and duration of use. For the CPAPgroup adherence was recorded using device data. All participants responded to each video scroll and reported no problems with adherence to the intervention program.
The results, before and after the intervention period between the groups, anthropometric characteristics, body composition and pulmonary function test, respiratory muscle strength, and physical fitness tests are presented in Table 1. The results of the cardiopulmonary and hemodynamic parameters during the 30 s StS test are presented in Table 2. Figure 2a–d shows the results of breathing reserve, oxygen breath, ventilation to body surface area ratio, and pulse respiratory quotient before and after the intervention period between the groups.

Table 1.
Analysis of results between groups before and after the intervention period. Data are expressed as mean ± standard deviation or percentage.

Table 2.
Analysis of results between groups before and after the intervention period for strength and cardiopulmonary and hemodynamic parameters during the 30 s Sit-to-Stand (StS) test. Data are expressed as mean ± standard deviation or percentage.
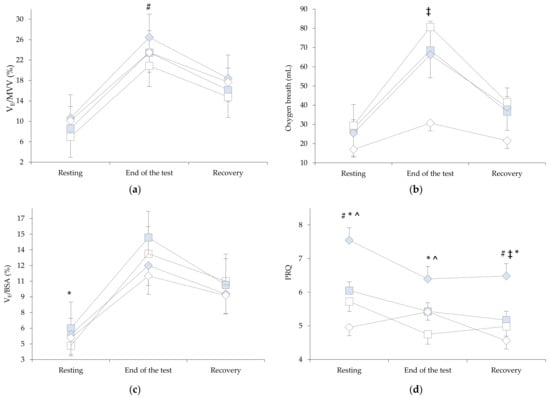
Figure 2.
Results before and after the intervention period between groups in three stages of the 30 s Sit-to-Stand: (a) breathing reserve , (b) oxygen breath, (c) ratio between ventilation (VE, L/min) and body surface area (BSA, m2), (d) and pulse respiration quotient. Abbreviations:  Baseline RMTgroup;
Baseline RMTgroup;  After 4-week RMTgroup;
After 4-week RMTgroup;  Baseline CPAPgroup;
Baseline CPAPgroup;  After 4-week CPAPgroup. # p < 0.001, * p < 0.05 between baseline and after 4-week, ^ p < 0.001, ‡ p < 0.05 between groups.
After 4-week CPAPgroup. # p < 0.001, * p < 0.05 between baseline and after 4-week, ^ p < 0.001, ‡ p < 0.05 between groups.
 Baseline RMTgroup;
Baseline RMTgroup;  After 4-week RMTgroup;
After 4-week RMTgroup;  Baseline CPAPgroup;
Baseline CPAPgroup;  After 4-week CPAPgroup. # p < 0.001, * p < 0.05 between baseline and after 4-week, ^ p < 0.001, ‡ p < 0.05 between groups.
After 4-week CPAPgroup. # p < 0.001, * p < 0.05 between baseline and after 4-week, ^ p < 0.001, ‡ p < 0.05 between groups.
The results showed a statistically significant effect with time F(1.0, 26.0) = 11.78, p = 0.002. The planned contrast test showed that there were statistically significant differences between the first and second measurements. The Bonferroni pairwise comparisons showed that the RMTgroup had changes in VE/MVV at the end of the test before and after the intervention period (24.3 ± 7.8 versus 20.1 ± 6.9, p < 0.001, 95% CI: 2.237 to 6.049, Figure 2a) compared to the CPAPgroup (26.8 ± 13.4 versus 26.4 ± 14.2, p = 0.703, 95% CI: −1.549 to 2.263, Figure 2a). The results of the oxygen breath at the end of test showed a statistically significant effect with time F(1.0, 26.0) = 13.169, p = 0.001. The Bonferroni pairwise comparisons showed that the RMTgroup had changes before and after the intervention period (68.5 ± 27.6 versus 80.7 ± 30.6, p = 0.002, 95% CI: 61.194 to 88.035, Figure 2b) compared to the CPAPgroup (66.21 ± 19.5 versus 72.6 ± 22.1, p = 0.088, 95% CI: 56.001 to 82.842, Figure 2b). The results of the VE/BSA in resting showed a statistically significant effect with time F(1.0, 26.0) = 7.93, p = 0.035. The Bonferroni pairwise comparisons showed that the RMTgroup had changes before and after the intervention period (5.4 ± 1.8 versus 4.3 ± 1.6, p = 0.024, 95% CI: 4.080 to 5.699, Figure 2c) compared to the CPAPgroup (5.4 ± 2.1 versus 5.1 ± 1.2, p = 0.467, 95% CI: 4.430 to 6.049, Figure 2c). The results of PRQ in resting showed a statistically significant effect with time and groups F(1.0, 26.0) = 32.104, p < 0.001 (RMTgroup: 5.5 ± 2.5 versus 7.0 ± 2.3, p < 0.001, 95% CI: 5.281 to 7.305; CPAPgroup: 5.2 ± 1.3 versus 4.5 ± 1.0, p = 0.011, 95% CI: 3.831 to 5.855, Figure 2d), at the end of test in time F(1.0, 26.0) = 18.169, p < 0.001 (RMTgroup: 4.9 ± 1.6 versus 5.9 ± 1.6, p = 0.001, 95% CI: 4.729 to 6.085; CPAPgroup: 4.3 ± 0.1 versus 4.9 ± 1.0, p = 0.020, 95% CI: 3.911 to 5.268, Figure 2d), and at the first minute of recovery in time and groups F(1.0, 26.0) = 18.893, p < 0.001 (RMTgroup: 4.7 ± 1.8 versus 5.9 ± 1.8, p < 0.001, 95% CI: 4.566 to 6.098; CPAPgroup: 4.5 ± 1.2 versus 4.1 ± 0.1, p = 0.136, 95% CI:3.516 to 5.048, Figure 2d).
Cognitive function assessment showed differences in both groups after a 4-week intervention. The RMTgroup showed improvement in the total MoCA score (23.4 ± 3.0 versus 25.3 ± 2.4 score, t(13) = −4.941, p < 0.001, 95% CI: −2.033 to −0.582) and in the attention–working memory domain (5.1 ± 0.9 versus 5.8 ± 0.3 score, t(13) = −3.667, p = 0.001, 95% CI: −1.248 to 0.323, Figure 3). The CPAPgroup showed an improvement in the short-term memory recall domain (2.4 ± 1.5 versus 3.4 ± 1.2 score, t(13) = −3.789, p = 0.001, 95% CI: −1.458 to 0.399, Figure 3) and a decrease in the visuospatial domain (2.9 ± 0.7 versus 2.4 ± 0.9 score, t(13) = 2.121, p = 0.027, 95% CI: −0.007 to 0.865, Figure 3). There were no differences between the groups before and after the intervention.
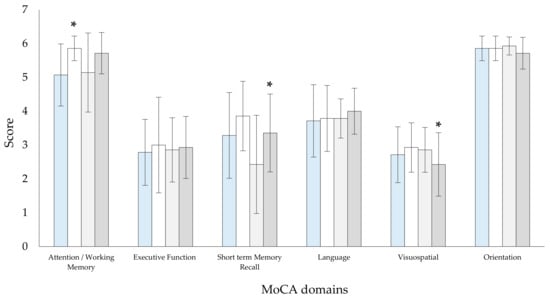
Figure 3.
Results before and after the intervention period between the groups in the domains of the Montreal Cognitive Assessment (MoCA) questionnaire. Abbreviations:  Baseline RMTgroup;
Baseline RMTgroup;  After 4-week RMTgroup;
After 4-week RMTgroup;  Baseline CPAPgroup;
Baseline CPAPgroup;  After 4-week CPAPgroup. * p < 0.05 between baseline and after 4-week.
After 4-week CPAPgroup. * p < 0.05 between baseline and after 4-week.
 Baseline RMTgroup;
Baseline RMTgroup;  After 4-week RMTgroup;
After 4-week RMTgroup;  Baseline CPAPgroup;
Baseline CPAPgroup;  After 4-week CPAPgroup. * p < 0.05 between baseline and after 4-week.
After 4-week CPAPgroup. * p < 0.05 between baseline and after 4-week.
Sleep quality assessment showed differences in both groups after a 4-week intervention. The RMTgroup showed an improvement in the total PSQI score (11.1 ± 5.8 versus 9.1 ± 5.1 score, t(13) = −4.941, p = 0.013, 95% CI: 0.082 to 1.248) and a decrease in “cannot breathe comfortably” domain (0.9 ± 1.1 versus 0.4 ± 0.9 score, t(13) = 2.527, p = 0.013, 95% CI: 0.290 to 3.710). The CPAPgroup showed an increase in the “wake up in the middle of the night or early morning awakening” domain (1.3 ± 1.4 versus 1.9 ± 1.5 score, t(13) = −1.847, p = 0.044, 95% CI: −1.240 to 0.097). There were no significant differences between the groups before and after the intervention.
4. Discussion
The aim of our study was to investigate potential differences in cardiopulmonary parameters and cognitive function in patients with OSA after 4 weeks of respiratory muscle training compared with CPAP treatment. Our findings showed different adjustments in cardiopulmonary parameters, cognitive function, and sleep quality in both groups before and after the intervention period.
Stavrou et al. [7,16,17,22,36,37,38] have shown that exercise programs improve cardiopulmonary indicators in patients with chronic respiratory diseases. The current study showed that RMTgroup after the 4-week intervention period improved the lower limb strength via a 30 s StS test by 7% (CPAPgroup ↑ 6.5%) and reduced the MAP after a 30 s StS test by 8.7% (CPAPgroup ↑ 6.8%). OSA is associated with impaired hemodynamic and chronotropic responses during exercise, with the main causative factor being impaired cardiovascular autonomic function resulting from structural downregulation of cardiac β-receptors and/or altered baroreflex set-point [39]. The increased MAP is related to sympathetic vasoconstriction, endothelial dysfunction, or a blunted response to beta-2 receptor stimulation, mechanisms that have been shown to be active in patients with sleep-disordered breathing [27]. After the intervention period and 30 s StS test, opposite MAP responses were observed in the two groups. Changes in MAP affected systemic vascular resistance and cardiac output, resulting in blood vessels being influenced by both local mediators and the autonomic nervous system and the endothelial cells respond to vasoactive depending on the body’s needs [28]. According to DeMers and Wachs [28] when MAP is elevated, a decrease in sympathetic output and an increase in parasympathetic tone will decrease myocardial chronotropy and dromotropy, resulting in a decrease in cardiac output and a subsequent decrease in MAP. In the current study, the oxygen uptake after a 30 s StS test was approximately at 55% of predicted values of maximal oxygen uptake in both groups before and after the intervention period while the RMTgroup after the intervention period showed higher values in RMS by 12.5% (CPAPgroup ↑ 3.1%). Respiratory muscle training may improve the respiratory muscle function during submaximal exercise and promote benefits for cardiac autonomic control [40]. According to de Abreu et al. [40], the effects of RMT on cardiac autonomic control are related to the respiratory metaboreflex, and the generated strengthening of the respiratory musculature can increase the threshold of metaboreflex activation, reducing limiting factors (e.g., dyspnea, peripheral fatigue) and improving physical performance, while the effects on the autonomic nervous system reflex control systems involve the main baroreceptors and chemoreceptors [41], embedding the theory of competitiveness between the diaphragm and the quadriceps muscle. The autonomic nervous system also plays a vital role in regulating MAP via the baroreceptor reflex and vagus nerve, determining the sympathetic or parasympathetic tone to either raise or lower MAP [28].
In the current study, the intervention period consisted of 4 weeks of daily training of approximately 35 min at 80–90% of MIP and MEP (submaximal interval exercise). Our results showed an increase in performance (repetitions in the 30 s StS test) and changes in respiratory function with the RMTgroup having lower breath frequency values at the end of the 30 s StS and after the intervention period (↓ 17%) compared to the CPAPgroup (↓ 8%). In addition, the RMTgroup showed changes in respiratory function with lower levels of VE/BSA before the 30 s StS test after the intervention period and lower levels of VE/MVV at the end of the test, and changes were also observed in PRQ in three stages. The PRQ (ratio of pulse rate to respiratory rate) is a parameter that captures the complex state of cardiorespiratory interactions, and the changes (increase, decrease) are related to conditions such as body position, anxiety, respiratory, and/or heart disease [42], is dependent on the vagus nerve, which plays a crucial role in maintaining homeostasis between these systems. Respiratory muscle training can positively influence the vagus nerve by increasing parasympathetic activity and improving vagal tone, while this interaction between the respiratory system and the autonomic nervous system (especially through respiratory rhythms) influences the vagal pathways [43]. The respiratory pattern influences cardiac vagal outflow, while the respiratory control system integrates complex signals from the drive to breathe generated by the central pattern with spontaneous breathing, which can be modulated by changes in respiratory cycle duration and HR dynamics [44].
Our results showed that respiratory muscle training improved cognitive function (via the MoCA score) and memory. Our previous study showed that the combination of aerobic exercise–resistance exercise–exercise with new technologies improves blood flow to the brain and has a positive effect on hippocampal function [45]. The current results are probably due to the intervention program with activation of anaerobic metabolism and activation of the lactic acid neutralization mechanism (Haldane effect) and facilitated cardio–pulmonary–metabolic processes, which is consistent with the results of our study and the adjustment of oxygen breathing [46]. A previous study has shown that transcutaneous vagus nerve stimulation correlates with cognitive performance on cognitive tasks and with neural activity in the brain, activating the fusiform region, which is associated with cognitive changes [47]. Vagal activation reduces anxiety levels and increases vagal outflow, which appears to have a greater effect on parasympathetic activity (depending on age), through a process of activating physiological and psychobiological mechanisms [48].
Another outcome of the current study was changes in sleep quality. We found an improvement in sleep quality (via the PSQI score) of −2.00 for the RMTgroup and −0.93 for the CPAPgroup, which is not clinically relevant for the CPAP group according to previous studies [49]. The CPAPgroup showed an increase in the “waking in the middle of the night or early in the morning” domain, probably due to lack of familiarity with the CPAP device, such as noise, mask fitting, dry nose or throat, etc., while the RMTgroup decreased the “cannot breathe comfortably” domain, results consistent with previous studies [50]. The regulation of breathing during sleep is mainly under the control of chemoreceptors, while the chemoreflex is fully stimulated, increasing sympathetic nervous system tone during both sleep and wakefulness [51]. Vagus nerve stimulation is a neuroregulatory technique in several cognitive domains [52] and is beneficial in sleep disorders [53] and affected from respiratory muscle training [44]. Previous studies have shown that patients with OSA who train the respiratory muscles (main and accessory respiratory muscles) have fewer arousals, fewer periodic limb movements (↓ blood pressure and plasma norepinephrine levels) and better overall sleep quality [54] and reduce stress on the respiratory muscles during intermittent breath-holding during hypoxia reoxygenation, leading to an increase in intrathoracic pressure [11]. The inspiratory muscles play a more important role in this improvement [55] and may be influenced by the Venturi effect—Bernoulli’s principle as derived from computational fluid dynamics and the Starling Resistor model [56].
However, our results should be interpreted in the light of certain limitations. The relatively small sample size, influenced by stringent inclusion criteria, limited the statistical power of the correlations. Additionally, our study excluded women by design, as including this group would have required a different study framework to address potential confounding factors such as hormonal fluctuations, menstruation, and their impact on breathing patterns. Another significant limitation was the recruitment of adults without comorbidities, as well as the absence of post-intervention polysomnography to assess sleep quality in the RMTgroup.
Our study was the first to evaluate the effects of respiratory muscle training using a smart device in patients with OSA. Improvements were seen in several indices of functionality and cognitive performance, suggesting that targeted rehabilitation with respiratory muscle training may be beneficial in the recovery of patients with OSA who cannot tolerate treatment with CPAP.
5. Conclusions
We investigated the effects of a 4-week respiratory muscle training program in patients with OSA who were unable to tolerate treatment with CPAP. We found significant changes in hemodynamic and respiratory parameters, cognitive indicators and sleep quality. Accordingly, we propose that RMT could be an effective and beneficial practice to promote exercise with intelligent devices and reduce symptoms of sleep disorders and a novel approach to the treatment of OSA.
Author Contributions
V.T.S. conceived the idea and designed the study. V.T.S., G.T., S.B. and M.S. performed patient recruitment and data collection. V.T.S. contributed to data analysis. V.T.S. designed the respiratory muscle training program. V.T.S., G.D.V., P.B., G.H. and K.I.G. contributed to drafting and editing the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board/Ethics Committee of the Medical School of the University of Thessaly (2229/13.04.2022), by the National Bioethics Committee of Cyprus (EEBK/EP 2023/60), the State Health Services Organization of Cyprus (05.34.001.002/44/23) and ClinicalTrials.gov (NCT06467682).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
Acknowledgments
The authors sincerely appreciate the invaluable cooperation of the patients who participated in this study. We are also express our gratitude to the ONISILOS—Co-funding International, Interdisciplinary and Intersectoral research excellence at the University of Cyprus. This research is part of Stavrou’s ONISILOS Postdoctoral Project, funded by the European Union’s Horizon 2020 Research and Innovation Program under the Marie Skłodowska-Curie Grant Agreement No. 101034403.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Eckert, D.J.; Malhotra, A. Pathophysiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.; Hunter, M.; Budgeon, C.; Murray, K.; Knuiman, M.; Hui, J.; Hillman, D.; Singh, B.; James, A. The prevalence and comorbidities of obstructive sleep apnea in middle-aged men and women: The Busselton Healthy Ageing Study. J. Clin. Sleep. Med. 2021, 17, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef]
- Punjabi, N.M. The epidemiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 136–143. [Google Scholar] [CrossRef]
- Crivelli, L.; Palmer, K.; Calandri, I.; Guekht, A.; Beghi, E.; Carroll, W.; Frontera, J.; García-Azorín, D.; Westenberg, E.; Winkler, A.S.; et al. Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis. Alzheimers Dement. 2022, 18, 1047–1066. [Google Scholar] [CrossRef]
- Krysta, K.; Bratek, A.; Zawada, K.; Stepańczak, R. Cognitive deficits in adults with obstructive sleep apnea compared to children and adolescents. J. Neural Transm. 2017, 124 (Suppl. S1), 187–201. [Google Scholar] [CrossRef]
- Stavrou, V.T.; Vavougios, G.D.; Astara, K.; Mysiris, D.S.; Tsirimona, G.; Papayianni, E.; Boutlas, S.; Daniil, Z.; Hadjigeorgiou, G.; Bargiotas, P.; et al. The Impact of Different Exercise Modes in Fitness and Cognitive Indicators: Hybrid versus Tele-Exercise in Patients with Long Post-COVID-19 Syndrome. Brain Sci. 2024, 14, 693. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Simpson, L.; McArdle, N.; Eastwood, P.R.; Ward, K.L.; Cooper, M.N.; Wilson, A.C.; Hillman, D.R.; Palmer, L.J.; Mukherjee, S. Physical Inactivity Is Associated with Moderate-Severe Obstructive Sleep Apnea. J. Clin. Sleep. Med. 2015, 11, 1091–1099. [Google Scholar] [CrossRef]
- Stavrou, V.T.; Pitris, K.; Constantinidou, F.; Adamide, T.; Frangopoulos, F.; Bargiotas, P. The impact of a 12-week tele-exercise program on cognitive function and cerebral oxygenation in patients with OSA: Randomized controlled trial-protocol study. Front. Sports Act. Living 2024, 6, 1418439. [Google Scholar] [CrossRef]
- Stavrou, V.T.; Astara, K.; Karetsi, E.; Daniil, Z.; Gourgoulianis, K.I. Respiratory Muscle Strength as an Indicator of the Severity of the Apnea-Hypopnea Index: Stepping Towards the Distinction Between Sleep Apnea and Breath Holding. Cureus 2021, 13, e14015. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, I.H.; Kline, C.E.; Youngstedt, S.D. Effects of exercise training on sleep apnea: A meta-analysis. Lung 2014, 192, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Weaver, T.E.; Grunstein, R.R. Adherence to continuous positive airway pressure therapy: The challenge to effective treatment. Proc. Am. Thorac. Soc. 2008, 5, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep. Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Weaver, T.E.; Sawyer, A.M. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: Implications for future interventions. Indian. J. Med. Res. 2010, 131, 245–258. [Google Scholar] [PubMed]
- Stavrou, V.T.; Astara, K.; Tourlakopoulos, K.N.; Papayianni, E.; Boutlas, S.; Vavougios, G.D.; Daniil, Z.; Gourgoulianis, K.I. Obstructive Sleep Apnea Syndrome: The Effect of Acute and Chronic Responses of Exercise. Front. Med. 2021, 8, 806924. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, V.; Karetsi, E.; Daniil, Z.; Gourgoulianis, K.I. 4 weeks exercise in obstructive sleep apnea syndrome patient with type 2 diabetes mellitus and without continuous positive airway pressure treatment: A case report. Sleep Med. Res. 2019, 10, 54–57. [Google Scholar] [CrossRef]
- Resneck, J.S., Jr. Revisions to the Declaration of Helsinki on Its 60th Anniversary: A Modernized Set of Ethical Principles to Promote and Ensure Respect for Participants in a Rapidly Innovating Medical Research Ecosystem. JAMA 2025, 333, 15–17. [Google Scholar] [CrossRef]
- Gleeson, M.; McNicholas, W.T. Bidirectional relationships of comorbidity with obstructive sleep apnoea. Eur. Respir. Rev. 2022, 31, 210256. [Google Scholar] [CrossRef]
- Stavrou, V.T.; Astara, K.; Daniil, Z.; Gourgoulianis, K.I.; Kalabakas, K.; Karagiannis, D.; Basdekis, G. The reciprocal association between fitness indicators and sleep quality in the context of recent sport injury. Int. J. Environ. Res. Public Health 2020, 17, 4810. [Google Scholar] [CrossRef]
- Stavrou, V.T.; Tourlakopoulos, K.N.; Daniil, Z.; Gourgoulianis, K.I. Respiratory Muscle Strength: New Technology for Easy Assessment. Cureus 2021, 13, e14803. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, V.T.; Astara, K.; Ioannidis, P.; Vavougios, G.D.; Daniil, Z.; Gourgoulianis, K.I. Tele-Exercise in Non-Hospitalized versus Hospitalized Post-COVID-19 Patients. Sports 2022, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Mosteller, R.D. Simplified Calculation of Body-Surface Area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar]
- Rauter, S.; Simenko, J. Morphological Asymmetries Profile and the Difference between Low- and High-Performing Road Cyclists Using 3D Scanning. Biology 2021, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, I.M.; Houri Neto, M.; Montemezzo, D.; Silva, L.A.; Andrade, A.D.; Parreira, V.F. Predictive equations for respiratory muscle strength according to international and Brazilian guidelines. Braz. J. Phys. Ther. 2014, 18, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, V.; Boutou, A.K.; Vavougios, G.D.; Pastaka, C.; Gourgoulianis, K.I.; Koutedakis, Y.; Daniil, Z.; Karetsi, E. The use of cardiopulmonary exercise testing in identifying the presence of obstructive sleep apnea syndrome in patients with compatible symptomatology. Respir. Physiol. Neurobiol. 2019, 262, 26–31. [Google Scholar] [CrossRef] [PubMed]
- DeMers, D.; Wachs, D. Physiology, Mean Arterial Pressure. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, V.T.; Karetsi, E.; Gourgoulianis, K.I. The Effect of Growth and Body Surface Area on Cardiopulmonary Exercise Testing: A Cohort Study in Preadolescent Female Swimmers. Children 2023, 10, 1608. [Google Scholar] [CrossRef]
- Scholkmann, F.; Wolf, U. The Pulse-Respiration Quotient: A Powerful but Untapped Parameter for Modern Studies About Human Physiology and Pathophysiology. Front. Physiol. 2019, 10, 371. [Google Scholar] [CrossRef]
- Stavrou, V.; Vavougios, G.D.; Bardaka, F.; Karetsi, E.; Daniil, Z.; Gourgoulianis, K.I. The effect of exercise training on the quality of sleep in national-level adolescent finswimmers. Sports Med. Open 2019, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699, Erratum in J. Am. Geriatr. Soc. 2019, 67, 1991. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, K.; Baril, A.A.; Gagnon, J.F.; Fortin, M.; Décary, A.; Lafond, C.; Desautels, A.; Montplaisir, J.; Gosselin, N. Cognitive impairment in obstructive sleep apnea. Pathol. Biol. 2014, 62, 233–240. [Google Scholar] [CrossRef]
- Stavrou, V.T.; Papayianni, E.; Astara, K.; Vavougios, G.D.; Kontogianni, M.D.; Bargiota, A.; Pastaka, C.; Daniil, Z.; Gourgoulianis, K.I. Tele-Pulmonary Rehabilitation and Mediterranean-like Lifestyle, Adjunctively to Continuous Positive Airway Pressure in Obstructive Sleep Apnea Patients: Effects in Fitness and Oxidative Indicators. Appl. Sci. 2024, 14, 8424. [Google Scholar] [CrossRef]
- Stavrou, V.T.; Griziotis, M.; Vavougios, G.D.; Raptis, D.G.; Bardaka, F.; Karetsi, E.; Kyritsis, A.; Daniil, Z.; Tsarouhas, K.; Triposkiadis, F.; et al. Supervised Versus Unsupervised Pulmonary Rehabilitation in Patients with Pulmonary Embolism: A Valuable Alternative in COVID Era. J. Funct. Morphol. Kinesiol. 2021, 6, 98. [Google Scholar] [CrossRef]
- Stavrou, V.T.; Tourlakopoulos, K.N.; Vavougios, G.D.; Papayianni, E.; Kiribesi, K.; Maggoutas, S.; Nikolaidis, K.; Fradelos, E.C.; Dimeas, I.; Daniil, Z.; et al. Eight Weeks Unsupervised Pulmonary Rehabilitation in Previously Hospitalized of SARS-CoV-2 Infection. J. Pers. Med. 2021, 11, 806. [Google Scholar] [CrossRef]
- Somers, V.K.; Dyken, M.E.; Mark, A.L.; Abboud, F.M. Sympathetic-nerve activity during sleep in normal subjects. N. Engl. J. Med. 1993, 328, 303–307. [Google Scholar] [CrossRef]
- de Abreu, R.M.; Rehder-Santos, P.; Minatel, V.; Dos Santos, G.L.; Catai, A.M. Effects of inspiratory muscle training on cardiovascular autonomic control: A systematic review. Auton. Neurosci. 2017, 208, 29–35. [Google Scholar] [CrossRef]
- Stavrou, V.; Voutselas, V.; Karetsi, E.; Gourgoulianis, K.I. Acute responses of breathing techniques in maximal inspiratory pressure. Sport Sci. Health 2018, 14, 91–95. [Google Scholar] [CrossRef]
- Matić, Z.; Kalauzi, A.; Moser, M.; Platiša, M.M.; Lazarević, M.; Bojić, T. Pulse respiration quotient as a measure sensitive to changes in dynamic behavior of cardiorespiratory coupling such as body posture and breathing regime. Front. Physiol. 2022, 13, 946613. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, R.J.S.; Band, G.P.H. Breath of Life: The Respiratory Vagal Stimulation Model of Contemplative Activity. Front. Hum. Neurosci. 2018, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.D.; Dal Lago, P.; da Silva Soares, P.P. Inspiratory muscle training improves breathing pattern and sympatho-vagal balance but not spontaneous baroreflex sensitivity in older women. Respir. Physiol. Neurobiol. 2021, 290, 103672. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, V.T.; Vavougios, G.D.; Hadjigeorgiou, G.M.; Bargiotas, P. The Effect of Physical Exercise on Patients With Mild Cognitive Impairment: A Scoping Review. Cureus 2024, 16, e73265. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, V.T.; Kyriaki, A.; Vavougios, G.D.; Fatouros, I.G.; Metsios, G.S.; Kalabakas, K.; Karagiannis, D.; Daniil, Z.; IGourgoulianis, K.; Βasdekis, G. Athletes with mild post-COVID-19 symptoms experience increased respiratory and metabolic demands: A cross-sectional study. Sports Med. Health Sci. 2023, 5, 106–111. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Z.; Qu, Y.; Zhao, Y.; Yang, Y.; Du, J.; Yang, C. Cognitive function and brain activation before and after transcutaneous cervical vagus nerve stimulation in healthy adults: A concurrent tcVNS-fMRI study. Front. Psychol. 2022, 13, 1003411. [Google Scholar] [CrossRef] [PubMed]
- Magnon, V.; Dutheil, F.; Vallet, G.T. Benefits from one session of deep and slow breathing on vagal tone and anxiety in young and older adults. Sci. Rep. 2021, 11, 19267. [Google Scholar] [CrossRef]
- Hughes, C.M.; McCullough, C.A.; Bradbury, I.; Boyde, C.; Hume, D.; Yuan, J.; Quinn, F.; McDonough, S.M. Acupuncture and reflexology for insomnia: A feasibility study. Acupunct. Med. 2009, 4, 163–168. [Google Scholar] [CrossRef]
- Silva de Sousa, A.; Pereira da Rocha, A.; Brandão Tavares, D.R.; Frazão Okazaki, J.É.; de Andrade Santana, M.V.; Fernandes Moça Trevisani, V.; Pereira Nunes Pinto, A.C. Respiratory muscle training for obstructive sleep apnea: Systematic review and meta-analysis. J. Sleep Res. 2024, 33, e13941. [Google Scholar] [CrossRef]
- Stavrou, V.; Bardaka, F.; Karetsi, E.; Daniil, Z.; Gourgoulianis, K.I. Brief Review: Ergospirometry in Patients with Obstructive Sleep Apnea Syndrome. J. Clin. Med. 2018, 7, 191. [Google Scholar] [CrossRef]
- Wang, W.; Li, R.; Li, C.; Liang, Q.; Gao, X. Advances in VNS efficiency and mechanisms of action on cognitive functions. Front. Physiol. 2024, 15, 1452490. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Song, L.; Wang, X.; Li, N.; Zhan, S.; Rong, P.; Wang, Y.; Liu, A. Transcutaneous Vagus Nerve Stimulation Could Improve the Effective Rate on the Quality of Sleep in the Treatment of Primary Insomnia: A Randomized Control Trial. Brain Sci. 2022, 12, 1296. [Google Scholar] [CrossRef] [PubMed]
- Vranish, J.R.; Bailey, E.F. Inspiratory Muscle Training Improves Sleep and Mitigates Cardiovascular Dysfunction in Obstructive Sleep Apnea. Sleep 2016, 39, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.; Emperumal, C.P.; Grbach, V.X.; Padilla, M.; Enciso, R. Effects of respiratory muscle therapy on obstructive sleep apnea: A systematic review and meta-analysis. J. Clin. Sleep Med. 2020, 16, 785–801. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Tanuma, T. The effect of nasal and oral breathing on airway collapsibility in patients with obstructive sleep apnea: Computational fluid dynamics analyses. PLoS ONE 2020, 15, e0231262. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).