Abstract
High-frequency electrotome (HFE) encompassing rapid cutting speed, effective hemostasis, and simple operation plays a critical role in the electrosurgical field. However, the blood and tissue adhesion on HFE can cause secondary tissue tearing and bleeding. Constrained by the blood and tissue adhesion of conventional HFEs during surgical procedure, aside from prolonged surgical time, secondary tissue tear and bleeding may occur. Available methods have been proposed to alleviate these problems, including additional devices, coating methods, and surface patterning. However, practical applications of existing HFEs with their high cost, low conductivity, and instability remain a challenge. To solve these challenges, we proposed a facile and eco-friendly superhydrophobic high-frequency electrotome (SH-HFE) based on surface patterning, which has blood anti-adhesion and tissue anti-adhesion. The mechanism contributing to superhydrophobicity was investigated by scanning electron microscopy, X-ray photoelectron spectroscopy, and Fourier-transform infrared spectroscopy. The blood adhesion experiments and tissue adhesion experiments were conducted to verify the effectiveness of SH-HFE with surface patterning in practical applications. Compared with conventional HFEs, blood adhesion and tissue adhesion on SH-HFE were reduced by 89.7% and 94.8%, respectively, while still maintaining excellent conductivity. The proposed SH-HFE would provide significant benefits to the electrosurgical field.
1. Introduction
Medical high-frequency electrotome (HFE) is an indispensable precision tool in surgery. It achieves rapid tissue cutting and effective hemostasis through the thermal effect of electric current, and is known for its advantages of fast cutting speed, good hemostatic effect, easy operation, and postoperative pain reduction [1,2,3]. However, during surgery, the phenomenon of HFE overheating may lead to the dehydration, crusting, and even charring of blood and soft tissues, which can adhere and cover the surface of the HFE. This situation not only makes cutting difficult and hemostasis incomplete, but also may cause secondary injuries to the patient and, in severe cases, affect the overall outcome of the surgery [4,5]. Therefore, the development of medical HFEs with significant anti-adhesion blood, anti-adhesion tissue, and harmlessness to the human body has become a research hotspot in this field and an important direction for future development [6,7].
Many scholars proposed a number of methods to improve the anti-adhesion of surface, such as additional auxiliary devices [8], coating methods [9,10,11], surface patterning [12,13,14], and so on. Inspired by these methods, researchers have proposed many strategies for HFE to enhance its ability to prevent blood adhesion and tissue adhesion. The additional auxiliary equipment type is mainly used to reduce the temperature of the working end to achieve anti-adhesion through the addition of a cold circulation system [15], saline infusion [16], etc., or the use of ultrasonic devices [17] to reduce adhesion through the energy of oscillation. This kind of HFE that achieves anti-adhesion by adding auxiliary devices not only increases the size of the original HFE, which affects the surgical field of view, but also increases the cost and difficulty of processing and manufacturing. To ensure the lightweight and compactness of the HFE, surface modification methods, including coatings and surface patterning, have been proposed. Ceviker et al. [18] applied PTFE coatings to bipolar electrocoagulation forceps in the form of stripes to reduce tissue adhesion. The results showed that the PTFE coating not only affects the electrical conductivity, but also tends to decompose toxic substances at high temperatures, which poses a certain risk to the patient’s health [19]. Ou et al. [20] effectively reduced the mass of tissue adhesion by 66.8% by depositing a chromium nitride film of about 1 μm on the surface of electrosurgical needles. Kang et al. [21] utilized atmospheric-pressure radiofrequency-driven plasma to deposit hexamethyldisiloxane on the jaws of surgical instruments to form a non-stick coating. The results showed that the surface coatings significantly reduced the adhesion of soft tissues on the instruments. However, they have some limitations in that they are difficult to accurately regulate and control throughout the process, and the coatings are usually unstable during use and may flake off at high temperatures, affecting the surgical results. Different from coating methods, surface patterning increases the surface area of the HFE by processing a pattern involving micro-/nanostructures to reduce the actual contact area between the tissue and the HFE surface while enhancing heat dissipation. Zhou et al. [22] prepared a longitudinal micro-channel pattern on the surface of the HFE. The results indicated that the pattern of longitudinal micro-channel could decrease the adhesion of tissue to the HFE, with the tissue adhesion quality reduced by 55% compared to an HFE without a pattern. The mechanism of reduced tissue adhesion was verified by COMSOL, which was attributed to reduced heat transfer by patterning. Liu et al. [23] prepared a hexagonal pit microstructure based on the surface of leaf-cutting ants, and the adhesion quality of bionic HFEs was reduced by about 36% on average compared to ordinary HFEs. Lin et al. [24] utilized femtosecond pulsed laser processing to process micro-/nanostructures on the surface of the HFE, which reduced thermal damage and improved anti-adhesive properties. Li et al. [25], inspired by pangolin scales, prepared a bionic scale structure on an HFE by means of a long-pulsed laser, and the water contact angle was increased from 68° to 94°. The coefficient of friction and the mass of tissue adhesion of the bionic HFE were reduced by 14.9% and 16.5%, respectively, compared to the initial surface. However, HFE’s anti-adhesion ability relying on microstructures is still limited. Han et al. [26] were inspired by corn blades to fabricate a groove structure arranged equidistantly along the direction of the blade and coated it with a TiO2 film to develop a novel HFE with a coupled biomimetic anti-adhesive surface, which increased the water contact angle from 71° to 148° and reduced the tissue adhesion quality by 71.43%. However, it is worth noting that the TiO2 film coating may have some impact on the electrical conductivity of the HFE, which is a factor to be considered in practical applications. Zhang et al. [27] constructed uniformly arranged micro-pit structures on the surface of the HFE and injected silicone oils after silanization to form a liquid-infused micro-structured surface to reduce tissue adhesion. However, silicone oil is easily lost at high temperatures, which may result in the inability of the HFE to perform surgical operations consistently and efficiently, thus affecting the continuity and effectiveness of the procedure [28,29]. Therefore, a safe, stable, conductive, and low-cost method is needed to improve the anti-adhesion performance of HFEs.
In this paper, we propose a simple and environmentally friendly new superhydrophobic high-frequency electrotome (SH-HFE) based on surface patterning, which was fabricated by nanosecond laser and stearic acid modification with blood anti-adhesion and tissue anti-adhesion. We first studied the influence of laser processing parameters, such as laser power, scanning speed, number of processing, and line spacing, on the surface wettability and surface roughness of the HFE. We then analyzed the surface chemical compositions of the ordinary HFE and SH-HFE. Then, inspired by the excellent hydrodynamic properties and anti-adhesion properties of shark skin due to its structure being naturally selected over a long period of time, we fabricated three different patterned microstructures on the HFE to mimic the micro- and nanostructures of shark skin, and compared the performance of the ordinary HFE to that of SH-HFEs with three different patterned microstructures in meat-cutting and blood-dipping experiments. The SH-HFE with arrowhead patterns had the best performance in blood anti-adhesion and pork tissue anti-adhesion. This provides a new technology for the anti-adhesion performance of HFEs and is expected to improve the efficiency and safety of surgeries.
2. Materials and Methods
2.1. Materials
Anhydrous ethanol (analytically pure) was purchased from Tianjin Damao Chemical Reagent Factory (Tianjin, China). Stearic acid (analytically pure) was purchased from Tianjin Chemical Reagent Factory No. 3 Co., Ltd. (Tianjin, China). HFEs were purchased from Shenzhen Life Discovery Medical Systems Co., Ltd. (Shenzhen, China). Sterile pig blood was purchased from Zhengzhou Pingrui Biotechnology Co., Ltd. (Zhengzhou, China). Pork was bought from the China Resources Vanguard supermarket in Dalian.
2.2. Fabrication of Anti-Adhesion SH-HFEs
Figure 1a shows the fabrication processes of the SH-HFEs. The HFEs were cleaned successively by anhydrous alcohol and deionized water for 5 min, and then dried in a pollution-free environment at room temperature. The cleaned HFEs were processed with a nanosecond laser (SK-CX30, Shanghai Sanke Laser Technology Co., Ltd., Shanghai, China) along a raster scanning path (10 μm scan line spacing in the 0° and 90° directions) at a power of 9 W and a scanning speed of 500 mm/s to fabricate HFEs with a flat pattern (Figure 1b) [30]. The second laser processing process was then conducted on the HFEs with a flat pattern with the same laser processing parameters to prepare triangle patterns (P1 Figure 1c), rhombus patterns (P2 Figure 1d), and arrowhead patterns (P3 Figure 1e). Stearic acid and anhydrous ethanol (0.018:1 mass ratio) were magnetically stirred for 2 h at room temperature and configured as a 0.05 mol/L mixture. The HFEs with different patterns were immersed in stearic acid solution (0.05 mol/L) for 20 min and then cured in the oven at 80 °C for 20 min to form solid-state coatings. The three patterned microstructures after modification were named SAP1, SAP2, and SAP3.
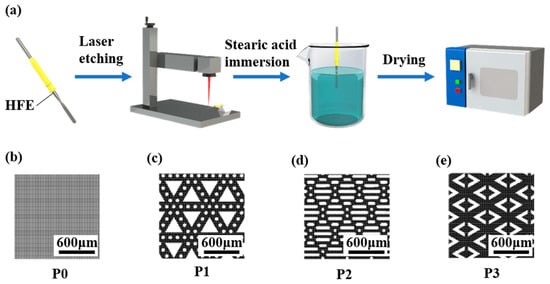
Figure 1.
(a) Schematic diagram of the preparation process of anti-adhesion SH-HFEs. Laser-etched patterns, including (b) flat pattern, (c) triangle patterns, (d) rhombus patterns, and (e) arrowhead patterns.
2.3. Characterization
Contact angle and rolling angle were measured by an optical measurement instrument (SL200KS, Kino, Boston, MA, USA) with 8 μL of water droplet. The contact angle was measured based on the droplet reaching equilibrium on the surface, while the roll angle was recorded by rotating the substrate after the droplet had reached equilibrium until the droplet slid off. The contact angle and rolling angle were measured five times for each sample. A scanning electron microscope (SEM, JSM-7900F, Akishima, Japan) and a Zygo 9000 (NewView 9000, Middlefield, OH, USA) were used to observe the morphology of different microstructures. To ensure the reliability and repeatability of the surface roughness measurements, five measurements were taken for each sample. X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250Xi, Waltham, MA, USA) and Fourier-transform infrared spectroscopy (FTIR, iS50, Waltham, MA, USA) were used to detect and analyze the chemical compositions of the HFE surfaces. X-ray photoelectron spectroscopy was performed with the C1s peak at 284.8 eV as a reference for all binding energies. Electrocutting tests were performed using a medical high-frequency power supply (ICC200INT, Albo Electronic Medical Instruments, Tubingen, Germany). The quality of the HFEs was evaluated using an electronic balance (FA2004B, Shanghai Yue Ping Scientific Instrument Co., Ltd., Shanghai, China) with a weighing accuracy of 0.1 mg.
2.4. Blood Adhesion Experiment
Ordinary HFE and the SH-HFEs with three different patterns were immersed in fresh sterile porcine blood for 1 min and subsequently removed, and the mass of blood adhering on the surfaces was tested. All blood adhesion experiments were performed at least four times.
2.5. Tissue Adhesion Experiment
Fresh pork tissue was cut using ordinary HFE and the SH-HFEs with three different patterns. A self-designed displacement platform was used to realize the directional movement of the HFEs, as shown in Figure 2a. Fresh pork was selected and cut into rectangles with dimensions of 180 mm × 80 mm × 30 mm, which were fixed on a self-designed fixture to ensure the uniformity of each cutting process. The output power of the electrotome system was set to 40 W. The electric knife was driven by the screw to cut the tissue at a speed of 5 mm/s and a depth of 10 mm, as shown in Figure 2b. After the cutting was completed, the weight of the tissue adhering on the blade was tested [31,32]. All cutting experiments were performed at least four times.
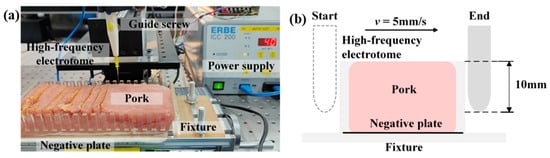
Figure 2.
(a) Tissue cutting displacement platform. (b) Schematic diagram of cut tissue.
3. Results and Discussion
We fabricated SH-HFEs by laser processing and stearic acid modification. In this study, we first explored the influence of laser scanning parameters on the surface wettability and morphology of HFEs. Since the adjustment of the laser parameters changes the surface morphology, if the morphology of the patterned microstructure and the surface structure change at the same time, it will complicate the study of the effect of the patterned microstructure on the hydrophobicity and anti-adhesive properties alone. Therefore, we first determined the optimal processing parameters and then used these parameters to process different patterned microstructures to ensure the consistency of the structure morphology. As shown in Figure 3a, the superhydrophobicity of the sample reaches a steady state when the laser power is 9 W, the contact angle is 159°, and the surface roughness Sa is 1.13 μm. Further investigating the influence of scanning speed, as shown in Figure 3b, we found that the sample maintains a higher superhydrophobicity (contact angle of 161°) when the scanning speed is 500 mm/s. The surface roughness Sa decreased to 1.08 μm, which exhibited a lower surface roughness. To achieve better superhydrophobicity and uniform morphology, we also explored the influence of line spacing on wettability and morphology. As shown in Figure 3d, when the line spacing is 10 μm, the contact angle is the highest, reaching 162°, while at the same time, the laser-etched morphology is the most homogeneous at the smallest laser line spacing, and the surface roughness Sa decreases to 1.0 μm. In addition, the number of scans does not have much effect on the surface wettability and morphology, as shown in Figure 3c; with the increase in the number of scans, the superhydrophobicity tends to be stabilized, and the surface roughness only slightly increases. The water contact angle and Zygo image of the untreated HFE surface are shown in Figures S1 and S2. Detailed data can be found in Tables S1–S4. Therefore, we chose the laser processing parameters of 9 W laser power, 500 mm/s scanning speed, 10 μm line spacing, and one repeat scan. After processing the patterned microstructures with optimal parameters, as shown in Figure 3e, we observed that SAP1, SAP2, and SAP3 could be superhydrophobic.
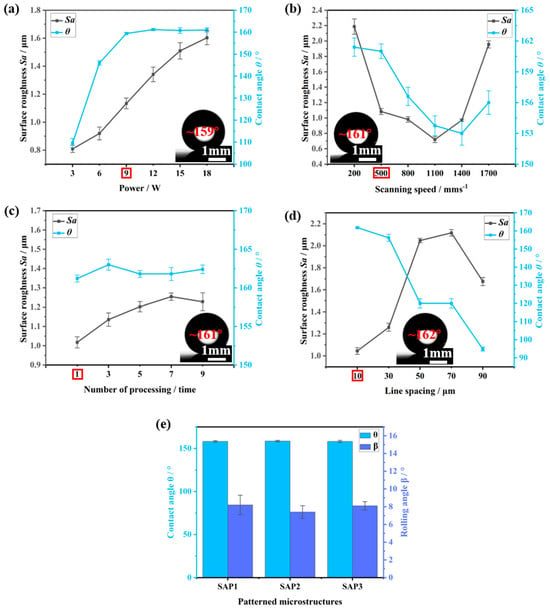
Figure 3.
Influence of different (a) laser powers, (b) scanning speeds, (c) processing times, and (d) line spacing on contact angle and surface roughness. (e) Influence of different patterned microstructures on surface wettability. The optimal parameters are circled in Figures (a–d).
The combination of surface micromorphology and low surface energy is a key factor in conferring superhydrophobicity and excellent anti-adhesion properties to HFE surfaces [33,34,35,36]. The surface prepared by laser treatment was shown to be homogeneous and consisted of many papillary microscale structures, which favored the surface, as shown in Figure 4a, by scanning electron microscopy images. With the Zygo image, as shown in Figure 4b, we visualized the height distribution of the surface design pattern. This image clearly presented the three-dimensional characteristics of the microstructure formed by laser processing.
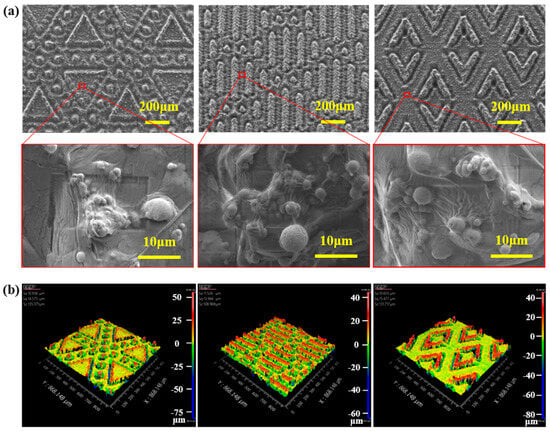
Figure 4.
(a) Microscopic morphology of SH-HFEs. (b) Zygo images of SH-HFEs.
The chemical groups on the surface of the ordinary HFE and SH-HFEs were analyzed using FTIR spectroscopy. As shown in Figure 5a, in the high-frequency region, the absorption peaks of the SH-HFE are located near 2913 cm−1 and 2846 cm−1, which belong to the asymmetric and symmetric stretching vibrations of C–H, respectively. In the low-frequency region, an absorption peak of 1697 cm−1 appears on the surface of the SH-HFEs compared with that of the ordinary HFE, which belong to the C=O stretching vibrations in the carboxyl group. Considering the chemical modifications, the appearance of the C peak proves the presence and bonding of CH3 (CH2)16COO– on the surface of the HFE [37]. The chemical composition information provided by the XPS spectra was then analyzed. As shown in Figure 5b, we found that the main elemental compositions of the ordinary HFE and the SH-HFEs remained the same, i.e., they mainly contained elements such as C, O, Fe, and Si, but the relative contents of the elements were quite different. The content of Fe was significantly reduced after modification with stearic acid, where the reduction in Fe content indicated that stearic acid had successfully modified and covered the surface of the HFE. Further observation of the differences before and after, as shown in Table 1, revealed that C and O showed significant changes, with the content of C significantly increasing from 42.2% to 86.6%, while the content of O significantly decreasing from 41.2% to 11.3%. In order to deeply investigate the effect of the chemical composition of the HFE surface on wettability, we fitted the high-resolution C1s peaks on the ordinary HFE and SH-HFE surfaces. The C1s at 284.8 eV was used as the reference peak, and the full-spectrum charge was corrected. XPS Peak v4.1 software was used to fit the C1s peak. The spectral line shape was set to 20% Lorentzian/Gaussian, and the FWHM of each peak was fixed at 1–2.5; the results are shown in Figure 5c and 5d. The peaks at 284.8 eV, 286 ± 0.2 eV, and 288.4 ± 0.3 eV in the high-resolution peaks of the C1s correspond to the C–C/C–H/C–Si moiety, the C–O moiety, and the C=O moiety, respectively. The atomic percentage of each sample was determined using the integrated peak area of each component. The relative content of groups on the surface of the HFE before and after treatment is shown in Table 2. The relative content of oxygen-containing groups, such as C–O and C=O, on the surface of the SH-HFE was reduced, from 13.2% and 6.8% to 4.9% and 4.1%, respectively. Meanwhile, the relative content of nonpolar groups such as C–C/C–H/C–Si increased from 80.0% to 91.0%. The relative decrease of oxygen-containing groups and the relative increase in nonpolar groups such as C–C/C–H/C–Si further confirm that stearic acid effectively reduces the surface energy by adsorbing its hydrophobic long-chain alkane structure onto the surface of the HFE [38,39,40]. The adsorption of stearic acid on the metal surface should be due to the fact that the oxide layer formed by the laser treatment is rich in reactive groups such as hydroxyl (–OH), which can react with the carboxyl group of stearic acid to form a chemical bond, so that its long-chain alkyl group is chemisorbed on the metal surface. Under the dual effect of the microstructure of the laser etching technique and the low surface energy modification provided by stearic acid, the surface of the HFE exhibits superhydrophobic properties.
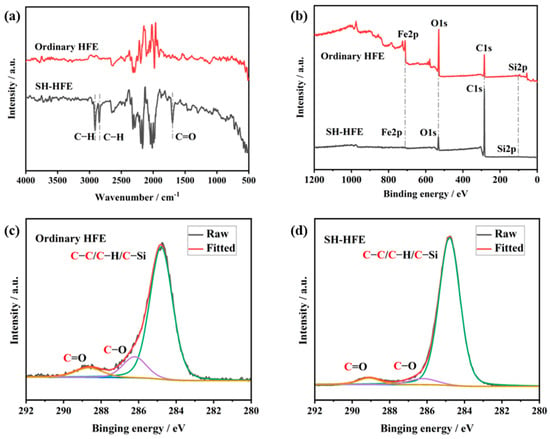
Figure 5.
(a) FTIR spectroscopy of ordinary and SH HEFs. (b) XPS spectroscopy of ordinary and SH HEFs. High-resolution C1s fitted peaks of (c) ordinary HFE and (d) SH-HFE surfaces.

Table 1.
Relative content of elements.

Table 2.
Relative contents of functional groups.
In electrosurgical operations, superior electrical conductivity is essential to achieve precise cutting and effective hemostasis. SH-HFE significantly reduces the resistance value to approximately 1.98 Ω (as shown in Figure 6b), which is only one-third of the resistance value of the ordinary HFE. This not only enhances the electrical conductivity of the HFE, but also provides a good basis for subsequent meat-cutting experiments.
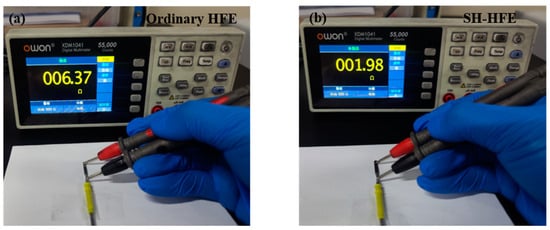
Figure 6.
Resistance tests of (a) ordinary HFE and (b) SH-HFE surfaces.
In this experiment, we selected porcine blood and pork tissues as the experiment materials to evaluate the anti-adhesion performance of HFE and SH-HFE. The experiments were conducted to investigate the anti-adhesion capability of HFE and SH-HFE by immersing these samples into the pig blood and measuring the mass of blood remaining on the surface, as shown in Figure 7a–f. In addition, we also tested HFE with only laser patterning treatment, without stearic acid modification, and HFE with only stearic acid modification, without laser patterning treatment, as shown in Figures S3 and S4. As shown in Figure 7c, a large amount of crimson blood with a weight of about 967 mg adhered to the surface of the ordinary HFE. In contrast, the amount of blood residue on the surface of SH-HFE was significantly reduced except for the SAP1 structure. In particular, the SH-HFE with the SAP3 structure showed almost no visible blood residue, as shown in Figure 7f and Video S1, and the mass of adhered blood was only 50 mg, which is an 89.7% reduction in the adhered mass, a result that proves its good blood anti-adhesion properties. When SH-HFE is fully immersed in blood, the unique structures on the SH-HFE surface are able to stably trap the air layer, effectively preventing the blood from fully penetrating into the surface. This design ensures that when HFE is removed from blood, it is difficult for the blood to adhere to the surface of HFE. The SEM of the ordinary HFE and these three SH-HFEs with different patterns after the blood adhesion experiments are shown in Figure S5. In addition, we used pork to simulate human tissues and conducted cutting experiments on fresh pork tissues using HFE and SH-HFE to investigate the tissue anti-adhesion ability of the different samples. In these experiments, we used a medical high-frequency power supply with a set output power of 40 W to cut the pork tissue at a speed of 5 mm/s and a depth of 10 mm. By measuring the quality of pork tissue adhering to the different surfaces after cutting, we compared the tissue anti-adhesion capacity of the different surfaces. As shown in Figure 7b, the mass of pork adhered after cutting with the ordinary HFE was about 18.4 g, whereas the mass of pork adhered after cutting with SH-HFE was reduced in all cases. The mass of adhered tissue on the SH-HFE with the SAP3 structure was only 1.9 g, which was a 94.8% reduction in adherent mass compared with the ordinary HFE, showing a significant reduction in tissue adhered, as shown in Figure 7j and Video S2. The SEM of the ordinary HFE and these three SH-HFEs with different patterns after the tissue adhesion experiments are shown in Figure S6. The microstructures enhance the anti-adhesion performance of SH-HFE by reducing the contact area with soft tissues and leveraging the effect of air within the microtextures.
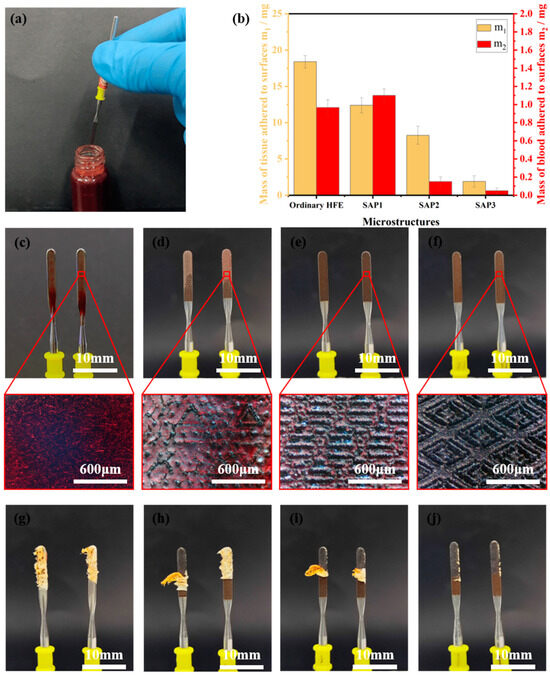
Figure 7.
(a) Blood adhesion experiment. (b) Mass of blood and tissue adhering to the surface of ordinary HFE and SH-HFE. Photographs of blood adhesion experiments on (c) ordinary HFE, (d) SAP1, (e) SAP2, and (f) SAP3 surfaces. Photographs of tissue adhesion experiments on (g) ordinary HFE, (h) SAP1, (i) SAP2, and (j) SAP3 surfaces.
4. Conclusions
In this paper, we proposed a facile method to fabricate SH-HFEs with superior anti-adhesion properties and excellent conductivity. The HFEs with the flat pattern, triangle patterns, rhombus patterns, and arrowhead patterns fabricated by nanosecond laser were immersed in stearic acid solution to obtain a superhydrophobic surface. The prepared HFEs exhibited good hydrophobicity property, where the water contact angle and the water rolling angle were about 158° and 8°, respectively. XPS and FTIR indicated that due to the successful modification of stearic acid on the HFE surface, the long-chain saturated group reduced the surface energy of the HFE. The SEM images showed that the patterns could be clearly observed, which meant that these embossed patterns would decrease the contact area of the HFE and tissue. According to the blood adhesion experiment and tissue adhesion experiment, compared with the ordinary HFE, the mass of blood adhesion and tissue adhesion on the SH-HFE was reduced by 89.7% and 94.8%, respectively, which showed that the SH-HFE had excellent anti-adhesion properties. Therefore, the proposed method would improve the surgical efficiency of HFE in the electrosurgical field.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15052363/s1, Video S1: Anti-adhesion blood experiment of SH-HFE with SAP3 structure; Video S2: Anti-adhesion tissue experiment of SH-HFE with SAP3 structure; Table S1: Surface roughness data measured at different laser powers; Table S2: Surface roughness data measured at different scanning speeds; Table S3: Surface roughness data measured at different processing numbers; Table S4: Surface roughness data measured at different line spacings; Figure S1: Contact angle of untreated HFE surface; Figure S2: Zygo images of untreated HFE surfaces; Figure S3: Blood adhesion and tissue adhesion in HFE after laser patterning treatment; Figure S4: Blood adhesion and tissue adhesion in stearic acid-treated HFE; Figure S5: SEM images of blood adhesion on the surface of (a) ordinary HFE, (b) SAP1, (c) SAP2 and (d) SAP3; Figure S6: SEM images of tissue adhesion on the surface of (a) ordinary HFE, (b) SAP1, (c) SAP2 and (d) SAP3.
Author Contributions
Conceptualization, H.F. and X.L.; methodology, H.F. and Y.L. (Yuheng Li); validation, H.F. and H.L.; formal analysis, Y.L. (Yuheng Li) and D.Y.; investigation, Y.L. (Yuheng Li), D.Y. and H.L.; data curation, H.F., H.L., Y.Z. and Y.L.(Yun Li); writing—original draft preparation, H.F. and Y.L. (Yuheng Li); writing—review and editing, D.Y., J.L. and X.L.; visualization, H.F., H.L., Y.Z. and Y.L. (Yun Li); funding acquisition, X.L. and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This project was financially supported by the Fundamental Research Funds for the Central Universities (DUT23YG118) and the National Natural Science Foundation of China (NSFC, 52405461).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We would like to thank Junyi Lin for his guidance and help in the use of laser equipment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Messenger, D.; Carter, F.; Noble, E.; Francis, N. Electrosurgery and energized dissection. Surgery 2020, 38, 133–138. [Google Scholar]
- Malis, L.I. Electrosurgery—Technical note. J. Neurosurg. 1996, 85, 970–975. [Google Scholar] [CrossRef]
- Taheri, A.; Mansoori, P.; Sandoval, L.F.; Feldman, S.R.; Pearce, D.; Williford, P.M. Electrosurgery: Part I. Basics and principles. J. Am. Acad. Dermatol. 2014, 70, 591. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wan, J.; Long, Y.; Fu, H.; Zheng, J.; Zhou, Z. Effect of high-frequency electric field on the tissue sticking of minimally invasive electrosurgical devices. R. Soc. Open Sci. 2018, 5, 180125. [Google Scholar] [CrossRef] [PubMed]
- Sutton, P.A.; Awad, S.; Perkins, A.C.; Lobo, D.N. Comparison of lateral thermal spread using monopolar and bipolar diathermy, the Harmonic Scalpel™ and the Ligasure™. Br. J. Surg. 2010, 97, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Tenjimbayashi, M.; Muto, J.; Shiratori, S. Antiadhesion Function between a Biological Surface and a Metallic Device Interface at High Temperature by Wettability Control. ACS Biomater. Sci. Eng. 2018, 4, 1891–1899. [Google Scholar] [CrossRef]
- Li, K.; Xie, Y.; Tang, B.; Yu, M.; Ding, H.; Li, C.; Lu, L. Evolution of electro-induced blood plasma droplets on a superhydrophobic microstructured surface. Appl. Phys. Lett. 2022, 121, 113701. [Google Scholar] [CrossRef]
- Yao, G.; Zhang, D.; Geng, D.; Jiang, X. Improving anti-adhesion performance of electrosurgical electrode assisted with ultrasonic vibration. Ultrasonics 2018, 84, 126–133. [Google Scholar] [CrossRef]
- Alzadjali, S.; Matouk, Z.; Alshehhi, A.; Rajput, N.; Mohammedture, M.; Guttierrez, M. Simple, Scalable Route to Produce Transparent Superhydrophobic/Hydrophilic Film Surfaces. Appl. Sci. 2023, 13, 1707. [Google Scholar] [CrossRef]
- Piscitelli, F. Superhydrophobic and Icephobic Coatings as Passive Ice Protection Systems for Aeronautical Applications. Appl. Sci. 2024, 14, 1288. [Google Scholar] [CrossRef]
- Chen, G.; Yan, D.; Liu, J.; Xu, Y.; Zhou, Y.; Wu, B.; Song, J. Self-cleaning coating for exterior walls of concrete buildings. Surf. Innov. 2022, 11, 453–463. [Google Scholar] [CrossRef]
- Yan, D.; Lin, J.; Zhang, B.; Zhang, S.; Ling, S.; Song, J. Drag reduction and antifouling of a spontaneous fast moving air film. J. Mater. Chem. A 2024, 12, 19268–19276. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Xue, Y.; Sui, X.; Yuan, B.; Wang, Y.; Liang, W. Functional surfaces with reversibly switchable wettability: Fundamentals, progresses, applications and challenges. Prog. Org. Coat. 2024, 188, 108167. [Google Scholar] [CrossRef]
- Yan, D.; Lu, Y.; Lin, J.; Li, W.; Song, J. Enhancing water transportation capacity by asymmetrical patterned surface with super-wettability. Appl. Phys. Lett. 2024, 125, 71601. [Google Scholar] [CrossRef]
- Dodde, R.E.; Gee, J.S.; Geiger, J.D.; Shih, A.J. Monopolar electrosurgical thermal management for minimizing tissue damage. IEEE. Trans. Biomed. Eng. 2012, 59, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.P.; Sabharwal, T.; Georganas, M.; Dourado, R.; Cahill, D.; Adam, A. Cold saline irrigation of the renal pelvis during Radiofrequency Ablation of a central renal neoplasm: A case report. J. Med. Case Rep. 2008, 2, 40. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Zhang, D.; Geng, D.; Wang, L. Novel ultrasonic vibration-assisted electrosurgical cutting system for minimizing tissue adhesion and thermal injury. Road Mater. Pavement Des. 2021, 201, 109528. [Google Scholar] [CrossRef]
- Ceviker, N.; Keskil, S.; Baykaner, K. A new coated bipolar coagulator: Technical note. Acta Neurochir. 1998, 140, 619–620. [Google Scholar] [CrossRef]
- Waritz, R.S. An industrial approach to evaluation of pyrolysis and combustion hazards. Environ. Health Perspect. 1975, 11, 197–202. [Google Scholar] [CrossRef]
- Ou, K.L.; Chu, J.S.; Hosseinkhani, H.; Chiou, J.F.; Yu, C.H. Biomedical nanostructured coating for minimally invasive surgery devices applications: Characterization, cell cytotoxicity evaluation and an animal study in rat. Surg. Endosc. 2014, 28, 2174–2188. [Google Scholar] [CrossRef]
- Kang, S.K.; Kim, P.Y.; Koo, I.G.; Kim, H.Y.; Jung, J.C.; Choi, M.Y.; Lee, J.K.; Collins, G.J. Non-stick polymer coatings for energy-based surgical devices employed in vessel sealing. Plasma Process. Polym. 2012, 9, 446–452. [Google Scholar] [CrossRef]
- Zhou, C.; Lu, J.; Wang, X. Adhesion behavior of textured electrosurgical electrode in an electric cutting process. Coatings 2020, 10, 596. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, F.; Gu, H.; Feng, J.; Huang, D.; Zheng, L.; Yao, G.; Chen, Z.; Wang, C. Adhesion failure and anti-adhesion bionic structure optimization of surgical electrodes in soft tissue cutting. J. Manuf. Process. 2023, 89, 444–457. [Google Scholar] [CrossRef]
- Lin, C.C.; Lin, H.J.; Lin, Y.H.; Sugiatno, E.; Ruslin, M.; Su, C.Y.; Ou, K.L.; Cheng, H.Y. Micro/nanostructured surface modification using femtosecond laser pulses on minimally invasive electrosurgical devices. J. Biomed. Mater. Res. Part B 2017, 105, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, Y.; Yang, L.; Shi, Z. Biomimetic anti-adhesive surface microstructures on electrosurgical blade fabricated by long-pulse laser inspired by pangolin scales. Micromachines 2019, 10, 816. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Fu, J.; Feng, X.; Niu, S.; Zhang, J.; Ren, L. Bionic anti-adhesive electrode coupled with maize leaf microstructures and TiO2 coating. RSC Adv. 2017, 7, 45287–45293. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, G.; Zhang, D.; Chen, H. Liquid-Infused Surfaces on Electrosurgical Instruments with Exceptional Antiadhesion and Low-Damage Performances. ACS Appl. Mater. Interfaces 2018, 10, 33713–33720. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Song, J. Multifunctional slippery photothermal coating. J. Colloid Interf. Sci. 2024, 653, 1548–1556. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, C.; Wong, W.; Song, J. Facile, scalable and Substrate-Independent omniphobic surface. Appl. Surf. Sci. 2025, 682, 161726. [Google Scholar] [CrossRef]
- Chen, K.; Xiao, X.; Hao, C.; Sun, F.; Zhang, H.; Tan, Y.; Zhu, J.; Peng, H.; Zhan, T.; Lyu, J.; et al. Innovative Janus wood membranes: Harnessing wood anisotropy for superior liquid separation and transport. Chem. Eng. J. 2025, 506, 160185. [Google Scholar] [CrossRef]
- Li, K.; Lu, L.; Chen, H.; Jiang, G.; Ding, H.; Yu, M.; Xie, Y. Cutting performance of surgical electrodes by constructing bionic microstriped structures. Front. Mech. Eng. 2023, 18, 12. [Google Scholar] [CrossRef]
- Li, K.; Lu, L.; Xie, Y.; Yu, M.; Jiang, G.; Kou, J.; Gao, J. Enhanced cutting performance of electrosurgical units by oil-infused laser-textured surfaces. Int. J. Mech. Sci. 2023, 254, 108422. [Google Scholar] [CrossRef]
- Yan, D.; Lin, J.; Yang, X.; Lu, Y.; Song, J. High-efficiency water collection of superhydrophobic condensation absorber. Adv. Sci. 2025, 2417024. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, G.; Zhu, D.; Tian, Z.; Chen, C.; Hu, X.; Peng, R.; Li, D.; Zhang, H.; Zhao, H.; et al. Self-Driven Droplet Motions Below their Icing Points. Small 2023, 19, 2302339. [Google Scholar] [CrossRef]
- Chen, K.; Zhu, J.; Tan, Y.; Sun, F.; Gan, J.; Peng, H.; Zhan, T.; Lyu, J. Development of gradient-wetting Janus wood membrane with high-efficiency fog collection and oil-water separation. Chem. Eng. J. 2023, 470, 144356. [Google Scholar] [CrossRef]
- Wu, B.; Yan, D.; Lin, J.; Song, J. Wire Electrochemical Etching of Superhydrophobic Nickel Surfaces with Enhanced Corrosion Protection. Materials 2023, 16, 7472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Zhong, L.; Wang, J.; Jiang, Q.; Guo, X. Super-hydrophobic surface on pure magnesium substrate by wet chemical method. Appl. Surf. Sci. 2010, 256, 3837–3840. [Google Scholar] [CrossRef]
- Boisier, G.; Lamure, A.; Pébère, N.; Portail, N.; Villatte, M. Corrosion protection of AA2024 sealed anodic layers using the hydrophobic properties of carboxylic acids. Surf. Coat. Technol. 2009, 203, 3420–3426. [Google Scholar] [CrossRef]
- Salman, S.A.; Okido, M. Self-assembled monolayers formed on AZ31 Mg alloy. J. Phys. Chem. Solids 2012, 73, 863–866. [Google Scholar] [CrossRef]
- Yao, C.; Ding, Y.; Li, W.; Wang, M.; Lu, L.; Wang, X. Effect of Organic Modification Temperature on the Microstructure of Nanoscale Titania. J. Inorg. Mater. 2009, 24, 438–442. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).