316L Austenitic Stainless Steel Deformation Organization and Nitriding-Strengthened Layer Relationships
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pre-Treatment
2.3. Low-Temperature Plasma Nitriding Treatment
2.4. Microstructure Characterization
2.5. Property Test
3. Results
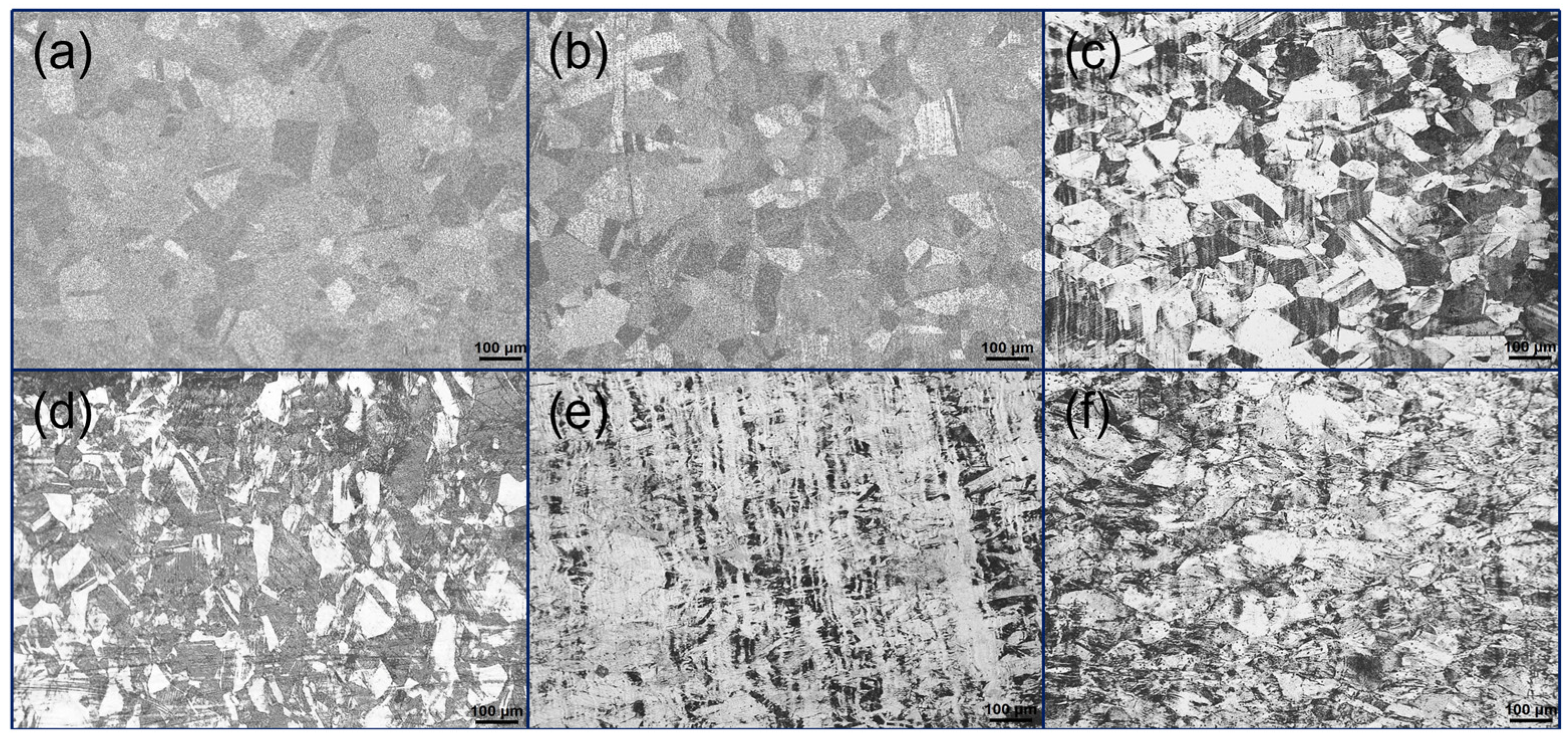
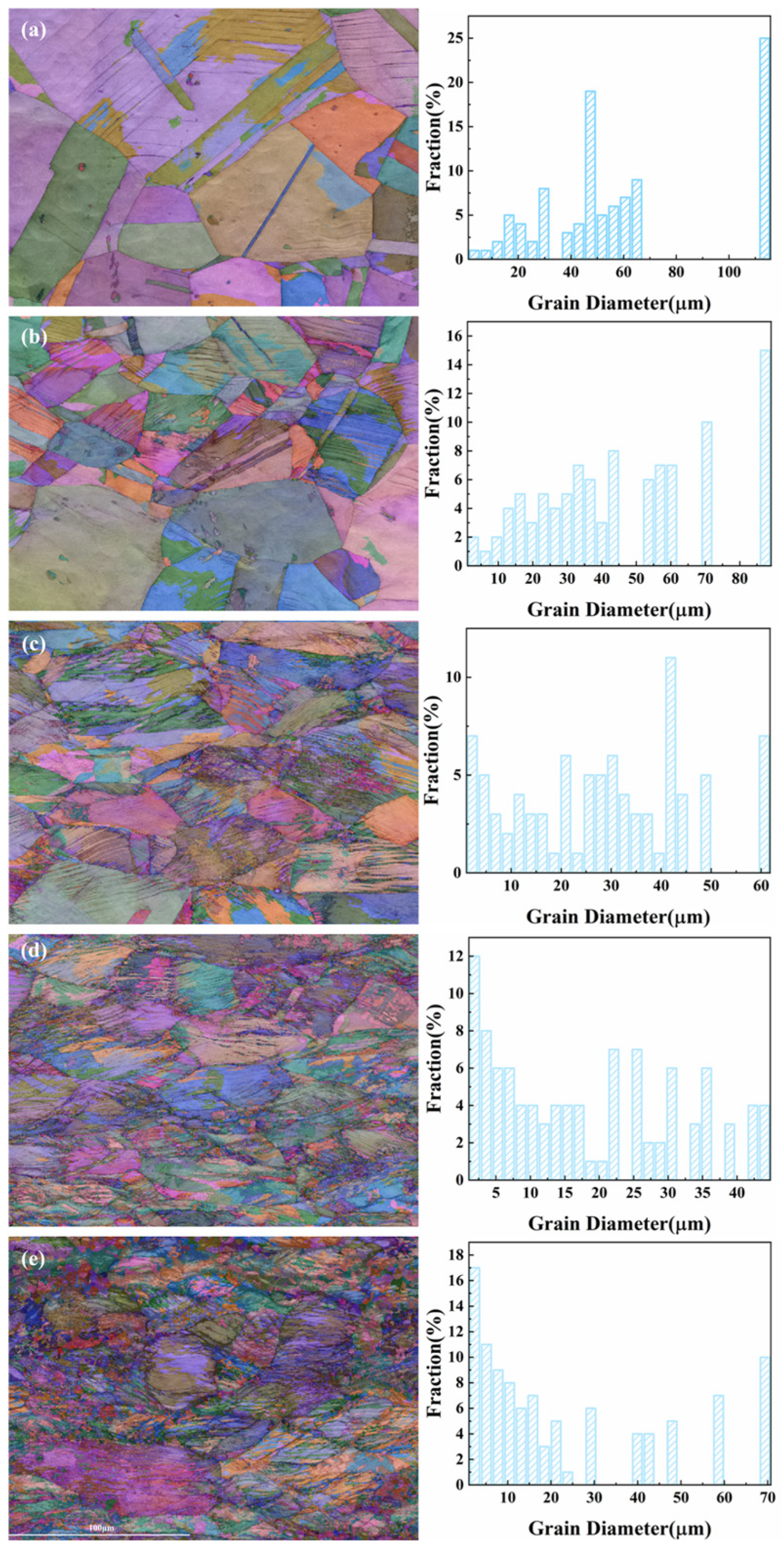
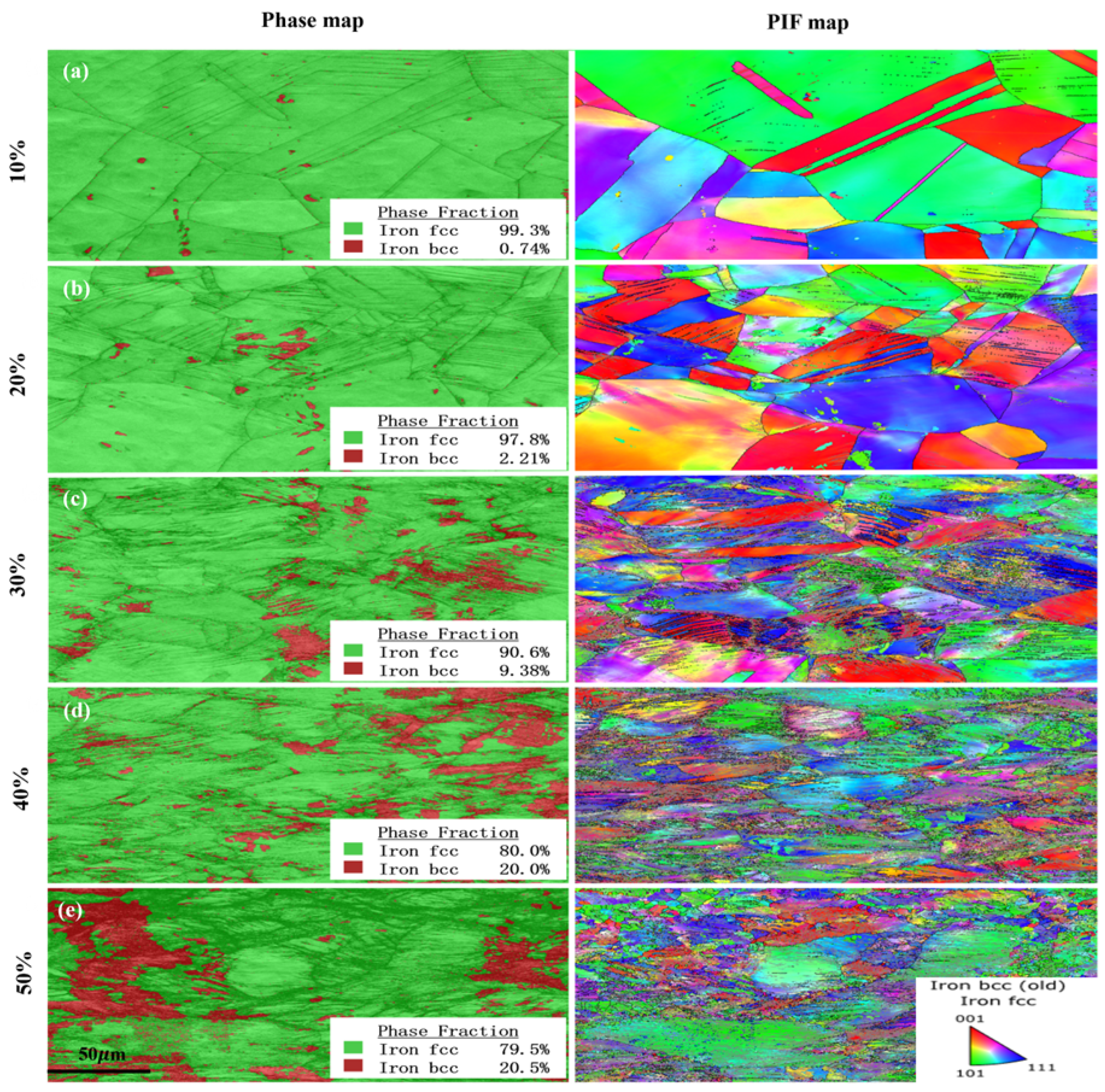
3.1. Microstructure of 316L Austenitic Stainless Steel After Different Deformation Treatment
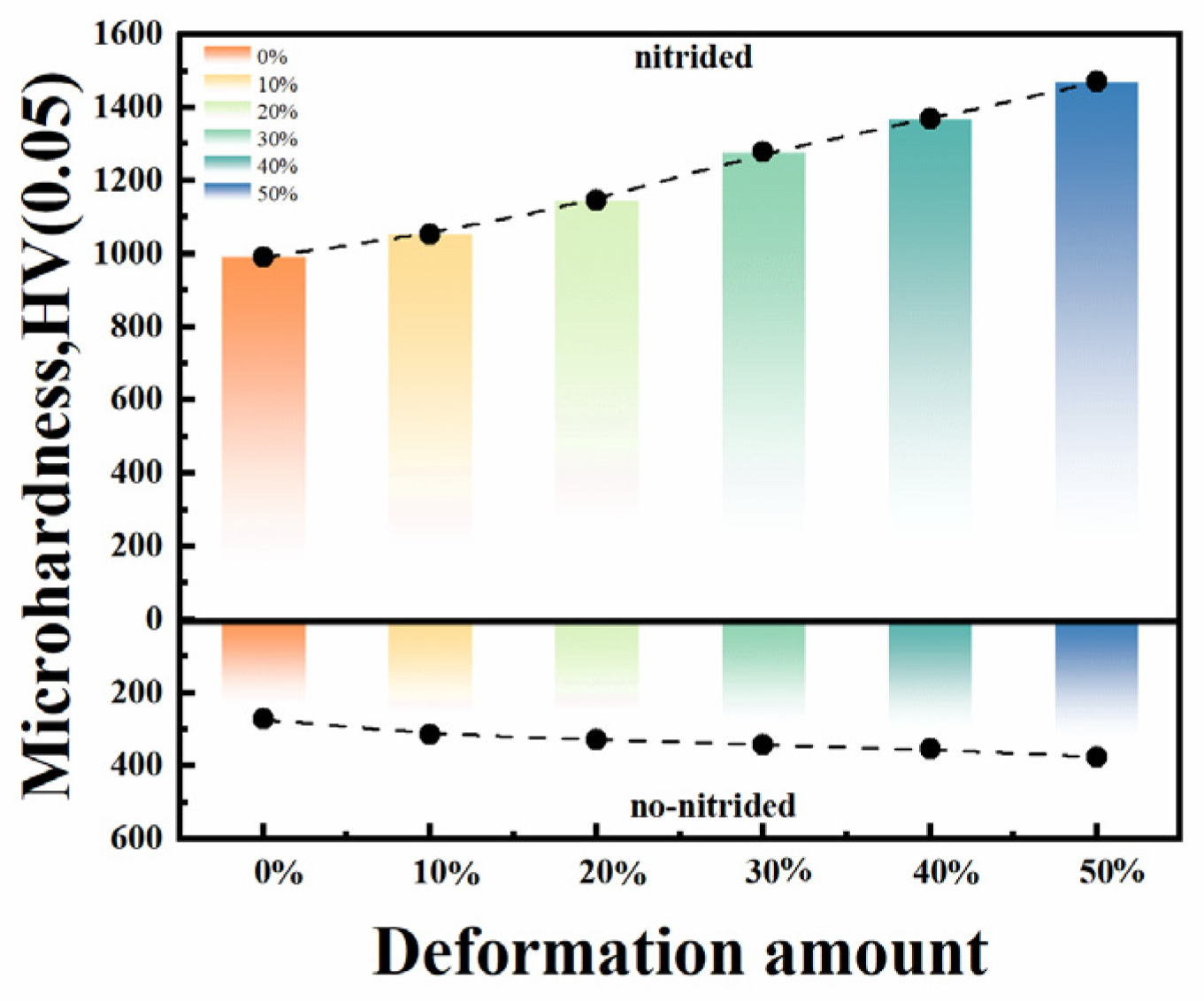
3.2. Microhardness
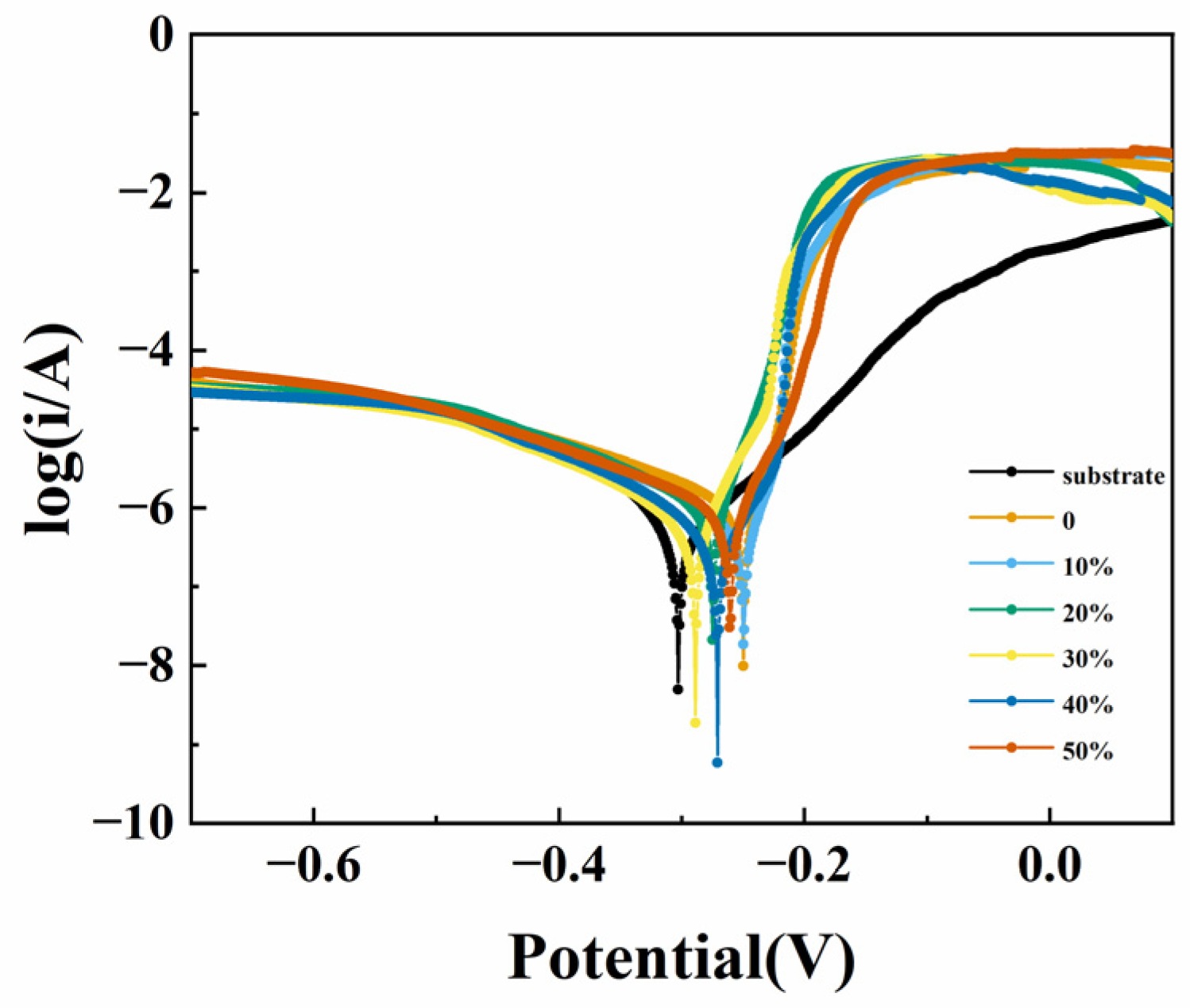
3.3. Corrosion Resistance
4. Discussion
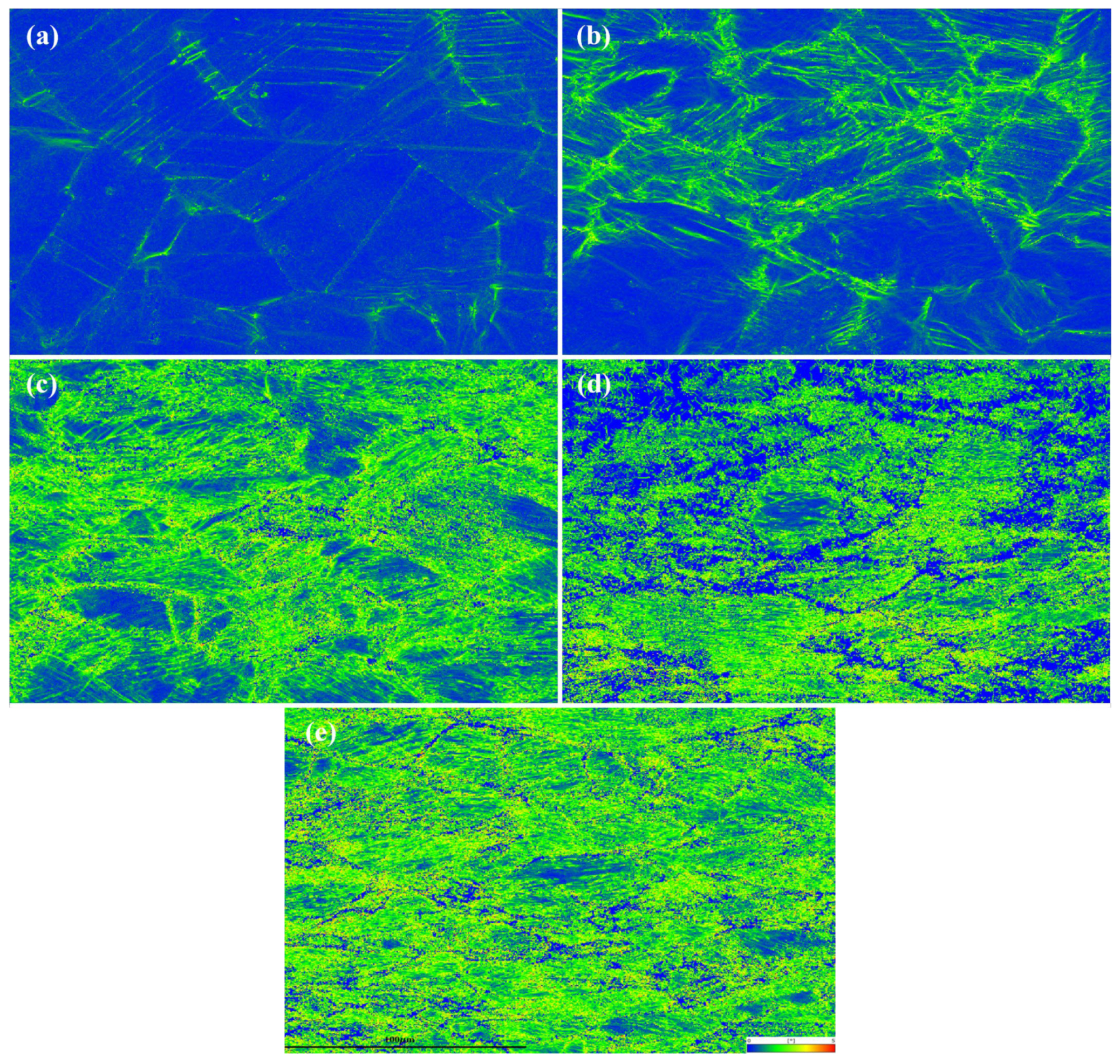
4.1. Phase Transformation Behavior During Deformation
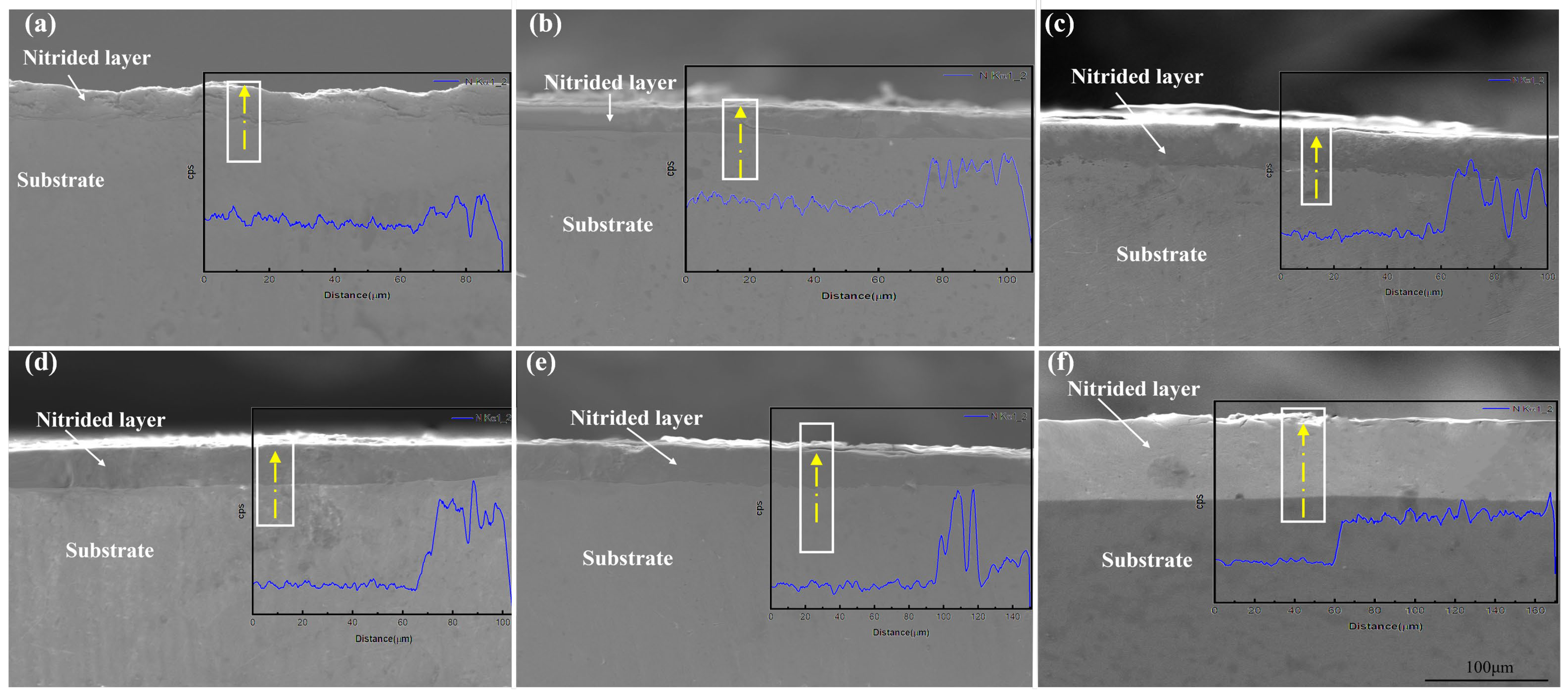
4.2. The Diffusion Behavior of Nitrogen Atoms During the Nitriding Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SEM | Scanning electron microscope |
| EBSD | Electron backscatter diffraction |
| EDS | Energy-dispersive X-ray spectroscopy |
| XRD | X-ray diffraction |
| BCC | Body-centered cubic |
| BCT | Body-centered tetragonal |
| FCC | Face-centered cubic |
| KAM | Kernel Average Misorientation |
References
- Bielawski, J.; Baranowska, J.; Szczecinski, K. Microstructure and properties of layers on chromium steel. Surf. Coat. Technol. 2006, 200, 6572–6577. [Google Scholar] [CrossRef]
- Manova, D.; Gerlach, J.; Scholze, F.; Mändl, S.; Neumann, H. Nitriding of austenitic stainless steel by pulsed low energy ion implantation. Surf. Coat. Technol. 2010, 204, 2919–2922. [Google Scholar] [CrossRef]
- Yang, W.J.; Zhang, M.; Zhao, Y.H.; Shen, M.L.; Lei, H.; Xu, L.; Xiao, J.Q.; Gong, J.; Yu, B.H.; Sun, C. Enhancement of mechanical property and corrosion resistance of 316 L stainless steels by low temperature arc plasma nitriding. Surf. Coat. Technol. 2016, 298, 64–72. [Google Scholar] [CrossRef]
- Ichimura, S.; Takashima, S.; Tsuru, I.; Ohkubo, D.; Matsuo, H.; Goto, M. Application and evaluation of nitriding treatment using active screen plasma. Surf. Coat. Technol. 2019, 374, 210–221. [Google Scholar] [CrossRef]
- Hoshiyama, Y.; Mizobata, R.; Miyake, H. Mechanical properties of austenitic stainless steel treated by active screen plasma nitriding. Surf. Coat. Technol. 2016, 307, 1041–1044. [Google Scholar] [CrossRef]
- Rocha, M.R.; Oliveira, C.A.S. Evaluation of the martensitic transformations in austenitic stainless steels. Mater. Sci. Eng. A 2009, 517, 281–285. [Google Scholar] [CrossRef]
- Cao, B.; Iwamoto, T.; Bhattacharjee, P.P. An experimental study on strain-induced martensitic transformation behavior in SUS304 austenitic stainless steel during higher strain rate deformation by continuous evaluation of relative magnetic permeability. Mater. Sci. Eng. A 2020, 774, 138927. [Google Scholar] [CrossRef]
- Zhang, R.H.; Yang, C.; Shi, N.; Guan, Y.; Ma, J.; Zhang, Y.; Chen, L. Research progress in plastic deformation characteristics of high nitrogen austenitic steel. Mater. Rep. 2021, 35, 11155. [Google Scholar]
- GB/T 7314-2017; Metallic Materials—Compression Test Method at Room Temperature. Standardization Administration of China: Beijing, China; China Standards Press: Beijing, China, 2017.
- Kaoumi, K.; Liu, J. Deformation induced martensitic transformation in 304 austenitic stainless steel: In-situ vs. ex-situ transmission electron microscopy characterization. Mater. Sci. Eng. A 2018, 715, 73–82. [Google Scholar] [CrossRef]
- Li, G.; Peng, Q.; Li, C.; Wang, Y.; Gao, J.; Chen, S.-Y.; Wang, J.; Shen, B.-L. Effect of DC plasma nitriding temperature on microstructure and dry-sliding wear properties of 316L stainless steel. Surf. Coat. Technol. 2008, 202, 2749–2754. [Google Scholar] [CrossRef]
- Fossati, A.; Borgioli, F.; Galvanetto, E.; Bacci, T. Glow-discharge nitriding of AISI 316L austenitic stainless steel: Influence of treatment time. Surf. Coat. Technol. 2006, 200, 3511–3517. [Google Scholar] [CrossRef]
- Wang, B.; Hong, C.; Winther, G.; Christiansen, T.L.; Somers, M.A. Deformation mechanisms in meta-stable and nitrogen-stabilized austenitic stainless steel during severe surface deformation. Materialia 2020, 12, 100751. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Y.; Zhou, R.; Feng, J. Mechanical properties and electronic structures of M23C6 (M= Fe, Cr, Mn)-type multicomponent carbides. J. Alloys Compd. 2015, 648, 874–880. [Google Scholar] [CrossRef]
- Luiz, L.A.; Kurelo, B.C.E.S.; de Souza, G.B.; de Andrade, J.; Marino, C.E.B. Effect of nitrogen plasma immersion ion implantation on the corrosion protection mechanisms of different stainless steels. Mater. Today Commun. 2021, 28, 102655. [Google Scholar] [CrossRef]
- Cheng, M.; He, P.; Lei, L.; Tan, X.; Wang, X.; Sun, Y.; Li, J.; Jiang, Y. Comparative studies on microstructure evolution and corrosion resistance of 304 and a newly developed high Mn and N austenitic stainless steel welded joints. Corros. Sci. 2021, 183, 109338. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Bell, T. Structure and corrosion resistance of plasma nitrided stainless steel. Surf. Eng. 1985, 1, 131–136. [Google Scholar] [CrossRef]
- Zhang, P.C. Strain induced martensite behavior of 316L stainless steel subjected to warm deformation. Heat Treat. Met. 2019, 44, 44. [Google Scholar]
- Mandal, A.; Morankar, S.; Sen, M.; Samanta, S.; Singh, S.B.; Chakrabarti, D. A Descriptive Model on the Grain Size Dependence of Deformation and Martensitic Transformation in Austenitic Stainless Steel. Met. Mater. Trans. A 2020, 51, 3886–3905. [Google Scholar] [CrossRef]
- Das, A.; Tarafder, S. Experimental investigation on martensitic transformation and fracture morphologies of austenitic stainless steel. Int. J. Plast. 2009, 25, 2222–2247. [Google Scholar] [CrossRef]
- Tao, X.; Liu, X.; Matthews, A.; Leyland, A. The influence of stacking fault energy on plasticity mechanisms in triode-plasma nitrided austenitic stainless steels: Implications for the structure and stability of nitrogen-expanded austenite. Acta Mater. 2019, 164, 60–75. [Google Scholar] [CrossRef]
- Lei, M.K.; Zhu, X.M. In vitro corrosion resistance of plasma source ion nitrided austenitic stainless steels. Biomaterials 2001, 22, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.Y.; Dong, H.; Li, B.; Bao, X.Y.; Ning, X.Z. Effect of nitrogen content on microstructure and properties of high nitrogen CrMnMo austenitic stainless steel. Heat Treat. Met. 2020, 45, 160. [Google Scholar]
- Naghizadeh, M.; Mirzadeh, H. Modeling the kinetics of deformation-induced martensitic transformation in AISI 316 metastable austenitic stainless steel. Vacuum 2018, 157, 243–248. [Google Scholar] [CrossRef]
- Kundu, A.; Field, D.P.; Chakraborti, P.C. Effect of strain and strain rate on the development of deformation heterogeneity during tensile deformation of a solution annealed 304 LN austenitic stainless steel: An EBSD study. Mater. Sci. Eng. A 2020, 773, 138854. [Google Scholar] [CrossRef]


| Si | Mn | Ni | Cr | Mo | Fe |
|---|---|---|---|---|---|
| ≤1.00 | ≤2.00 | ≤10.00~14.00 | 16.00~18.5 | 2.00~3.00 | Balance |
| Temperature (°C) | Pressure (Pa) | Time (min) | P (H2):P (N2) | Voltage (V) |
|---|---|---|---|---|
| 500 | 300–400 | 1000 | 4:3 | 500–600 |
| Sample | Ecorr/V | icorr/A.cm−2 |
|---|---|---|
| Substrate | −0.303 | 8.913 × 10−7 |
| 0 | −0.25 | 5.687 × 10−6 |
| 10% | −0.25 | 8.769 × 10−6 |
| 20% | −0.272 | 8.18 × 10−7 |
| 30% | −0.289 | 9.183 × 10−7 |
| 40% | −0.271 | 2.164 × 10−6 |
| 50% | −0.261 | 1.1007 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, L.; Ma, C.; Hai, X.; Song, K. 316L Austenitic Stainless Steel Deformation Organization and Nitriding-Strengthened Layer Relationships. Appl. Sci. 2025, 15, 2352. https://doi.org/10.3390/app15052352
Zhang X, Zhang L, Ma C, Hai X, Song K. 316L Austenitic Stainless Steel Deformation Organization and Nitriding-Strengthened Layer Relationships. Applied Sciences. 2025; 15(5):2352. https://doi.org/10.3390/app15052352
Chicago/Turabian StyleZhang, Xuedi, Lulu Zhang, Chunxiao Ma, Xiaofei Hai, and Kaihong Song. 2025. "316L Austenitic Stainless Steel Deformation Organization and Nitriding-Strengthened Layer Relationships" Applied Sciences 15, no. 5: 2352. https://doi.org/10.3390/app15052352
APA StyleZhang, X., Zhang, L., Ma, C., Hai, X., & Song, K. (2025). 316L Austenitic Stainless Steel Deformation Organization and Nitriding-Strengthened Layer Relationships. Applied Sciences, 15(5), 2352. https://doi.org/10.3390/app15052352





