Residues of St. John’s Wort (Hypericum perforatum) Tea Infusions/Water Extracts as a Valuable Source of Tocotrienols: An Extraction Study
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Material
2.3. Saponification and n-Hexane–Ethyl Acetate Extraction Protocol
2.4. Preparation of H. perforatum Tea Infusions
2.5. Ultrasound-Assisted Extraction (UAE) of Tocochromanols from Residues of H. perforatum Tea Infusions
2.6. Tocochromanol Determination by RP-HPLC-FLD
2.7. LC-MS Confirmation of Presence of Tocochromanols in St. John’s Wort Inflorescences
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| dw | dry weight |
| MS | mass spectrometry |
| nd | not detected |
| T | tocopherol |
| T3 | tocotrienol |
| RP-HPLC-FLD | reverse-phase high-performance liquid chromatography with fluorescent light detector |
| UAE | ultrasound-assisted extraction |
References
- Galeotti, N. Hypericum perforatum (St John’s wort) beyond depression: A therapeutic perspective for pain conditions. J. Ethnopharmacol. 2017, 200, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Carrubba, A.; Lazzara, S.; Giovino, A.; Ruberto, G.; Napoli, E. Content variability of bioactive secondary metabolites in Hypericum perforatum L. Phytochem. Lett. 2021, 46, 71–78. [Google Scholar] [CrossRef]
- Napoli, E.; Siracusa, L.; Ruberto, G.; Carrubba, A.; Lazzara, S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Phytochemical profiles, phototoxic and antioxidant properties of eleven Hypericum species—A comparative study. Phytochemistry 2018, 152, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Kladar, N.; Anačkov, G.; Srđenović, B.; Gavarić, N.; Hitl, M.; Salaj, N.; Jeremić, K.; Babović, S.; Božin, B. St. John’s wort herbal teas–Biological potential and chemometric approach to quality control. Plant Foods Hum. Nutr. 2020, 75, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Mišina, I.; Lazdiņa, D. Tocopherol and tocotrienol homologue recovery from Hypericum perforatum L. and extraction residues after hydroethanolic extraction. Ind. Crops Prod. 2025, 224, 120321. [Google Scholar] [CrossRef]
- Inoue, T.; Tatemori, S.; Muranaka, N.; Hirahara, Y.; Homma, S.; Nakane, T.; Takano, A.; Nomi, Y.; Otsuka, Y. The Identification of Vitamin E Homologues in Medicinal Plant Samples Using ESI (+)-LC-MS3. J. Agric. Food Chem. 2012, 60, 9581–9588. [Google Scholar] [CrossRef] [PubMed]
- Hosni, K.; Msaâda, K.; Taârit, M.B.; Marzouk, B. Fatty acid composition and tocopherol content in four Tunisian Hypericum species: Hypericum perforatum, Hypericum tomentosum, Hypericum perfoliatum and Hypericum ericoides ssp. Roberti. Arab. J. Chem. 2017, 10, S2736–S2741. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Morales, M.; Garcia, Q.S.; Siqueira-Silva, A.I.; Silva, M.C.; Munné-Bosch, S. Tocotrienols in Vellozia gigantea leaves: Occurrence and modulation by seasonal and plant size effects. Planta 2014, 240, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Yabuta, Y.; Tamoi, M.; Tanabe, N.; Shigeoka, S. Generation of transgenic tobacco plants with enhanced tocotrienol levels through the ectopic expression of rice homogentisate geranylgeranyl transferase. Plant Biotechnol. 2015, 32, 233–238. [Google Scholar] [CrossRef]
- Tan, X.; Zhong, F.; Teng, H.; Li, Q.; Li, Y.; Mei, Z.; Chen, Y.; Yang, G. Acylphloroglucinol and tocotrienol derivatives from the fruits of Garcinia paucinervis. Fitoterapia 2020, 146, 104688. [Google Scholar] [CrossRef] [PubMed]
- Lavaud, A.; Richomme, P.; Gatto, J.; Aumond, M.-C.; Poullain, C.; Litaudon, M.; Andriantsitohaina, R.; Guilet, D. A tocotrienol series with an oxidative terminal prenyl unit from Garcinia amplexicaulis. Phytochemistry 2015, 109, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Lavaud, A.; Richomme, P.; Litaudon, M.; Andriantsitohaina, R.; Guilet, D. Antiangiogenic tocotrienol derivatives from Garcinia amplexicaulis. J. Nat. Prod. 2013, 76, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, R.; Kruk, J. Novel and rare prenyllipids—Occurrence and biological activity. Plant Physiol. Biochem. 2018, 122, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A. Tocopherols, tocotrienols and tocomonoenols: Many similar molecules but only one vitamin E. Redox Biol. 2019, 26, 101259. [Google Scholar] [CrossRef]
- Peh, H.Y.; Tan, W.S.D.; Liao, W.; Wong, W.S.F. Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Sailo, B.L.; Banik, K.; Padmavathi, G.; Javadi, M.; Bordoloi, D.; Kunnumakkara, A.B. Tocotrienols: The promising analogues of vitamin E for cancer therapeutics. Pharmacol. Res. 2018, 130, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Luo, P.; Zeng, Z.; Wang, H.; Malafa, M.; Suh, N. Vitamin E and cancer prevention: Studies with different forms of tocopherols and tocotrienols. Mol. Carcinog. 2020, 59, 365–389. [Google Scholar] [CrossRef]
- Aggarwal, V.; Kashyap, D.; Sak, K.; Tuli, H.S.; Jain, A.; Chaudhary, A.; Garg, V.K.; Sethi, G.; Yerer, M.B. Molecular mechanisms of action of tocotrienols in cancer: Recent trends and advancements. Int. J. Mol. Sci. 2019, 20, 656. [Google Scholar] [CrossRef]
- Alahmad, A.; Alghoraibi, I.; Zein, R.; Kraft, S.; Dräger, G.; Walter, J.-G.; Scheper, T. Identification of major constituents of Hypericum perforatum L. extracts in Syria by development of a rapid, simple, and reproducible HPLC-ESI-Q-TOF MS analysis and their antioxidant activities. ACS Omega 2022, 7, 13475–13493. [Google Scholar] [CrossRef]
- Jarzębski, M.; Smułek, W.; Baranowska, H.M.; Masewicz, Ł.; Kobus-Cisowska, J.; Ligaj, M.; Kaczorek, E. Characterization of St. John’s wort (Hypericum perforatum L.) and the impact of filtration process on bioactive extracts incorporated into carbohydrate-based hydrogels. Food Hydrocolloids 2020, 104, 105748. [Google Scholar] [CrossRef]
- Hernandez, M.F.; Falé, P.L.V.; Araújo, M.E.M.; Serralheiro, M.L.M. Acetylcholinesterase inhibition and antioxidant activity of the water extracts of several Hypericum species. Food Chem. 2010, 120, 1076–1082. [Google Scholar] [CrossRef]
- Górnaś, P.; Segliņa, D.; Lācis, G.; Pugajeva, I. Dessert and crab apple seeds as a promising and rich source of all four homologues of tocopherol (α, β, γ and δ). LWT-Food Sci. Technol. 2014, 59, 211–214. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Waśkiewicz, A.; Perkons, I.; Pugajeva, I.; Segliņa, D. Simultaneous extraction of tocochromanols and flavan-3-ols from the grape seeds: Analytical and industrial aspects. Food Chem. 2025, 462, 140913. [Google Scholar] [CrossRef]
- Górnaś, P.; Baškirovs, G.; Siger, A. Free and esterified tocopherols, tocotrienols and other extractable and non-extractable tocochromanol-related molecules: Compendium of knowledge, future perspectives and recommendations for chromatographic techniques, tools, and approaches used for tocochromanol determination. Molecules 2022, 27, 6560. [Google Scholar] [CrossRef] [PubMed]
- Mišina, I.; Lazdiņa, D.; Górnaś, P. Tocochromanols in the leaves of plants in the Hypericum and Clusia genera. Molecules 2025, 30, 709. [Google Scholar] [CrossRef]
- Lazdiņa, D.; Mišina, I.; Górnaś, P. Tocotrienols in eleven species of Hypericum genus leaves. Molecules 2025, 30, 662. [Google Scholar] [CrossRef] [PubMed]
- Ardra, S.; Barua, M.K. Halving food waste generation by 2030: The challenges and strategies of monitoring UN sustainable development goal target 12.3. J. Clean. Prod. 2022, 380, 135042. [Google Scholar] [CrossRef]
- Sinha, A.K.; Sharma, M.D.; Mishra, P.; Kulshrestha, S. Food waste and sustainable development goals. In Food Waste Valorization; Elsevier: Amsterdam, The Netherlands, 2024; pp. 19–31. [Google Scholar]
- Chaves, J.O.; de Souza Mesquita, L.M.; Strieder, M.M.; Contieri, L.S.; Pizani, R.S.; Sanches, V.L.; Viganó, J.; Bezerra, R.M.N.; Rostagno, M.A. Eco-friendly and high-performance extraction of flavonoids from lemon peel wastes by applying ultrasound-assisted extraction and eutectic solvents. Sustain. Chem. Pharm. 2024, 39, 101558. [Google Scholar] [CrossRef]
- Usman, M.; Nakagawa, M.; Cheng, S. Emerging trends in green extraction techniques for bioactive natural products. Processes 2023, 11, 3444. [Google Scholar] [CrossRef]
- Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Naumovski, N. Tocotrienols, health and ageing: A systematic review. Maturitas 2017, 95, 55–60. [Google Scholar] [CrossRef]
- Younes, M.; Loubnane, G.; Sleiman, C.; Rizk, S. Tocotrienol isoforms: The molecular mechanisms underlying their effects in cancer therapy and their implementation in clinical trials. J. Integr. Med. 2024, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lebiocka, M.; Montusiewicz, A.; Pasieczna–Patkowska, S.; Szaja, A. Pretreatment of herbal waste using sonication. Bioresour. Technol. 2023, 377, 128932. [Google Scholar] [CrossRef]
- Cho, S.-H.; Lee, T.; Cha, H.; Chen, W.-H.; Tsang, Y.F.; Kwon, E.E. Production of combustible gas via incorporating CO2 to pyrolysis of medicinal herbal waste. Ind. Crops Prod. 2024, 219, 119110. [Google Scholar] [CrossRef]
- Singh, D.; Suthar, S. Vermicomposting of herbal pharmaceutical industry solid wastes. Ecol. Eng. 2012, 39, 1–6. [Google Scholar] [CrossRef]
- Dewan, A.; Dawra, S.; Kaushik, N.; Singh, A.; Thakur, S.; Kaur, S.; Xavier, J.R. Process characterization for tisane development using pomegranate waste: An herbal drink optimization strategy. Sustain. Food Technol. 2024, 2, 806–815. [Google Scholar] [CrossRef]
- Gaid, M.; Biedermann, E.; Füller, J.; Haas, P.; Behrends, S.; Krull, R.; Scholl, S.; Wittstock, U.; Müller-Goymann, C.; Beerhues, L. Biotechnological production of hyperforin for pharmaceutical formulation. Eur. J. Pharm. Biopharm. 2018, 126, 10–26. [Google Scholar] [CrossRef] [PubMed]
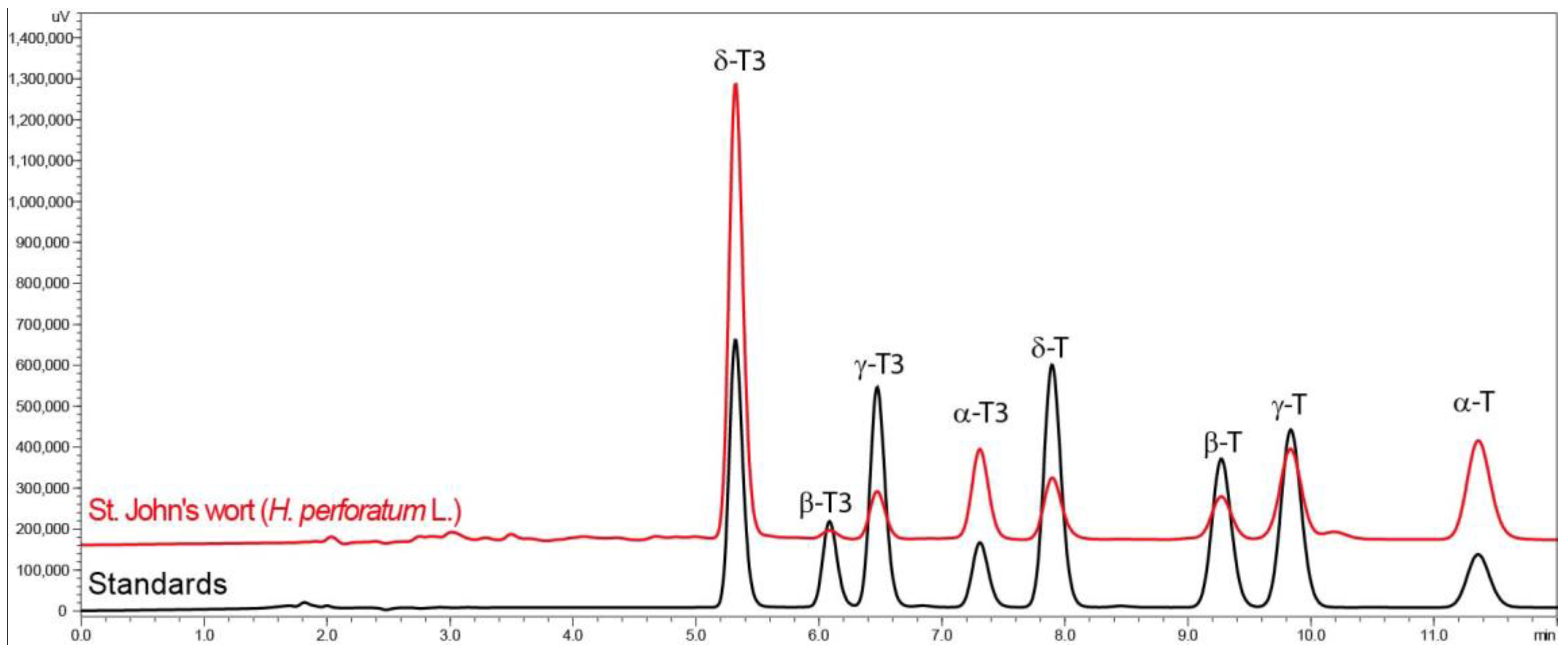
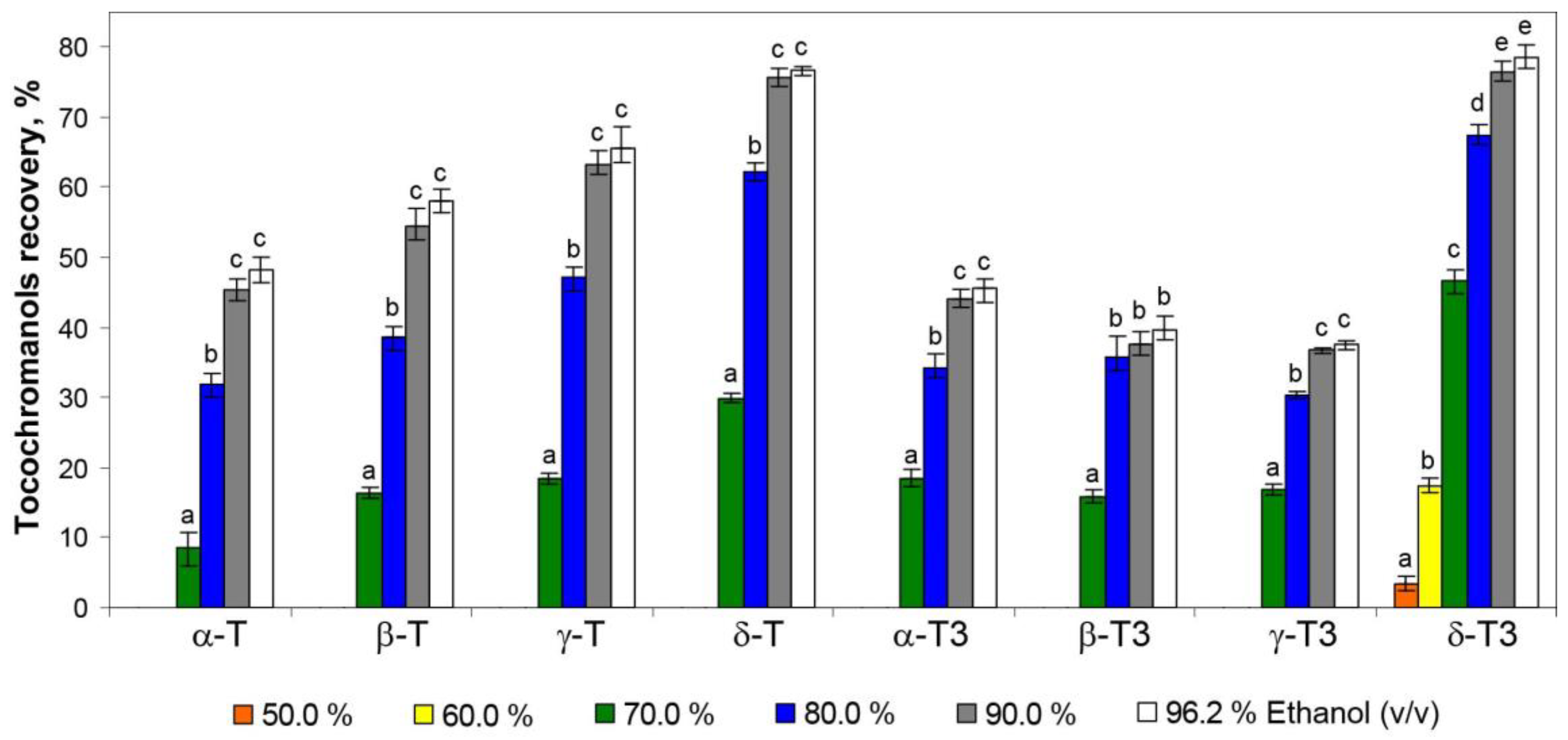
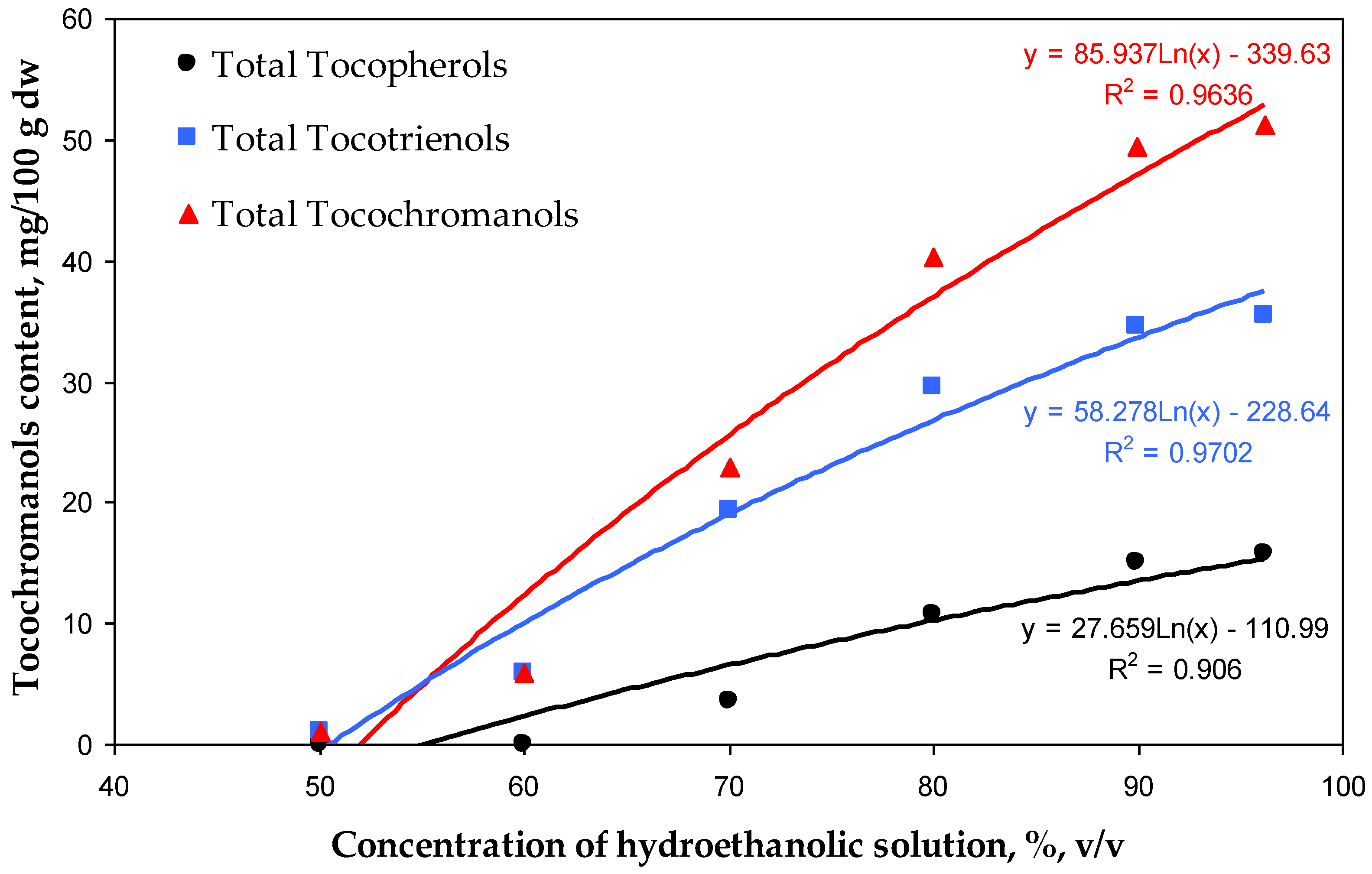
| Tocols | H. perforatum Powder | H. perforatum Tea Residues Extracted by Different Hydroethanolic (v/v) Solutions | |||||
|---|---|---|---|---|---|---|---|
| 50.0% | 60.0% | 70.0% | 80.0% | 90.0% | 96.2% | ||
| α-T | 22.29 ± 0.75 d | nd | nd | 2.02 ± 0.58 a | 7.08 ± 0.83 b | 10.12 ± 0.48 c | 10.75 ± 0.55 c |
| β-T | 0.94 ± 0.09 d | nd | nd | 0.15 ± 0.03 a | 0.36 ± 0.07 b | 0.53 ± 0.05 c | 0.54 ± 0.04 c |
| γ-T | 5.86 ± 0.36 d | nd | nd | 1.08 ± 0.23 a | 2.77 ± 0.30 b | 3.67 ± 0.16 c | 3.79 ± 0.19 c |
| δ-T | 0.86 ± 0.05 d | nd | nd | 0.26 ± 0.04 a | 0.53 ± 0.05 b | 0.65 ± 0.05 c | 0.67 ± 0.04 c |
| Total Ts | 29.94 ± 1.25 d | nd | nd | 3.50 ± 0.87 a | 10.75 ± 1.25 b | 14.98 ± 0.74 c | 15.75 ± 0.82 c |
| α-T3 | 17.58 ± 0.43 d | nd | nd | 3.23 ± 0.21 a | 6.03 ± 0.25 b | 7.74 ± 0.45 c | 8.01 ± 0.23 c |
| β-T3 | 0.41 ± 0.04 c | nd | nd | 0.06 ± 0.01 a | 0.15 ± 0.01 b | 0.15 ± 0.01 c | 0.16 ± 0.01 c |
| γ-T3 | 1.65 ± 0.07 d | nd | nd | 0.28 ± 0.04 a | 0.50 ± 0.04 b | 0.60 ± 0.07 c | 0.62 ± 0.06 c |
| δ-T3 | 33.98 ± 1.02 f | 1.14 ± 0.30 a | 5.94 ± 0.55 b | 15.82 ± 1.21 c | 22.92 ± 1.00 d | 26.01 ± 0.54 e | 26.72 ± 0.64 e |
| Total T3s | 53.62 ± 1.56 f | 1.14 ± 0.31 a | 5.94 ± 0.55 b | 19.39 ± 1.47 c | 29.59 ± 1.30 d | 34.51 ± 1.06 e | 35.51 ± 0.95 e |
| Total Ts + T3s | 83.56 ± 2.81 f | 1.14 ± 0.31 a | 5.94 ± 0.55 b | 22.88 ± 2.35 c | 40.33 ± 2.55 d | 49.49 ± 1.81 e | 51.26 ± 1.77 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mišina, I.; Perkons, I.; Siger, A.; Soliven, A.; Górnaś, P. Residues of St. John’s Wort (Hypericum perforatum) Tea Infusions/Water Extracts as a Valuable Source of Tocotrienols: An Extraction Study. Appl. Sci. 2025, 15, 2047. https://doi.org/10.3390/app15042047
Mišina I, Perkons I, Siger A, Soliven A, Górnaś P. Residues of St. John’s Wort (Hypericum perforatum) Tea Infusions/Water Extracts as a Valuable Source of Tocotrienols: An Extraction Study. Applied Sciences. 2025; 15(4):2047. https://doi.org/10.3390/app15042047
Chicago/Turabian StyleMišina, Inga, Ingus Perkons, Aleksander Siger, Arianne Soliven, and Paweł Górnaś. 2025. "Residues of St. John’s Wort (Hypericum perforatum) Tea Infusions/Water Extracts as a Valuable Source of Tocotrienols: An Extraction Study" Applied Sciences 15, no. 4: 2047. https://doi.org/10.3390/app15042047
APA StyleMišina, I., Perkons, I., Siger, A., Soliven, A., & Górnaś, P. (2025). Residues of St. John’s Wort (Hypericum perforatum) Tea Infusions/Water Extracts as a Valuable Source of Tocotrienols: An Extraction Study. Applied Sciences, 15(4), 2047. https://doi.org/10.3390/app15042047







