Featured Application
Extraction/isolation of tocotrienols from St. John’s wort water extract residues.
Abstract
Hypericum perforatum L., commonly known as St. John’s wort, is a widely distributed herbaceous plant utilized in traditional and phytomedicinal applications, particularly for its hydrophilic bioactive compounds. It is often used for treating early depressive states. In this study, we focused on reporting the tocotrienols—lipophilic phytochemicals with health-promoting properties—in St. John’s wort. H. perforatum flowerheads predominantly contained tocotrienols compared with tocopherols (54 and 30 mg/100 g dry weight, respectively). The major tocotrienols (T3) were δ-T3 and α-T3 (34.0 and 17.6 mg/100 g dry weight, respectively). Tocopherols and tocotrienols are lipophilic phytochemicals that cannot be present in St. John’s wort water extracts (tea infusions), but they can be recovered from the remaining residues of H. perforatum tea infusions by using hydroethanolic solutions. A 50.0% (v/v) hydroethanolic solution was not effective in the recovery of tocochromanols. The greatest increase in the extractability of tocochromanols was observed for 70.0–80.0% (v/v) hydroethanolic extracts, while increasing the ethanol concentration from 90.0% to 96.2% (v/v) only slightly improved extractability (not statistically significant). For each ethanol concentration, the recovery was proportionally higher for tocotrienols than for tocopherols. Residues of H. perforatum tea infusions can be proposed as valuable by-products rich in tocotrienols.
1. Introduction
Hypericum perforatum L., from Greek spiritual traditions known as St. John’s wort, is a common perennial herbaceous plant that thrives in temperate regions around the globe. This species is characterized by its distinctive yellow flowers and perforated leaves, which contain numerous translucent dots. The inflorescences of H. perforatum are rich in bioactive phytochemicals, including phloroglucinol derivatives, naphthodianthrones, and xanthones, all of which are unique to the Hypericum genus. These compounds contribute to the plant’s significant role in traditional and phytomedicinal practices, where it has been employed for its therapeutic properties. Extensive research has substantiated the efficacy of H. perforatum tea and extracts in alleviating symptoms of early depressive states, highlighting their importance in herbal medicine [1,2,3,4]. While most studies focus on the biological potential of hydrophilic molecules in St. John’s wort [1,2,3,4], there is limited knowledge about the presence of lipophilic bioactive compounds, e.g., tocopherols (T) and tocotrienols (T3), which are also not consistent with each other [5,6,7]. For the first time, the identification of tocopherol and tocotrienol homologues in different medicinal plants, including H. perforatum, was performed by using ESI(+)-LC-MS/MS over a decade ago (2012). The presence of α-T and δ-T3 was reported, without quantitative data. Unfortunately, the part of St. John’s wort that was investigated was not provided [6]. Five years later, only tocopherols (α-T, γ-T, and δ-T) were detected in the top two-thirds of the H. perforatum plant. The reported predominance of δ-T and low content of total tocopherols (<1 mg/100 g dw) were unusual. It is important to note that this report did not consider the utilization of tocotrienol standards [7]. Only in 2025, the presence and quantification of all four tocopherol and tocotrienol homologues (δ, γ, β, and α) were confirmed in the aerial parts of the plant—the top 5−10 cm (inflorescences with slight quantities of leaves and stems) of H. perforatum [5]. The inflorescences of St. John’s wort prevalently contained tocotrienols compared with tocopherols, with significant predominance of δ-T3 [5]. This finding is significant, as there are no known plants in the temperate climate zone that exhibit predominance of this tocochromanol. This makes St. John’s wort (a dicot species) the first plant in the temperate climate zone presenting predominantly δ-T3 compared with the main tocopherols—α-T and γ-T, found in leaves and seeds, respectively [8]. Tocochromanols occur in all photosynthetic organisms. In photosynthetic tissues, α-T is the dominant tocochromanol [8]. In the past (pre-2014), tocotrienols were not detected in considerable amounts in photosynthetic tissues. In 2014, tocotrienols were found in Vellozia gigantea N.L.Menezes and Mello-Silva leaves (monocot species), but their concentration was 2.5-fold lower than that of tocopherols. The concentrations of tocochromanols in Vellozia gigantea were influenced by both seasonal variations and the size of the plant [9]. As of 2025, there remains a scarcity of reports addressing the presence of tocotrienols in photosynthetic tissues, with the exception of genetically modified organisms (transgenic plants). To date (2025), there is a lack of reports focusing on the substantial tocotrienol content in photosynthetic tissues, except for genetically modified plants (transgenic) [10] and anti-angiogenic tocotrienol derivatives found in the Garcinia genus [11,12,13]. St. John’s wort, due to its prevalence at almost all latitudes, is an excellent candidate for obtaining these valuable phytochemicals—tocotrienols. Tocopherols, particularly the α and γ homologues, are widely prevalent in nature. In contrast, tocotrienols and other related tocochromanol compounds are not well characterized and identified within natural ecosystems. Consequently, there are fewer studies documenting their identification, determination, biosynthesis, taxonomic distribution, biological functions in plants and humans, and metabolism, despite the potential health benefits associated with these molecules [14]. Current knowledge is mainly focused only on α-T, the most well-studied tocochromanol for the prevention of human deficiency disease, specifically “Ataxia with Vitamin E Deficiency”. Therefore, the term “vitamin E” must not be used for expressing or used synonymously for any other tocochromanol other than α-T [15]. In 2016, it was estimated that fewer than 3% of investigations and clinical studies addressing the effects of tocochromanol supplementation on health focused on tocotrienols, whereas over 97% were centered on tocopherols [16].
Despite numerous reports suggesting that tocotrienols exhibit superior biological functions compared with tocopherols—such as enhanced anti-oxidant and anti-inflammatory properties and protective effects against cancer, cardiovascular diseases, diabetes, and neuro-degenerative disorders—these compounds remain less studied [16,17,18]. Tocotrienols are safe, health-beneficial molecules (non-toxic to normal cells), possess anti-metastatic and anti-angiogenic properties, and specifically target and eliminate cancer stem cells. Therefore, their inclusion in mono-targeted and/or combinatorial therapy with other chemotherapeutic substances is encouraged [19].
For α-T, there is the absence of evidence supporting its cancer-preventive activity. Regarding δ-T, γ-T, and tocotrienols, their impact on cancer risk remains ambiguous and necessitates further investigation [20]. Well-designed clinical trials combining tocotrienols, nanotechnology (micelle complexes, liposomes, and nano emulsions), and quantitative structure–activity relationship models could lead to the emergence of nature-based therapeutic agents (tocotrienols as selective, cost-effective drugs) for the management of various malignancies or, at a minimum, their prevention [21]. Consequently, it is crucial to identify natural sources of tocotrienols that entail low costs for isolation and purification while also enhancing their delivery efficiency and bioavailability, in order to achieve the next milestone in cancer therapy via tocotrienols.
Within the current market, several tea products under the name St. John’s wort can be found. A recent investigation into the chemical profiling of St. John’s wort products revealed significant variations in the quantified secondary metabolites in St. John’s wort tea products [4]. To obtain extracts rich in hydrophilic phytochemicals from St. John’s wort, at lab and industrial scales, pure water was typically applied [22,23,24]. Simultaneously, the home-made, lab, and industrial production of water extracts of hydrophilic bioactive compounds from St. John’s wort does not consider the re-use of the generated plant residues (by-products) for the future re-extraction of other valuable phytochemicals with different properties, e.g., lipophilic tocochromanols. Therefore, obtaining tocotrienols from sustainable/natural sources is important. Hence, the objective of this study is to demonstrate that the medical potential of St. John’s wort is higher than previously believed, attributable to the presence of rare prenyllipids such as tocotrienols, and enhance the value of the processing chain to achieve the extraction of lipophilic bioactive phytochemicals after the extraction of hydrophilic compounds by water (tea infusions).
2. Materials and Methods
2.1. Reagents
The chemicals used in this study were acquired from several suppliers. Ethanol, ethyl acetate, methanol, and HPLC-grade n-hexane, along with pyrogallol, reagent-grade potassium hydroxide, and sodium chloride, were purchased from Sigma-Aldrich (Steinheim, Germany). Lower-cost 96.2% ethanol, used for the saponification of H. perforatum plant powder, was supplied by Ltd. Kalsnavas Elevators (Jaunkalsnava, Latvia); this ethanol was also used to prepare hydroethanolic solutions to extract tocochromanols from residues of St. John’s wort tea infusions/water extracts. Tocopherol and tocotrienol standards (α, β, γ, and δ) of >98% HPLC purity were obtained from Extrasynthese (Genay, France) and Cayman Chemical (Ann Arbor, MI, USA), respectively.
2.2. Plant Material
Twenty-five plants of wild H. perforatum were collected from the Institute of Horticulture garden premises in Dobele, Latvia (GPS location: N: 56°36′39″ E: 23°17′50″), in July 2023. The Hypericum plants were grown on clay soil with the following parameters: pH—7.6; organic matter—2.1%; P2O5—95.0; K2O—180.0; Mg—1515.0; Ca—3592.0; B—0.8; Cu—3.9; Mn—107.0; Zn—4.0; S-SO4—2.8; Fe—490; Na—2.5; N-NH4—1.0; N-NO3—27.3 (mg/kg), according to the soil analysis performed by the State Plant Protection Service of the Republic of Latvia (Riga, Latvia). Wild St. John’s wort was identified according to a taxonomic guide provided by the Royal Botanic Gardens, Kew (https://powo.science.kew.org (accessed on 15 June 2022)). Furthermore, the identification of harvested plants of wild H. perforatum was supported by the plant comparison to cultivated specimens. Cultivated St. John’s wort was grown at the Institute of Horticulture, Dobele, Latvia, from the seeds obtained from the Botanical Garden of Medicinal Plants, Department of Biology and Pharmaceutical Sciences, University of Medicine, Wrocław, Poland. The harvested wild H. perforatum aerial parts are presented in Figure S1A,B (Supplementary Materials). The top 5–10 cm of the plant was cut to collect inflorescences, immediately frozen at −80 ± 2 °C for 2 h, and freeze-dried by using a FreeZone freeze–dry system (Labconco, Kansas City, MO, USA) at a temperature of −51 ± 1 °C under vacuum of below 0.01 mbar for 48 h. Freeze-dried aerial parts (50 ± 5 g) were powdered by using an MM 400 mixer mill (Retsch, Haan, Germany). The St. John’s wort sample was placed in screw-top 50 mL grinding jars from stainless steel and milled with the following parameters: a frequency of 30 Hz and a time of 30 s. The obtained 5 μm final fineness (according to the manufacturer) St. John’s wort powder was well mixed and used directly for tocopherol and tocotrienol homologue extraction as described below in Section 2.3 and Section 2.4, and the dry mass was measured gravimetrically. The remnants of the powdered samples were transferred into polypropylene bags and stored at −18 °C.
2.3. Saponification and n-Hexane–Ethyl Acetate Extraction Protocol
Saponification was conducted by using a previously established protocol, as it provides the best extraction efficiency for tocochromanols [25].
2.4. Preparation of H. perforatum Tea Infusions
H. perforatum powder was weighed (0.25 g) into a 15 mL centrifuge tube and supplemented with 5 mL of boiled water (95 ± 5 °C), mixed with a vortex mixer for 30 s, and subsequently left for 10 min to cool down. Then, the samples were centrifuged (11,000× g, 10 min, 21 °C). The obtained water extract was immediately removed, and the residues were used for tocochromanol recovery by hydroethanolic solutions via ultrasound-assisted extraction (UAE) as described below in Section 2.5.
2.5. Ultrasound-Assisted Extraction (UAE) of Tocochromanols from Residues of H. perforatum Tea Infusions
The extractability of tocochromanols from residues of H. perforatum tea infusions was tested under different concentrations of hydroethanolic solutions. Briefly, the obtained residues of H. perforatum tea infusions were supplemented with 5 mL of one specific hydroethanolic solution concentration. Six different hydroethanolic solution concentrations were systematically studied (50.0%, 60.0%, 70.0%, 80.0%, 90.0%, and 96.2%, v/v), mixed for 1 min on the vortex REAX top (Heidolph, Schwabach Germany) with vibration frequency rates of up to 2500 rpm, and subsequently subjected for 15 min to ultrasonic bath (USB) treatment (Sonorex RK 510 H, Bandelin electronic, Berlin, Germany) at the frequency of 35 kHz, nominal ultrasonic power of 160 W, and temperature thermostated at 60 °C. After 15 min of USB, the samples were immediately mixed again for 1 min by the vortex REAX top at the maximum vibration frequency rate and then centrifuged at 11,000× g for 10 min at 21 °C. The obtained extract was transferred to a 2 mL tube and then centrifuged at 21,300× g for 5 min at 4 °C to remove potential plant material particles and high-molecular-weight compounds, e.g., proteins. The obtained supernatant was transferred to a 2 mL analytical glass vial and subsequently analyzed by a RP-HPLC-FLD system.
2.6. Tocochromanol Determination by RP-HPLC-FLD
A reversed-phase high-performance liquid chromatography (RP-HPLC) system, coupled with fluorescence detection (FLD), was implemented for the separation and analysis of tocochromanols. The chromatography instrument is consistent with the module configuration in the previously published paper [5]. The chromatographic analyses were carried out as follows: A 150 mm × 4.6 mm Epic PFP-LB column (PerkinElmer, Waltham, MA, USA), with 3 µm fully porous particles, was used to separate tocochromanols. A Phenomenex guard column (4 mm length, 3 mm ID) from Torrance, CA, USA, was used. The isocratic mobile phase was methanol–water (91:9, volume/volume), delivered at 1.0 mL/min. The column oven was set to 45 ± 1 °C. The time of the analysis was 13 min. Fluorescence detection (excitation: 295 nm; emission: 330 nm) was used to quantify tocochromanols based on standard calibration curves.
The linearity of the RPLC method was evaluated by injecting a range of volumes (0.2–10 μL) of a standard solution containing four tocopherols and four tocotrienols. Calibration curves were generated by using tocopherol and tocotrienol standards within a concentration range of 0.2–106.0 ng (injected amount). The limit of detection (LOD) was determined based on a signal-to-noise ratio (S/N) of 3, while the limit of quantification (LOQ) was calculated by using the formula LOQ = 3.3 × LOD. The S/N ratio was calculated by using LabSolutions software (version 5.124; Shimadzu Corporation, Kyoto, Japan) and verified by injecting the obtained concentrations of tocopherol and tocotrienol standards as the calculated LOD values. The LOD values ranged from 0.01 ng to 0.03 ng, and the LOQ values ranged from 0.03 ng to 0.10 ng, with δ-T3 exhibiting the lowest LOD and LOQ values and α-T the highest.
2.7. LC-MS Confirmation of Presence of Tocochromanols in St. John’s Wort Inflorescences
Mass spectrometry (MS) was employed only to confirm the identification of tocotrienols and tocopherols in St. John’s wort inflorescences obtained by RP-HPLC-FLD analysis according to the previously developed method [26]. In summary, the analytical workflow was conducted by using a Q-Exactive Orbitrap MS system (Thermo Scientific, Dreieich, Germany) coupled with an Ultimate 3000 HPLC system (Dionex, Sunnyvale, CA, USA). The separation was achieved on a Kinetex PFP column (1.7 µm, 100 × 3 mm; Phenomenex, Torrance, CA, USA) with a binary mobile phase system of water (A) and methanol (B). The gradient elution protocol included 20% B for 1 min, a linear increase from 20% to 95% B between 1 and 9.5 min, a hold at 95% B from 9.5 to 25 min, and a return to 20% B from 25.1 to 28 min. The flow rate was set to 0.3 mL/min. Tocochromanols were quantified in full scan mode at a resolution of 70,000 FWHM (at m/z 200) over a range of 100 to 1000 m/z, using negative atmospheric pressure chemical ionization (APCI) mode. The identification of compounds was based on comparing peak areas of corresponding deprotonated [M-H]− ions, with a mass accuracy tolerance of ±5 ppm and a retention time tolerance of ±0.1 min. The LOQ was determined by analyzing standard solutions at low concentrations (0.01 to 0.25 ng/μL), with the lowest concentration producing an S/N ratio of ≥10 designated as the LOQ. Data acquisition and processing were carried out by using Thermo Scientific Xcalibur software (version 4.1).
2.8. Statistical Analysis
The results are presented as means ± standard deviations (n = 3) from three independent replications of the extraction process. A p-value ≤ 0.05 was used to denote significant differences between the mean values of tocochromanol extractability via different hydroethanolic solutions determined by the one-factorial analysis of variance (ANOVA). Tukey’s post hoc tests were performed to determine homogenous groups for significant results performed with Statistica 13.0 (TIBCO Software Inc., Palo Alto, CA, USA) software.
3. Results and Discussion
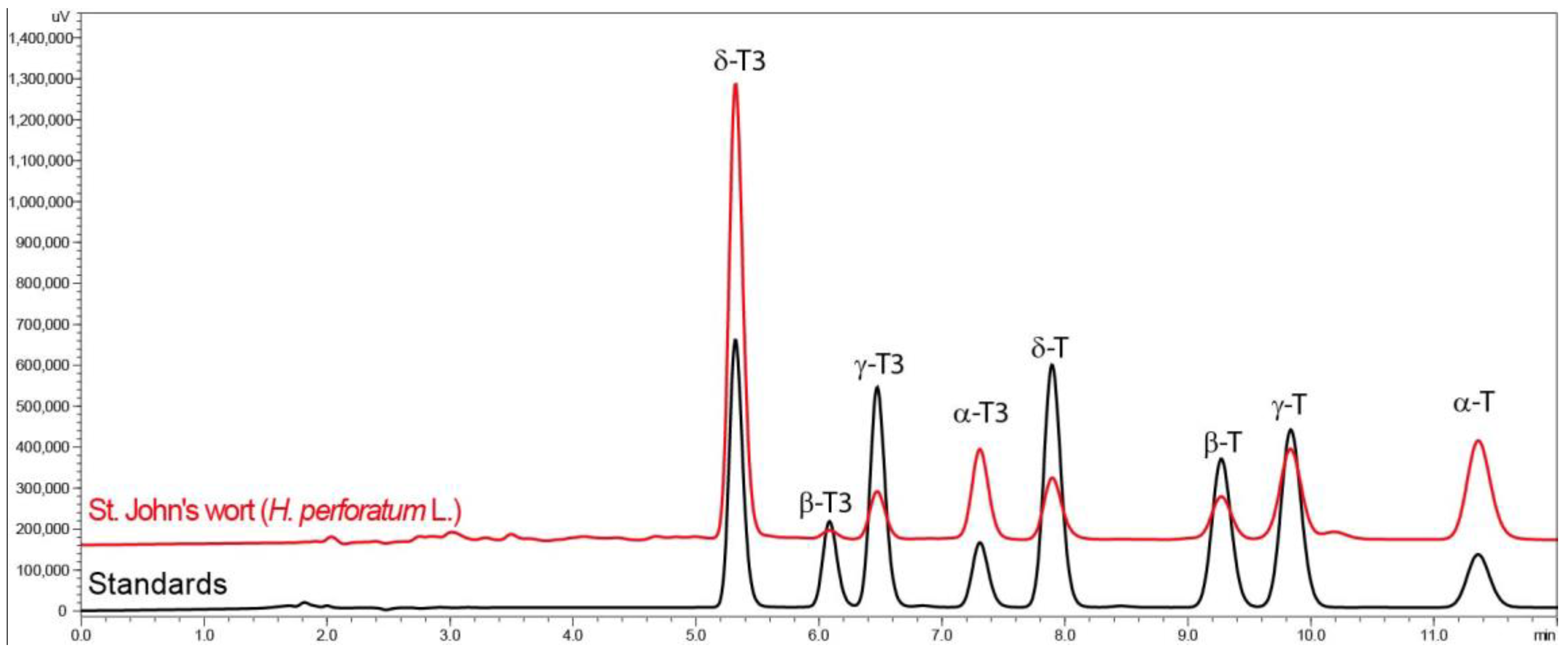
The profile (RP-HPLC-FLD chromatogram) of tocochromanols in the H. perforatum inflorescence samples obtained by the saponification protocol can be seen in Figure 1. The chromatograms of hydroethanolic extracts from residues of St. John’s wort tea infusions/water extracts can be found in the Supplementary Material (Figure S2).
Figure 1.
Chromatograms of tocotrienol (T3) and tocopherol (T) homologue (δ, γ, β, and α) separation by RP-HPLC-FLD in St. John’s wort (H. perforatum L.) and standards.
St. John’s wort inflorescences contained all eight analyzed tocochromanols (four tocopherol and four tocotrienol homologues—δ, γ, β, and α), where tocotrienols were the dominant part (64%). The percentages of individual tocotrienol homologues (α/β/γ/δ%) amounted to 21/0.5/2/41%, while in case of tocopherol homologues (α/β/γ/δ%), they were 27/1/7/1%. Similar findings in St. John’s wort inflorescences were reported previously [5]. Thus, the current study confirms the presence of eight tocochromanols in St. John’s wort. The data obtained with the fluorescence detector were confirmed by a mass spectrometry (MS) study following the previous methodology [26]. The predominance of δ-T3 is rare in natural products. The exceptions include annatto (Bixa orellana L.) and lychee (Litchi chinensis Sonn.) seeds and their oils, the richest known sources of δ-T3 [27]. While hydrophilic compounds in general seem well studied [3], we know relatively little about lipophilic phytochemicals in the Hypericum genus, even when they are the subject of research. In four Tunisian Hypericum species—H. perforatum, H. tomentosum, H. perfoliatum, and H. ericoides—the composition of fatty acid and tocopherols was investigated; however, the occurrence of tocotrienols, as well as the occurrence of tocopherols, was not considered, despite the earlier discovery of δ-T3 occurrence in H. perforatum [6]. The uniqueness of the Hypericum genus has been previously reported, due to the presence of a relatively high concentration of tocotrienols in the leaves of seven species in one study [28] and in nine species and two hybrids in another investigation [29]. The latest study and the present study on H. perforatum show that this medical plant is a relatively good source of tocotrienols [5]. The quantity of tocochromanols (total content) in the dry powder of H. perforatum inflorescences was 83.56 mg/100 g dry weight (dw), where tocotrienols accounted for 53.62 mg/100 g dw (Table 1).

Table 1.
Tocopherol and tocotrienol contents (mg/100 g dw) in H. perforatum plant powder and its water extract (tea residues).
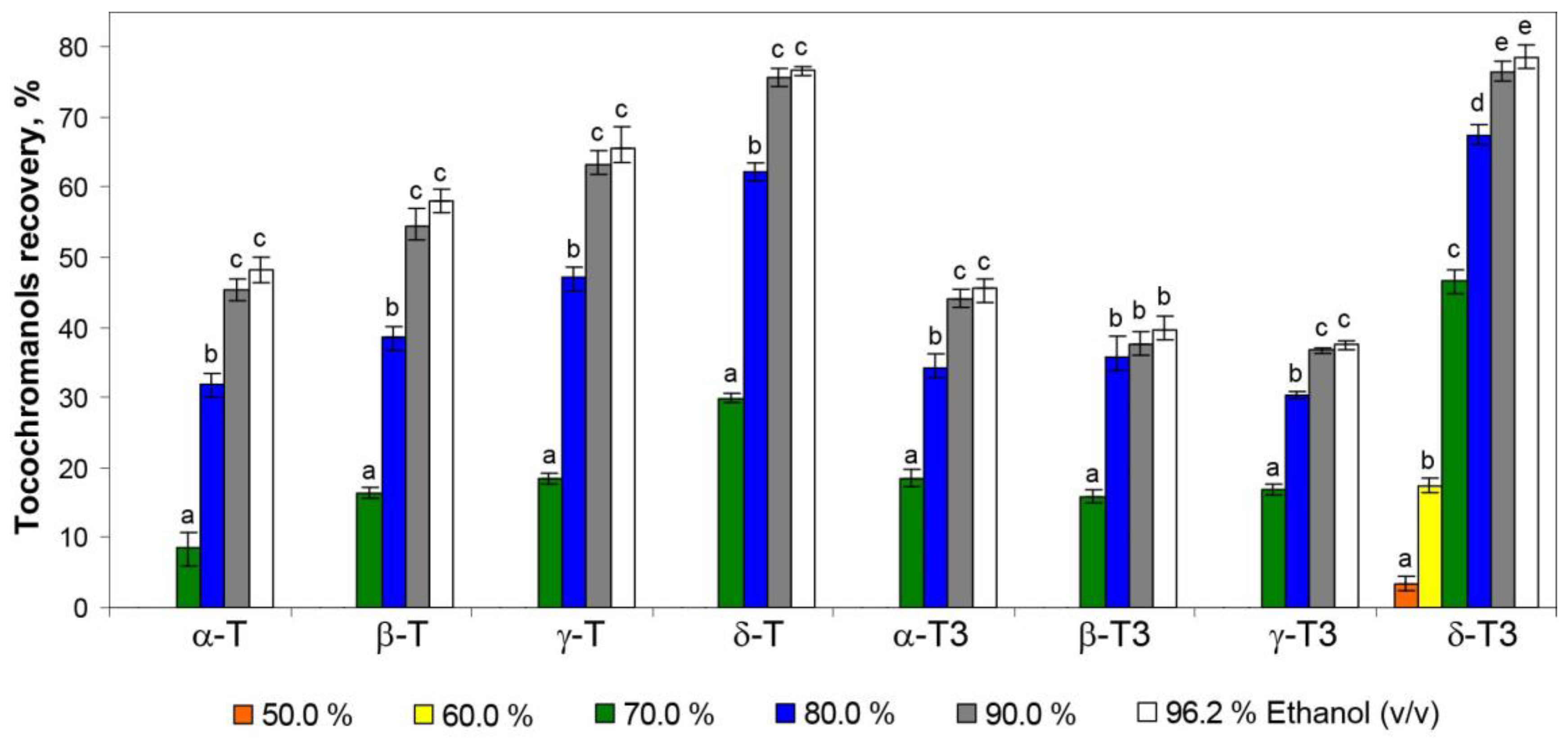
To the best of our understanding, no tocotrienols have been reported to be dominant over the tocopherols in any of the medicinal plants, as a result of their rarity in nature. The water extracts (tea infusions) of St. John’s wort did not contain tocochromanols as a result of their lipophilic character; however, the application of hydroethanolic solutions allowed for their recovery from the residues remaining after tea infusions. The 50.0% hydroethanolic solutions were not effective in tocochromanol recovery, and only a low content of δ-T3 (3% and 17% of the original content in plant material, respectively) was obtained. A breakthrough was obtained with the 70.0% hydroethanolic solution, which allowed for the recovery of all tocochromanols, however in a low origin content (10–20%), except for δ-T3 (almost 50%) (Table 1, Figure 2).
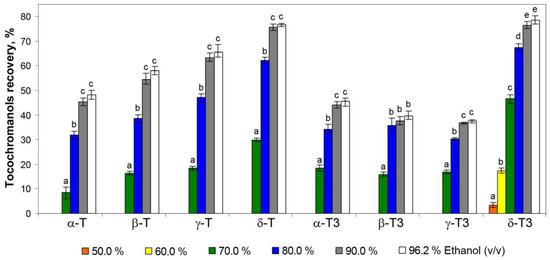
Figure 2.
The recovery of tocopherol (T) and tocotrienol (T3) homologues (δ, γ, β, and α) from residues of St. John’s wort tea infusions by using different concentrations of hydroethanolic solutions (50.0–96.2%). As a reference (100% recovery), a saponification protocol was used. Different letters within the same tocochromanol indicate statistically significant differences at p ≤ 0.05.
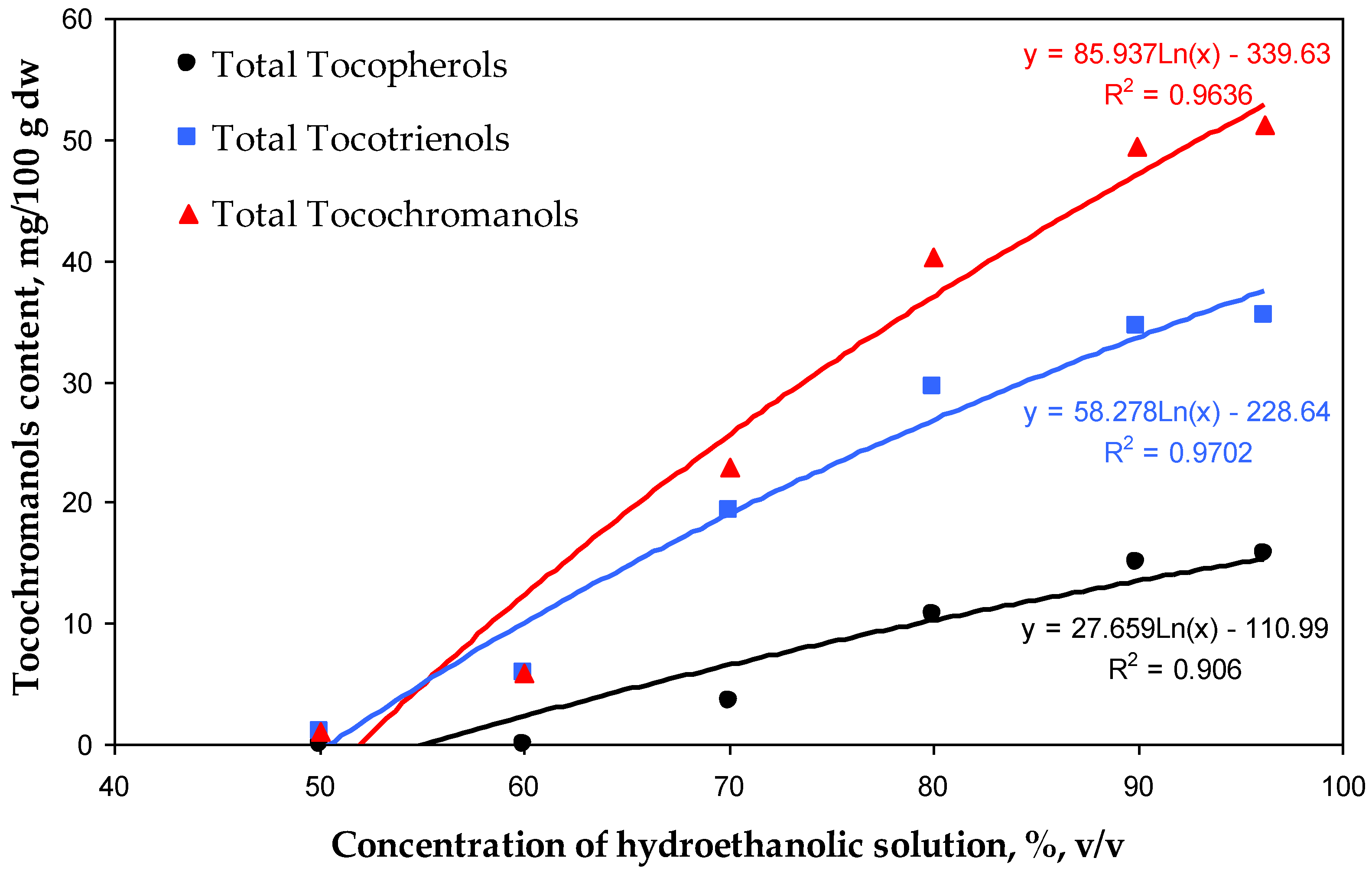
The most rapid increment in the recovery of tocochromanols was observed in a relatively short window of difference in ethanol concentration, 60–70% for tocotrienols and 70–80% for tocopherols. The highest recovery values of tocopherols and tocotrienols amounted to 15.75 mg/100 g dw (53%) and 35.51 mg/100 g dw (66%), respectively, for 96.2% hydroethanolic solutions. However, the differences between the 90.0% and 96.2% hydroethanolic solutions were not statistically significant (α < 0.05) (Table 1, Figure 2). Comparing the present study with the previous one, where extraction with pure water was not tested [5], it can be seen that the hydration of the H. perforatum sample, during extraction by water, decreases the future re-extraction of tocopherols and tocotrienols. The recovery of all tocochromanols was directly proportional to the concentration of ethanol used; however, an inhibition of tocochromanol recovery can be clearly observed at a concentration of 90% hydroethanolic solutions, especially for tocotrienols (Figure 3).
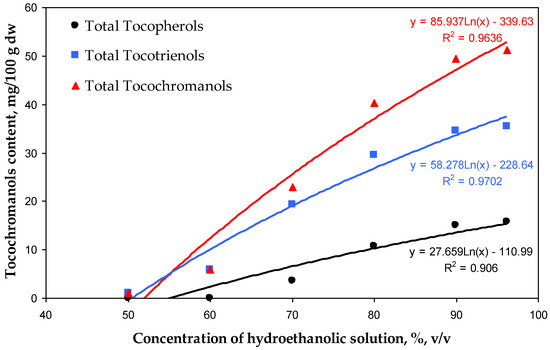
Figure 3.
Correlations between the concentration of hydroethanolic solutions (50.0–96.2%) and the contents (mg/100 g dw) of total tocopherols, tocotrienols, and tocochromanols recovered by those solutions from residues of H. perforatum tea infusions.
For each ethanol concentration, the recovery was proportionally higher for tocotrienols than for tocopherols. The same tendency was observed during tocochromanol extraction from the seeds of different crossbreeds of grape [26]. This phenomenon can be attributed to the relatively higher polarity of tocotrienols (characterized by an unsaturated carbon chain) compared with tocopherols (which possess a saturated carbon chain). The polarity of tocochromanols is further influenced by the number and positioning of methyl substituents on the chromanol ring [26]. This characteristic of tocochromanols explains the highest recovery of the δ homologue resulting in 79% of δ-T3 and 77% of δ-T extractability by the 96.2% hydroethanolic solution. Nevertheless, aspects such as strong binding/interactions of individual tocochromanols in conjunction with the plant matrix, the occurrence of tocochromanol esters, and losses during saponification can affect the obtained differences [26]. Those factors could explain the relatively low recovery of β-T3 and γ-T3 (40% and 38%, respectively) of all tested hydroethanolic solutions. This aligns with the United Nations Sustainable Development Goals (SDGs), particularly Goal 12—Responsible Consumption and Production. According to its definition, sustainable consumption and production is a concept that involves the efficient use of natural resources, minimizing negative environmental impacts and supporting quality of life for both current and future generations. It is based on the responsible creation and use of products, considering their life cycle and striving to reduce waste and pollution [30,31]. Resource recovery and utilization are essential components of sustainable waste management. Residues from St. John’s wort infusions hold significant potential for valorization through the application of innovative, eco-friendly extraction technologies. These processes facilitate the extraction of valuable lipophilic phytochemicals, which have broad applications in the agro-food and pharmaceutical industries. Ultrasound-assisted extraction (UAE) is an efficient technique for extracting bioactive compounds from plant sources and offers numerous advantages over traditional solvent-based extraction methods [32]. Ultrasound induces cavitation, which disrupts plant cell walls and facilitates the release of intracellular bioactive compounds. This process can significantly enhance extraction efficiency, reduce extraction time, minimize the use of organic solvents, and preserve the integrity of thermally sensitive bioactive compounds [33]. Incorporating extracts from plant-based raw materials, such as residues from St. John’s wort processing, into functional foods can significantly enhance their nutritional value and provide numerous health benefits stemming from the presence of tocotrienols. These benefits include analgesic, anti-bacterial, anti-aging, anti-diabetic, anti-hyperlipidemic, anti-cholesterolemic, anti-inflammatory, anti-oxidant, anti-cancer, and anti-pyretic properties [17,34,35]. Currently, there is an increasing demand for herbal raw materials, which are widely used in natural medicine and cosmetics and as food additives. This trend is simultaneously leading to a gradual increase in the volume of such waste, characterized by small particle sizes, which consequently creates challenges in their disposal. The most common methods for managing herbal waste are briquetting or granulation, resulting in the production of solid fuel [36,37]. Waste from the herbal industry can also undergo composting processes which allow it to be transformed into valuable organic fertilizer [38]. Alternatively, it can be used through the application of appropriate extraction methods to recover valuable bioactive phytochemicals, with potential applications in the food or pharmaceutical industries [39]. It is important to note that during ethanol extraction, other compounds are also extracted and present in St. John’s wort, such as hyperforin [40]. However, these were outside of the scope of this study and could pose a challenge in obtaining a purified extract enriched solely in tocotrienols.
4. Conclusions
This study demonstrated that St. John’s wort is a valuable novel source of tocotrienols, mainly δ-T3 and α-T3, lipophilic phytochemicals that can be efficiently recovered from residues of H. perforatum water extracts (tea infusions) by 80.0–96.2% hydroethanolic solutions. The tocochromanol homologue δ is effectively recovered from St. John’s wort tea residues by using hydroethanolic solutions in comparison to α. This is clearly indicated by the recovery numbers obtained for δ-T3 (79%) and α-T (48%) by using the 96.2% (v/v) hydroethanolic solution. The proper integration of tocotrienol extraction into existing St. John’s wort processing schemes could improve their value chain. The relatively high content of δ-T3 in residues of H. perforatum tea infusions can enable their use as an alternative source for δ-T3 extract production in temperate climate zones; however, developing sustainable purification techniques from other phytochemicals present in St. John’s wort hydroethanolic extracts may be required to obtain high-quality products.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15042047/s1, Figure S1: H. perforatum L. aerial parts, Figure S2: Chromatograms of tocotrienol (T3) and tocopherol (T) homologue (α, β, γ, and δ) separation by RP-HPLC-FLD in St. John’s wort (H. perforatum) extracted by saponification protocol and hydroethanolic solutions.
Author Contributions
I.M.: formal analysis, investigation, and resources; I.P.: formal analysis, investigation, and writing—review and editing; A.S. (Aleksander Siger): writing—original draft preparation and writing—review and editing; A.S. (Arianne Soliven): writing—original draft preparation and writing—review and editing; P.G.: data curation, methodology, investigation, conceptualization, software, visualization, writing—original draft preparation, writing—review and editing, supervision, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Latvian Council of Science, project “Genus Hypericum as a new valuable source of tocotrienols and tocochromanol-related molecules—from ornamental crop to industrial applications”, No. lzp-2021/1-0651.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available in the Supplementary Material and from the corresponding author upon request.
Acknowledgments
Thanks go to the Latvian Council of Science for project funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| dw | dry weight |
| MS | mass spectrometry |
| nd | not detected |
| T | tocopherol |
| T3 | tocotrienol |
| RP-HPLC-FLD | reverse-phase high-performance liquid chromatography with fluorescent light detector |
| UAE | ultrasound-assisted extraction |
References
- Galeotti, N. Hypericum perforatum (St John’s wort) beyond depression: A therapeutic perspective for pain conditions. J. Ethnopharmacol. 2017, 200, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Carrubba, A.; Lazzara, S.; Giovino, A.; Ruberto, G.; Napoli, E. Content variability of bioactive secondary metabolites in Hypericum perforatum L. Phytochem. Lett. 2021, 46, 71–78. [Google Scholar] [CrossRef]
- Napoli, E.; Siracusa, L.; Ruberto, G.; Carrubba, A.; Lazzara, S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Phytochemical profiles, phototoxic and antioxidant properties of eleven Hypericum species—A comparative study. Phytochemistry 2018, 152, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Kladar, N.; Anačkov, G.; Srđenović, B.; Gavarić, N.; Hitl, M.; Salaj, N.; Jeremić, K.; Babović, S.; Božin, B. St. John’s wort herbal teas–Biological potential and chemometric approach to quality control. Plant Foods Hum. Nutr. 2020, 75, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Mišina, I.; Lazdiņa, D. Tocopherol and tocotrienol homologue recovery from Hypericum perforatum L. and extraction residues after hydroethanolic extraction. Ind. Crops Prod. 2025, 224, 120321. [Google Scholar] [CrossRef]
- Inoue, T.; Tatemori, S.; Muranaka, N.; Hirahara, Y.; Homma, S.; Nakane, T.; Takano, A.; Nomi, Y.; Otsuka, Y. The Identification of Vitamin E Homologues in Medicinal Plant Samples Using ESI (+)-LC-MS3. J. Agric. Food Chem. 2012, 60, 9581–9588. [Google Scholar] [CrossRef] [PubMed]
- Hosni, K.; Msaâda, K.; Taârit, M.B.; Marzouk, B. Fatty acid composition and tocopherol content in four Tunisian Hypericum species: Hypericum perforatum, Hypericum tomentosum, Hypericum perfoliatum and Hypericum ericoides ssp. Roberti. Arab. J. Chem. 2017, 10, S2736–S2741. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Morales, M.; Garcia, Q.S.; Siqueira-Silva, A.I.; Silva, M.C.; Munné-Bosch, S. Tocotrienols in Vellozia gigantea leaves: Occurrence and modulation by seasonal and plant size effects. Planta 2014, 240, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Yabuta, Y.; Tamoi, M.; Tanabe, N.; Shigeoka, S. Generation of transgenic tobacco plants with enhanced tocotrienol levels through the ectopic expression of rice homogentisate geranylgeranyl transferase. Plant Biotechnol. 2015, 32, 233–238. [Google Scholar] [CrossRef]
- Tan, X.; Zhong, F.; Teng, H.; Li, Q.; Li, Y.; Mei, Z.; Chen, Y.; Yang, G. Acylphloroglucinol and tocotrienol derivatives from the fruits of Garcinia paucinervis. Fitoterapia 2020, 146, 104688. [Google Scholar] [CrossRef] [PubMed]
- Lavaud, A.; Richomme, P.; Gatto, J.; Aumond, M.-C.; Poullain, C.; Litaudon, M.; Andriantsitohaina, R.; Guilet, D. A tocotrienol series with an oxidative terminal prenyl unit from Garcinia amplexicaulis. Phytochemistry 2015, 109, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Lavaud, A.; Richomme, P.; Litaudon, M.; Andriantsitohaina, R.; Guilet, D. Antiangiogenic tocotrienol derivatives from Garcinia amplexicaulis. J. Nat. Prod. 2013, 76, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, R.; Kruk, J. Novel and rare prenyllipids—Occurrence and biological activity. Plant Physiol. Biochem. 2018, 122, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A. Tocopherols, tocotrienols and tocomonoenols: Many similar molecules but only one vitamin E. Redox Biol. 2019, 26, 101259. [Google Scholar] [CrossRef]
- Peh, H.Y.; Tan, W.S.D.; Liao, W.; Wong, W.S.F. Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Sailo, B.L.; Banik, K.; Padmavathi, G.; Javadi, M.; Bordoloi, D.; Kunnumakkara, A.B. Tocotrienols: The promising analogues of vitamin E for cancer therapeutics. Pharmacol. Res. 2018, 130, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Luo, P.; Zeng, Z.; Wang, H.; Malafa, M.; Suh, N. Vitamin E and cancer prevention: Studies with different forms of tocopherols and tocotrienols. Mol. Carcinog. 2020, 59, 365–389. [Google Scholar] [CrossRef]
- Aggarwal, V.; Kashyap, D.; Sak, K.; Tuli, H.S.; Jain, A.; Chaudhary, A.; Garg, V.K.; Sethi, G.; Yerer, M.B. Molecular mechanisms of action of tocotrienols in cancer: Recent trends and advancements. Int. J. Mol. Sci. 2019, 20, 656. [Google Scholar] [CrossRef]
- Alahmad, A.; Alghoraibi, I.; Zein, R.; Kraft, S.; Dräger, G.; Walter, J.-G.; Scheper, T. Identification of major constituents of Hypericum perforatum L. extracts in Syria by development of a rapid, simple, and reproducible HPLC-ESI-Q-TOF MS analysis and their antioxidant activities. ACS Omega 2022, 7, 13475–13493. [Google Scholar] [CrossRef]
- Jarzębski, M.; Smułek, W.; Baranowska, H.M.; Masewicz, Ł.; Kobus-Cisowska, J.; Ligaj, M.; Kaczorek, E. Characterization of St. John’s wort (Hypericum perforatum L.) and the impact of filtration process on bioactive extracts incorporated into carbohydrate-based hydrogels. Food Hydrocolloids 2020, 104, 105748. [Google Scholar] [CrossRef]
- Hernandez, M.F.; Falé, P.L.V.; Araújo, M.E.M.; Serralheiro, M.L.M. Acetylcholinesterase inhibition and antioxidant activity of the water extracts of several Hypericum species. Food Chem. 2010, 120, 1076–1082. [Google Scholar] [CrossRef]
- Górnaś, P.; Segliņa, D.; Lācis, G.; Pugajeva, I. Dessert and crab apple seeds as a promising and rich source of all four homologues of tocopherol (α, β, γ and δ). LWT-Food Sci. Technol. 2014, 59, 211–214. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Waśkiewicz, A.; Perkons, I.; Pugajeva, I.; Segliņa, D. Simultaneous extraction of tocochromanols and flavan-3-ols from the grape seeds: Analytical and industrial aspects. Food Chem. 2025, 462, 140913. [Google Scholar] [CrossRef]
- Górnaś, P.; Baškirovs, G.; Siger, A. Free and esterified tocopherols, tocotrienols and other extractable and non-extractable tocochromanol-related molecules: Compendium of knowledge, future perspectives and recommendations for chromatographic techniques, tools, and approaches used for tocochromanol determination. Molecules 2022, 27, 6560. [Google Scholar] [CrossRef] [PubMed]
- Mišina, I.; Lazdiņa, D.; Górnaś, P. Tocochromanols in the leaves of plants in the Hypericum and Clusia genera. Molecules 2025, 30, 709. [Google Scholar] [CrossRef]
- Lazdiņa, D.; Mišina, I.; Górnaś, P. Tocotrienols in eleven species of Hypericum genus leaves. Molecules 2025, 30, 662. [Google Scholar] [CrossRef] [PubMed]
- Ardra, S.; Barua, M.K. Halving food waste generation by 2030: The challenges and strategies of monitoring UN sustainable development goal target 12.3. J. Clean. Prod. 2022, 380, 135042. [Google Scholar] [CrossRef]
- Sinha, A.K.; Sharma, M.D.; Mishra, P.; Kulshrestha, S. Food waste and sustainable development goals. In Food Waste Valorization; Elsevier: Amsterdam, The Netherlands, 2024; pp. 19–31. [Google Scholar]
- Chaves, J.O.; de Souza Mesquita, L.M.; Strieder, M.M.; Contieri, L.S.; Pizani, R.S.; Sanches, V.L.; Viganó, J.; Bezerra, R.M.N.; Rostagno, M.A. Eco-friendly and high-performance extraction of flavonoids from lemon peel wastes by applying ultrasound-assisted extraction and eutectic solvents. Sustain. Chem. Pharm. 2024, 39, 101558. [Google Scholar] [CrossRef]
- Usman, M.; Nakagawa, M.; Cheng, S. Emerging trends in green extraction techniques for bioactive natural products. Processes 2023, 11, 3444. [Google Scholar] [CrossRef]
- Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Naumovski, N. Tocotrienols, health and ageing: A systematic review. Maturitas 2017, 95, 55–60. [Google Scholar] [CrossRef]
- Younes, M.; Loubnane, G.; Sleiman, C.; Rizk, S. Tocotrienol isoforms: The molecular mechanisms underlying their effects in cancer therapy and their implementation in clinical trials. J. Integr. Med. 2024, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lebiocka, M.; Montusiewicz, A.; Pasieczna–Patkowska, S.; Szaja, A. Pretreatment of herbal waste using sonication. Bioresour. Technol. 2023, 377, 128932. [Google Scholar] [CrossRef]
- Cho, S.-H.; Lee, T.; Cha, H.; Chen, W.-H.; Tsang, Y.F.; Kwon, E.E. Production of combustible gas via incorporating CO2 to pyrolysis of medicinal herbal waste. Ind. Crops Prod. 2024, 219, 119110. [Google Scholar] [CrossRef]
- Singh, D.; Suthar, S. Vermicomposting of herbal pharmaceutical industry solid wastes. Ecol. Eng. 2012, 39, 1–6. [Google Scholar] [CrossRef]
- Dewan, A.; Dawra, S.; Kaushik, N.; Singh, A.; Thakur, S.; Kaur, S.; Xavier, J.R. Process characterization for tisane development using pomegranate waste: An herbal drink optimization strategy. Sustain. Food Technol. 2024, 2, 806–815. [Google Scholar] [CrossRef]
- Gaid, M.; Biedermann, E.; Füller, J.; Haas, P.; Behrends, S.; Krull, R.; Scholl, S.; Wittstock, U.; Müller-Goymann, C.; Beerhues, L. Biotechnological production of hyperforin for pharmaceutical formulation. Eur. J. Pharm. Biopharm. 2018, 126, 10–26. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).