Positron Emission Tomography with Fibroblast Activation Protein-Targeted Radiopharmaceuticals in Primary Hepatic Tumors: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Quality of Selected Studies
3. Results
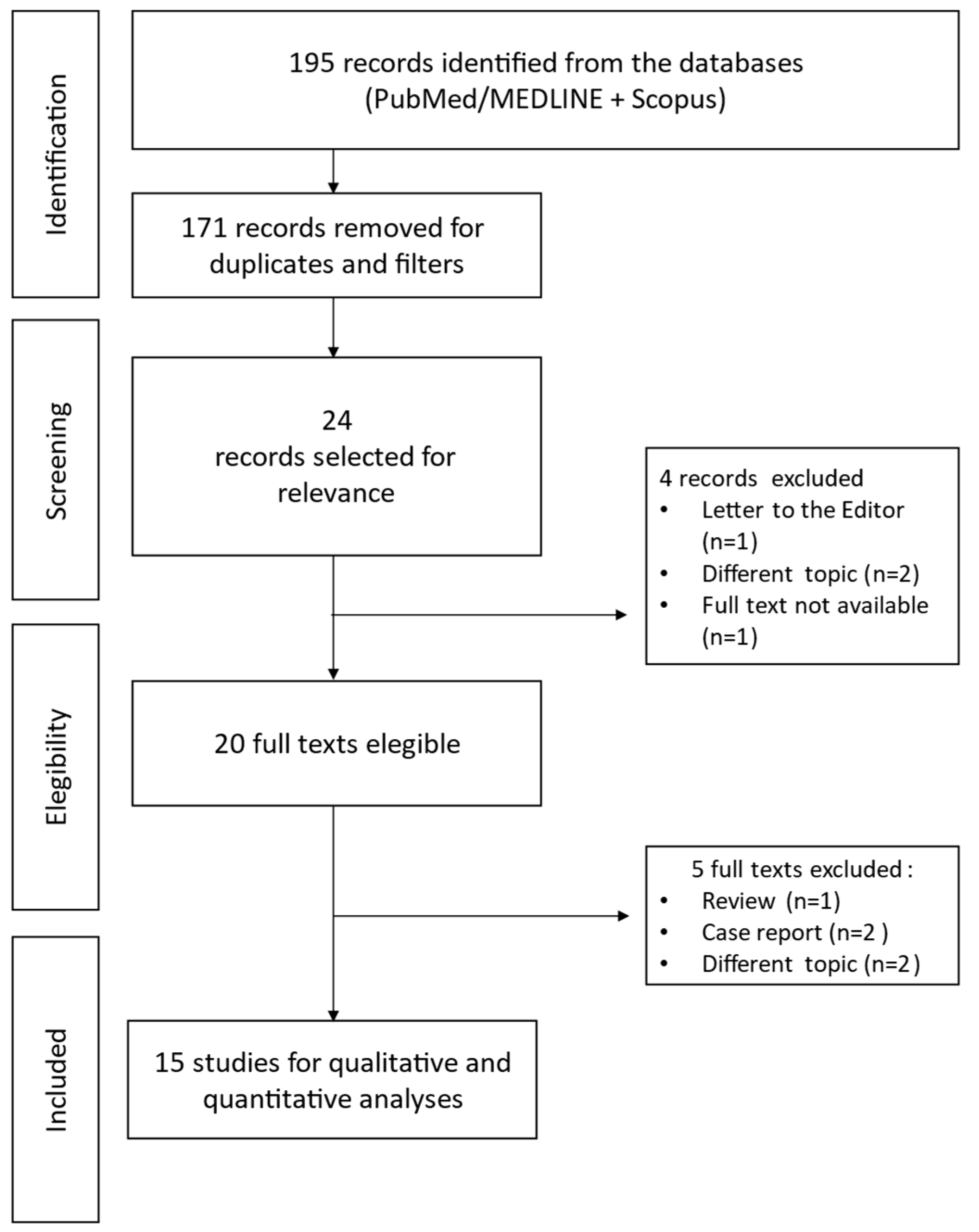
3.1. Search Results
3.2. Characteristics of Selected Studies
3.3. Methodological Quality
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Liver Cancer Collaboration; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, C.; Zhang, H.; Zhang, L.; Zhu, A.X.; Bernards, R.; Qin, W.; Wang, C. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.I. Hepatocellular carcinoma: Current management and future trends. Gastroenterology 2004, 127 (Suppl. 1), S218–S224. [Google Scholar] [CrossRef] [PubMed]
- Sirica, A.E.; Campbell, D.J.; Dumur, C.I. Cancer-associated fibroblasts in intrahepatic cholangiocarcinoma. Curr. Opin. Gastroenterol. 2011, 27, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Sirica, A.E. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 9, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koustoulidou, S.; Hoorens, M.W.H.; Dalm, S.U.; Mahajan, S.; Debets, R.; Seimbille, Y.; de Jong, M. Cancer-Associated Fibroblasts as Players in Cancer Development and Progression and Their Role in Targeted Radionuclide Imaging and Therapy. Cancers 2021, 13, 1100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hartel, M.; Di Mola, F.F.; Gardini, A.; Zimmermann, A.; Di Sebastiano, P.; Guweidhi, A.; Innocenti, P.; Giese, T.; Giese, N.; Büchler, M.W.; et al. Desmoplastic reaction influences pancreatic cancer growth behavior. World J. Surg. 2004, 28, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.J.; Alpaugh, R.K.; Palazzo, I.; Meropol, N.J.; Rogatko, A.; Xu, Z.; Hoffman, J.P.; Weiner, L.M.; Cheng, J.D. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas 2008, 37, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Räsänen, K.; Vaheri, A. Activation of fibroblasts in cancer stroma. Exp. Cell Res. 2010, 316, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, M.; Mellody, K.T.; Orimo, A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin. Cell Dev. Biol. 2010, 21, 19–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lamarca, A.; Barriuso, J.; Chander, A.; McNamara, M.G.; Hubner, R.A.; ÓReilly, D.; Manoharan, P.; Valle, J.W. 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) for patients with biliary tract cancer: Systematic review and meta-analysis. J. Hepatol. 2019, 71, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, H.J.; Park, J.H.; Park, D.I.; Cho, Y.K.; Sohn, C.I.; Jeon, W.K.; Kim, B.I. Clinical usefulness of 18F-FDG PET-CT for patients with gallbladder cancer and cholangiocarcinoma. J. Gastroenterol. 2010, 45, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Lan, B.Y.; Kwee, S.A.; Wong, L.L. Positron emission tomography in hepatobiliary and pancreatic malignancies: A review. Am. J. Surg. 2012, 204, 232–241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; He, Q.; Jiang, S.; Li, M.; Xue, H.; Zhang, D.; Li, S.; Peng, H.; Liang, J.; Liu, Z.; et al. [18F]FAPI PET/CT in the evaluation of focal liver lesions with [18F]FDG non-avidity. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zhao, L.; Meng, T.; Xu, W.; Lin, Q.; Wu, H.; Zhang, J.; Chen, X.; Sun, L.; Chen, H. PET Imaging of Fibroblast Activation Protein in Various Types of Cancer Using 68Ga-FAP-2286: Comparison with 18F-FDG and 68Ga-FAPI-46 in a Single-Center, Prospective Study. J. Nucl. Med. 2023, 64, 386–394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mu, X.; Huang, X.; Li, M.; Sun, W.; Fu, W. Comparison of physiological uptake of normal tissues in patients with cancer using 18F-FAPI-04 and 18F-FAPI-42 PET/CT. Front. Nucl. Med. 2022, 2, 927843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roustaei, H.; Kiamanesh, Z.; Askari, E.; Sadeghi, R.; Aryana, K.; Treglia, G. Could Fibroblast Activation Protein (FAP)-Specific Radioligands Be Considered as Pan-Tumor Agents? Contrast Media Mol. Imaging 2022, 2022, 3948873. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sidrak, M.M.A.; De Feo, M.S.; Corica, F.; Gorica, J.; Conte, M.; Filippi, L.; Schillaci, O.; De Vincentis, G.; Frantellizzi, V. Fibroblast Activation Protein Inhibitor (FAPI)-Based Theranostics-Where We Are at and Where We Are Heading: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 3863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Evangelista, L.; Frantellizzi, V.; Schillaci, O.; Filippi, L. Radiolabeled FAPI in pancreatic cancer: Can it be an additional value in the management of patients? Expert. Rev. Anticancer. Ther. 2023, 23, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Pu, Y.; Huang, S.; Yang, C.; Yang, F.; Pu, Y.; Li, J.; Chen, L.; Huang, Y. FAPI-PET/CT in Cancer Imaging: A Potential Novel Molecule of the Century. Front. Oncol. 2022, 12, 854658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arçay Öztürk, A.; Flamen, P. FAP-targeted PET imaging in gastrointestinal malignancies: A comprehensive review. Cancer Imaging 2023, 23, 79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Singh, J. Critical appraisal skills programme. J. Pharmacol. Pharmacother. 2013, 4, 76–77. [Google Scholar] [CrossRef]
- Pabst, K.M.; Trajkovic-Arsic, M.; Cheung, P.F.Y.; Ballke, S.; Steiger, K.; Bartel, T.; Schaarschmidt, B.M.; Milosevic, A.; Seifert, R.; Nader, M.; et al. Superior Tumor Detection for 68Ga-FAPI-46 Versus 18F-FDG PET/CT and Conventional CT in Patients with Cholangiocarcinoma. J. Nucl. Med. 2023, 64, 1049–1055. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jinghua, L.; Kui, X.; Deliang, G.; Bo, L.; Qian, Z.; Haitao, W.; Yaqun, J.; Dongde, W.; Xigang, X.; Ping, J.; et al. Clinical prospective study of Gallium 68 (68Ga)-labeled fibroblast-activation protein inhibitor PET/CT in the diagnosis of biliary tract carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2152–2166, Erratum in Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2230. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, V.; Meenakshi, L.A.; Selvaraj, A.J.; Pottakkat, B.; Halanaik, D. Role of 68Ga-FAPI PET/CT in Assessing Hepatobiliary Malignancies: A Prospective Pilot Study. Clin. Nucl. Med. 2023, 48, e281–e288. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, W.; Ren, S.; Kong, Y.; Huang, Q.; Zhao, J.; Guan, Y.; Jia, H.; Chen, J.; Lu, L.; et al. 68Ga-FAPI-04 Versus 18F-FDG PET/CT in the Detection of Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 693640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, X.; Xing, H.; Yang, X.; Li, F.; Yao, S.; Congwei, J.; Zhao, H.; Hacker, M.; Huo, L.; Li, X. Comparison of PET imaging of activated fibroblasts and 18F-FDG for diagnosis of primary hepatic tumours: A prospective pilot study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Siripongsatian, D.; Promteangtrong, C.; Kunawudhi, A.; Kiatkittikul, P.; Boonkawin, N.; Chinnanthachai, C.; Jantarato, A.; Chotipanich, C. Comparisons of Quantitative Parameters of Ga-68-Labelled Fibroblast Activating Protein Inhibitor (FAPI) PET/CT and [18F]F-FDG PET/CT in Patients with Liver Malignancies. Mol. Imaging Biol. 2022, 24, 818–829. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, H.; Pang, Y.; Wu, J.; Zhao, L.; Hao, B.; Wu, J.; Wei, J.; Wu, S.; Zhao, L.; Luo, Z.; et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1820–1832. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xing, H.; Yang, X.; Li, F.; Yao, S.; Zhang, H.; Zhao, H.; Hacker, M.; Huo, L.; Li, X. Fibroblast imaging of hepatic carcinoma with 68Ga-FAPI-04 PET/CT: A pilot study in patients with suspected hepatic nodules. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Liu, H.; Wang, Y.; Deng, J.; Peng, D.; Feng, Y.; Wang, L.; Chen, Y.; Qiu, L. The potential utility of [68Ga]Ga-DOTA-FAPI-04 as a novel broad-spectrum oncological and non-oncological imaging agent-comparison with [18F]FDG. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 963–979. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, W.; Pang, Y.; Yao, L.; Zhao, L.; Fan, C.; Ke, J.; Guo, P.; Hao, B.; Fu, H.; Xie, C.; et al. Imaging fibroblast activation protein in liver cancer: A single-center post hoc retrospective analysis to compare [68Ga]Ga-FAPI-04 PET/CT versus MRI and [18F]-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1604–1617. [Google Scholar] [CrossRef] [PubMed]
- Geist, B.K.; Xing, H.; Wang, J.; Shi, X.; Zhao, H.; Hacker, M.; Sang, X.; Huo, L.; Li, X. A methodological investigation of healthy tissue, hepatocellular carcinoma, and other lesions with dynamic 68Ga-FAPI-04 PET/CT imaging. EJNMMI Phys. 2021, 8, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kosmala, A.; Serfling, S.E.; Schlötelburg, W.; Lindner, T.; Michalski, K.; Schirbel, A.; Higuchi, T.; Hartrampf, P.E.; Buck, A.K.; Weich, A.; et al. Impact of 68Ga-FAPI-04 PET/CT on Staging and Therapeutic Management in Patients with Digestive System Tumors. Clin. Nucl. Med. 2023, 48, 35–42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chou, R.; Cuevas, C.; Fu, R.; Devine, B.; Wasson, N.; Ginsburg, A.; Zakher, B.; Pappas, M.; Graham, E.; Sullivan, S.D. Imaging Techniques for the Diagnosis of Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2015, 162, 697–711, Erratum in Ann. Intern. Med. 2015, 162, 880. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, J.M.; Lee, J.S.; Lee, H.Y.; Park, B.H.; Kim, Y.H.; Han, J.K.; Choi, B.I. Hepatocellular carcinoma: Diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology 2015, 275, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Romagnuolo, J.; Bardou, M.; Rahme, E.; Joseph, L.; Reinhold, C.; Barkun, A.N. Magnetic resonance cholangiopancreatography: A meta-analysis of test performance in suspected biliary disease. Ann. Intern. Med. 2003, 139, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Patel, T. Cholangiocarcinoma--controversies and challenges. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 189–200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kato, T.; Tsukamoto, E.; Kuge, Y.; Katoh, C.; Nambu, T.; Nobuta, A.; Kondo, S.; Asaka, M.; Tamaki, N. Clinical role of 18F-FDG PET for initial staging of patients with extrahepatic bile duct cancer. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Martin, A.; Zerizer, I. Positron emission tomography-computed tomography in liver imaging. Semin. Ultrasound CT MRI. 2013, 34, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Aznar, D.L.; Ojeda, R.; Garcia, E.U.; Aparici, F.; Sánchez, P.A.; Flores, D.; Martínez, C.; Sopena, R. Focal nodular hyperplasia (FNH): A potential cause of false-positive positron emission tomography. Clin. Nucl. Med. 2005, 30, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kim, J.H.; Kim, S.K.; Kang, K.W.; Park, K.W.; Choi, J.I.; Lee, W.J.; Kim, C.M.; Nam, B.H. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J. Nucl. Med. 2008, 49, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Eo, J.S.; Paeng, J.C.; Lee, D.S. Nuclear imaging for functional evaluation and theragnosis in liver malignancy and transplantation. World J. Gastroenterol. 2014, 20, 5375–5388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kluge, R.; Schmidt, F.; Caca, K.; Barthel, H.; Hesse, S.; Georgi, P.; Seese, A.; Huster, D.; Berr, F. Positron emission tomography with [18F]fluoro-2-deoxy-D-glucose for diagnosis and staging of bile duct cancer. Hepatology 2001, 33, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Yun, M.; Lee, W.J.; Kim, K.S.; Lee, J.D. Usefulness of 18F-FDG PET in intrahepatic cholangiocarcinoma. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Siveke, J.T. Fibroblast-Activating Protein: Targeting the Roots of the Tumor Microenvironment. J. Nucl. Med. 2018, 59, 1412–1414. [Google Scholar] [CrossRef] [PubMed]
- Puré, E.; Blomberg, R. Pro-tumorigenic roles of fibroblast activation protein in cancer: Back to the basics. Oncogene 2018, 37, 4343–4357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, Z.; Peng, H.; Li, W.; Liu, Z. Head-to-head study of [18F]FAPI-04 PET/CT and [18F]FDG PET/CT for non-invasive assessment of liver cancer and its immunohistochemical markers. BMC Cancer 2024, 24, 1378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pang, Y.; Zhao, L.; Luo, Z.; Hao, B.; Wu, H.; Lin, Q.; Sun, L.; Chen, H. Comparison of 68Ga-FAPI and 18F-FDG Uptake in Gastric, Duodenal, and Colorectal Cancers. Radiology 2021, 298, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.F.L.; Blecha, J.; Rosenberg, O.; Ohliger, M.; Flavell, R.R.; Wilson, D.M. Cyclic 68Ga-Labeled Peptides for Specific Detection of Human Angiotensin-Converting Enzyme 2. J. Nucl. Med. 2021, 62, 1631–1637. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zboralski, D.; Hoehne, A.; Bredenbeck, A.; Schumann, A.; Nguyen, M.; Schneider, E.; Ungewiss, J.; Paschke, M.; Haase, C.; von Hacht, J.L.; et al. Preclinical evaluation of FAP-2286 for fibroblast activation protein targeted radionuclide imaging and therapy. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3651–3667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herrmann, K.; Schwaiger, M.; Lewis, J.S.; Solomon, S.B.; McNeil, B.J.; Baumann, M.; Gambhir, S.S.; Hricak, H.; Weissleder, R. Radiotheranostics: A roadmap for future development. Lancet Oncol. 2020, 21, e146–e156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferdinandus, J.; Costa, P.F.; Kessler, L.; Weber, M.; Hirmas, N.; Kostbade, K.; Bauer, S.; Schuler, M.; Ahrens, M.; Schildhaus, H.U.; et al. Initial Clinical Experience with 90Y-FAPI-46 Radioligand Therapy for Advanced-Stage Solid Tumors: A Case Series of 9 Patients. J. Nucl. Med. 2022, 63, 727–734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Kaghazchi, F.; Aghdam, R.A.; Haghighi, S.; Vali, R.; Adinehpour, Z. 177Lu-FAPI Therapy in a Patient with End-Stage Metastatic Pancreatic Adenocarcinoma. Clin. Nucl. Med. 2022, 47, e243–e245. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Kramer, V.; Moon, E.S.; Roesch, F.; Tripathi, M.; Mallick, S.; ArunRaj, S.T.; Bal, C. A theranostic approach of [68Ga]Ga-DOTA.SA.FAPi PET/CT-guided [177Lu]Lu-DOTA.SA.FAPi radionuclide therapy in an end-stage breast cancer patient: New frontier in targeted radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 942–944. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Pabst, K.M.; Kessler, L.; Fragoso Costa, P.; Ferdinandus, J.; Weber, M.; Lippert, M.; Lueckerath, K.; Umutlu, L.; Kostbade, K.; et al. Safety and Efficacy of 90Y-FAPI-46 Radioligand Therapy in Patients with Advanced Sarcoma and Other Cancer Entities. Clin. Cancer Res. 2022, 28, 4346–4353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Author/Year/Country | Study Design | Radiopharmaceutical | Sample Size | Main Results |
|---|---|---|---|---|
| Rajaraman V, et al. [30] 2023, India | Prospective Pilot Study | 68Ga-FAPI-04 vs. 18F-FDG | 41 patients (28 m, 13 f) (18 CCC; 6 HCC; 10 non-neoplastic lesions; 15 metastatic) | In CCC, FAPI PET has a sensitivity of 94%, a specificity of 100%, and an accuracy of 95% compared to 50%, 100%, and 57% of FDG. For HCC, the sensitivity, specificity, and accuracy of FAPI-PET were 100%, 85%, and 92% compared to 33%, 100%, and 69% of FDG. Nevertheless, the diagnostic accuracy of FAPI-PET was 61.54% for metastatic HCC compared with 84.62% for FDG PET. |
| Zhang J, et al. [18] 2022, China | Prospective study | 18F-FAPI vs. 18F-FDG | 37 patients (34 m; 3 f) (20 HCC; 5 CCC; 4 Inflammatory focal liver lesions (FLLs); 8 non-inflammatory FLLs) | Patients with liver malignancies showing 18F-FDG non-avidity can be differentiated from benign liver disease on 18F-FAPI PET with high accuracy (83.8%) and high detection rate of 98.1%. |
| Wang H, et al. [31] 2021, China | Retrospective study | 68Ga-FAPI-04 vs. 18F-FDG | 29 patients (24 m) HCC | 68Ga-FAPI-04 was more sensitive than 18F-FDG in detecting small HCCs (≤2 cm) and well- or moderately differentiated HCCs (both p < 0.005), but there were no significant sensitivity differences in the detection of HCCs > 2 cm and poorly differentiated or undifferentiated HCCs (both p > 0.05). |
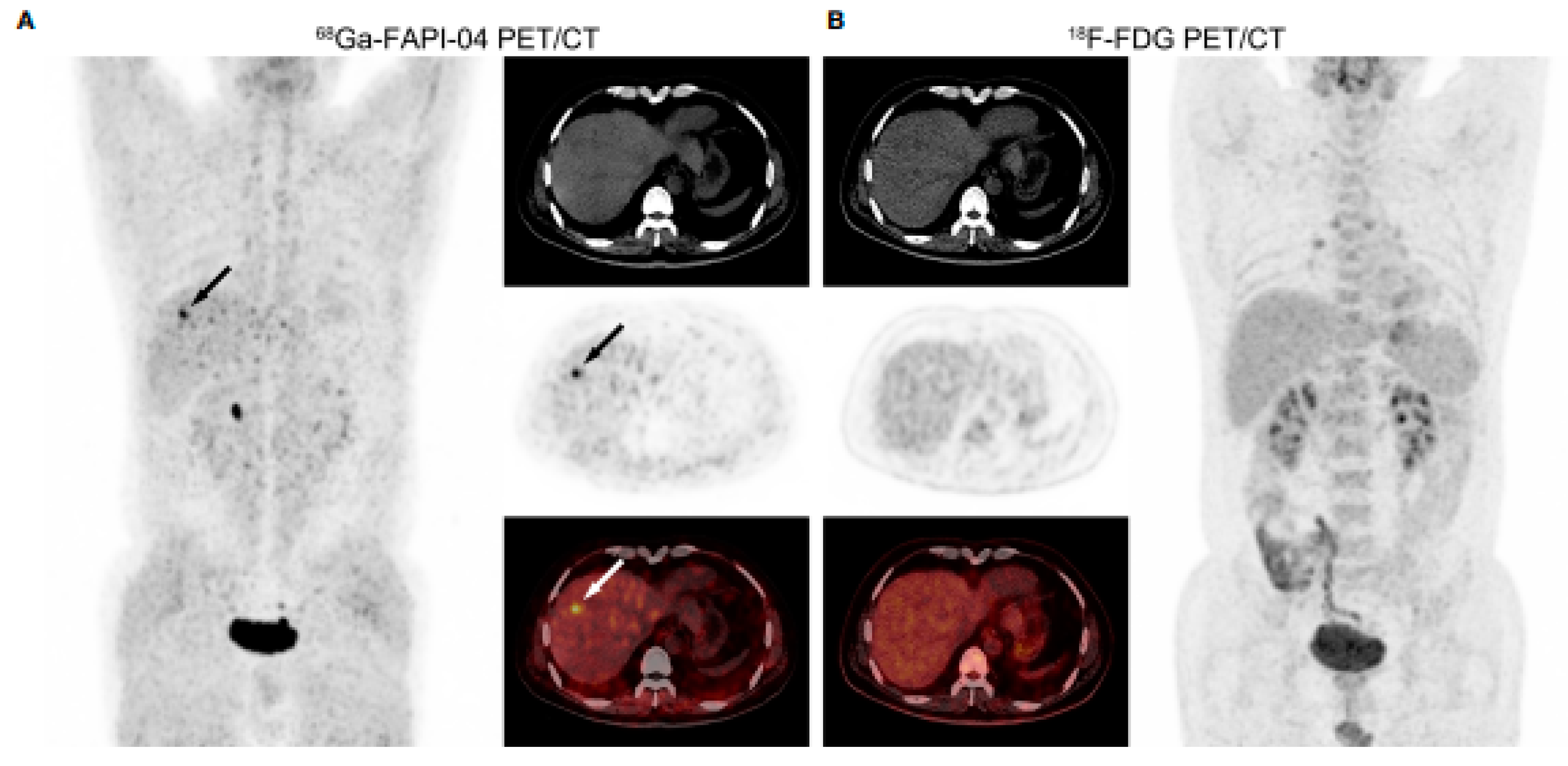
| Shi X, et al. [32] 2020, China | Prospective pilot study | 68Ga-FAPI-04 vs. 18F-FDG | 20 patients (18 m) (16 HCC; 4 ICC) | In per-lesion analysis of 20 intrahepatic malignancies, both scans presented high diagnostic specificity (100%). 68Ga-FAPI PET/CT has, however, higher sensitivity (100%) compared with FDG (55.0%). |
| Pang Y, et al. [19] 2022, China | Prospective Study | 68Ga-FAP-2286 vs. 68Ga-FAPI-46 vs. 18F-FDG | 12 patients with HCC | The SUVmax of all primary tumor lesions derived from 68Ga-FAP-2286 PET/CT was significantly higher than that derived from 18F-FDG PET/CT (11.1 vs. 6.9, p< 0.001). 68Ga-FAP-2286 and 68Ga-FAPI-46 yielded comparable clinical results. |
| Siripongsatian D, et al. [33] 2022, Thailand | Retrospective study | 68Ga-FAPI-46 vs. 18F-FDG | 27 patients (21 m) (13 CCA; 14 HCC) | For HCC, the sensitivity of 18F-FDG and 68Ga-FAPI-46 PET/CT were 71% and 100%, respectively, while for CCA, they were 50% and 100%, respectively. |
| Pabst KM, et al. [28] 2023, Germany | Prospective observational trial | 68Ga-FAPI-46 vs. 18F-FDG | 10 CCC (6 m; 4 f) | Grade 3 tumors demonstrated a significantly higher 68Ga-FAPI-46 uptake than grade 2 tumors. SUVmax was significantly higher for 68Ga-FAPI-46 PET/CT than for 18F-FDG PET/CT for primary lesions and distant metastases. No significant difference was noted for lymph node metastases. |
| Chen H, et al. [34] 2020, China | Prospective study | 68Ga-DOTA-FAPI-04 vs. 18F-FDG | 8 patients (4 HCC; 4 CCC) | The sensitivity of 18F-FDG PET/CT was 82.1%, compared with 98.2% for 68Ga-DOTA-FAPI-04. Of these, 2 CCC and 1 HCC were not visualized by 18F-FDG PET/CT (<1 cm). In 68Ga-DOTA-FAPI-04 PET/CT images, most primary lesions demonstrated a higher TBR than 18F-FDG, especially for liver cancer. |
| Shi X, et al. [35] 2021, China | Pilot study | 68Ga-FAPI-04 | 17 patients (13 m) (11 HCC, 2 ICC, 3 metastatic lesions, 1 FLL) | A benign tumor presented a neglected uptake. In contrast, a total of 28 intrahepatic lesions presented an elevated uptake, with histology individually revealing malignant hepatic lesions in each patient. Thus, 68Ga-FAPI-04 PET/CT revealed a sensitivity of 100% in detecting intrahepatic malignancy. |
| Lan L, et al. [36] 2022, China | Prospective phase II clinical study | 68Ga-DOTA-FAPI-04 vs. 18F-FDG | 16 HCC | SUVmax, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of 68Ga-DOTA-FAPI-04 PET were significantly higher than 18F-FDG PET. 68Ga-DOTA-FAPI-04 PET/CT detected almost all primary tumors (86/88), whereas 18F-FDG PET/CT missed 13 of them (including 4 HCC). |
| Jinghua L., et al. [29] 2023, China | Clinical prospective study | 68Ga-DOTA-FAPI-04 vs. 18F-FDG | 47 patients (21 m 26 f) (38 CCC, 4 Gallbladder carcinoma, 5 Benign) | In the semi-quantitative parameter analysis, the uptake of 68Ga-DOTA-FAPI in the lesion was higher than 18F-FDG, with a primary lesion detection rate of 97.62% compared to 85.71%. An association between 68Ga PET/CT-DOTA-FAPI indices and the expression of FAP, CEA, PLT, and CA19.9 was revealed. |
| Kratochwil C, et al., 2019 [37] 2019, Germany | Retrospective study | 68Ga-FAPI-04 vs. 18F-FDG | 17 (5 HCC, 12 CCC) | The highest average SUVmax (>12) was found in CCC. The average SUVmax of HCC was intermediate: (6–12). A significantly lower hepatic background for 68Ga-FAPI (SUV 1.7) than for 18F-FDG (SUV 2.8) was revealed. This may be advantageous for liver metastasis detection. |
| Guo W, et al. [38] 2021, China | Retrospective study | 68Ga-FAPI-04 vs. 18F-FDG | 34 patients (25 m, 9 f) (20HCC, 12 CCI, 2 FLLs) | 68Ga-FAPI-04 PET/CT visualized most primary lesions with higher median SUVmax and clearer tumor delineation (median TBR) compared with 18F-FDG, especially in ICC, with a positive association between the severity of the corresponding pathological grade. 68Ga-FAPI-04 PET/CT detected 96% of primary liver tumors with favorable TBR, showing a comparable detection rate with CE-CT (96%) and liver MRI (100%). Contrastingly, 18F-FDG PET/CT could only detect 65% of primary liver tumors. 68Ga-FAPI-04 detected more metastatic lesions than 18F-FDG, especially peritoneal carcinomatosis and bone metastases. |
| Geist, B.K, et al. [39] 2021, China | Methodological investigation | 68Ga-FAPI-04 | 8 patients (7 HCC lesions, 2 ICC lesions) | The study investigated kinetic models for FAPI PET in liver lesions, showing that the consideration of the SUVmax values for artery and venous image-derived input function is suitable to distinguish between healthy regions, HCC lesions, and non-HCC lesions in model-derived macro parameters. |
| Kosmala A, et al. [40] 2023, Germany | Retrospective study | 68Ga-FAPI-04 | 6 HCC, 2 liver metastases | 68Ga-FAPI showed increased sensitivity than FDG due to favorable TBR FAPI PET identified otherwise occult local recurrence in 3/6 patients with HCC and additional liver metastases in 2 more patients with extrahepatic malignancies. Overall, upstaging occurred in 4/6 (66.7%) patients with HCC, leading to treatment changes in 3/6 cases. |
| 1. Was there a clear question for the study to address? | 2. Was there a comparison with an appropriate reference standard? | 3. Did all patients receive the diagnostic test and reference standard? | 4. Could the results of the test have been influenced by the results of the reference standard? | 5. Is the disease status of the tested population clearly described? | 6. Were the methods for performing the test described insufficient detail? | 7. What are the results? | 8. How sure are we about the results? Consequences and cost of alternatives performed? | 9. Can the results be applied to your patients/the population of interest? | 10. Can the test be applied to your patient or population of interest? | 11. Were all outcomes important to the individual or population considered? | 12. What would be the impact of using this test on your patients/population? | |
| Rajaraman V, et al., 2023 [30] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | It may have a positive outcome to evaluate HCC and CCA but it has lower accuracy for metastatic HCC. |
| Zhang J, et al., 2022 [18] | ☺ | ☺ | ☹ | ? | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | It may be useful for the differential diagnosis of benign and malignant liver masses without 18F-FDG avidity. |
| Wang H, et al., 2021 [31] | ☺ | ☺ | ☹ | ? | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | It may have a positive outcome in the detection of small and well- or moderately differentiated HCCs. |
| Shi X, et al., 2020 [32] | ☺ | ☺ | ☹ | ? | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | It can be used as a diagnostic, theranostic, or prognostic target in patients with hepatic malignancies. |
| Pang Y, et al., 2022 [19] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ? | ☺ | ☺ | ☺ | 68Ga-FAP-2286 is a promising FAP-inhibitor derivative as a diagnostic, theranostic, or prognostic cancer target. |
| Siripongsatian D, et al., 2022 [33] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ? | ☺ | ☺ | ☺ | It may have a better outcome for detecting hepatic malignancies than [18F]FDG PET/CT or MRI alone. |
| Pabst KM, et al., 2023 [28] | ☺ | ☹ | ☹ | ? | ☺ | ☺ | ☺ | ? | ☺ | ☺ | ☺ | It could be a promising new test to stage and treat CCA with FAP-targeted radiopharmaceuticals. |
| Chen, H., et al., 2020 [34] | ☺ | ☺ | ? | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | It could well visualize various primary tumors and metastatic lesions with better sensitivity and accuracy for primary tumors but lower specificity for metastatic lesions. |
| Shi, X., et al., 2021 [35] | ☺ | ☺ | ☺ | ? | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | It can be used to characterize FAP expression in less-advanced HCC and ICC lesions, and it shows high sensitivity in detecting hepatic malignancies, particularly if poorly differentiated. |
| Lan, L., et al., 2022 [36] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | It could be used to detect broad-spectrum oncological and non-oncological lesions, such as inflammatory lesions. |
| Jinghua, L., et al., 2023 [29] | ☺ | ☺ | ☹ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | It may have a positive outcome in evaluating primary and metastatic BTC lesions. |
| Kratochwil C, et al., 2019 [37] | ☺ | ☺ | ☹ | ? | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | It may open new applications for non-invasive tumor characterization, staging examinations, and theranostic approach. |
| Guo, W., et al., 2021 [38] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | It could correctly identify primary liver tumors and metastatic lesions with high sensitivity, equivalent to that of CE-CT and liver MRI, and it could better identify liver lesions than [18F]-FDG PET/CT. |
| Geist, B.K, et al., 2021 [39] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ? | ? | ☺ | ☺ | ☺ | It could distinguish between healthy regions, HCC lesions, and non-HCC lesions. |
| Kosmala A, et al., 2023 [40] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | It may lead to changes in the staging of people with digestive system cancers, HCC, and pancreatic cancers, thus inducing changes in therapeutic management. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semeraro, L.; Frantellizzi, V.; Filippi, L.; Palumbo, B.; De Vincentis, G.; De Feo, M.S. Positron Emission Tomography with Fibroblast Activation Protein-Targeted Radiopharmaceuticals in Primary Hepatic Tumors: A Systematic Review. Appl. Sci. 2025, 15, 2025. https://doi.org/10.3390/app15042025
Semeraro L, Frantellizzi V, Filippi L, Palumbo B, De Vincentis G, De Feo MS. Positron Emission Tomography with Fibroblast Activation Protein-Targeted Radiopharmaceuticals in Primary Hepatic Tumors: A Systematic Review. Applied Sciences. 2025; 15(4):2025. https://doi.org/10.3390/app15042025
Chicago/Turabian StyleSemeraro, Lucia, Viviana Frantellizzi, Luca Filippi, Barbara Palumbo, Giuseppe De Vincentis, and Maria Silvia De Feo. 2025. "Positron Emission Tomography with Fibroblast Activation Protein-Targeted Radiopharmaceuticals in Primary Hepatic Tumors: A Systematic Review" Applied Sciences 15, no. 4: 2025. https://doi.org/10.3390/app15042025
APA StyleSemeraro, L., Frantellizzi, V., Filippi, L., Palumbo, B., De Vincentis, G., & De Feo, M. S. (2025). Positron Emission Tomography with Fibroblast Activation Protein-Targeted Radiopharmaceuticals in Primary Hepatic Tumors: A Systematic Review. Applied Sciences, 15(4), 2025. https://doi.org/10.3390/app15042025







