Abstract
Background: This systematic review was performed to investigate the potential diagnostic role of fibroblast activation protein (FAP)-targeted radiopharmaceuticals in hepatocarcinoma (HCC) and cholangiocarcinoma (CCA). Methods: Relevant studies published between 2019 and 2023 were selected by searching PubMed and Scopus. The following data were extracted: authors, radiopharmaceuticals, sample size, country and year of publication, study design, and main results. Selected studies were analyzed using a modified version of the Critical Appraisal Skills Programme (CASP). Results: A total of 15 papers were finally selected, where 5 (33%) were retrospective, and 10 (67%) were prospective, with an overall number of 331 involved patients. Most of the studies (14/15, 93%) employed the FAP inhibitor series (FAPI), while only one research study used cyclic peptides as FAP-binding motifs. Twelve papers (80%) compared these FAP-targeted radiopharmaceuticals with 18F-FDG. The other 3/15 (20%) were not comparative studies and used exclusively 68Ga-FAPI-04. 68Ga-FAPI-04 is the most used radiopharmaceutical in analyzed studies (11/15, 73%), while other tracers, including 18F-FAPI, 68Ga-FAPI-46, and 68Ga-FAP-2286, were used in the remaining ones. Conclusions: FAP-targeted radiopharmaceuticals have good diagnostic accuracy in HCC and CCA, with potential and promising theragnostic applications.
1. Introduction
The two most common malignant liver neoplasms, hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA) represent significant risks for global health. In 2022, around 757,948 people died from liver cancer, making it the third most common cause of cancer-related death and the sixth most prevalent kind of cancer to be diagnosed. Among them, intrahepatic CCA makes up 10–15% of instances in comparison to HCC, which makes up 75–85% [1].
The risk factors of these two tumors have characteristics in common, such as chronic liver diseases due to HBV, HCV, alcohol abuse, non-alcoholic fatty liver disease (NAFLD), and subsequent cirrhosis [2,3,4]. CCA, beyond these factors, presents a more complex etiology, of which risk factors comprise primary sclerosing cholangitis, gallstones, or biliary tract parasitosis contribute [5,6].
CCA is characterized by a conspicuous hypovascular desmoplastic stroma. Cancer-associated fibroblasts (CAFs), extracellular matrix proteins, and growth factors make up this special setting, which is essential for tumor growth and treatment resistance [7,8,9].
The tumor microenvironment (TME) of HCC is also made up, in addition to tumor cells, of various elements such as immune cells, vascular cells, extracellular matrix, and CAFs, which represent more than 50% of the tumor mass. This TME, including CAFs, promotes the proliferation of neoplastic cells, as well as local and distant invasion. Fibroblast activation protein (FAP) is a membrane glycoprotein expressed on CAFs and is a marker associated with tumor invasion and neoangiogenesis. Its overexpression is therefore associated with a poor prognosis in several neoplasms, including HCC, pancreatic tumors, and colon–rectal cancer [10,11,12,13,14].
Early diagnosis of both HCC and CCA is often difficult. The diagnosis of biliary tract tumors (BTCs) is frequently delayed by nonspecific symptoms, necessitating a multimodal diagnostic approach based on imaging, specifically magnetic resonance imaging (MRI), contrast-enhanced (CE) computed tomography (CT), and ultrasound followed by biopsies. Although positron emission tomography (PET) using 18Fluoro-2-deoxyglucose (18F-FDG) may be helpful in staging, a significant false-positive rate brought on by conditions including local infections and biliary stenting limits its usage [15]. 18F-FDG-PET is useful for identifying metastases and recurrences in HCC, even though its sensitivity is lower for well-differentiated tumors [16,17].
Through the last few years, innovative technologies like PET-CT with FAP-targeted radiopharmaceuticals have shown significant potential in tumor imaging. This approach can selectively identify FAP expressed by CAFs [10].
Most of the published studies are based on the use of FAP inhibitors (FAPI) radiolabelled with 68-Gallium (68Ga). A minority of published studies have been performed by using other isotopes rather than 68Ga, mostly 18F [18], as well as FAP binding motifs rather than FAPI inhibitors [19].
FAP-targeted radiopharmaceuticals have a physiological biodistribution and a high uptake in the salivary glands, thyroid, gallbladder, uterus, and renal pelvis. Among these tissues, the gallbladder and renal pelvis serve as the excretion route. A moderate uptake is present in the cervical, respiratory, abdominal, and pelvic organs [20]. These radiotracers have been demonstrated to be particularly effective in a variety of cancers, including HCC, CCA, and various gastrointestinal tumors such as those of the pancreas, stomach, colorectal region, esophagus, duodenum, and anus. It has also been successfully used in gynecological cancers (e.g., cervix and ovary), breast cancer, head and neck cancers (including nasopharyngeal carcinoma), non-small cell lung cancer, sarcomas, and cancers of unknown primary origin [21,22,23,24].
The first advantage of this diagnostic method is represented by its incredibly high target-to-background ratio (TBR), showing excellent image quality even for small tumors (<1 cm), which are difficult to identify using other approaches like 18F-FDG PET-CT. Additionally, the low background activity of the tracer increases contrast and sensitivity, giving more reliable results even in complicated or ambiguous cases [21]. Other advantages include the possibility of early imaging, as soon as 10 min after tracer injection, simplifying clinical workflows and reducing scan times, thus improving patient comfort. Moreover, unlike 18F-FDG, 68Ga-FAPI is not affected by patient glycemia levels and does not require specific dietary preparations, enhancing its clinical applicability. In addition, with this radiopharmaceutical, no significant side effects were reported, being well tolerated by patients [21]. These characteristics make it particularly promising for managing HCC and CCA useful for early diagnosis, accurate staging, and personalized treatment planning.
To the best of our knowledge, a few review articles have looked at FAP-targeted PET imaging in gastrointestinal malignancies without a specific focus on primary hepatic tumors [25].
The objective of this systematic review is to collect literature data regarding nuclear imaging of HCC and CCA with FAP-targeted radiopharmaceuticals, discussing their advantages compared to conventional imaging or common tracers such as 18F-FDG and underlining their diagnostic and therapeutic potential.
2. Materials and Methods
2.1. Search Strategy
With the purpose of evaluating the applications of FAP-targeted radiopharmaceuticals, a systematic search was conducted in PubMed and Scopus databases using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [26]. Specifically, the search was focused on studies that included patients with CCA or HCC.
The keywords “Positron Emission Tomography” OR “PET” AND “FAPI OR FAP” AND “primary hepatic tumours” AND “hepatocarcinoma OR HCC”, AND “cholangiocarcinoma OR CCA/CCC OR intrahepatic cholangiocarcinoma ICC”, were used to develop the search strategy. The following kinds of research were taken into consideration: clinical trials, prospective or retrospective studies, and head-to-head comparison series.
Studies without abstracts, studies on healthy volunteers or xenograft studies, case reports/case series, reviews, meta-analyses, conference proceedings, editorial remarks, interesting pictures, letters to the editor, as well as studies that did not include CCA or HCC or that lacked a radiologic and/or histologic final diagnosis of these tumor entities were all disqualified. We also examined the references of pan-tumor FAPI reviews, as well as case reports of CCA or HCC, thus including additional clinical studies that might be incorporated into our research.
The English language was mandatory for inclusion. Two reviewers (V.F., M.S.D.F.) conducted the literature search and independently examined the titles and abstracts of the results obtained from all sources using a standard procedure and data extraction. The full texts of selected papers were carefully examined for ultimate selection. To find any further pertinent literature, the reference lists of selected articles were thoroughly examined. The following characteristics were collected from each included study: authors, radiopharmaceuticals, sample size, country and year of publication as well as study design (prospective, retrospective, etc.). Studies having insufficient clinical or technical data were not given any more consideration.
2.2. Quality of Selected Studies
We analyzed included studies using a modified version of the Critical Appraisal Skills Program (CASP) checklist for diagnostic test studies [27]. Two independent reviewers (V.F. and M.S.D.F.) conducted the critical evaluation, and any disagreements were resolved by consensus.
3. Results
3.1. Search Results
A total of 195 articles were selected by reviewing each abstract to potentially identify eligible studies. From the overall group of 195, 171 articles were removed because they were duplicates or were not related to the object of this systematic review. Four of the first selected twenty-four articles were disqualified because they were reviews or letters, dealt with distinct subjects, or lacked full texts. A total of 20 full texts were reviewed, of which 5 were further excluded. Therefore, from the systematic literature search, 15 articles were ultimately selected. The resulting PRISMA search strategy is shown in Figure 1.
Figure 1.
Schematic representation of PRISMA workflow for manuscripts’ selection.
3.2. Characteristics of Selected Studies
Of the 15 selected studies published between 2019 and 2023, 5 (33%) were retrospective, while 10 (67%) were prospective. Overall, the number of patients involved was 331. The majority of the research (14/15, 93%) employed the FAP inhibitor series (FAPI), with cyclic peptides as FAP-binding motifs being used in only one study [19]. Overall, 12 out of 15 studies (80%) compared these novel radiopharmaceuticals with 18F-FDG, while the other 3/15 (20%) were not comparative studies and used only 68Ga-FAPI-04 as radiopharmaceutical. The latter is the most used radiopharmaceutical in analyzed studies (11/15, 73%), while other tracers, including 18F-FAPI, 68Ga-FAPI-46, and 68Ga-FAP-2286, were used in the remaining ones.
Most of these studies were conducted in China (10/15, 66%), 3/15 in Germany (20%), 1/15 (7%) in India, and 1/15 (7%) in Thailand.
The searches carried out can be further divided based on the different neoplasms examined: 6 studies (40%) examined various neoplasms, including HCC and CCA. Overall, 9/15 papers looked at both HCC and CCA, while 2/15 focused exclusively on BTC and 4/15 exclusively on HCC [28,29].
Table 1 summarizes the main characteristics of selected studies and their main results.

Table 1.
Main findings of the selected papers.
3.3. Methodological Quality
The CASP technique was used to qualitatively evaluate each of the 15 research papers. A few possible sources of bias were noted based on the quality assessment. Of the 15 papers, only 8 (53%) provided both the test and the reference standard to all participating patients. Additionally, as illustrated in Table 2, in 14 research papers (93%), the test and the standard or reference were conducted independently.

Table 2.
Quality appraisal of selected articles using the CASP checklist for diagnostic studies. ☺ Low risk; ? Unknown; ☹ High risk.
4. Discussion
Imaging plays a crucial role in the diagnosis of HCC and CCA. CT and MRI are the gold standard for non-invasive diagnosis and correct staging of the primary tumor. For HCC, MRI has greater sensitivity and specificity than CT [41], particularly for small lesions [42]. Similarly, MR cholangiography is used to identify CCA with a sensitivity of 100% and specificity of 95% [43,44].
Currently, PET-CT imaging techniques are used in the oncology field for the diagnosis and staging of various tumors, being also useful in detecting distant metastases [45]. The most commonly used PET radiotracer is 18F-FDG. Its mechanism is based on increased glucose metabolism in tumor cells, which have high levels of glucose transporter proteins (GLUT) and intracellular enzymes that promote glycolysis, leading to an accumulation of FDG in the tumor cell, especially if poorly differentiated [46]. For this reason, 18F-FDG PET is also useful for distinguishing benign cases such as focal nodular hyperplasia (FNH), where FDG accumulation is similar to underlying physiological liver activity [47].
However, the role of 18F-FDG PET in liver lesions is controversial, as it reports a sensitivity of 60.9% depending on the degree of differentiation and size of the tumor [48]. In fact, the liver is physiologically involved in glucose metabolism, leading to a notable uptake of FDG [49] with high background activity that does not allow a clear distinction in terms of TBR, particularly in well-differentiated HCCs, whose cells are similar to normal hepatocytes [46]. Therefore, 18F-FDG PET is not appropriate for discriminating well-differentiated or small lesions.
In ICC, the sensitivity of 18F-FDG PET is 91–95%, dropping, however, to 60% in extrahepatic cholangiocarcinoma [50,51].
Given the poor sensitivity of 18F-FDG PET in some types of tumors, new targets have been identified, including TME and, specifically, the expression of FAP. FAP-targeted radiopharmaceuticals have therefore been developed to allow the detection of cancer types characterized by a strong desmoplastic reaction, such as breast, ovarian, lung, pancreatic, colorectal, and head-neck carcinomas [37,52]. Also, in the immunohistochemical analysis of HCC and CCA, a significantly higher expression of FAP was observed compared to normal liver tissue [53], making FAP-targeted radiopharmaceuticals an attractive alternative to 18F-FDG in terms of sensitivity. A recent study by Liang et al. reported a sensitivity of 84.6% and 76.9% for the detection of malignant liver lesions with 18F-FAPI-04 and 18F-FDG, respectively [54].
The aim of this review is therefore to collect and describe published studies on the use of FAP-targeted radiopharmaceuticals in HCC and CCA.
Overall, most studies have shown that 68Ga-FAPI is more sensitive than 18F-FDG for HCC and CCA with a sensitivity ranging from 85% to 100% rather than 51–87% of 18F-FDG [29,30,31,32,33,34,36,38], especially for small and well- and moderately differentiated lesions, as demonstrated by Wang et al. finding a higher sensitivity than 18F-FDG in detecting intrahepatic HCC lesions with a diameter ≤ 2 cm (68.8% vs. 18.8%) and well- or moderately differentiated tumors (83.3% vs. 33.3%). In this study, the maximum standardized uptake value (SUVmax) was comparable between 68Ga-FAPI-04 and 18F-FDG, but the TBR was significantly higher in the 68Ga-FAPI-04 group [31] (Figure 2).
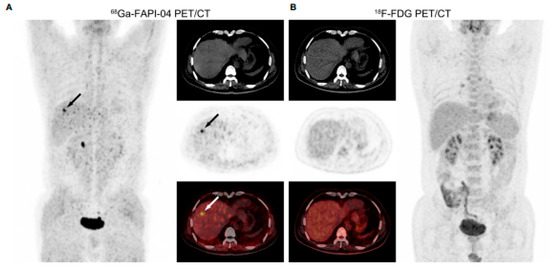
Figure 2.
From Wang et al., PET/CT images in a 53-year-old male patient with moderately differentiated HCC. (A) 68Ga-FAPI-04 PET/CT revealed a strongly FAPI-avid lesion (black and white arrows, SUVmax = 7.36, TBR = 6.03) in the right lobe of the liver. (B) No positive finding was observed within the liver in 18F-FDG PET/CT images (SUVmax = 2.36, TBR = 1.31) [31].
Even in terms of accuracy, the results seem promising [30,36]. Only three studies reported the specificity values. According to their results, the specificity ranges from 72 to 100% [29,30,32]. Only two studies reported a worse specificity compared to 18F-FGD, specifically 90% vs. 100% [30] and 72% vs. 76% [29].
Despite that, in the paper by Rajaraman et al., although the specificity of 68Ga FAPI is worse than 18F-FDG for HCC (85.71% vs. 100%), in CCA 68Ga FAPI is significantly more sensitive (94.4% vs. 50%) and accurate (95.24% vs. 57.14%), with a specificity reaching 100% [30].
The lower physiological hepatic absorption of 68Ga-FAPI allowed the detection of intrahepatic metastases [29,34,36,37]. Furthermore, unlike 18F-FDG, FAP-targeted radiopharmaceuticals are not absorbed in the gastrointestinal tract [55], contributing to better detection of peritoneal metastases [19,29,33,34,36,37,38]. Overall, encouraging results were obtained in the study by Jinghua et al., showing a sensitivity of 68Ga-FAPI higher than 18F-FDG with a few differences in terms of specificity and accuracy [29].
A sensitivity and specificity of 100% were found by Shi et al. [32]. According to their data, FAP-targeted radiopharmaceuticals are the best among currently used PET tracers. FAPI hybrid imaging presents advantages in discerning liver tumors and benign lesions, being able to overcome the limitations of conventional radiological imaging, especially in hypovascular nodules such as well-differentiated HCC. Moreover, FAP-targeted radiopharmaceuticals, being a molecular imaging method, could reflect the histopathological characteristics of the lesion. Furthermore, FAPI has great potential as a new theragnostic target in staging and detecting disease recurrence, as well as in therapeutic and prognostic evaluation of patients with hepatic tumors [32].
Another prospective pilot study by Shi et al. shows high sensitivity and accuracy of 68Ga-FAPI-04 in detecting malignant liver neoplasms, especially those that are poorly differentiated and have high immunohistochemical expression of FAP [35].
These characteristics were also investigated by Zhang J. et al. in a prospective study evaluating non-FDG-avid focal liver lesions. For primary lesions, the sensitivity, specificity, and accuracy were 96%, 58%, and 94%, respectively, in line with the results of the other studies examined. In semi-quantitative analysis, SUVmax and TBR of 18F-FAPI in hepatic neoplasms were significantly higher than in benign non-inflammatory focal liver lesions (FLL). However, there was no significant difference in SUVmax of 18F-FAPI PET between HCC lesions and inflammatory FLLs, but the TBR in HCC was significantly lower [18]. The results of this study therefore suggest that 18F-FAPI could be a promising radiopharmaceutical for the differential diagnosis of benign and malignant hepatic masses with non-avidity for 18F-FDG.
Similarly, Geist B.K., et al. sought to establish kinetic models to differentiate HCC lesions from non-HCC lesions and concluded that the SUVmax of 68Ga-FAPI-04 is sufficiently effective in distinguishing healthy regions, HCC lesions and non-HCC lesions [39].
This review includes studies carried out on different types of cancer, including HCC and CCA [19,34,36,37,40]. Pang Y et al. compared 68Ga-FAP-2286 and 68Ga-FAPI-46, confirming that they could both be a valid alternative to 18F-FDG for cancer types such as gastric, pancreatic, and liver cancers [19]. In particular, 68Ga-FAP-2286 is a promising FAPI derivative with improved biological properties compared to the small molecule FAPI series [56], including binding affinity and amplified receptor selectivity, due to major plasma stability and conformational rigidity. In preclinical studies, FAP-2286 demonstrated longer tumor retention than FAPI-46 [57].
Kratochwil et al. observed 68Ga-FAPI-04 uptake in 28 types of cancer. In the semi-quantitative analysis, the highest SUVmax (>12) was found in CCA, in sarcoma, as well as breast, esophageal, and lung cancers, while an intermediate SUVmax (6–12) was found in HCC, colorectal, pancreatic, prostatic, ovarian and head-neck carcinomas. The high TBR allowed the detection of liver metastases as small as 1 cm in diameter and the identification of peritoneal carcinomatosis, particularly frequent in ovarian cancer. This study therefore underlines the potential of FAPI tracers for the non-invasive theragnostic approach to tumors [37].
A similar prospective study was carried out by Chen et al., showing a greater sensitivity of 68Ga-FAPI-04 compared to 18F-FDG (98.2% vs. 82.1%). Lymph node uptake was also significantly higher in the neck, supraclavicular, abdomen, and mediastinum regions. Regarding metastatic lesions, SUVs derived from 68Ga-FAPI were 2–3 times greater than values derived from 18F-FDG. Indeed, more lesions were discovered in the regions of the liver, brain, axial skeleton, peritoneum, mesentery, and omentum, with higher sensitivity (83.8% vs. 59.5%) and accuracy (73.5% vs. 59.2%). In this study, 68Ga-FAPI-04 PET-CT was able to visualize very small tumors (diameter < 1.0 cm), which were not detected by 18F-FDG PET-CT. Furthermore, the study highlights the utility of the FAPI tracer in delineating gross tumor volume in preparation for external beam radiotherapy due to exceptionally clear tumor delineation and high TBR in nasopharyngeal carcinoma [34].
Lan et al. compared 68Ga-FAPI-04 and 18F-FDG PET-CT in various oncological and non-oncological lesions. 68Ga-FAPI-04 demonstrated a significantly higher uptake and detection rate for primary lesions (sensitivity 97.67 vs. 84.89%; and accuracy 96.59 vs. 82.95%), nodal metastasis (sensitivity 97.59 vs. 84.72%; and accuracy 97.34 vs. 84.31%) and distant metastasis (sensitivity 98.01 vs. 65.59%; and accuracy 97.04 vs. 65.51%) of solid tumor, while it showed a lower activity and detection rate (sensitivity 50.65 vs. 96.75% and accuracy 51.28 vs. 95.51%) for multiple myeloma and lymphoma compared to 18F-FDG. As regards non-oncological lesions and benign tumors, the two radiopharmaceuticals showed comparative detection activity and efficacy (sensitivity 86.52 vs. 72.34% and accuracy 84.83 vs. 72.41%) [36].
The study by Kosmala et al. analyzes tumors of the gastrointestinal tract, such as HCC, CCA, pancreatic cancer, colorectal cancer, and neuroendocrine tumors, comparing PET-CT with 68Ga-FAPI-04 to gold-standard conventional imaging for the specific pathology. In this study, 68Ga-FAPI-04 brought changes in half of patients with digestive system cancers, particularly HCC and pancreatic cancers, leading to changes in therapeutic management in 25% of these patients, identifying local and distant lesions that allowed the beginning of systemic chemotherapy [40].
There have also been studies comparing FAPI PET and FDG PET to MRI as the gold standard [33,38]. The results suggest that 68Ga-FAPI PET-CT can more accurately detect liver cancer than 18F-FDG PET-CT or MRI alone [33]. Indeed, 68Ga-FAPI-46 showed a favorable TBR, detecting 100% of intrahepatic tumors like MRI. In contrast, only 52% of lesions were detected with 18F-FDG. The main advantage of PET-CT over MRI is the detection of extrahepatic metastases. Positive uptake rates of regional lymph node metastases were 100% with 68Ga-FAPI-46 and 58% with 18F-FDG. More distant metastatic lesions were detected with 68Ga-FAPI-46 compared to 18F-FDG (96% vs. 89%, respectively), with 4 peritoneal metastases and 1 brain metastasis detected with 68Ga-FAPI-46 and not detectable with 18F-FDG PET-CT [33].
In the same way, the sensitivity of 68Ga-FAPI-04 PET-CT in the retrospective analysis by Guo et al. is equivalent to that of CE-CT and liver MRI to identify primary liver tumors and metastatic disease. The sensitivity of CE-CT and liver MRI for the detection of primary liver tumors is 96% and 100%, respectively, while PET-CT imaging with 18F-FDG and 68Ga-FAPI-04 has a sensitivity of 65% and 96%, respectively. Moreover, Ga-FAPI-04 detected more metastatic lesions than 18F-FDG, especially peritoneal carcinomatosis and bone metastasis, thus improving liver cancer evaluation and subsequent therapeutic management [38]. Given the superiority of MRI over CT in primary hepatic tumors and the emergent implementation of hybrid imaging techniques, PET/MRI with FAP-targeted radiopharmaceuticals may have a future central role in the detection and characterization of both HCC and CCA, thus further improving diagnostic performance.
Similar comparisons were also performed between 68Ga-FAPI-46 PET-CT, 18F-FDG PET-CT, and conventional CT, confirming superior detection efficacy and tumor-to-background uptake of PET-CT with 68Ga-FAPI-46 for the detection of primary tumor, lymph nodes, and distant metastases, especially regarding grade 3 CCA [28].
Treatment options for CCA are limited and dependent on the stage and location of the tumor. Surgical resection is curative in the early stages. However, CCA is often diagnosed in advanced stages, and chemotherapy treatment is very often required. Radiotherapy can be used as a palliative treatment or in case of an incomplete resection [6].
In the last decade, the so-called radiotheranostics, combining PET imaging with targeted radioligand therapy (RLT), has continuously expanded [58]. In this context, FAP-targeting agents can be bound to therapeutic isotopes such as the β − emitters 90-Yttrium (90Y) and Lutetium-177 (177Lu) for therapeutic purposes. Given the encouraging results of RLT with 90Y-FAPI and 177Lu-FAPI for other tumor entities such as sarcoma, pancreatic adenocarcinoma, and breast cancer [59,60,61,62,63], the study by Pabst et al. indicated promising therapeutic use in CCA, worthy of further research [28].
5. Limitations
A mention of some major limitations and drawbacks of this systematic review is needed.
First of all, it is worth highlighting that the number of selected studies is quite limited due to the specific focus on the role of FAP-targeted radiopharmaceuticals in HCC and CCA, without including other types of tumors. For the same reason, the number of patients is quite small and extremely variable. In addition, it is worth considering that new publications that emerged after the completion of the systematic review might not have been included.
Moreover, one of the major concerns in the analyzed literature is represented by the use of different types of FAP-targeted compounds radiolabeled with different isotopes, making a direct comparison between the performances of the radiopharmaceuticals extremely challenging.
An additional non-negligible limitation is represented by the lack of a meta-analysis of the results.
6. Conclusions
FAP-targeted radiopharmaceuticals show better sensitivity than 18F-FDG with an excellent TBR in HCC and CCA. Specificity is also good, especially for diagnosing primary lesions. The theragnostic potential of these new-generation radiopharmaceuticals deserves further and intensive research in these tumor entities.
Author Contributions
Conceptualization, V.F. and M.S.D.F.; methodology, M.S.D.F. and G.D.V.; validation, B.P. and L.F. formal analysis, L.S. and G.D.V.; investigation, L.S.; writing—original draft preparation, L.S.; writing—review and editing, V.F.; visualization, B.P.; supervision, G.D.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Liver Cancer Collaboration; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, C.; Zhang, H.; Zhang, L.; Zhu, A.X.; Bernards, R.; Qin, W.; Wang, C. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.I. Hepatocellular carcinoma: Current management and future trends. Gastroenterology 2004, 127 (Suppl. 1), S218–S224. [Google Scholar] [CrossRef] [PubMed]
- Sirica, A.E.; Campbell, D.J.; Dumur, C.I. Cancer-associated fibroblasts in intrahepatic cholangiocarcinoma. Curr. Opin. Gastroenterol. 2011, 27, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Sirica, A.E. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 9, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koustoulidou, S.; Hoorens, M.W.H.; Dalm, S.U.; Mahajan, S.; Debets, R.; Seimbille, Y.; de Jong, M. Cancer-Associated Fibroblasts as Players in Cancer Development and Progression and Their Role in Targeted Radionuclide Imaging and Therapy. Cancers 2021, 13, 1100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hartel, M.; Di Mola, F.F.; Gardini, A.; Zimmermann, A.; Di Sebastiano, P.; Guweidhi, A.; Innocenti, P.; Giese, T.; Giese, N.; Büchler, M.W.; et al. Desmoplastic reaction influences pancreatic cancer growth behavior. World J. Surg. 2004, 28, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.J.; Alpaugh, R.K.; Palazzo, I.; Meropol, N.J.; Rogatko, A.; Xu, Z.; Hoffman, J.P.; Weiner, L.M.; Cheng, J.D. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas 2008, 37, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Räsänen, K.; Vaheri, A. Activation of fibroblasts in cancer stroma. Exp. Cell Res. 2010, 316, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, M.; Mellody, K.T.; Orimo, A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin. Cell Dev. Biol. 2010, 21, 19–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lamarca, A.; Barriuso, J.; Chander, A.; McNamara, M.G.; Hubner, R.A.; ÓReilly, D.; Manoharan, P.; Valle, J.W. 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) for patients with biliary tract cancer: Systematic review and meta-analysis. J. Hepatol. 2019, 71, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, H.J.; Park, J.H.; Park, D.I.; Cho, Y.K.; Sohn, C.I.; Jeon, W.K.; Kim, B.I. Clinical usefulness of 18F-FDG PET-CT for patients with gallbladder cancer and cholangiocarcinoma. J. Gastroenterol. 2010, 45, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Lan, B.Y.; Kwee, S.A.; Wong, L.L. Positron emission tomography in hepatobiliary and pancreatic malignancies: A review. Am. J. Surg. 2012, 204, 232–241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; He, Q.; Jiang, S.; Li, M.; Xue, H.; Zhang, D.; Li, S.; Peng, H.; Liang, J.; Liu, Z.; et al. [18F]FAPI PET/CT in the evaluation of focal liver lesions with [18F]FDG non-avidity. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zhao, L.; Meng, T.; Xu, W.; Lin, Q.; Wu, H.; Zhang, J.; Chen, X.; Sun, L.; Chen, H. PET Imaging of Fibroblast Activation Protein in Various Types of Cancer Using 68Ga-FAP-2286: Comparison with 18F-FDG and 68Ga-FAPI-46 in a Single-Center, Prospective Study. J. Nucl. Med. 2023, 64, 386–394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mu, X.; Huang, X.; Li, M.; Sun, W.; Fu, W. Comparison of physiological uptake of normal tissues in patients with cancer using 18F-FAPI-04 and 18F-FAPI-42 PET/CT. Front. Nucl. Med. 2022, 2, 927843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roustaei, H.; Kiamanesh, Z.; Askari, E.; Sadeghi, R.; Aryana, K.; Treglia, G. Could Fibroblast Activation Protein (FAP)-Specific Radioligands Be Considered as Pan-Tumor Agents? Contrast Media Mol. Imaging 2022, 2022, 3948873. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sidrak, M.M.A.; De Feo, M.S.; Corica, F.; Gorica, J.; Conte, M.; Filippi, L.; Schillaci, O.; De Vincentis, G.; Frantellizzi, V. Fibroblast Activation Protein Inhibitor (FAPI)-Based Theranostics-Where We Are at and Where We Are Heading: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 3863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Evangelista, L.; Frantellizzi, V.; Schillaci, O.; Filippi, L. Radiolabeled FAPI in pancreatic cancer: Can it be an additional value in the management of patients? Expert. Rev. Anticancer. Ther. 2023, 23, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Pu, Y.; Huang, S.; Yang, C.; Yang, F.; Pu, Y.; Li, J.; Chen, L.; Huang, Y. FAPI-PET/CT in Cancer Imaging: A Potential Novel Molecule of the Century. Front. Oncol. 2022, 12, 854658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arçay Öztürk, A.; Flamen, P. FAP-targeted PET imaging in gastrointestinal malignancies: A comprehensive review. Cancer Imaging 2023, 23, 79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Singh, J. Critical appraisal skills programme. J. Pharmacol. Pharmacother. 2013, 4, 76–77. [Google Scholar] [CrossRef]
- Pabst, K.M.; Trajkovic-Arsic, M.; Cheung, P.F.Y.; Ballke, S.; Steiger, K.; Bartel, T.; Schaarschmidt, B.M.; Milosevic, A.; Seifert, R.; Nader, M.; et al. Superior Tumor Detection for 68Ga-FAPI-46 Versus 18F-FDG PET/CT and Conventional CT in Patients with Cholangiocarcinoma. J. Nucl. Med. 2023, 64, 1049–1055. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jinghua, L.; Kui, X.; Deliang, G.; Bo, L.; Qian, Z.; Haitao, W.; Yaqun, J.; Dongde, W.; Xigang, X.; Ping, J.; et al. Clinical prospective study of Gallium 68 (68Ga)-labeled fibroblast-activation protein inhibitor PET/CT in the diagnosis of biliary tract carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2152–2166, Erratum in Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2230. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, V.; Meenakshi, L.A.; Selvaraj, A.J.; Pottakkat, B.; Halanaik, D. Role of 68Ga-FAPI PET/CT in Assessing Hepatobiliary Malignancies: A Prospective Pilot Study. Clin. Nucl. Med. 2023, 48, e281–e288. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, W.; Ren, S.; Kong, Y.; Huang, Q.; Zhao, J.; Guan, Y.; Jia, H.; Chen, J.; Lu, L.; et al. 68Ga-FAPI-04 Versus 18F-FDG PET/CT in the Detection of Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 693640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, X.; Xing, H.; Yang, X.; Li, F.; Yao, S.; Congwei, J.; Zhao, H.; Hacker, M.; Huo, L.; Li, X. Comparison of PET imaging of activated fibroblasts and 18F-FDG for diagnosis of primary hepatic tumours: A prospective pilot study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Siripongsatian, D.; Promteangtrong, C.; Kunawudhi, A.; Kiatkittikul, P.; Boonkawin, N.; Chinnanthachai, C.; Jantarato, A.; Chotipanich, C. Comparisons of Quantitative Parameters of Ga-68-Labelled Fibroblast Activating Protein Inhibitor (FAPI) PET/CT and [18F]F-FDG PET/CT in Patients with Liver Malignancies. Mol. Imaging Biol. 2022, 24, 818–829. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, H.; Pang, Y.; Wu, J.; Zhao, L.; Hao, B.; Wu, J.; Wei, J.; Wu, S.; Zhao, L.; Luo, Z.; et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1820–1832. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xing, H.; Yang, X.; Li, F.; Yao, S.; Zhang, H.; Zhao, H.; Hacker, M.; Huo, L.; Li, X. Fibroblast imaging of hepatic carcinoma with 68Ga-FAPI-04 PET/CT: A pilot study in patients with suspected hepatic nodules. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Liu, H.; Wang, Y.; Deng, J.; Peng, D.; Feng, Y.; Wang, L.; Chen, Y.; Qiu, L. The potential utility of [68Ga]Ga-DOTA-FAPI-04 as a novel broad-spectrum oncological and non-oncological imaging agent-comparison with [18F]FDG. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 963–979. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, W.; Pang, Y.; Yao, L.; Zhao, L.; Fan, C.; Ke, J.; Guo, P.; Hao, B.; Fu, H.; Xie, C.; et al. Imaging fibroblast activation protein in liver cancer: A single-center post hoc retrospective analysis to compare [68Ga]Ga-FAPI-04 PET/CT versus MRI and [18F]-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1604–1617. [Google Scholar] [CrossRef] [PubMed]
- Geist, B.K.; Xing, H.; Wang, J.; Shi, X.; Zhao, H.; Hacker, M.; Sang, X.; Huo, L.; Li, X. A methodological investigation of healthy tissue, hepatocellular carcinoma, and other lesions with dynamic 68Ga-FAPI-04 PET/CT imaging. EJNMMI Phys. 2021, 8, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kosmala, A.; Serfling, S.E.; Schlötelburg, W.; Lindner, T.; Michalski, K.; Schirbel, A.; Higuchi, T.; Hartrampf, P.E.; Buck, A.K.; Weich, A.; et al. Impact of 68Ga-FAPI-04 PET/CT on Staging and Therapeutic Management in Patients with Digestive System Tumors. Clin. Nucl. Med. 2023, 48, 35–42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chou, R.; Cuevas, C.; Fu, R.; Devine, B.; Wasson, N.; Ginsburg, A.; Zakher, B.; Pappas, M.; Graham, E.; Sullivan, S.D. Imaging Techniques for the Diagnosis of Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2015, 162, 697–711, Erratum in Ann. Intern. Med. 2015, 162, 880. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, J.M.; Lee, J.S.; Lee, H.Y.; Park, B.H.; Kim, Y.H.; Han, J.K.; Choi, B.I. Hepatocellular carcinoma: Diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology 2015, 275, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Romagnuolo, J.; Bardou, M.; Rahme, E.; Joseph, L.; Reinhold, C.; Barkun, A.N. Magnetic resonance cholangiopancreatography: A meta-analysis of test performance in suspected biliary disease. Ann. Intern. Med. 2003, 139, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Patel, T. Cholangiocarcinoma--controversies and challenges. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 189–200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kato, T.; Tsukamoto, E.; Kuge, Y.; Katoh, C.; Nambu, T.; Nobuta, A.; Kondo, S.; Asaka, M.; Tamaki, N. Clinical role of 18F-FDG PET for initial staging of patients with extrahepatic bile duct cancer. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Martin, A.; Zerizer, I. Positron emission tomography-computed tomography in liver imaging. Semin. Ultrasound CT MRI. 2013, 34, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Aznar, D.L.; Ojeda, R.; Garcia, E.U.; Aparici, F.; Sánchez, P.A.; Flores, D.; Martínez, C.; Sopena, R. Focal nodular hyperplasia (FNH): A potential cause of false-positive positron emission tomography. Clin. Nucl. Med. 2005, 30, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kim, J.H.; Kim, S.K.; Kang, K.W.; Park, K.W.; Choi, J.I.; Lee, W.J.; Kim, C.M.; Nam, B.H. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J. Nucl. Med. 2008, 49, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Eo, J.S.; Paeng, J.C.; Lee, D.S. Nuclear imaging for functional evaluation and theragnosis in liver malignancy and transplantation. World J. Gastroenterol. 2014, 20, 5375–5388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kluge, R.; Schmidt, F.; Caca, K.; Barthel, H.; Hesse, S.; Georgi, P.; Seese, A.; Huster, D.; Berr, F. Positron emission tomography with [18F]fluoro-2-deoxy-D-glucose for diagnosis and staging of bile duct cancer. Hepatology 2001, 33, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Yun, M.; Lee, W.J.; Kim, K.S.; Lee, J.D. Usefulness of 18F-FDG PET in intrahepatic cholangiocarcinoma. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Siveke, J.T. Fibroblast-Activating Protein: Targeting the Roots of the Tumor Microenvironment. J. Nucl. Med. 2018, 59, 1412–1414. [Google Scholar] [CrossRef] [PubMed]
- Puré, E.; Blomberg, R. Pro-tumorigenic roles of fibroblast activation protein in cancer: Back to the basics. Oncogene 2018, 37, 4343–4357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, Z.; Peng, H.; Li, W.; Liu, Z. Head-to-head study of [18F]FAPI-04 PET/CT and [18F]FDG PET/CT for non-invasive assessment of liver cancer and its immunohistochemical markers. BMC Cancer 2024, 24, 1378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pang, Y.; Zhao, L.; Luo, Z.; Hao, B.; Wu, H.; Lin, Q.; Sun, L.; Chen, H. Comparison of 68Ga-FAPI and 18F-FDG Uptake in Gastric, Duodenal, and Colorectal Cancers. Radiology 2021, 298, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.F.L.; Blecha, J.; Rosenberg, O.; Ohliger, M.; Flavell, R.R.; Wilson, D.M. Cyclic 68Ga-Labeled Peptides for Specific Detection of Human Angiotensin-Converting Enzyme 2. J. Nucl. Med. 2021, 62, 1631–1637. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zboralski, D.; Hoehne, A.; Bredenbeck, A.; Schumann, A.; Nguyen, M.; Schneider, E.; Ungewiss, J.; Paschke, M.; Haase, C.; von Hacht, J.L.; et al. Preclinical evaluation of FAP-2286 for fibroblast activation protein targeted radionuclide imaging and therapy. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3651–3667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herrmann, K.; Schwaiger, M.; Lewis, J.S.; Solomon, S.B.; McNeil, B.J.; Baumann, M.; Gambhir, S.S.; Hricak, H.; Weissleder, R. Radiotheranostics: A roadmap for future development. Lancet Oncol. 2020, 21, e146–e156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferdinandus, J.; Costa, P.F.; Kessler, L.; Weber, M.; Hirmas, N.; Kostbade, K.; Bauer, S.; Schuler, M.; Ahrens, M.; Schildhaus, H.U.; et al. Initial Clinical Experience with 90Y-FAPI-46 Radioligand Therapy for Advanced-Stage Solid Tumors: A Case Series of 9 Patients. J. Nucl. Med. 2022, 63, 727–734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Kaghazchi, F.; Aghdam, R.A.; Haghighi, S.; Vali, R.; Adinehpour, Z. 177Lu-FAPI Therapy in a Patient with End-Stage Metastatic Pancreatic Adenocarcinoma. Clin. Nucl. Med. 2022, 47, e243–e245. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Kramer, V.; Moon, E.S.; Roesch, F.; Tripathi, M.; Mallick, S.; ArunRaj, S.T.; Bal, C. A theranostic approach of [68Ga]Ga-DOTA.SA.FAPi PET/CT-guided [177Lu]Lu-DOTA.SA.FAPi radionuclide therapy in an end-stage breast cancer patient: New frontier in targeted radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 942–944. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Pabst, K.M.; Kessler, L.; Fragoso Costa, P.; Ferdinandus, J.; Weber, M.; Lippert, M.; Lueckerath, K.; Umutlu, L.; Kostbade, K.; et al. Safety and Efficacy of 90Y-FAPI-46 Radioligand Therapy in Patients with Advanced Sarcoma and Other Cancer Entities. Clin. Cancer Res. 2022, 28, 4346–4353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).