New Insights into Common Bean (Phaseolus vulgaris L.) Sprouts: Pilot Studies on the Formulation of a Cosmeceutical Based on Micellar Extracts Bean Sprouts
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Development of Recipes for Creams with Common Bean (Phaseolus vulgaris L.) Sprout Extract and Selection of the Cream with the Most Favorable Physicochemical Properties
2.2.1. Preparation of the Extract
2.2.2. Preparation of Cosmetic Creams
2.3. Evaluation of Physicochemical and Organoleptic Properties
2.3.1. Evaluation of Centrifugal Stability
2.3.2. Organoleptic Evaluation of the Preparations
2.3.3. In Vitro Occlusion Assessment
2.3.4. Determination of Emulsion Type
2.3.5. Determination of Oil-Phase Droplet Size
2.3.6. Determination of pH
2.3.7. Rheological Analysis of the Creams
2.3.8. Spreadability Test for Creams
2.3.9. Slip Test for Creams
2.3.10. Long-Term Stability and Accelerated Aging Studies for C1 Cream
2.4. Evaluation of the Efficacy of Common Bean (Phaseolus vulgaris L.) Sprout Extract Cream Under In Vivo Conditions
Statistical Methodology
3. Results
3.1. Evaluation of Physicochemical and Organoleptic Properties
3.2. Long-Term Stability Analysis of the Selected Formulation for Volunteer Testing
3.3. Evaluation of the Efficacy of Common Bean (Phaseolus vulgaris L.) Sprout Micellar Extract Cream Under In Vivo Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devi, M.; Dhanalakshmi, G.E.; Govindarajan, T.; Tanisha, B.A.; Sonalika, T.; Ruth, J.E.; Avinash, C.; Sri, J.; Logeswaran, K.; Ramasamy, M. A review on Phaseolus vulgaris Linn. Pharmacogn. J. 2020, 12, 1160–1164. [Google Scholar] [CrossRef]
- Santos, E.; Marques, G.; Lino-Neto, T. Phaseolus vulgaris L. as a functional food for aging protection. Oxidative Stress and Dietary Antioxidants. Aging 2020, 2, 289–295. [Google Scholar] [CrossRef]
- Fonseca-Hernándezn, D.; Lugo-Cervantes, E.D.C.; Escobedo-Reyes, A.; Mojica, L. Black bean (Phaseolus vulgaris L.) polyphenolic extract exerts antioxidant and antiaging potential. Molecules 2021, 26, 6716. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-L.; Choi, J.-H.; Park, S.-Y.; Jeong, M.-Y.; Lee, H.-C.; Song, J.-H. The influences of Phaseolus radiatus L. Ethanol extracts and fractions on skin whitening and anti-inflammatory effects. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2018, 31, 39–49. [Google Scholar] [CrossRef]
- Oh, S.; Zheng, S.; Fang, M.; Kim, M.; Bellere, A.D.; Jeong, J.; Yi, T.-H. Anti-photoaging effect of Phaseolus angularis L. Extract on UVB-exposed HaCaT keratinocytes and possibilities as cosmetic materials. Molecules 2023, 28, 1407. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Usman, M.; Nawaz, H.A.; Rasheed, H.; Khan, R.R.; Anjum, S.M.M.; Khokhar, M.I.; Akhtar, N. Formulation of Phaseolus vulgaris L. Cream and its characterization. Pak. J. Pharm. Sci. 2020, 33, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Peñas, E.; Martínez-Villaluenga, C. Advances in production, properties and applications of sprouted seeds. Foods 2020, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Geyfman, M.; Chung, R.; Boissy, R.; Poloso, N.; Kadoya, K.; Maitra, P.; Mehta, R. Lotus sprout extract induces selective melanosomal autophagy and reduces pigmentation. J. Cosmet. Dermatol. 2024, 24, e16587. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.Y. A study on peanut sprouts extract as the anti-oxidant activity and the skin whitening cosmetic ingredients. KSBB J. 2016, 31, 14–19. [Google Scholar] [CrossRef]
- Schmid, D.; Sacher, R.; Belser, E.; Zülli, F. Vegetable sprouts: A potent source for cosmetic actives. Househ. Pers. Care Today 2011, 1, 50–52. [Google Scholar]
- Rostkowska, E.; Poleszak, E.; Przekora, A.; Wójcik, M.; Typek, R.; Wojciechowska, K.; Dos Santos Szewczyk, K. Novel insights into Phaseolus vulgaris L. sprouts: Phytochemical analysis and anti-aging properties. Molecules 2024, 29, 3058. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E. Cosmetics Emulsions; Wydawnictwo Politechniki Krakowskiej: Kraków, Poland, 2019. [Google Scholar]
- Wissing, S.A.; Lippacher, A.; Muller, R.H. Investigations on the occlusive properties of solid lipid nanoparticles (SLN). J. Cosmet. Sci. 2001, 52, 313–324. [Google Scholar] [PubMed]
- Garg, A.; Aggarwal, D.; Garg, S.; Singla, A.K. Spreading of semisolid formulations: An update. Pharm. Technol. 2002, 26, 84–105. [Google Scholar]
- Wojciechowska, K.; Walczak, A.; Rostowska, E.; Poleszak, E. Comparison of sensory and rheological properties of green cosmetic creams prepared on different natural, ECOCERT, and BDIH certificated self-emulsifying bases. Curr. Issues Pharm. Med. Sci. 2021, 34, 218–223. [Google Scholar] [CrossRef]
- Moravkova, T.; Stern, P. Rheological and textural properties of cosmetic emulsions. Appl. Rheol. 2011, 21, 35200. [Google Scholar] [CrossRef]
- Calixto, L.S.; Infante, V.H.P.; Maia Campos, P.M.B.G. Design and characterization of topical formulations: Correlations between instrumental and sensorial measurements. AAPS PharmSciTech 2018, 19, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Dapcević, T.; Hadnad, P.; Dokic, P.; Krstonos, V.; Hadnad, M. Influence of oil phase concentration on droplet size distribution and stability of oil-in-water emulsions. Eur. J. Lipid Sci. Technol. 2013, 115, 313–321. [Google Scholar] [CrossRef]
- Chanamai, R.; McClements, D.J. Dependence of creaming and rheology of monodisperse oil-in-water emulsions on droplet size and concentration. Colloid Surf. A 2006, 172, 79–86. [Google Scholar] [CrossRef]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Deuschle, V.C.K.N.; Deuschle, R.A.N.; Bortoluzzi, M.R.; Athayde, M.L. Physical chemistry evaluation of stability, spreadability, in vitro antioxidant, and photo-protective capacities of topical formulations containing Calendula officinalis L. leaf extract. Braz. J. Pharm. Sci. 2015, 51. [Google Scholar] [CrossRef]
- Moravkova, T.; Filip, P. The influence of thickeners on the rheological and sensory properties of cosmetic lotions. Acta Polytech. Hung. 2014, 11, 173–186. [Google Scholar]
- Adejokun, D.A.; Dodou, K. A novel method for the evaluation of the long-term stability of cream formulations containing natural oils. Cosmetics 2020, 7, 86. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Gloor, M.; Lazzerini, S.; Kleesz, P.; Grieshaber, R.; Berardesca, E. Comparative study of five instruments measuring stratum corneum hydration (Corneometer CM 820 and CM 825, Skicon 200, Nova DPM 9003, Dermalab). Part I: In vitro. Skin Res. Technol. 1999, 5, 161–170. [Google Scholar] [CrossRef]
- Courage Website; Khazaka Electric GmbH. Corneometer® CM 825. Available online: https://www.courage-khazaka.com/en/scientific-products/corneometer-cm-825 (accessed on 29 June 2024).
- Heinrich, U. Multicentre comparison of skin hydration in terms of physical-, physiological- and product-dependent parameters by the capacitance method (Corneometer CM 825). Int. J. Cosmet. Sci. 2003, 25, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Krueger, N.; Luebberding, S.; Oltmer, M.; Streker, M.; Kerscher, M. Age-related changes in skin mechanical properties: A quantitative evaluation of 120 female subjects. Skin Res. Technol. 2011, 17, 141–148. [Google Scholar] [CrossRef]
- Barcaui, E.; Carvalho, A.; Lopes, F.; Piñeiro-Maceira, J.; Barcaui, C. High-Frequency Ultrasound with Color Doppler in Dermatology. An. Bras. Dermatol. 2016, 91, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, F.; Wright, C.; Nester, C.; Lam, S. The reliability of non-invasive biophysical outcome measures for evaluating normal and hyperkeratotic foot skin. J. Foot Ankle Res. 2015, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kwon, S.; Hun Huh, C.; Park, K.; Woong Youn, S. The influences of skin viscoelasticity, hydration level, and aging on the formation of wrinkles: A comprehensive and objective approach. Skin Res. Technol. 2013, 19, e349–e355. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.; Na, J.; Choi, S.; Hun Huh, C.; Park, K. Regional and seasonal variations in facial sebum secretions: A proposal for the definition of combination skin type. Skin Res. Technol. 2005, 11, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Crowther, J.M. Method for quantification of oils and sebum levels on skin using the Sebumeter®. Int. J. Cosmet. Sci. 2016, 38, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Nayak, C.S.; Ansari, S.M.M.; Salve, V.; Patil, S. Effectiveness of a combination of anti-pigmentary products in facial post-inflammatory hyperpigmentation. Int. J. Res. Dermatol. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Neto, P.; Ferreira, M.; Bahia, F.; Costa, P. Improvement of the methods for skin mechanical properties evaluation through correlation between different techniques and factor analysis. Skin Res. Technol. 2013, 19, 4. [Google Scholar] [CrossRef]
- Watanabe, F.; Hashizume, E.; Chan, G.; Kamimura, A. Skin-whitening and skin-condition-improving effects of topical oxidized glutathione: A double-blind and placebo-controlled clinical trial in healthy women. Clin. Cosmet. Investig. Dermatol. 2014, 7, 267–274. [Google Scholar] [CrossRef]
- Rostkowska, E.; Poleszak, E.; Wojciechowska, K.; Dos Santos Szewczyk, K. Dermatological management of aged skin. Cosmetics 2023, 10, 55. [Google Scholar] [CrossRef]
- Trojahn, C.; Dobos, G.; Lichterfeld, A.; Blume-Peytavi, U.; Kottner, J. Characterizing facial skin ageing in humans: Disentangling extrinsic from intrinsic biological phenomena. Hindawi BioMed Res. Int. 2015. [Google Scholar] [CrossRef]
- Fluhr, J.; Darlenski, R. Transepidermal Water Loss (TEWL). In Non-Invasive Diagnostic Techniques in Clinical Dermatology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 353–356. [Google Scholar]
- Zięba, M.; Czerwonka, D.; Ruszkowska, M. Micellar extracts obtained from Bistortae rhizoma, Fraxinus excelsior, and Romex crispus as components of hair shampoos. De Gruyter 2024. [Google Scholar] [CrossRef]

| C1 | C2 | C3 | |||
| Phase A—oil | |||||
| GSC | 3.0 | MSG | 3.0 | Cetyl palmitate | 2.5 |
| Emulgade succro | 1.5 | MG | 1.25 | Cetyl alcohol | 2.0 |
| Sucrose stearate | 0.7 | Sucrose stearate | 0.25 | Behenyl alcohol | 1.0 |
| Cetyl alkohol | 1.5 | Cetyl alkohol | 1.5 | Heptyl undecylate | 0.75 |
| Heptyl undecylate | 0.7 | Heptyl undecylate | 1.0 | Isopropyl Mirystate | 0.75 |
| Isopropyl Mirystate | 0.7 | Isopropyl Mirystate | 0.5 | C/CT | 2.0 |
| C/CT | 3.5 | C/CT | 3.5 | C12-15 alkane | 0.7 |
| C12-15 alkane | 0.7 | C12-15 alkane | 0.75 | Ethylhexyl Olivate | 1.0 |
| Ethylhexyl Olivate | 1.5 | Shea light | 0.75 | Shea butter | 2.0 |
| Shea butter | 1.5 | Shea butter | 1.5 | Sweet almond oil | 3.0 |
| Sweet almond oil | 3.0 | Sweet almond oil | 3.0 | Argania spinosa oil | 0.5 |
| Argania spinosa oil | 0.5 | Argania spinosa oil | 0.5 | Shea light | 1.0 |
| Dicapryl propanediol | 0.4 | Dicapryl propanediol | 0.5 | ||
| Phase B—water | |||||
| Propanediol | 2.2 | Propanediol | 2.2 | Propanediol | 2.0 |
| Sodium phytate | 0.1 | Sodium phytate | 0.1 | L-arginine | 1.5 |
| Panthenol | 1.0 | Panthenol | 1.0 | Sodium phytate | 0.1 |
| Seppiplus S | 1.5 | Seppiplus S | 1.5 | Panthenol | 1.0 |
| Aqua | do 100.0 | Aqua | do 100.0 | Seppiplus S | 1.20 |
| Aqua | do 100.0 | ||||
| Phase C—additives | |||||
| Phaseolus vulgaris L. sprotus micellar extract | 4.0 | ||||
| α-tocopherol | 1.0 | ||||
| Phenoxyethanol, Caprylyl Glycol | 1.0 | ||||
| C4 | C5 | C6 | |||
| Phase A—oil | |||||
| GSC | 3.63 | Lipowax NF | 2.0 | GSC | 6.0 |
| MG | 1.73 | Emulgade sucro | 1.5 | Cetearyl alcohol | 2.5 |
| Sucrose stearate | 0.75 | MG | 1.5 | Behenyl alcohol | 0.5 |
| Cetyl alcohol | 1.5 | Sucrose stearate | 0.5 | Heptyl undecylate | 3.0 |
| Behenyl alcohol | 0.5 | Cetyl alcohol | 1.5 | Isopropyl palmitate | 2.0 |
| Heptyl undecylate | 3.0 | Behenyl alcohol | 0.5 | C/CT | 2.0 |
| Isopropyl mirystate | 2.0 | Heptyl undecylate | 0.5 | C12-15 alkane | 1.0 |
| C/CT | 2.0 | Isopropyl Mirystate | 1.0 | Shea butter | 3.0 |
| C12-15 alkane | 0.7 | C/CT | 2.5 | Dulcis amygdalarus oil | 4.0 |
| Shea butter | 1.5 | Shea butter | 1.5 | Argania spinosa oil | 0.5 |
| Sweet almond oil | 3.0 | Sweet almond oil | 3.0 | ||
| Argania spinosa oil | 0.5 | Argania spinosa oil | 0.5 | ||
| Phase B—water | |||||
| Propanediol | 2.2 | Propanediol | 2.0 | Propanediol | 2.2 |
| Sodium phytate | 0.1 | Sodium phytate | 0.1 | Sodium phytate | 0.1 |
| Panthenol | 1.0 | Panthenol | 1.0 | Panthenol | 1.0 |
| Simulgel 600 | 1.5 | Simulgel 600 | 1.0 | Cosmedia SP | 1.5 |
| Aqua | do 100.0 | Aqua | do 100.0 | Aqua | do 100.0 |
| Faza C | |||||
| Phaseolus vulgaris L. sprotus micellar extract | 4.0 | ||||
| α-tocopherol | 1.0 | ||||
| Phenoxyethanol, Caprylyl Glycol | 1.0 | ||||
| Reference cream (CR) | |||||
| Aqua, Glycerin, Octocrylene, Cetearyl Alcohol, Methylpropanediol, Caprylic/Capric Triglyceride, Ethylhexyl Salicylate, Glyceryl Stearate Citrate, Isopropyl Palmitate, Butyl Methoxydibenzoylmethane, Octyldodecanol, Dimethicone, Synthetic Beeswax, Argania Spinosa Kernel Oil, Ubiquinone, Creatine, 1-Methylhydantoin-2-Imide, Vitis Vinifera Seed Oil, Calcium Pantothenate, Tocopherol, Ethylhexylglycerin, Xanthan Gum, Carbomer, Trisodium EDTA, Sodium Hydroxide, Phenoxyethanol, Benzyl Alcohol, Limonene, Parfum | |||||
| C1 | C2 | C3 | C4 | C5 | C6 | CR | |
|---|---|---|---|---|---|---|---|
| F | 55.1 ± 1.4 | 51.1 ± 0.5 | 49.1 ± 4.7 | 58.8 ± 1.5 | 52.5 ± 4.8 | 41.7 ± 2.3 | 49.36 ± 4.3 |
| Φ | 3.63 ± 1.56 | 3.31 ± 0.71 | 2.98 ± 0.52 | 3.01 ± 0.62 | 3.04 ± 0.95 | 3.42 ± 0.89 | 4.4 ± 1.14 |
| η5s−1 | 18.09 | 27.01 | 7.13 | 30.75 | 27.7 | 34.38 | 17.73 |
| η50s−1 | 2.41 | 3.56 | 1.36 | 4.19 | 3.41 | 4.55 | 2.58 |
| A × 103 | 692 | 1366.3 | 463.51 | 2490 | 1833 | 1532.6 | 1099 |
| K | 73.39 | 113 | 25.15 | 127.48 | 115.7 | 144.48 | 67.22 |
| p | 0.13 | 0.12 | 0.25 | 0.12 | 0.1 | 0.11 | 0.1 |
| Ʈr0 | 49.2 | 133.67 | --- | 90.17 | ---- | 125.63 | 84.54 |
| k | 25.98 | 6.35 | ----- | 55.75 | ---- | 30.6 | 3.5 |
| n [-] | 0.25 | 0.5 | ---- | 0.19 | ---- | 0.31 | 0.65 |
| Sf | 2.95 ± 0.05 | 2.59 ± 0.04 | 3.59 ± 0.02 | 2.88 ± 0.03 | 2.85 ± 0.02 | 2.56 ± 0.03 | 3.11 ± 0.04 |
| S | 76 ± 3 | 105 ± 1 | 44 ± 3 | 80 ± 0.5 | 79 ± 0.2 | 108 ± 1 | 64 ± 4 |
| pH | 5.65 ± 0.01 | 6.02 ± 0.02 | 7.06 ± 0.04 | 5.55 ± 0.02 | 6.76 ± 0.02 | 5.15 ± 0.01 | 6.54 ± 0.03 |
| Days | 1 d. | 7 d. | 30 d. | 90 d. | 180 d. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| temp. | ---- | 4 °C | 20 °C | 40 °C | 4 °C | 20 °C | 40 °C | 4 °C | 20 °C | 40 °C | 4 °C | 20 °C | 40 °C | |
| C1 | η5s−1 | 18.09 | 19.21 | 19.22 | 20.86 | 17.76 | 19.84 | 20.78 | 23.08 | 22.14 | 17.49 | 23.084 | 19.81 | 17.129 |
| η50s−1 | 2.4 | 2.62 | 2.65 | 2.73 | 2.64 | 3.07 | 3.17 | 2.86 | 3.02 | 2.68 | 2.86 | 2.86 | 2.67 | |
| d | 0.986 ± 0.002 | 0.992 ± 0.001 | 0.990 ± 0.002 | 0.997 ± 0.001 | 0.996 ± 0.001 | 0.999 ± 0.003 | 0.995 ± 0.002 | 1.002 ± 0.001 | 0.998 ± 0.004 | 1.012 ± 0.003 | 1.000 ± 0.001 | 1.001 ± 0.001 | 1.001 ± 0.001 | |
| Sf | 2.95 ± 0.05 | 2.81 ± 0.02 | 2.85 ± 0.0 | 2.81 ± 0.04 | 2.83 ± 0.03 | 2.98 ± 0.03 | 2.78 ± 0.02 | 3.01 ± 0.03 | 3.16 ± 0.05 | 3.01 ± 0.04 | 3.16 ± 0.04 | 3.29 ± 0.05 | 3.31 ± 0.04 | |
| pH | 6.65 ± 0.04 | 6.45 ± 0.02 | 6.50 ± 0.0 | 6.53 ± 0.0 | 6.51 ± 0.02 | 6.66 ± 0.0 | 6.63 ± 0.02 | 6.76 ± 0.02 | 6.60 ± 0.02 | 6.32 ± 0.04 | 6.52 ± 0.01 | 6.68 ± 0.02 | 6.65 ± 0.01 | |
| CR | η5s−1 | 17.73 | 19.7 | 20.23 | 16.58 | 15.67 | 21.97 | 13.82 | 21.05 | 21.59 | 19.85 | 23.95 | 15.87 | 18.62 |
| η50s−1 | 2.58 | 2.58 | 2.75 | 2.36 | 2.38 | 2.81 | 2.18 | 2.51 | 2.73 | 2.64 | 2.7 | 2.38 | 2.61 | |
| d | 0.986 ± 0.002 | 0.968 ± 0.001 | 0.975 ± 0.001 | 0.975 ± 0.002 | 0.986 ± 0.001 | 0.989 ± 0.002 | 0.995 ± 0.001 | 0.998 ± 0.002 | 0.989 ± 0.001 | 0.992 ± 0.002 | 0.996 ± 0.001 | 1.002 ± 0.001 | 1.006 ± 0.002 | |
| Sf | 2.75 ± 0.03 | 2.79 ± 0.05 | 2.89 ± 0.04 | 2.77 ± 0.03 | 2.820.04 | 2.9 ± 0.05 | 2.84 ± 0.04 | 2.970.05 | 2.95 ± 0.03 | 2.92 ± 0.02 | 3.36 ± 0.04 | 3.09 ± 0.03 | 3.11 ± 0.05 | |
| pH | 5.65 ± 0.01 | 5.76 ± 0.02 | 5.56 ± 0.05 | 5.76 ± 0.03 | 5.81 ± 0.04 | 5.76 ± 0.04 | 5.63 ± 0.04 | 5.80 ± 0.03 | 5.76 ± 0.02 | 5.55 ± 0.02 | 5.72 ± 0.3 | 5.68 ± 0.03 | 5.65 ± 0.04 | |
| Days | 1 d. | 7 d. | 30 d. | 90 d. | 180 d. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| temp. | ---- | 4 °C | 20 °C | 40 °C | 4 °C | 20 °C | 40 °C | 4 °C | 20 °C | 40 °C | 4 °C | 20 °C | 40 °C | |
| C1 | average | 3.63 | 3.66 | 3.68 | 3.63 | 3.65 | 3.67 | 3.71 | 3.67 | 3.77 | 3.73 | 3.67 | 3.75 | 3.76 |
| min. | 1.72 | 1.54 | 1.02 | 1.65 | 1.58 | 1.84 | 2.05 | 2.04 | 1.8 | 1.27 | 1.6 | 1.94 | 2.29 | |
| max | 11.98 | 11.98 | 11.94 | 11.15 | 12.10 | 11.97 | 11.88 | 12.64 | 12.06 | 12.90 | 13.51 | 13.67 | 13.73 | |
| sd | 1.56 | 1.55 | 1.67 | 1.43 | 1.80 | 1.53 | 1.22 | 1.74 | 1.31 | 1.5 | 1.75 | 1.48 | 1.89 | |
| CR | average | 4.10 | 4.13 | 4.14 | 4.05 | 4.07 | 4.15 | 4.07 | 4.15 | 4.17 | 4.43 | 4.47 | 4.45 | 4.76 |
| min. | 2.12 | 2.05 | 2.43 | 2.08 | 2.36 | 2.2 | 2.09 | 2.24 | 2.58 | 2.57 | 2.6 | 2.99 | 3.29 | |
| max | 8.98 | 9.19 | 6.94 | 9.15 | 6.10 | 9.97 | 13.88 | 10.94 | 9.67 | 9.79 | 10.51 | 10.67 | 17.73 | |
| sd | 1.16 | 1.58 | 0.97 | 1.53 | 0.86 | 1.32 | 1.79 | 1.34 | 1.28 | 1.15 | 1.85 | 1.18 | 1.68 | |
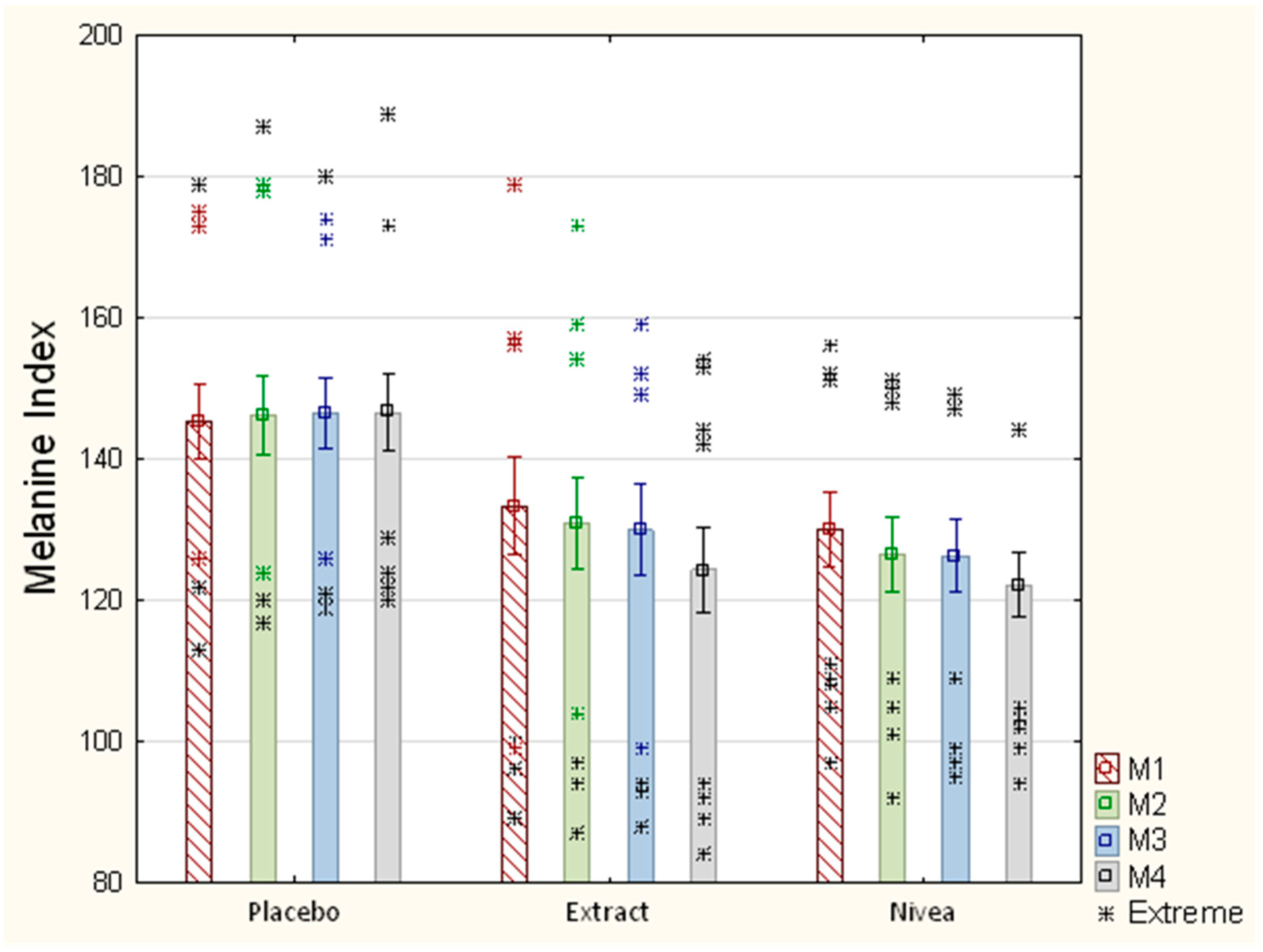
| Placebo n = 15 | Extract n = 15 | CR n = 15 | Summary n = 45 | p = Value/ Statistically Significant Between Group | |
|---|---|---|---|---|---|
| Measurement 1 (M1) | |||||
| Average (±SD) | a 49.38 (11.1) | a 48.81 (8.45) | a 50.55 (9.07) | No | |
| Measurement 2 (M2) | |||||
| Average (±SD) | b 50.14 (10.65) | b 53.50 (10.63) | b 51.72 (11.35) | No | |
| ∆% | +1.54 | +9.61 | +2.31 | ||
| Measurement 3 (M3) | |||||
| Average (±SD) | c 50.74 10.48 | c 57.48 10.09 | c 54.34 9.48 | No | |
| ∆% | +2.75 | +17.76 | +7.5 | ||
| Measurement 4 (M4) | df 2.42, F = 4.74 | ||||
| Average (±SD) | d 51.12 1 9.56 | d 59.70 2 9.07 | d 58.68 3 9.06 | 1,2 0.04 1,3 0.04 | |
| ∆% | +3.52 | +22.31 | +16.08 | ||
| p–value Statistical significant within the group. | a.d 0.03 | a.b 0.012 a.c 0.0012 a.d 0.0008 | a.c 0.026 a.d 0.001 | ||
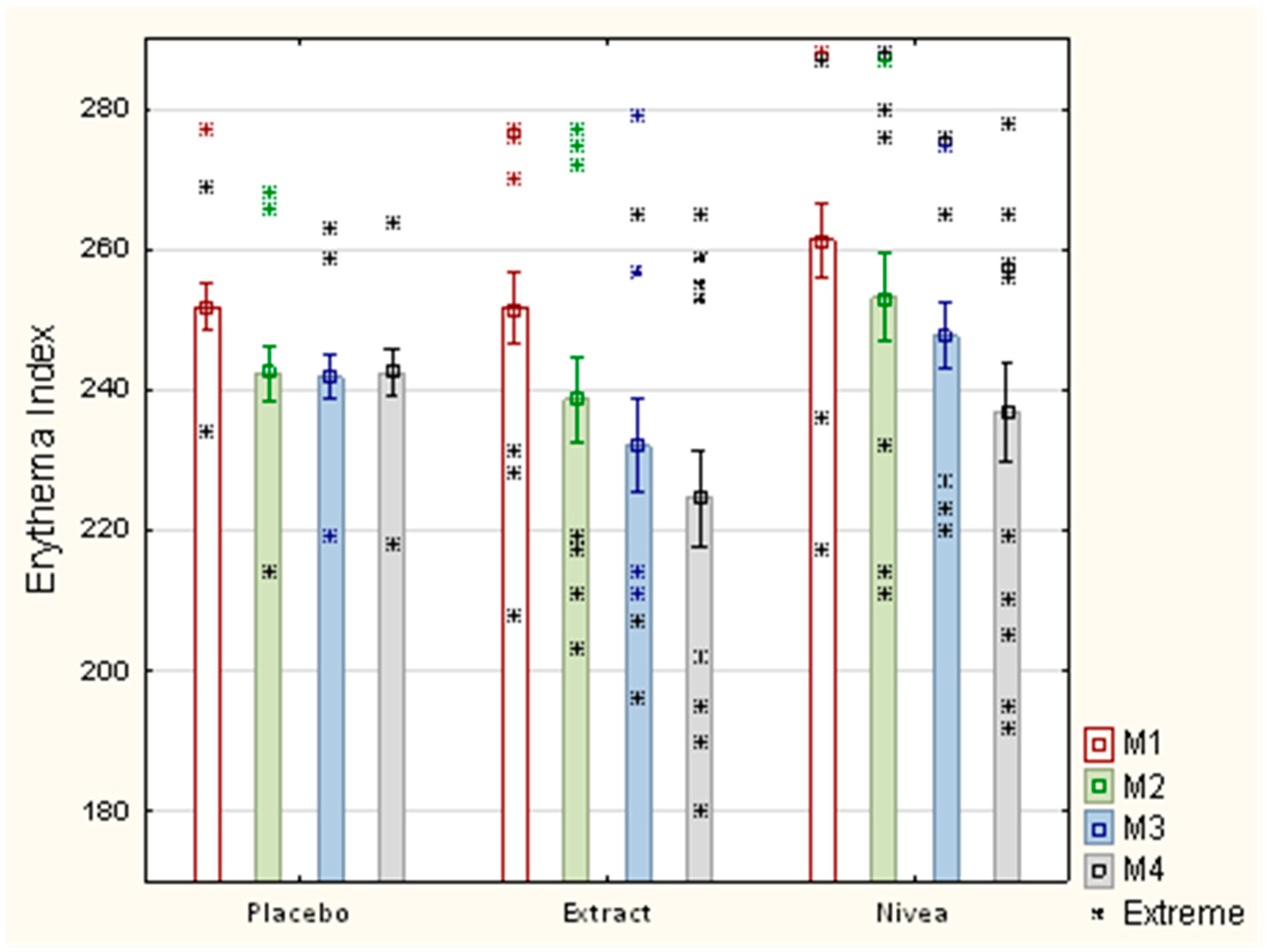
| Placebo n = 15 | Extract n = 15 | CR n = 15 | Statistically Significant Between Group | |
|---|---|---|---|---|
| Measurement 1–parameter R2 | ||||
| Average ± SD | a 70.53 ± 6.49 | a 71.41 ± 7.24 | a 71.93 ± 9.07 | No |
| Measurement 2–parameter R2 | ||||
| Average ± SD | b 73.02 ± 5.33 | b 74.22 ± 7.17 | b 72.76 ± 4.75 | No |
| ∆% | +3.53 | +3.93 | +1.15 | |
| Measurement 3–parameter R2 | ||||
| Average ± SD | c 75.90 ± 3.37 | c 77.76 ± 5.63 | c 75.18 ± 5.28 | No |
| ∆% | +7.61 | +8.89 | +4.52 | |
| Measurement 4–parameter R2 | ||||
| Average ± SD | d 76.53 ± 3.4 1 | d 80.91 ± 5.07 2 | d 76.52 ± 5.01 3 | df(2.42), F = 4.35 1,2 0.04 |
| ∆% | +8.5 | +13.3 | +6.38 | 2,3 0.04 |
| p–value within the group | a.c 0.04 a.d 0.01 | a.c 0.005 a.d 0.0002 | a.d 0.048 | |
| Measurement 1–parameter R5 | ||||
| Average ± SD | a 62.41 ± 7.22 | a 61.94 ± 7.96 | a 67.17 ± 7.12 | No |
| Measurement 2–parameter R5 | ||||
| Average ± SD | b 63.02 ± 8.57 | b 64.28 ± 5.76 | b 63.38 ± 6.47 | No |
| ∆% | +1.76 | +3.78 | −5.66 | |
| Measurement 3–parameter R5 | ||||
| Average ± SD | c 60.54 ± 8.281 | c 67.34 ± 8.13 2 | c 65.57 ± 5.47 3 | df(2.42), F = 3.34 1,2 0.04 |
| ∆% | −3.01 | +8.71 | −2.39 | |
| Measurement 4–parameter R5 | ||||
| Average ± SD | d 63.86 ± 8.37 | d 69.58 ± 6.86 | d 69.74 ± 7.32 | No |
| ∆% | +2.32 | +12.33 | +3.82 | |
| p–value within the group | a.d 0.008 | b.d 0.01 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojciechowska, K.; Rostkowska, E.; Ginalska, G.; Zimmer, Ł.; Poleszak, E. New Insights into Common Bean (Phaseolus vulgaris L.) Sprouts: Pilot Studies on the Formulation of a Cosmeceutical Based on Micellar Extracts Bean Sprouts. Appl. Sci. 2025, 15, 1831. https://doi.org/10.3390/app15041831
Wojciechowska K, Rostkowska E, Ginalska G, Zimmer Ł, Poleszak E. New Insights into Common Bean (Phaseolus vulgaris L.) Sprouts: Pilot Studies on the Formulation of a Cosmeceutical Based on Micellar Extracts Bean Sprouts. Applied Sciences. 2025; 15(4):1831. https://doi.org/10.3390/app15041831
Chicago/Turabian StyleWojciechowska, Katarzyna, Ewelina Rostkowska, Grażyna Ginalska, Łukasz Zimmer, and Ewa Poleszak. 2025. "New Insights into Common Bean (Phaseolus vulgaris L.) Sprouts: Pilot Studies on the Formulation of a Cosmeceutical Based on Micellar Extracts Bean Sprouts" Applied Sciences 15, no. 4: 1831. https://doi.org/10.3390/app15041831
APA StyleWojciechowska, K., Rostkowska, E., Ginalska, G., Zimmer, Ł., & Poleszak, E. (2025). New Insights into Common Bean (Phaseolus vulgaris L.) Sprouts: Pilot Studies on the Formulation of a Cosmeceutical Based on Micellar Extracts Bean Sprouts. Applied Sciences, 15(4), 1831. https://doi.org/10.3390/app15041831








