1. Introduction
Developmental dyslexia (DD) is a specific learning difficulty primarily affecting reading and writing. Characterized by difficulties with decoding words, spelling, reading fluency, and comprehension, dyslexia is not caused by a lack of intelligence or effort but rather by neurological differences that affect how the brain processes language. Dyslexia, a heritable neurodevelopmental disorder, affects approximately 10% of the population, significantly impacting their acquisition of reading skills. The neurological model of reading [
1] emphasizes the crucial role of functional connections between the left angular gyrus and visual processing areas in the occipital and temporal lobes. The angular gyrus links visual information to language representations in areas like Wernicke’s area. In dyslexia, functional networks within brain regions associated with phonological processing (frontal, occipital, angular, inferior temporal, fusiform, supramarginal gyrus, and cerebellum) exhibit significantly reduced activation compared to good readers [
2]. Impaired automatic visual word processing is a hallmark of dyslexia, evidenced by functional deficits within the “visual word form area” (VWFA) in the left occipitotemporal system [
3]. Disrupted connectivity between the VWFA and crucial language areas in the left frontal and parietal lobes emerges early in reading development, coinciding with deficits in orthographic and phonological processing, highlighting its connections in the development of fluent reading. Difficulties in perceiving letters and their order may stem from abnormal development of magnocellular (M) nerve cells, crucial for rapid letter identification, visual attention, and eye fixations [
4]. Evidence for M-cell impairment has been found throughout the visual system, from the retina and lateral geniculate nucleus to the primary visual cortex, and the dorsal “where” pathway, which processes spatial visual information. This impairment destabilizes visual perception, with the severity of M-cell weakness correlating with the severity of reading difficulties. Genetic vulnerabilities may contribute to this abnormality by disrupting the proper migration of cortical neurons during development. Altered visual perception in reading difficulties, specifically a preference for local over global visual processing, suggests a strong link between global-before-local perception and reading proficiency [
5].
A recent study suggests that dyslexia may have a different neurological basis in women and men [
6]. In healthy men, strong functional connectivity exists between the left angular gyrus and extrastriate occipital and temporal regions during single-word reading [
1]. This functional connectivity between the left angular gyrus and the reading network is disrupted in men with DD. Understanding DD requires examining sex differences in neurobiological research. This variation could influence current models of dyslexia and the treatment strategies used for reading difficulties. For instance, global-before-local perceptual training has shown promise in improving reading skills in dyslexic children, and a strong preference for local processing in pre-readers is a significant predictor of future reading difficulties [
5]. These findings challenge the traditional view of dyslexia as primarily a left-hemisphere, phonological-based deficit. They highlight the importance of an efficient right-hemisphere network for global visual scene analysis in successful reading acquisition. These new insights into the multifaceted nature of dyslexia have important implications for early identification and development of effective prevention and intervention programs.
A review of recent evidence on sex differences in dyslexia discusses their impact on understanding the brain basis of DD, providing a framework for development of differential neuroanatomical profiles [
7]. Over the past two decades, research has produced conflicting results concerning sex differences in the relationship between sex, neurobiology, and behavior. An important question is whether observed differences are attributed to biology (biological sex) or social constructions (social sex). Sex differences are primarily identified through genetic studies that explore the genetic predispositions associated with each sex. Factors such as genes, hormones, and environment influence brain morphology. It remains uncertain whether there are sex differences in the adolescent personalized network or significant individual variations in the spatial distribution of functional networks or their cortical topology.
The brain’s associative networks, including the ventral and dorsal attention networks, as well as frontoparietal networks, are particularly effective in distinguishing the sex of young individuals [
8] because sex hormones (progesterone, estrogen) impact connectivity within these networks. Additionally, these networks are connected to mood and fear symptoms linked to psychopathology. Research indicates that females exhibit greater abnormalities in connectivity related to fear symptoms within the frontoparietal, ventral attentional, and default networks. Sex differences in multivariate functional topology patterns are spatially associated with X chromosome gene expression [
8,
9].
Variation in brain topology among individuals is particularly evident in associative networks (default mode, ventral attention networks). Motor and sensory networks typically demonstrate more consistency among individuals [
8]. Group map similarity of functional topology across 17 networks was lowest in the associative network and highest in the sensorimotor network, indicating greater interindividual differences in the topology of associative networks [
8]. Regions in the frontoparietal network demonstrate the highest degree of interindividual heterogeneity in various characteristics, which can explain why variability in functional topology in association cortices is closely linked to individual differences in cognitive abilities particularly with reading. While it is impossible to discriminate among individuals solely based on sex, research has shown normative sex differences in the functional topology of adolescent personalized associative networks, suggesting that interindividual differences in functional topology are partially influenced by sex. Individual differences in cognitive abilities during childhood influence adult life’s physical, social, and mental outcomes.
The cortical surface during development reflects the functional reorganization of the brain. Determining changes in the topology of functional brain networks in the growing cortex of children aged 9 and 10 provides insight into their individual variation and differences in cognitive abilities before the crucial transition to adolescence [
7,
10]. These variations are best predicted by the frontoparietal and ventral attentional networks, while the visual and somatomotor networks have the lowest predictive accuracy. Considering the degree of maturation of the central nervous system and age-related functional changes, even in normally developed children, it is essential to establish the relationship between a child’s age and the frequency functional networks (α, θ, and δ rhythms of the EEG, most clearly reflecting age-related changes in brain activity), as well as the peculiarities of the development of reading abilities in childhood. In children with learning disabilities, high θ and low α frequency activities suggest a delay in the development of cognitive neural networks [
11,
12]. However, the effect of increased effort on discriminating a more difficult task on the activity of stimulus-related brain regions is poorly understood. In achieving fully automated multisensory integration, behavioral assessments are not as sensitive as they could be [
13]. There is a growing search for new, more effective methods to modify the brain’s neural networks and promote the development of reading abilities.
In our previous study [
14], we characterized functional brain networks using graph theory, analyzing their small-world propensity (SWP,
ϕ). SWP quantifies a network’s efficiency in transferring information [
15], reflecting a balance between high clustering (strong local connections) and short path lengths (fast global communication). This analysis revealed differences in network integration and segregation of functional networks between a control group of children and one with DD [
14]. By focusing on tasks that highlight deficits in the selection of visual stimuli (low-spatial-frequency illusion), the research explored visual perception problems using a functional analysis method that identifies the deactivation or suppression of certain brain centers and their connections within networks affected by DD. During contrast discrimination of this specific visual task, the control group exhibited greater segregation in the δ, α, β2 (low-contrast illusion), and θ, γ1 networks (high-contrast illusion), as well as greater integration in the γ2 network (both contrasts) compared to children with DD. The present study investigates interindividual variations in children with DD and their typically developing peers, focusing on changes in functional brain networks related to sex in 8- to 9-year-old children This study also explores the functional brain connectivity in children with DD, using EEG measurements during specific visual task. Unsolved issues in DD include the research of possible pathological changes in weak and strong connections within functional networks during this specific visual task and their impact on the overall functioning of the investigated neural network influenced by sex and inter-individual variations. The challenges of interpreting results within the specific context of this visual task should be carefully balanced with the need for robust methods to characterize functional networks, identify key hubs, and analyze their interactions within the network.
The main research questions concern the following: (i) whether brain network analysis can provide new insights into the neurophysiological origins of DD; (ii) how specific tasks affect the activity of neural frequency networks in children within a particular age group; (iii) the extent to which the deficits observed in DD can be attributed to variations in the strength and connectivity of different brain regions, particularly hubs within these networks; (iv) the relationship between the functional visual network and the oscillatory activity of specific local brain hubs.
The study aims to study the functional brain connectivity in children with DD using graph theory and the small-world propensity technique. Our goals are to provide an objective interpretation of the findings, demonstrate the potential and limitations of these techniques, and determine whether any significant variability in brain networks among control children and those with DD is associated with a specific experimental paradigm.
We hypothesize the following: (1) compared to typically developing children (normolexics), children with DD have different “small-world properties” and weaker connections in various frequency sub-networks which may vary by sex; (2) the personalized frequency functional networks within separate sex groups during the experimental task will not change if they do not associate with the age-related functional brain organization; (3) rather, the changes of specific local hubs in certain frequency networks will be related to the brain’s functional network during the task, if the functional networks of children with DD differ from those of normolexics.
3. Results
Behavioral parameters for the task were compared within groups (controls and children with DD) to ensure that there were no large differences among children. There were no significant differences between the children in the groups regarding the number of correct answers when completing the task. The children in the groups were also well sorted by age. There were some sex differences in both reaction time and some network parameters of functional networks at certain frequencies. However, the functional analysis appears to “eliminate this effect”; the sex was included in the analysis below of both behavioral parameters in this task and functional characteristics.
3.1. Within-Group Design of Behavioral Parameters in Contrast Illusion Discrimination
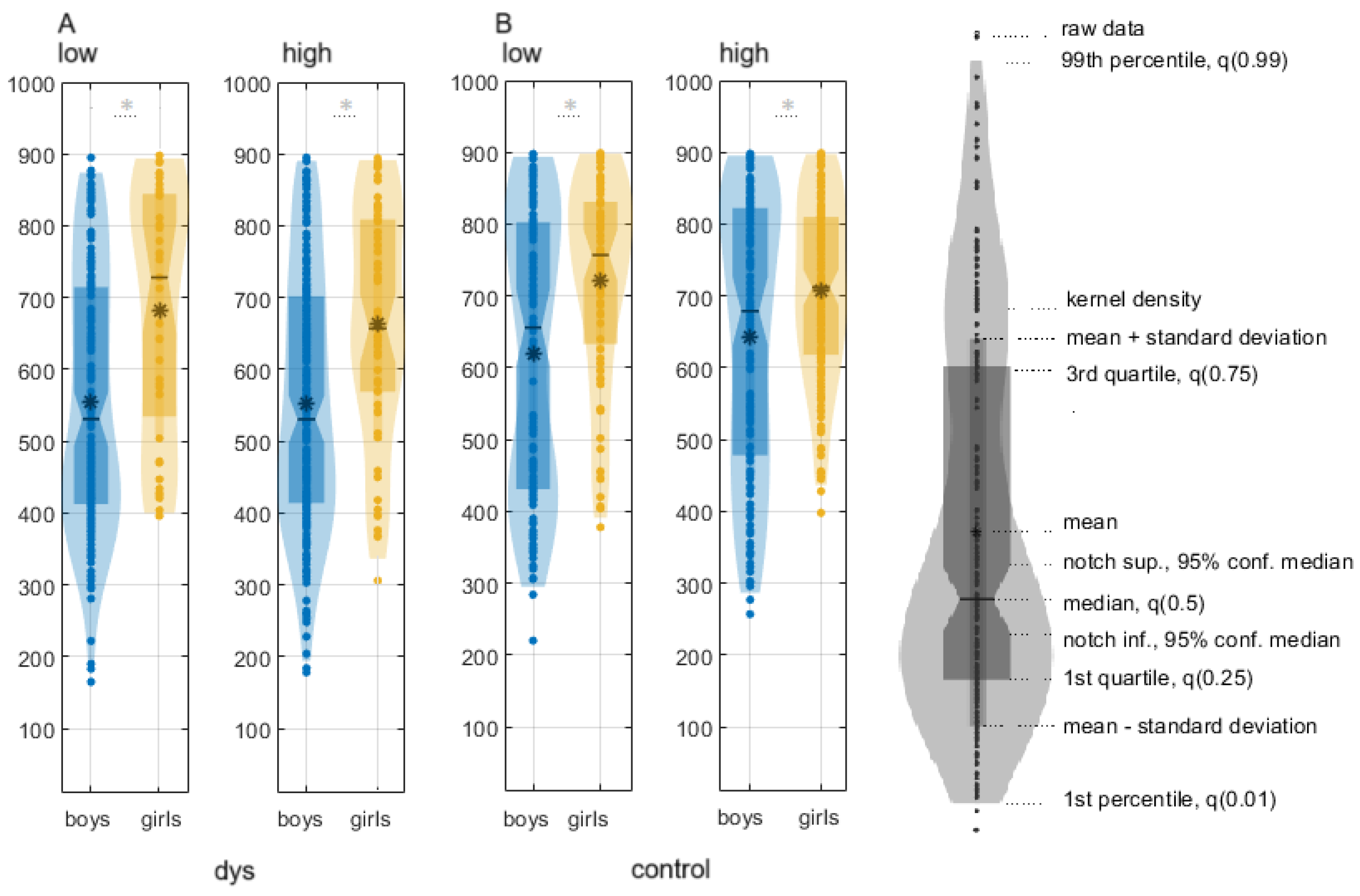
Boys with DD are faster than girls with DD for both conditions:
(1) low-contrast illusion: boys with DD, median ± CI, 544.28 (520.28, 562.43), girls with DD, 695.72 (673.97, 728), p = 1.42 × 10−16; χ2 = 68.27, Q = 0.85;
(2) high-contrast illusion: boys with DD, 555.61 (547.01, 564.23), girls with DD, 661.33 (653.27, 669.4),
p = 3.54 × 10
−8, χ
2 = 30.39, Q = 0.7 (
Figure 1;
Table S2).
Control boys were faster than control girls:
(1) low-contrast illusion: 624.9207 (614.77, 635.14) ms; 727.31 (719.93, 734.68) ms; χ2 = 24.69, p = 7.03 × 10−7, Q = 0.3;
(2) high-contrast illusion: 645.4887 (635.67, 655.31) ms; 714.4453 (708, 720.9) ms; χ
2 = 62,
p = 3.4178 × 10
−15, Q = 0.3 (
Table S2).
3.2. Within-Group Comparison of Global SWP Measures of Functional Connectivity
There were no statistically significant differences in network measures between boys with DD and girls with DD in both task conditions (
Figure 2A,B;
Tables S3 and S4).
Control boys exhibited statistically higher ∆C and smaller SWP (ϕ) than control girls in low-contrast LSFI:
(1) The α-frequency network: ∆C of the (∆C: χ
2 = 8.48;
p = 0.001; Q = 0.48;
Table S3);
(2) the β2-frequency network:
ϕ, ∆C of only in the low-contrast (
ϕ, ∆C: χ
2 > 8.07;
p < 0.005, Q = 0.6;
Table S3).
Sex differences were found in the high-contrast SLF at:
(1) β-frequencies
ϕ, ∆C, ∆L (β1:
ϕ, ∆C: χ
2 > 13.86
p = 0.002, Q > 0.58; β2:
ϕ, ∆C, ∆L: χ
2 > 8.87,
p < 0.003, Q > 0.43;
Figure 3,
Table S4);
3.3. Within-Group Comparison of Local Measures of Frequency Networks
3.3.1. Sex Differences in DD Group
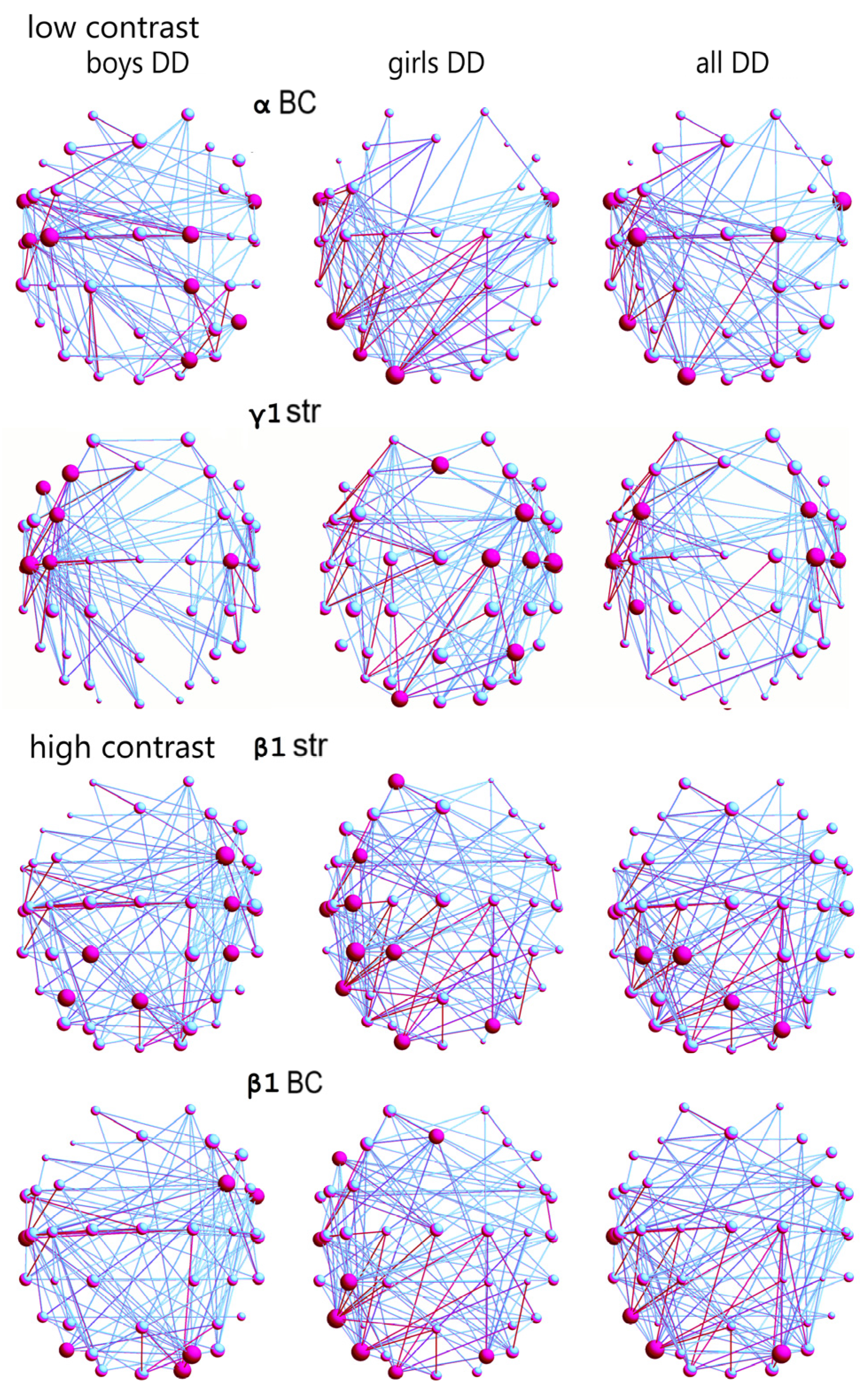
There were statistically significant differences between the distribution of the hubs with the greatest betweenness centrality (BC) of the boys and girls with DD in the α-frequency network (
Figure 4,
Table S5) in the low-contrast illusion; χ
2 = 5.84,
p = 0.015, Q = 0.3. Boys with DD had more hubs with the greatest BC in this network, and they were in areas related to language, auditory, motor, motion-sensitivity, and visual attentional processing (FT9-10, C3, C5, C2, CP2, PO4, P8;
Figure S2); and for the girls with DD, in areas related to language processing, sensitivity to movement, and visual attention (FT9-10, P7, PO7, O1; ref.
Figures S2 and S3).
The hub distribution between boys and girls with DD was statistically different in the γ1-frequency network (according to hub strength; χ
2 = 7.26,
p = 0.007, Q = 0.4;
Table S5); as in girls with DD, it was in areas related to language processing, reading, motor, auditory processing, sensitivity to visual information and that to optic flow in the right hemisphere (Fz, FC4, C2, C4, C6, P4, O1), and in boys, in language processing, reading, motor, and auditory sub-networks in the left hemisphere (F7, F3, FC3, C3-C4, C5; ref.
Figures S2 and S3).
The hub distribution between the two DD sex groups was statistically different in the β1-frequency network, relative to hub strength, in the high-contrast illusion (χ
2 = 9.65,
p = 0.001, Q = 0.3;
Table S6): for boys with DD, in areas related to motor processing, language comprehension, expression, and reading (FC4, C4, CP4, P3, Pz, Oz); for the girls with DD, in more areas related to language comprehension, expression, reading, basic and complex auditory, visual motion processing, and visual attention, mostly in the left hemisphere (AF3, FC3, C3, CP1, CP3, T7, PO4, P7, O1;
Figure 4).
In the β1-frequency network of boys and girls with DD, the hubs distributions with the greatest BC were statistically different (χ
2 = 8.37,
p = 0.003, Q = 0.3;
Table S6). In the boys with DD, the hubs were related to language comprehension, expression, reading, integration of auditory and visual information, auditory feedback, and sensitivity to visual attention, areas predominantly in the right hemisphere (FC4, FT10, T7, PO4, PO7, O2), compared to the hubs for girls in the left hemisphere, in areas for language, writing, auditory, and sensitivity to motion and visual attention (Fz, F7, CP3, T7, PO4, PO7, P7, O1;
Figure 4).
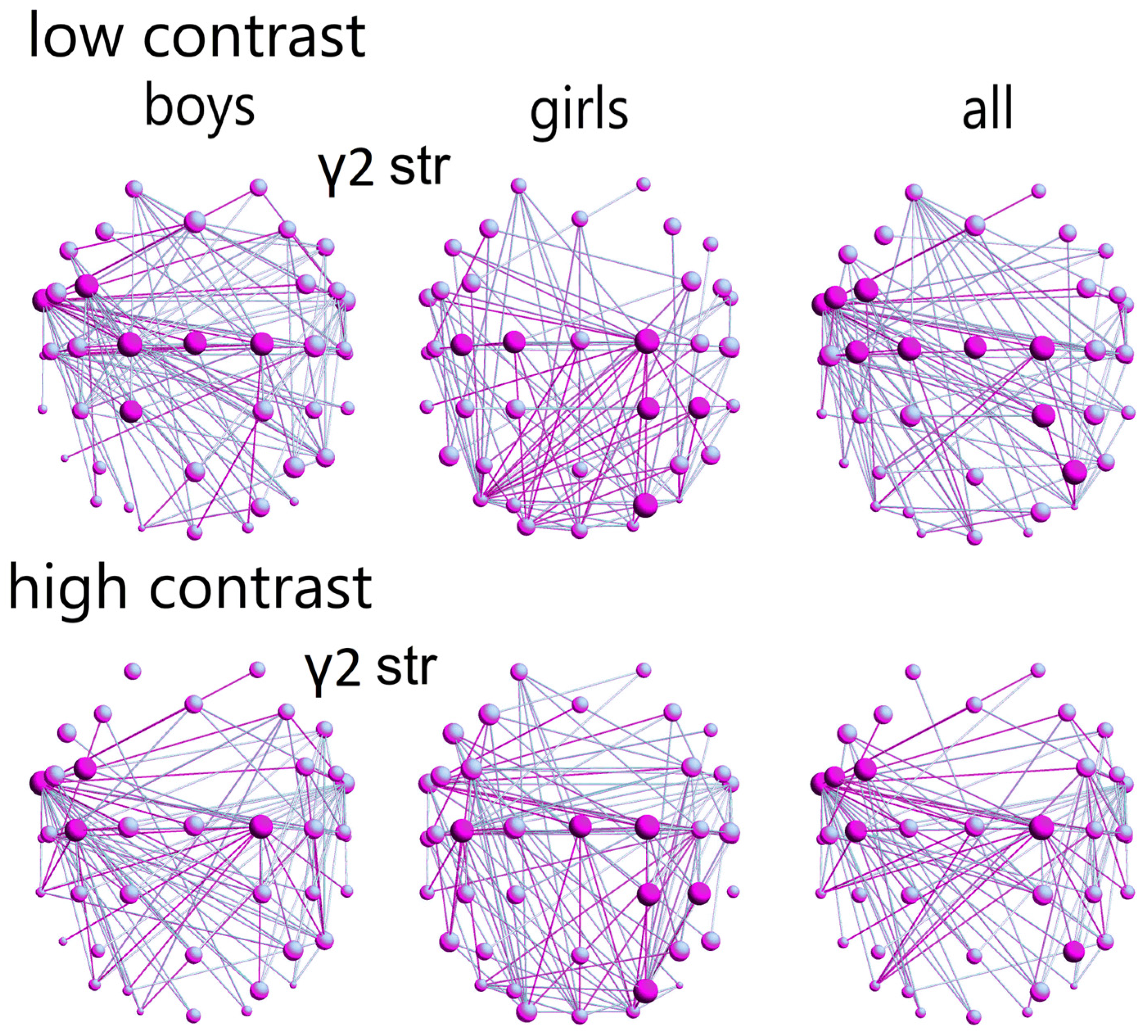
3.3.2. Sex Differences in the Control Group
In the γ2-network (according to the hub strength; low-contrast illusion), the control boys demonstrated a greater distribution of hubs across areas related to language (comprehension, expression, reading), visual motion processing, and visual attention, mostly in the left hemisphere (FC3, FT9, C1-2, Cz, CP1;
Figure 5; ref.
Figures S2 and S3). Control girls exhibited a more concentrated hub distribution within motor areas, visuomotor, and visuospatial attention-related regions, predominantly in the right hemisphere (C1-2, C3, CP2, CP4, PO4;
Figure 5). This difference in hub distribution between sexes was statistically significant (χ
2 = 9.8,
p = 0.002, Q = 0.3;
Table S5).
In the high-contrast LSF illusion, control girls showed a stronger γ2-frequency network with higher hub strength in motor areas, visuomotor, and visuospatial attention-related regions, predominantly in the right hemisphere (C2, Cz, C3, CP2, CP4, PO4). Control boys exhibited a greater network strength in areas related to language (comprehension, expression, reading), primarily in the left hemisphere (FC3, FT9, C2, C3); this sex difference in network strength was also statistically significant (
p = 0.001, χ
2 = 10.9, Q = 0.3;
Table S6).
3.4. Exploring Interindividual Variation of Functional Networks
Interindividual heterogeneity in functional brain networks for 9–10-year-olds is also related to individual differences in their cognition, according to the model for the contribution of different networks along the axis of somatosensory association to cognitive functioning [
10,
43,
44,
45,
46].
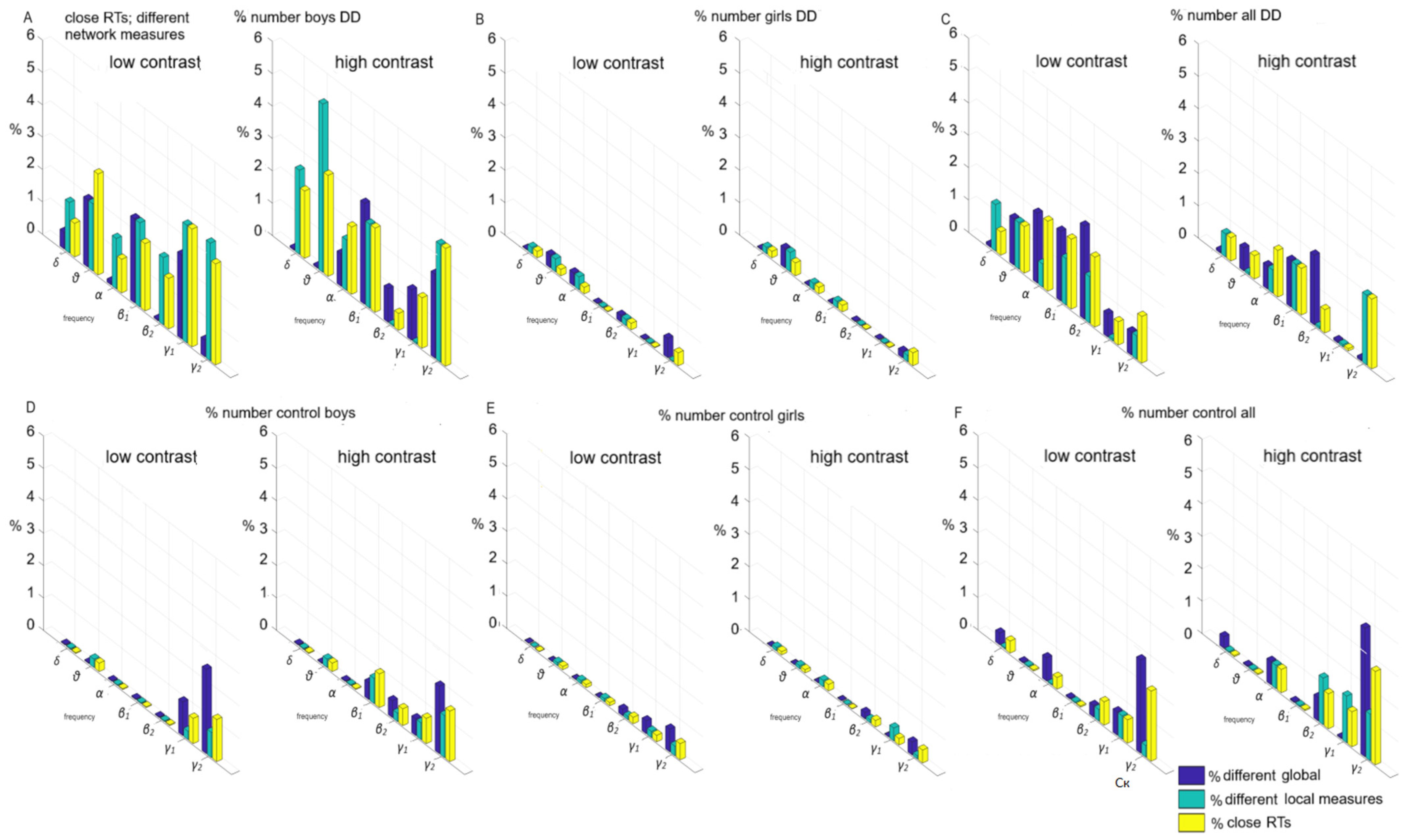
Individual variations of global and local measures at the level of 8-year-old single individuals were examined for two cases. Each bar on the subplots illustrates the proportion of significantly different pairs of functional (global, local) measures within each comparison (boys; girls; boys vs. girls), expressed as a percentage. This percentage is derived by dividing the number of pairs exhibiting significant differences by the total number of pairs examined.
Without sensorimotor network contribution (children had close reaction times, no statistical difference of RTs,
p ≥ 0.05,
Figure 6), i.e., the influence of frontoparietal networks was investigated: (a) the subgroup of boys with DD (both contrasts) in 3–5% of children (
Figure 6A); (b) the subgroup of girls with DD—up to 1% of children (
Figure 6B); (c) the subgroup of boys versus girls with DD—up to 2% (
Figure 6C); (d) the subgroup of controls boys—up to 2% (
Figure 6D); (e) the subgroup of controls, girls—1% (
Figure 6E); (f) the subgroup of control boys vs. girls—up to 3% (
Figure 6F).
With an equal contribution of the sensorimotor network in both sex groups, the contribution of the frontoparietal and associative networks is equally underdeveloped in all sex subgroups as yet. Associative networks have not undergone plasticity compared to other parts of the cortex under the influence of the environment, children’s experiences, and interventions targeting these systems effective in supporting cognitive development.
The contribution of the sensorimotor network to the individual variations of global and local measures at the level of personalized networks (
Figure 7); statistically different reaction times were found among the children;
p ≤ 0.05: (a) a subgroup of boys with DD—between 10–12% (
Figure 7A); (b) a subgroup of girls with DD—1% (
Figure 7B); (c) subgroup of boys versus girls with DD—up to 8% (
Figure 7C); (d) a subgroup of control boys—up to 3% (
Figure 7D); (e) a subgroup of controls girls—1% (
Figure 7E); (f) a subgroup of control boys vs. girls—up to 5% (
Figure 7F).
The sex is preferably related to sensorimotor network. These associations, related to individual functional connectivity, may underlie the sex differences that exist in an immature brain in DD. Children with DD (mostly boys) have not reached the development of the sensorimotor network, which later leads to a decrease in interindividual heterogeneity. At this age, associative and sensorimotor networks have not yet matured enough to observe individual and sex differences.
4. Discussion
Functional brain topology is affected by sex, a biological characteristic that leads to differences in the organization of individual cortical and subcortical networks [
46]. These variations can explain normative differences in cognitive or socioemotional abilities, executive functions, and sex differences in psychopathology at a given age, likely linked to gene expression [
8,
9]. A limitation of these studies is that the relationship between sex differences in brain topology and gene expression was assessed at the group level rather than the individual level [
8,
9].
The sensorimotor association axis in the hierarchical cortical organization, passing from the unimodal visual and somatomotor cortex to the transmodal association cortex, shows the greatest variability in functional topology among these networks that support cognition [
10]. The observed individual variances in associative network topology may be crucial for predicting variations in cognition, as different networks probably contribute differently to cognitive processes. Functional brain topology is affected by sex, a biological characteristic that leads to differences in the organization of individual cortical and subcortical networks [
46].
4.1. Relationship Between Variability in Network Connectivity and Sex
The present study investigated global and local functional connections related to sexual differences. Our findings indicated a significant overlap in the association networks for control boys and girls, with only 4% of them (see
Figure 6F). The sex of 8-year-old children is preferentially linked to sensorimotor, visual, and control networks in the brain and more closely linked to individualized functional connectivity. The observed sex differences between the children with DD are related more to specific brain deficits.
Sex-related variations in functional networks were evident in sensorimotor networks among children with DD (up to 8%,
Figure 7C). In the control group, sex-related differences were more pronounced in γ2 frequency association cortical networks (up to 5%,
Figure 6F).
4.2. Relationship Between Variability in Network Connectivity During the Low-Contrast LSF Illusion and the Development in the Control Group
Control boys’ global α, β, and γ2-networks exhibited a reduced small-world propensity, indicating a deviation from the typical balance of high clustering and short path lengths observed in small-world architecture (
Figure 3). A greater deviation in the clustering coefficient suggested greater variability in the interconnectivity of neighboring brain areas. Smaller deviations in characteristic path lengths implied a more efficient and integrated network with shorter average distances between brain regions. This type of network topology of α, β1, β2, and γ2-frequency networks for the control boys were more integrated than those for the control girls.
Within the γ2-network, a sex-dependent pattern of hub connectivity emerged. Boys exhibited stronger connectivity within anterior hubs, primarily associated with left-hemisphere language and visual motion processing, across both high- and low-contrast LSF illusions. Notably, posterior hubs, implicated in visuospatial attention, were specifically involved in the low-contrast illusion for boys. Within both conditions, girls demonstrated enhanced connectivity within posterior brain regions, predominantly linked to right-hemispheric visuospatial and visuomotor attention.
In control boys, local γ2-network variations mainly involved connections within and between the frontal, temporal and motor networks in the left hemispheres (both contrast LSF illusions,
Figure 5 and
Figure 7A). Local functional γ2- (low-contrast), θ-, and β2-frequency differences (high-contrast) within motor networks among control subgroups were attributed to a sex difference within the control group (up to 5%;
Figure 7). Global functional differences in α-frequency networks and variations in γ-networks between sexes involved connections within and between frontoparietal and associative networks, affecting up to 3% of children (both contrast LSF illusions,
Figure 6F). Additionally, θ-, β2-, and γ-frequency network sex differences were observed within and between motor networks among 5% of children in the control group (high-contrast,
Figure 7F).
4.3. Relationship Between Variability in Network Connectivity During Low-Contrast LSF Illusion to DD Deficits
In boys with DD, individual differences in local α network characteristics (
Figure 6A and
Figure 7A) were primarily driven by variations in hub betweenness centrality within and between temporal, parietal, dorsal attentional, and sensorimotor networks in the right hemisphere. In contrast, these differences in girls with DD (
Figure 6B and
Figure 7B) were observed primarily within temporal and visual networks in the left hemisphere (low-contrast,
Figure 4). Local γ1 network variations in boys with DD were associated with changes in hub strength within frontal, motor, and auditory networks of the left hemisphere (low-contrast). Conversely, in girls with DD, these variations were linked to changes within and between frontoparietal, motor, somatosensory, auditory, and visual networks in the right hemisphere (low-contrast,
Figure 4).
Within motor networks in individuals with DD, local α network variations across subgroups were primarily driven by sex differences in hub betweenness centrality (BC), accounting for less than 5% of the children. Individual differences in hub strength within the group of boys with DD had a minimal impact (low-contrast; compare
Figure 7A with
Figure 7C). In contrast, local γ1 network differences across subgroups were mainly attributable to individual variations in frontoparietal and associative networks within the group of boys with DD.
For θ- and β-frequency networks, subgroup differences in network connectivity (low-contrast) were primarily driven by the following: (1) sex differences in connections between hubs with high strength or BC within and between motor networks, frontoparietal, and associative networks, observed in 10% of individuals with DD (low-contrast,
Figure 6 and
Figure 7); (2) individual variations in connections among hubs with high strength or BC within and between motor networks, observed in 8% of boys with DD.
4.4. Relationship Between Variability in Network Connectivity During High-Contrast LSF Illusion and DD Deficits
In boys with DD, local functional variations within the β1-frequency network (high-contrast,
Figure 4) were observed within the right hemisphere’s frontoparietal, motor, visual, and dorsal attention networks, as well as within temporal networks in the left hemisphere. In girls with DD, similar variations were observed within and between lateral frontoparietal, motor, and temporal networks in the left hemisphere and within visual networks in both hemispheres (high-contrast,
Figure 4). These findings suggest that sex-related differences may exist in functional connectivity within and between local unimodal and heteromodal networks, affecting up to 8% of children with DD.
In motor networks of individuals with DD, local β1-network variations between subgroups were primarily attributed to sex differences in hub strength and BC, accounting for up to 8% of the children. These variations were not significantly influenced by individual differences within the group of boys with DD (high-contrast; compare
Figure 7A with
Figure 7C).
In other frequency networks, subgroup differences were largely driven by the following: (1) individual variations in connections among hubs with high strength or BC within and between frontoparietal and association networks in 10% of boys with DD; (2) individual variations in connections between hubs with high strength or BC within motor networks in 8% of these boys (
Figure 7).
In θ- and α-frequency networks (high-contrast LSF,
Figure 7), network differences between subgroups of individuals with DD were primarily influenced by the following: (1) sex differences in the connectivity between hubs with high strength, or high BC, within and between motor networks and other brain networks, observed in 10% of children with DD; and (2) individual variations in the connectivity between hubs with high strength, or high BC, within and between motor networks, observed in 8% of boys with DD. For β2-frequency networks, subgroup differences primarily arose from sex differences in the connectivity of hubs within and between frontoparietal and associative networks (high contrast,
Figure 6).
4.5. Limitation of Variability in Functional Connectivity Across Sex
A variability in global functional connectivity was observed in associative networks among girls and boys for both groups (
Figure 6C,F), but a greater variability in local functional connectivity was found in motor networks among boys and girls within both controls and children with DD (
Figure 7), as reported by Long et al., 2017 [
46].
These findings indicate that the influence of sex on functional brain connectivity may be more intricate than initially anticipated. It is possible that sex-related differences in brain function, as reflected in functional cortical networks, may not yet be fully manifested in 8-year-old children. The lack of significant associations between sex and functional connectivity could be attributed to the limited variability in these measures, which may arise from the immature development of cortical areas at age 8 or a relatively small sample size of children (DD and controls, 114). It is not surprising that the proportion of internal variation in boys with DD (unrelated to sex, rather related to different maturation) was relatively small (12%). In the girls, it was even lower (1%), likely due to the smaller subgroup. Our findings indicate that the associations between functional connectivity and sex of 8-year-old children influence the organization of brain networks in a minority of children because functional brain networks mature unevenly in cortical networks across genders during childhood [
47].
Stimulus-responsive unimodal sensory networks within a given sensory modality mature first, followed by heteromodal associative networks involved in cognitive processes. All networks with different sets of functional connections contribute to local functional variation between sexes.
The relationship between sex and connectivity within and between sensorimotor, visual, and motor networks is stronger than other connections. In girls, the strongest connections and main hubs were found within and between temporal, parietal, and attentional networks, predominantly located in the right hemisphere for typically developing children, and in different brain sides among the significantly different frequency networks for dyslexics. In contrast, boys exhibited a more diffuse connectivity pattern that spanned several heteromodal networks and sensorimotor networks, primarily in the left hemisphere for controls and with varying hemispheric dominance in dyslexic boys across different frequency bands. Sex-specific differences in functional capacities between the association and sensorimotor cortices may stem from regional variations in functional connectivity profiles across hemispheres. These variations could arise from differential refinement of connectivity during brain development. A study of the development of cortico-cortical functional connectivity confirmed that this variability varies across the cortical hierarchy [
46].
Children’s sex expression from 9- to 10-year-olds undergoes significant changes during puberty, marked by both structural and functional maturation of the brain [
47].
The interplay of childhood development before puberty with brain maturation and their relationship to emerging developmental disorders influences brain network sensitivity to individual and sex differences. Understanding this variation in childhood brain development can help capture the biological variation associated with emerging psychopathology. Moreover, findings related to psychopathological associations indicate sensitivity to subtle manifestations of psychopathological issues that may not yet reach clinical thresholds. It is important to note that brain maturation and sex differences introduce further variation into these outcomes.