Featured Application
Purification of Produced Water in Oil Production.
Abstract
In crude oil production, large volumes of produced water are generated as a highly polluting waste byproduct. On average, at least two barrels of produced water are generated for every barrel of oil. This water contains oil traces in stable and complex emulsions. To purify it, a method is proposed based on breaking these emulsions using solvents that induce the coalescence of oil droplets, facilitating their separation from the water. The method has two main objectives: (1) To identify the characteristics a solvent must have to effectively break oil emulsions according to the Hansen solubility parameter (HSP) model. (2) To select, from 40 solvents of different chemical families, the most suitable ones based on efficiency, low toxicity, industrial availability, and cost. The experimental procedure included the following steps: (1) Contacting the solvent with produced water containing 150 ppm of oil under agitation. (2) Allowing the mixture to rest until a layer of recovered oil formed. (3) Spectrophotometric analysis of the residual oil. Three distinct HSP solubility spheres were identified, within which the most effective solvents were xylene (99.4% recovery), cyclohexane (99.5% recovery), and tetrahydrofuran (100% recovery). Their high efficiency not only facilitated oil separation but also made the recovered oil suitable for commercialization.
1. Introduction
Due to the significance of the oil industry in ensuring a continuous supply of energy for the development of nations, this sector has maintained an uninterrupted extraction rate of over 4 billion metric tonnes annually over the past decade [1]. This equates to approximately 80 million barrels of crude oil per day. The extraction process generates large volumes of water contaminated with traces of oil in the form of small emulsified droplets, referred to in this technical field as “produced water”.
On average, it is estimated that the volume of produced water is double that of the crude oil extracted, with this volume increasing as the oil well becomes depleted. The concentration of oil in the produced water typically ranges from 300 ppm to 1000 ppm, levels which should not be underestimated. Although a significant portion of the produced water is reused for reinjection into oil wells, the remainder must be discharged on land or into the sea after treatment. None of the methods currently employed have proven fully satisfactory, particularly considering the stricter water quality requirements expected to be implemented soon.
The treatment of produced water to remove residual oil typically involves primary separation, secondary separation, and, in some cases, a final cleaning treatment. Produced water enters primary treatment with up to 1000 ppm of oil and exits with concentrations between 100 ppm and 200 ppm. In secondary treatment, the water enters with concentrations of 100–200 ppm and exits with oil levels reduced to between 10 ppm and 40 ppm. Following secondary treatment, current environmental regulations can generally be met in most oil-producing regions. However, future trends, as exemplified by Norway’s regulations for oil platforms, aim to achieve zero harmful discharges—an objective that remains unattainable with the current technology [2].
The state-of-the-art final cleaning treatments currently available in the industry can reduce the residual oil content to approximately 15 ppm, a level that is still far from the desired goal of complete elimination.
In the industry, various traditional techniques are used for treating produced water, but their results are not optimal, as they do not achieve 100% efficiency or even values close to it. For this reason, new methods are being researched; however, they are currently developed only at the laboratory level. These methods also are not sufficiently effective and, in some cases, even lead to additional environmental problems.
The next two sections briefly outline a significant number of methods, including both those commonly used in the industry and those proposed at the laboratory level. To keep the text concise, only their names are mentioned, along with a few characteristics and/or drawbacks that make them insufficiently suitable.
1.1. Traditional Industrial Methods for Produced Water Treatment
Primary treatment can be carried out using skimming tanks, API separators, corrugated plate interceptors (CPIs), and coalescence. Secondary treatment may employ hydrocyclones, induced gas flotation (IGF), dissolved gas flotation (DGF), compact flotation units (CFUs), or membranes, among other technologies. The final cleaning or refining treatment uses walnut shell filters or dual media filters [3].
Hydrocyclones require energy to pressurise the inlet, do not separate solids, are prone to fouling, and incur high maintenance costs [4]. Corrugated plate interceptors are inefficient due to the time required to retain the fine oil particles and their maintenance requirements [5]. Dissolved air flotation involves dissolving pressurised air into produced water and subsequently depressurising it under atmospheric pressure, releasing air bubbles that attach to oil droplets and make them float. This technique has the drawback of requiring a large volume of air, long retention times for separation, and producing a high volume of skimmed waste [6]. Flotation has the disadvantage that, at high temperatures, high pressure is needed to dissolve the gas in the water [7,8].
Hydrophilic membrane filtration (MF) suffers from fouling caused by the formation of colloidal scaling, requiring elaborate multi-stage pretreatment, large base areas, and complex designs for the treatment system. It consumes a significant amount of energy due to the high power per unit surface area of the membrane.
Antifouling chemicals and other pretreatment agents require handling and storage [7,9].
Microfiltration (MF) demands high energy, exhibits low efficiency for divalent and monovalent salts and viruses, and is prone to fouling from iron deposits [7]. Ultrafiltration (UF) also requires high energy; iron fouling and membrane fouling are problematic, as is its lower efficiency for low-molecular-weight organic compounds. The salt rejections may also contain radioactive materials [2,7]. Nanofiltration similarly requires high energy, is less effective for monovalent salts and low-molecular-weight organic compounds, and, like ultrafiltration, its salt rejections may contain radioactive materials [7].
The catalytic method for treating produced water requires high temperatures and pressures, which can promote emulsification [10].
Using produced water from oil fields for irrigation also requires desalination methods, to address the high salt concentrations, as well as the removal of crude oil droplets [11,12].
Media formed from alternating hydrophilic and hydrophobic fibres are convenient for the purification of liquids containing certain organic materials, such as oil, but the interactions between crude oil droplets and water present complex correlations [13].
Ceramic membranes suffer from internal scaling [11,14].
Oxidation processes treat only small volumes of water, which is disproportionate to the massive quantities requiring purification [15]. A similar issue arises with methods utilising microalgae [16] or activated carbon: the volumes of water requiring treatment are so large that proportionality is unfeasible [11,17].
1.2. Laboratory Methods for Produced Water Treatment
In addition to the traditional processes for separating crude oil droplets from produced water, there are numerous laboratory-based methods and attempts characterised by promising ideas. However, these are not without their pros and cons when scaled to real industrial dimensions. Below, we summarise some of these methods.
The method of using melamine sponges with silica granules has the drawback of a complex manufacturing process for these sponges. Furthermore, crude oil is removed from produced water by absorption into the sponge, making subsequent separation of the crude oil from the sponge, as well as its recovery and commercial utilisation, difficult [17,18,19,20,21,22,23,24].
Another method involves flooding the oil well with a thickening polymer to reduce water leakage and improve crude oil extraction. The required polymer concentrations are on the order of thousands of parts per million, which is excessive. Moreover, this method is not specifically designed for the removal of oil droplets from produced water, which would be preferable [25].
Other separation operations rely on the use of cotton and lotus plants, which are limited to specific operations such as offshore oil spills. Porous cotton fabrics are used to absorb oil from spills. However, the subsequent treatment of the oil-saturated cotton involves burning it, which generates another type of environmental pollution [26,27].
Membrane-based methods using carbon and graphene do not mention the percentage of crude oil recovered, which is a crucial metric for evaluating a treatment’s effectiveness [28,29].
Another alternative is the use of cationic surfactants with a double fatty chain, such as esterquats. These have limited applications and achieve a recovery rate of 81.31% to 83.75% of oil, both for removing crude oil from produced water and for extracting water traces from crude oil [30].
Another absorption method involves using sawdust at an ideal temperature of 27 °C. This is not feasible for seawater or large volumes of water. This method, cited in [31], does not indicate the percentage of oil separated. While sawdust absorbs oil effectively [32], it provides poor separation rates, not exceeding 70%. Additionally, separating the crude oil from the saturated sawdust is difficult. Saturated sawdust could be burned to produce energy, but this would shift the pollution problem elsewhere. Sawdust is useful for cleaning stains from essentially dry surfaces but has limited utility when mixed with produced water.
The use of superhydrophilic Al₂O₃ particle beds is another proposed method. If the pore width in the layers is approximately 0.3 µm, they are effective for removing crude oil from oil-in-water (O/W) emulsions. Pores of approximately 40 µm are effective for removing water from water-in-oil (W/O) emulsions. However, the issue with produced water is that it is not a simple emulsion, but rather a complex mixture of multiple emulsions, rendering this system ineffective [33].
The carbon steel method is suitable for treating produced water but has the disadvantage of high manufacturing costs and suboptimal oil removal efficiency [34].
Polyaluminium chloride has also been proposed as a method for treating produced water. High salinity and an increase in temperature can improve oil separation [35]. However, this is illogical, as increasing the temperature enhances molecular movement. It has been demonstrated that emulsification begins at as low as 47 °C, which is detrimental to the oil separation process [32].
In summary, both traditional industrial processes and recent laboratory-scale attempts at purifying produced water have significant flaws. These include low efficiency, high procedural costs, the inability to recover crude oil for commercialisation (and thus offset the purification costs), or the fact that solving one environmental issue may create another. Laboratory methods often lack scalability to industrial levels.
Therefore, a simple procedure is needed that is easy to implement in the industry, scalable to large production volumes, and highly efficient at purifying produced water. Moreover, it should be cost-effective, avoiding additional expenses in crude oil extraction and instead providing a means to economically benefit from the crude oil recovered from the produced water.
1.3. Produced Water Stream in Real Oil Production
Figure 1 illustrates a flowchart of the general oil extraction and produced water purification procedure. Initially, wet oil is gathered from wells via pipelines converging at point 1, leading to gas isolation stations. The oil undergoes four stages at a pressure of 28 bar, as depicted in the diagram. Upon entry into the separator, it impacts deflector plates and separating the gas, water, and oil. Level control is maintained through level and pressure control valves. After the second stage, a dehydrator is installed to extract water, which also contains dissolved salts. This process is conducted with the addition of a demulsifier and the application of a 380-volt electric current through graphite electrodes submerged in the oil above the water level. Subsequently, a desalter is installed to remove residual salts by introducing steam or pure wash water. This setup prevents conduction and disrupts the water–oil bonds, causing the residual droplets of produced water, laden with oil droplets and forming complex emulsions, to settle at the bottom. These emulsions, not effectively broken down in the earlier stages, have oil concentrations varying between 300 and 1000 ppm, which is a substantial amount necessitating treatment; therefore, purification operations commence from point 2, where the produced water, a byproduct requiring treatment due to its environmental impact and the presence of oil droplets, is extracted. Previously discussed were the traditional and laboratory processes and the shortcomings of most of these methods. An integral solution to this problem is proposed, based on the use of demulsifying agents consisting of solvents capable of interacting with oil droplets and breaking the emulsions. For this purpose, a selection of optimal materials is necessary, adhering to established principles and standards. At point 3, following the prior addition of the solvent, oil is separated in settling tanks.
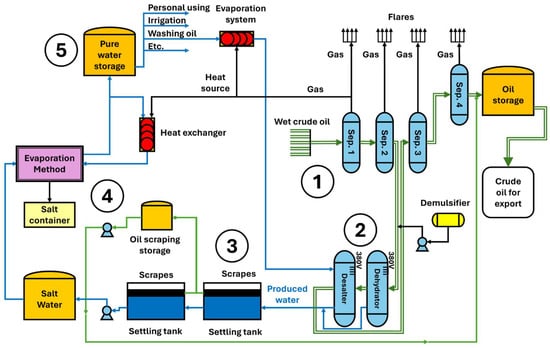
Figure 1.
Example of a flowchart for crude oil production and produced water treatment (Rumaila oil field, Iraq).
Resolving the treatment of produced water superficially is insufficient, because crude oil production involves huge amounts of produced water generated daily worldwide. Therefore, establishing a standard solution is challenging, considering the diverse conditions of oil-producing nations and the existence of wells in marine, onshore, and offshore environments. The adopted approach also includes a salt removal process at point 4 using an evaporation method and collecting the salts from the bottom through sedimentation. Condensed pure water, in the form of steam, is collected in a tank at point 5 for uses such as irrigation or general purposes, or it is re-evaporated for use in washing salts in the desalting stage. All these processes utilize the energy from gas combustion to capitalise on energy that would otherwise be wasted.
1.4. Objective
Although numerous industrial techniques are available and innovative proposals at the laboratory level for the purification of produced water exist, the use of solvents has not yet been studied. These solvents should be capable of breaking emulsions to induce the coalescence of oil droplets, and thus, their separation as a supernatant. The oil thus recovered can be commercialised and, therefore, will pay for the water purification costs. Depending on the solvent used, the recovery may involve a standard additional refining process to recycle the solvent.
The primary aim of this study is to propose a solvent-based method for purifying produced water that allows for the recovery of residual oil for future commercialisation. Specifically, the first goal is to identify the essential characteristics a solvent must have to effectively disrupt oil emulsions, using the Hansen solubility parameters (HSPs) as a framework. The second goal is to select, from the tested solvents that meet the HSP criteria, those that are most suitable based on their high efficiency, low toxicity, industrial availability, and cost-effectiveness.
2. Materials and Methods
2.1. Crude Oil
The crude oil used comes from the Rumaila oil field in Basrah (Iraq). It is a medium-density crude and has the characteristics shown in Table 1.

Table 1.
Characteristics of crude oil.
The residual resins and asphaltenes remaining in the produced water from crude oil are responsible for the stability of oil emulsions as they have surfactant properties.
2.2. Preparation of Produced Water and Purification Procedure
Laboratory-prepared produced water under conditions simulating real-world scenarios is used. The procedure involves adding sodium chloride to water until it reaches a concentration of 80,000 ppm. Under magnetic stirring (at 300 rpm) and in a water bath at 45 °C, 150 ppm of crude oil is added until it emulsifies. The oil comes from oil wells in Iraq. Exactly 100 cm3 of the prepared produced water is placed into a beaker. Solvent volumes of 5, 10, 15, 20, and 25 cm3, respectively, are added for each of the five experiments conducted with each solvent. The contents of the beaker are stirred at 300 rpm to ensure thorough mixing and the coalescence of emulsion droplets. Subsequently, it is allowed to stand for 10 min to allow the oil with solvents less dense than water to float to the surface. In this case, 10 cm3 of the purified produced water is extracted from the bottom of the beaker. Conversely, when the solvents are denser than water, they settle at the bottom, and the extraction of the purified produced water is performed halfway up.
2.3. Determination of Residual Oil Concentration in Treated Produced Water
The concentration of residual oil present in the previously treated produced water is determined spectrophotometrically using liquid–liquid extraction. For this purpose, 5 cm3 of tetrachloroethylene from Labkem (Barcelona, Spain), Tetrachloroethylene GLR > 99.9%, CAS 204-825-9) are added to 5 cm3 of produced water. Tetrachloroethylene is used because it is superior to other solvents as a reference for measuring the amount of oil in water [36]. Afterwards, the oil concentration in tetrachloroethylene is measured using a UV spectrophotometer (Cary 100 BIO from Varian) based on a standard curve previously prepared at 311 nm:
where Cp is the concentration of residual oil in the produced water, expressed in ppm, and A is the absorbance.
Cp = 105.39 A
The wavelength of 311 nm was chosen because it allows for a perfectly linear standard curve. This curve was established beforehand using concentrations of produced water ranging from 5 to 120 ppm. The fit is excellent since R2 = 0.9999.
2.4. Solvents
Data on the solvents used are shown in Table 2.

Table 2.
Solvents used for the purification of produced water through emulsion destabilisation and oil recovery. The term “unspecified” refers to a value that is either unavailable or poorly defined within a range.
2.5. Software
To determine the Hansen solubility parameters of the emulsions in produced water, the Techné Solubility 3S software 1.0, developed by the Techné research group at the University of Granada, was employed. This software is characterised by its ability to determine the solubility parameters of the centres of up to three solubility spheres as well as their radii.
3. Results
In Table 3, the concentrations of residual oil, Cp, in ppm, after the treatment of produced water using the methodology previously described, are presented. Additionally, the percentages of oil recovery, denoted as R, determined according to the following expression, are also presented:
R is expressed as a percentage (%), and the higher its value, the more effective the oil recovery from produced water has been.

Table 3.
Emulsion destabilisation tests for the purification of produced water using solvents.
Table 3.
Emulsion destabilisation tests for the purification of produced water using solvents.
| Chemical Family | Solvent | 5 mL Solvent | 10 mL Solvent | 15 mL Solvent | 20 mL Solvent | 25 mL Solvent | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cp, ppm | R, % | Cp, ppm | R, % | Cp, ppm | R, % | Cp, ppm | R, % | Cp, ppm | R, % | ||
| Linear aliphatic hydrocarbons | n-Heptane | 86.7 | 42.2 | 84.5 | 43.7 | 81.2 | 45.9 | 72.7 | 51.5 | 17.9 | 52.1 |
| Isooctane | 95.9 | 36.1 | 93.4 | 37.1 | 85.1 | 43.3 | 77.8 | 48.1 | 73.6 | 50.9 | |
| Petroleum ether | 129.1 | 13.9 | 126.8 | 15.5 | 120.5 | 19.7 | 114.7 | 23.5 | 99.0 | 34.0 | |
| Vaseline (liquid) | 145.9 | 2.7 | 146.8 | 2.1 | 148.1 | 1.3 | 149.3 | 0.5 | 149.7 | 0.2 | |
| Liquid paraffin | 147.1 | 0.2 | 148.1 | 0.1 | 149.7 | 0.0 | 149.8 | 0.0 | 150.0 | 0.0 | |
| Cyclic hydrocarbons | Xylene | 11.6 | 92.2 | 9.4 | 93.7 | 4.4 | 97.1 | 1.1 | 99.3 | 0.9 | 99.4 |
| Cyclohexane | 22.4 | 85.1 | 11.1 | 92.6 | 5.6 | 96.3 | 3.2 | 97.9 | 0.7 | 99.5 | |
| Benzene | 43.4 | 71.1 | 31.8 | 78.8 | 24.3 | 83.8 | 15.9 | 89.4 | 11.8 | 92.1 | |
| Toluene | 59.3 | 60.5 | 52.1 | 65.3 | 45.9 | 69.4 | 35.5 | 76.3 | 26.7 | 82.2 | |
| Limonene | 98.0 | 34.7 | 96.2 | 35.9 | 89.9 | 40.1 | 73.9 | 50.7 | 66.7 | 55.5 | |
| Chlorinated hydrocarbons | Tetrachloroethylene | 0.1 | 99.9 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 | 100.0 |
| Dichloromethane | 12.5 | 91.7 | 10.3 | 93.1 | 5.3 | 96.5 | 1.1 | 99.3 | 0.9 | 99.4 | |
| Chloroform | 13.6 | 90.9 | 10.7 | 92.9 | 9.5 | 93.6 | 5.4 | 96.6 | 2.2 | 98.5 | |
| Monohydric alcohols | Methanol | 34.0 | 77.3 | 22.3 | 85.0 | 15.7 | 89.5 | 10.7 | 92.9 | 8.4 | 94.4 |
| Ethanol | 39.4 | 73.7 | 24.6 | 83.6 | 18.3 | 87.8 | 15.8 | 89.5 | 11.0 | 92.7 | |
| 1-Propanol | 38.5 | 74.3 | 33.1 | 77.9 | 20.8 | 86.1 | 17.1 | 88.6 | 13.1 | 91.3 | |
| 2-Propanol | 40.2 | 73.2 | 34.2 | 77.2 | 22.4 | 85.1 | 18.3 | 87.8 | 14.1 | 90.1 | |
| 1-Butanol | 43.3 | 71.1 | 35.6 | 76.3 | 24.4 | 84.0 | 20.9 | 86.0 | 15.7 | 89.5 | |
| 2-Phenylethanol | 57.2 | 61.9 | 53.9 | 64.1 | 45.3 | 69.8 | 41.2 | 72.5 | 34.7 | 76.9 | |
| C3 alcohols | Ethylene glycol | 43.2 | 71.2 | 37.8 | 74.8 | 30.7 | 79.5 | 22.2 | 85.2 | 19.2 | 87.2 |
| Propylene glycol | 45.5 | 69.7 | 40.1 | 73.3 | 31.7 | 78.9 | 26.4 | 82.4 | 21.1 | 85.9 | |
| Dipropylene glycol | 52.2 | 65.2 | 45.2 | 69.9 | 38.8 | 74.1 | 31.8 | 78.8 | 24.4 | 83.7 | |
| Glycerin | 146.7 | 2.2 | 148.3 | 1.1 | 149.0 | 0.7 | 149.2 | 0.5 | 149.9 | 0.1 | |
| Ketones | Cyclohexanone | 27.1 | 81.9 | 15.7 | 89.5 | 11.2 | 92.5 | 5.9 | 96.1 | 2.7 | 98.2 |
| Acetone | 60.4 | 59.7 | 54.2 | 63.9 | 47.8 | 68.1 | 36.2 | 75.9 | 29.0 | 80.7 | |
| Butanone | 92.2 | 38.5 | 89.2 | 40.5 | 82.7 | 44.9 | 74.5 | 50.3 | 67.2 | 55.2 | |
| Ethers | Diethyl ether | 44.0 | 70.7 | 33.6 | 77.6 | 23.6 | 84.3 | 19.2 | 87.2 | 17.4 | 88.4 |
| Polyethylene glycol 400 | 51.1 | 65.9 | 43.5 | 71 | 37.4 | 75.1 | 33.1 | 77.9 | 26.1 | 82.6 | |
| Polypropylene glycol 2000 | 52.6 | 64.9 | 47.8 | 68.1 | 41.5 | 72.3 | 34.5 | 77 | 27.2 | 81.9 | |
| Esters | Decyl oleate | 47.9 | 68.1 | 42.7 | 71.5 | 33.9 | 77.4 | 31.1 | 79.3 | 26.7 | 82.2 |
| Isobutyl acetate | 82.5 | 45.0 | 74.2 | 50.5 | 65.7 | 56.2 | 57.1 | 61.9 | 48.7 | 67.5 | |
| Acetic acid | 82.9 | 44.7 | 77.7 | 48.2 | 74.4 | 50.4 | 69.2 | 53.9 | 53.3 | 64.5 | |
| Isopropyl myristate | 94.5 | 37.0 | 89.5 | 40.3 | 84.9 | 43.4 | 78.6 | 47.6 | 72.3 | 51.8 | |
| n-Butyl acrylate | 87.2 | 29.2 | 97.9 | 31.0 | 101.6 | 32.3 | 103.5 | 34.7 | 105.9 | 41.9 | |
| Ethyl acetate | 107.9 | 28.1 | 106.2 | 29.2 | 104.3 | 30.5 | 99.0 | 34.0 | 94.8 | 36.8 | |
| Miscellanea | Tetrahydrofuran | 0.4 | 99.8 | 0.2 | 99.9 | 0.1 | 100.0 | 0.1 | 100.0 | 0.0 | 100.0 |
| N,N-Dimethyl formamide | 45.2 | 69.9 | 36.0 | 76.0 | 26.7 | 82.2 | 22.2 | 85.2 | 18.6 | 87.6 | |
| Diethanolamine | 62.6 | 58.3 | 58.2 | 61.2 | 48.6 | 67.6 | 41.4 | 72.4 | 37.1 | 75.3 | |
| Acetonitrile | 79.3 | 47.1 | 75.0 | 50.0 | 70.7 | 52.9 | 64.8 | 56.9 | 59.5 | 60.3 | |
| Dimethyl sulfoxide | 85.8 | 42.8 | 96.4 | 35.7 | 98.2 | 34.5 | 102.8 | 31.5 | 105.1 | 29.9 | |
4. Discussion
4.1. Hydrocarbons and Chlorinated Hydrocarbons
4.1.1. Linear Aliphatic Hydrocarbons
Figure 2a illustrates the relationship between the volume of solvent per 100 mL of produced water and the percentage of residual oil removed, focusing on various aliphatic hydrocarbons. For hydrocarbons with low molecular weights (such as n-heptane, isooctane, and petroleum ether), oil recovery effectiveness remains moderate, peaking at 52%. Interestingly, a proportional increase in solvent volume correlates with enhanced oil recovery rates. However, substances like Vaseline (liquid) and liquid paraffin show no efficacy in this process. Notably, escalating Vaseline and paraffin volumes can inversely impact oil recovery, leading to a complete lack of recovery at 25 mL.
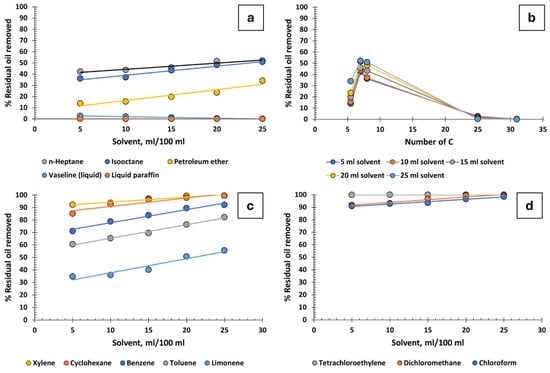
Figure 2.
Percentage of residual oil removed from produced water: (a) aliphatic hydrocarbons, (b) influence of the number of carbons in aliphatic hydrocarbons, (c) cyclic hydrocarbons, and (d) chlorinated hydrocarbons.
Figure 2b reveals that hydrocarbons with 7 to 8 carbon atoms exhibit the best recovery, whereas the effectiveness notably declines for those with higher molecular weights. This is explained by the ability of smaller molecules to destabilize the oil–water emulsion interface, facilitating oil droplet coalescence. On the contrary, Vaseline and liquid paraffin, being macromolecules, contribute to the stabilization of these emulsions. The increased viscosity of these substances further amplifies this effect and is known to enhance the stability of emulsions.
4.1.2. Cyclic Hydrocarbons
Cyclic hydrocarbons, encompassing both aliphatic and aromatic types, significantly outperform linear aliphatic hydrocarbons in oil recovery efficiency, as shown in Figure 2c. These cyclic molecules likely experience fewer steric hindrances compared with their linear counterparts, enhancing their ability to destabilize the oil–water interface. Consider limonene, which possesses a branched substituent within its cycle. Though not excessively large, this structure might introduce some steric hindrance, potentially limiting its effectiveness in destabilizing emulsions. Xylene, with its two methyl substituents positioned ortho, meta, or para, is small enough to facilitate droplet coalescence, seemingly acting as “destabilizing tweezers”. Both xylene and cyclohexane demonstrate high efficacy even in low volumes. Additionally, these substances are not only readily available and cost-effective but also recyclable and reusable in water purification processes, provided the right recovery system is in place. In terms of toxicity and handling, within the context of the oil extraction industry, they are not deemed hazardous, offering a practical advantage in this sector. Supplementary Document S1 presents data on the toxicological properties of cyclohexane and xylene as well as crude oil for reference.
4.1.3. Chlorinated Hydrocarbons
Incorporating chlorine atoms into hydrocarbons markedly enhances oil recovery in water purification processes. As depicted in Figure 2d, recovery rates soar to between 90% and 100%, with carbon tetrachloride being particularly notable. Dichloromethane and chloroform also show exceptional effectiveness in these processes. Despite their efficacy, the industrial application of these chlorinated hydrocarbons is advised against due to their associated risks. Tetrachloroethylene, for instance, decomposes into corrosive and toxic substances like hydrochloric acid and trichloroacetic acid in moist environments. Its violent reaction with aluminium coupled with its environmental hazards, particularly to aquatic systems, renders it a less favourable choice. Dichloromethane, which has a boiling point of 40 °C, is unsuitable for high-temperature applications without additional cooling, and the temperature of produced water often exceeds 45 °C. Chloroform, although less technologically challenging due to its higher boiling point of 61 °C, poses health risks including central nervous system effects and potential carcinogenicity.
4.2. Alcohols
4.2.1. Monohydric Alcohols
Monohydric alcohols with one to four carbon atoms are notably effective in recovering oil from produced water. In contrast, 2-phenylethanol exhibits moderate efficiency. The size of its aromatic ring appears to create steric hindrance, reducing its effectiveness compared with its counterparts with one to four carbon atoms (Figure 3a). It has also been observed that the position of the hydroxyl group within the carbon chain does not impact the efficiency of oil recovery: both 1-propanol and 2-propanol demonstrate identical effectiveness. Figure 3b illustrates oil recovery percentages as a function of the carbon atom count, revealing that the efficacy of monohydric alcohols is inversely proportional to the number of carbon atoms [37].
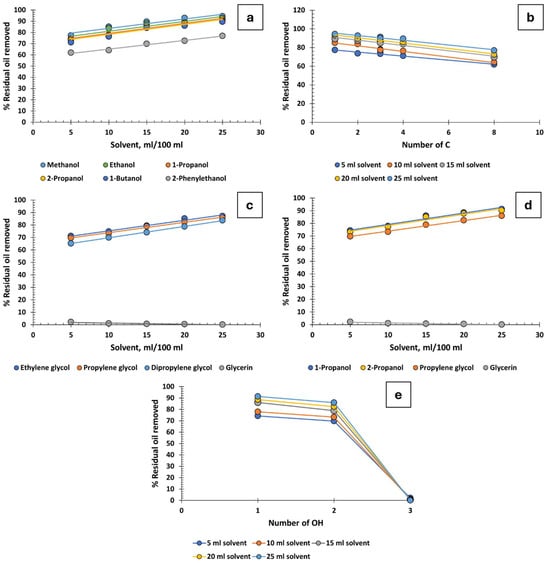
Figure 3.
Percentage of residual oil removed from produced water: (a) monohydric alcohols, (b) influence of carbon atom count in monohydric alcohols, (c) polyhydric alcohols, (d) C3 alcohols, (e) influence of the number of hydroxyl groups.
4.2.2. Polyhydric Alcohols
Glycols, possessing two OH groups and with two, three, or six carbon atoms, are slightly less effective than monohydric alcohols in oil recovery, as shown in Figure 3c. However, as with glycerin, the introduction of a third OH group leads to a stronger affinity for the aqueous phase over the oily phase. Despite high concentrations of salts in the water, particularly sodium chloride, glycerin dissolves well and becomes solvated. This solvated glycerin can encapsulate oil droplets, thereby increasing their zeta potential. Consequently, this results in electrostatic repulsion that prevents droplet aggregation and coalescence, potentially even stabilizing the emulsion. Indeed, the addition of just 5 mL of glycerin to 100 mL of produced water achieves a mere 2.2% oil recovery. When 25 mL is added, the recovery is virtually non-existent, at only 0.1%.
To assess the influence of the number of hydroxyl groups for a constant carbon number, four different C3 alcohols were compared: 1-propanol, 2-propanol (1OH), propylene glycol (2OH), and glycerin (3OH). As expected, and in line with the previous paragraph, alcohols with a single OH group are the most effective (regardless of whether the OH is in the 1 or 2 position), followed by propylene glycol (with 2OH), and almost complete inefficacy for glycerin with 3OH (Figure 3d,e).
Except for glycerin, the effectiveness of alcohols is high, though it does not reach the desired standards of near-complete purification of produced water. However, in their favour are highly advantageous factors such as widespread industrial availability, very low cost, and easy recyclability. Ethylene glycol and methanol are exceptions, as they are toxic to higher life forms. Nonetheless, the other alcohols analysed are minimally toxic to humans and the environment. If near-complete purification is not required, they could be a good alternative for industrial applications.
4.3. Ketones
Three ketones, cyclohexanone, acetone, and butanone, were tested, yielding varied results (Figure 4a,b). Cyclohexanone is the most effective, achieving high oil recovery percentages, while the performance of acetone and butanone is mediocre. Focusing on acetone (C3) and butanone (C4), it seems that increasing the number of carbon atoms leads to decreased recovery efficiency, but cyclohexanone, which is a C6, is much more effective. The reason must be the positive effect of the ring structure on oil recovery, as is the case with cyclic hydrocarbons (Figure 2c). Although cyclohexanone does not have as high an efficiency as some other solvents, it could be useful in the industry as it has several additional advantages. It is readily available, low in cost, can be easily recycled within the industrial process, and has low toxicity.
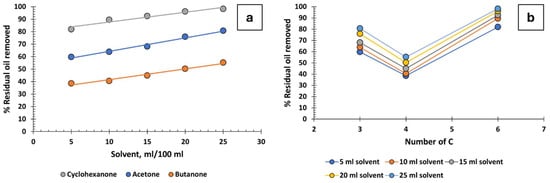
Figure 4.
Percentage of residual oil removed from produced water: (a) ketones and (b) influence of the number of carbons in ketones.
4.4. Ethers
Three ethers were tested: one of low molecular weight, diethyl ether, CH3-CH2-O-CH2-CH3, and two of high molecular weight, polyethylene glycol 400 (PEG 400), HO-(CH2-CH2-O)n-H and polypropylene glycol 2000 (PPG 2000), HO-(CH2-CH(CH3)-O)n-H. Their efficacy at oil recovery is medium to high, with diethyl ether performing slightly better (up to 90%) due to its small molecule size (Figure 5).
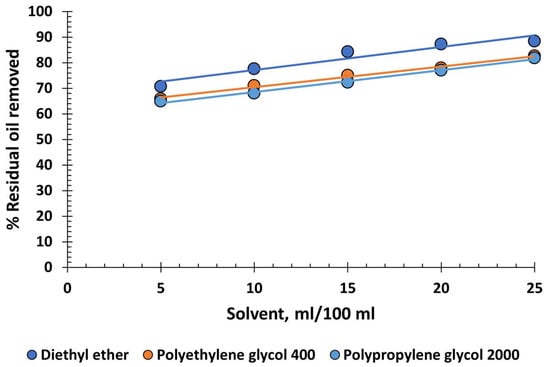
Figure 5.
Percentage of residual oil removed from produced water versus various volumes of ethers.
Surprisingly, the other two ethers, which are polymers, show acceptable performance, even though polymers usually tend to stabilize emulsions. Diethyl ether has a boiling point of 34.6 °C, making it unsuitable for industrial use, as the produced water typically exceeds 45 °C and the environment in oil fields can have high temperatures. If other alternatives do not require cooling of the produced water or special storage conditions, it is better not to consider it as a candidate. Moreover, it is toxic due to its narcotic effects and dangerous because it can form explosive mixtures. PEG 400 and PPG 2000, on the other hand, are completely harmless and not dangerous. However, their performance is not sufficient to recommend them for industrial use.
4.5. Esters
In Figure 6, the graphical representation of the percentage of residual oil removed from produced water by various esters and additionally by acetic acid is shown. Surprisingly, decyl oleate, a molecule significantly larger than the others, is the most effective. However, in no case is the efficacy sufficient to recommend its use in the purification of produced water. Also noteworthy is the stabilizing effect of butyl acrylate as its dosage increases. This behaviour may be due to its molecule having a certain surfactant capacity.
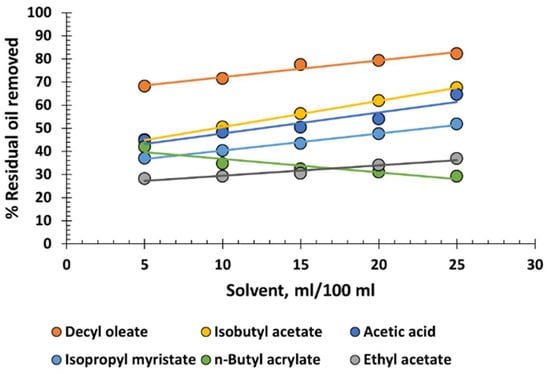
Figure 6.
Percentage of residual oil removed from produced water versus various volumes of esters.
The acrylate group, which includes a carboxyl and a conjugated double bond, acts as the hydrophilic group of the surfactant, and the butyl acts as the lipophilic group (Figure 7).
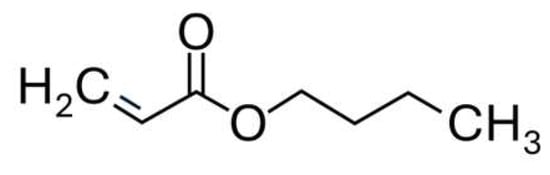
Figure 7.
Butyl acrylate.
Esters, although they are readily available, low-cost, and non-hazardous materials, have the disadvantage of low efficiency in oil removal, and therefore are not recommended for industrial use.
4.6. Miscellanea
To conclude, Figure 8 depicts a variety of solvents that cannot be classified into the chemical families previously analysed. Undoubtedly, the most notable is the exceptionally high performance of tetrahydrofuran, with virtually 100% recovery at all dosages. It is a small, cyclic molecule with an appropriate degree of ionization, thereby combining various advantages of xylene, cyclohexane, and the chlorinated hydrocarbons. In this respect, it is the most advantageous solvent of all. It has low viscosity and low density, which favours the flotation of the recovered oil. Additionally, with a boiling point of 66 °C, it can be easily distilled and reused in the process. It is not especially toxic, but it has the drawback of potentially forming explosive epoxides when distilled to dryness. This situation must be avoided. Supplementary Document S1 presents data on the toxicological properties of tetrahydrofuran as well as of crude oil for reference.
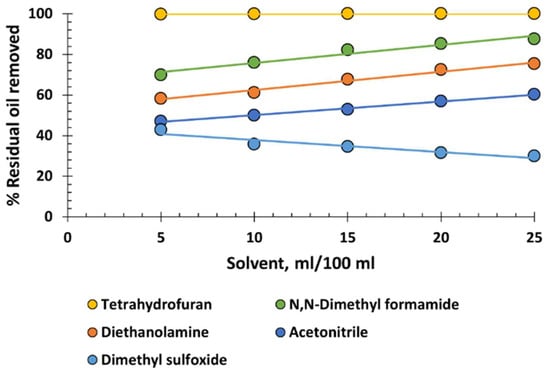
Figure 8.
Percentage of residual oil removed from produced water versus various volumes of solvents.
Regarding N,N-dimethyl formamide, diethanolamine, and acetonitrile, molecules with different nitrogenous groups do not exhibit sufficient oil recovery capacity to be recommended for use. The behaviour of dimethyl sulfoxide is striking as a very small and polarized molecule that stabilizes the oil emulsion as its dosage increases. This behaviour can be likened to that of glycerin, as previously discussed, as it can be interpreted as the effect of dimethyl sulfoxide’s affinity for water, its solvation, and probably its ability to increase the zeta potential of the oil droplets in the emulsion.
4.7. Hansen Solubility Parameters and Produced Water
A basic rule of chemistry is that “like dissolves like”. However, it is intriguing that substances with markedly different functional groups, such as xylene, cyclohexanone, methanol, or tetrahydrofuran, are capable of effectively dissolving the residual oil in produced water. Conversely, substances with the same functional group, such as cyclohexanone, acetone, and butanone—all ketones—exhibit markedly different capacities for dissolving the residual oil in produced water.
An approach that can explain these seemingly inexplicable behaviours is the Hansen solubility parameters (HSPs) [38]. According to Hansen, two substances interact with each other and dissolve in one another if their cohesion energies are similar. It is proposed that the cohesion energy of a substance, E, comprises three components: energy due to London dispersion forces, ED; energy due to the polarity of molecules, EP; and energy due to hydrogen bonding, EH:
If the total solubility parameter, δ, is defined as the square root of the cohesion energy density as follows,
and if the cohesion energy, E, is expressed in J/mol and the molar volume, v, in cm3/mol, then the units of the total solubility parameter are MPa1/2.
The parameters of dispersion, dD; polarity, dP; and hydrogen bonding, dH; respectively, are as follows:
where the units of the parameters thus defined are also MPa1/2.
Therefore:
In the three-dimensional Hansen space, the distance between two substances, 1 and 2, Ra, is defined as
where the units are MPa1/2.
This definition is like the definition of distance in Euclidean spaces, with the exception that the term related to the dispersion solubility parameters dD1 and dD2 is multiplied by 4. The Hansen solubility parameters of any substance form the centre of a “solubility sphere” with a radius Ro, where “good solvents” for the considered substance have a distance to the centre, Ra, equal to or less than the solubility radius, and “poor solvents” have a greater distance. The parameter Radius of Energy Difference, RED, is defined as follows:
Therefore, RED ≤ 1 for good solvents of the considered substance and RED > 1 for poor solvents. These conditions can be extended to any energetic interaction between substances, not only for solubilisation processes but also for adsorption, absorption, coating resistance, suspension stability, free surface energy [39], or for the specific case addressed in this article: the destabilization of oil emulsions in produced water by solvents.
From the perspective of Hansen solubility parameters, the present study considers the criterion that a solvent is an “effective destabilizer” of oil emulsions when, upon treating 100 mL of produced water with 25 mL of the solvent, the residual oil concentration, R, is reduced to less than 20 ppm. Table 4 presents the Hansen solubility parameters of the tested solvents. Solvents that are effective destabilisers are marked with a “1”, while ineffective ones are marked with a “0”.

Table 4.
Hansen solubility parameters of the tested solvents, MPa1/2, and ability to destabilise oil emulsions in produced water.
After entering this information into the Techné Solubility 3S software, it was found that, exceptionally, produced water exhibits three solubility spheres (Figure 9). This explains why solvents with very different cohesion energies are capable of effectively destabilising oil emulsions in produced water and purifying it. The most lipophilic solvents are grouped within the sphere with the lowest hydrogen-bonding solubility parameter (sphere L). In contrast, the most hydrophilic solvents, with the highest hydrogen-bonding parameter, are in sphere H, while those with an intermediate hydrogen-bonding parameter are found in sphere LH. The figure presents two- and three-dimensional representations of the spheres, while Table 5 displays the solubility parameters of the centres of the spheres and their radii.
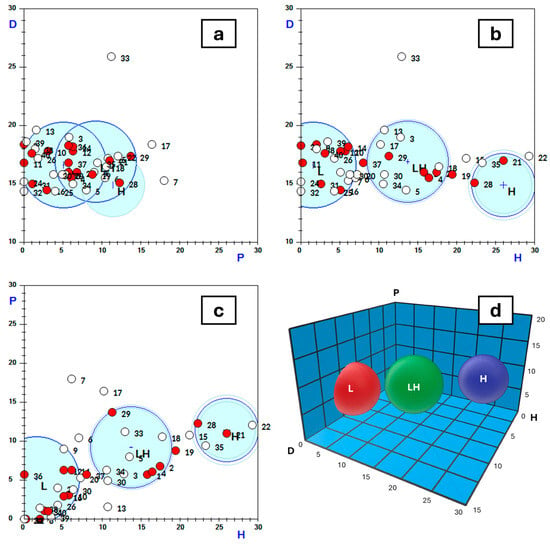
Figure 9.
Graphical representation of the solubility spheres of produced water. On the axes, according to the nomenclature provided by the Techné Solubility 3S software, D, P, and H represent δD, δP, and δH, respectively. The red points represent solvents that are effective destabilisers, while the white points represent those that are not. In the 2D representations, there are white points that may appear to be inside the spheres; however, other than a few exceptions, they are actually located in front of or behind the spheres.

Table 5.
HSPs and radii of the three solubility spheres of produced water. Units in MPa1/2. Abbreviations: L (lipophilic), LH (lipophilic–hydrophilic), and H (hydrophilic).
4.8. Stability of Oil Emulsions in Produced Water
The high stability of oil emulsions in produced water is due to the emulsifying capacity of the asphaltenes and resins that, together with hydrocarbons, make up crude oil. An example of an asphaltene molecule and an example of a resin molecule are shown in Figure 10 [40].
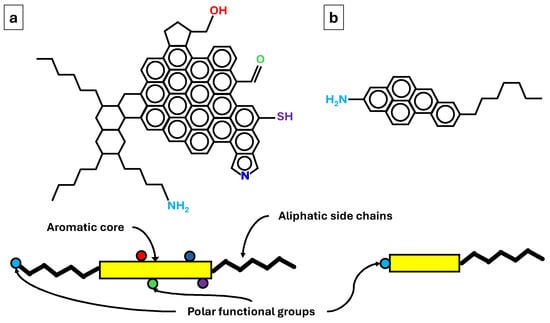
Figure 10.
Example of (a) asphaltene and (b) resin.
Asphaltenes are very complex molecules with a molecular weight greater than 1000 g/mol that are made up of a nucleus of aromatic rings, aliphatic side chains, and heteroatoms such as O, S, and N that act as polar functional groups. Resins are molecules like asphaltenes but with molecular weights of less than 1000 g/mol. These complex molecules combine lipophilic structures together with hydrophilic structures that give them amphiphilic characters, and therefore, they show surfactant behaviours. Figure 11 shows a graphical representation of a multiple emulsion of oil in produced water and a detail of the locations of molecules and ions at the oil–produced water interface.
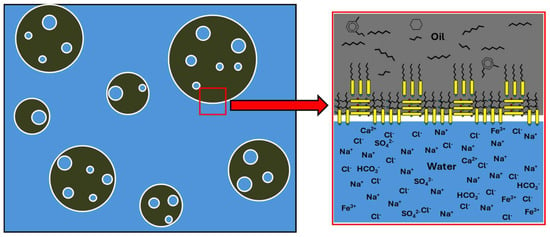
Figure 11.
Multiple emulsion of oil in produced water and detail of the positions of molecules and ions at the oil–produced water interface.
Asphaltene molecules, due to their flat structure, form overlapping layers in which their polar groups face each other. These layers, near the interface, also orient their hydrophilic polar groups towards the aqueous phase. Resins intercalate their lipophilic chains between the alkyl chains of the asphaltenes and position their polar groups towards the aqueous phase. They also align their polar groups with the polar groups of the asphaltenes through a solvation process. The result is a highly stable structure.
Therefore, to purify the produced water, it is necessary to induce the coalescence of the oil droplets, which can be achieved by destabilising these structures. One strategy is to add a solvent that, due to its molecular structure, can intercalate between the intermolecular bonds between asphaltenes and resins. Since asphaltenes and resins are bonded by both non-polar and polar groups, both non-polar and polar solvents can be used for this purpose.
This also justifies why the emulsions in produced water exhibit three solubility spheres, as demonstrated earlier.
5. Conclusions
This study proposes a novel procedure using solvents to purify produced water. Produced water contains high concentrations of salts, primarily sodium chloride, which, in the case of the Rumaila oil field (Iraq), taken as a reference, is approximately 80,000 ppm. It also contains oil droplets in the form of highly stable, complex emulsions that are difficult to break.
The procedure involves mixing a solvent with produced water containing an initial oil concentration of 150 ppm under agitation. The mixture is then left to rest for 10 min, allowing the oil to separate as a supernatant phase. This oil can be recovered and commercialised.
A total of 40 solvents from various chemical families were tested: linear aliphatic hydrocarbons, cyclic aliphatic and aromatic hydrocarbons, chlorinated hydrocarbons, alcohols and glycols, low- and high-molecular-weight ethers, ketones, esters, and a miscellaneous group of solvents with special characteristics.
The most effective solvents, with oil recovery rates exceeding 99%, were tetrachloroethylene (100%), tetrahydrofuran (100%), cyclohexane (99.5%), xylene (99.4%), and dichloromethane (99.4%). Tetrachloroethylene was excluded due to its suspected carcinogenicity [41], and dichloromethane was ruled out because its boiling point (40 °C) is below the industrial operating temperature of 45 °C. Therefore, cyclohexane, xylene, and tetrahydrofuran are preferred. These three solvents are relatively non-toxic compared with crude oil, are readily available at an industrial scale, and are cost-effective.
It was also found that solvents capable of reducing the oil content in produced water to below 20 ppm (oil recovery exceeding 87%) are grouped into three solubility spheres according to the Hansen solubility parameter (HSP) model:
- A lipophilic sphere with a centre at (16.6, 5.1, 1.6) and a radius of 5.5,
- A lipophilic–hydrophilic sphere with a centre at (16.9, 9.2, 13.7) and a radius of 5.2,
- A hydrophilic sphere with a centre at (14.9, 11.4, 26.0) and a radius of 4.1.
This grouping is explained by the amphiphilic nature of resins and asphaltenes, meaning that any solvent within these spheres can destabilise oil emulsions and purify produced water.
In conclusion, the application of solvents is an effective and viable alternative for the purification of produced water.
6. Patents
Part of the results of this manuscript are described in the Spanish patent application number P202431030, filed on 10 December 2024.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15041700/s1. Supplementary Document S1: Toxicity of Xylene, Cyclohexane, and Tetrahydrofuran.
Author Contributions
Conceptualization, A.S.A.A. and R.B.-M.; formal analysis, A.S.A.A. and R.B.-M.; investigation, A.S.A.A. and R.B.-M.; methodology, A.S.A.A. and R.B.-M.; software, R.B.-M.; writing—original draft, A.S.A.A. and R.B.-M.; writing—review and editing, A.S.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions in this study are included in the article. For further inquiries about these contributions and the Techné Solubility 3S software, please contact the corresponding author.
Acknowledgments
This work has been carried out as part of the research of the doctoral thesis of Aqeel Shaikhah Arafat Aljadiri, directed by Rafael Bailón-Moreno. The doctoral thesis belongs to the Doctoral Program in Chemistry of the University of Granada (codes ISCED 1 Chemistry and ISCED 2 Physical, Chemical, Geological Sciences), International Postgraduate School of the University of Granada).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Petróleo: Producción Mundial 1990–2022. Estatista. Available online: https://es.statista.com/estadisticas/635470/volumen-de-petroleo-producido-a-nivel-mundial/ (accessed on 12 March 2024).
- Dey, A.K. What is Produced Water?|Produced Water Treatment Processes. Available online: https://whatispiping.com/produced-water-treatment/ (accessed on 12 March 2024).
- Mastouri, R.; Nadim, F.; Kargari, N. 2010: A Time to Review the Produced Water Treatment Technologies, A Time to Look Forward for New Management Policies. In Proceedings of the 17th Annual Petroleum and Biofuels Environmental Conference [IPEC], San Antonio, TX, USA, 7–9 October 2010; Available online: https://www.avividwater.com/uploads/1/3/1/6/131696832/mastouri_2010_a_time_to_review_the_produced_water_treatment_technologies.pdf (accessed on 12 March 2024).
- Pangestu, N.L.; Zahra, N.L.; Sarwono, A.; Suryawan, I.W.K. Produced Water Treatment Planning Using Corrugated Plate. Serambi Eng. 2021, 6, 2286–2293. [Google Scholar]
- Abdul-Wahab, M.I.; Al-Shimmery, A.T. Oil Removal from Wastewater of Al -Al-Bezerqan Crude Oil Field by Air Flotation. Iraqi J. Chem. Petroelum 2004, 5, 41–47. [Google Scholar] [CrossRef]
- Alley, E.R. Water Quality Control Handbook Associates, 2nd ed.; The McGraw-Hill Companies, Inc.: New York, NY, USA, 2007. [Google Scholar]
- Hayes, T.; Arthur, D. Overview of Emerging Produced Water Treatment Technologies. In Proceedings of the 11th Annual International Petroleum Environmental Conference, Albuquerque, NM, USA, 12–15 October 2004. [Google Scholar]
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery Theory and Practice; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Salahia, A. Oily wastewater treatment using ultrafiltration. Desalination Water Treat. 2009, 6, 289–298. [Google Scholar] [CrossRef]
- Felch, C.L.; Patterson, M.R.; Kumfer, B.J. Catalytic Systems and Methods for Process Stream Treatment. Patent WO2015161187A2, 2 April 2015. [Google Scholar]
- Stewart, D.R.; Leong, L.Y. Purification of Oil Field Production Water for Beneficial Use. Patent US8097163B1, 17 January 2012. [Google Scholar]
- Chase, G. Mixed Hydrophilic/Hydrophobic Fiber Media for Liquid-Liquid. Coalescence. Patent US20100200512A1, 12 August 2010. [Google Scholar]
- Zhong, J.; Sun, X.; Wang, C. Treatment of oily wastewater produced from refinery processes using flocculation and ceramic membrane filtration. Sep. Purif. Technol. 2003, 32, 93–98. [Google Scholar] [CrossRef]
- Hassan, A.A.; Al-Zobai, K.M.M. Chemical oxidation for oil separation from oilfield produced water under UV irradiation using Titanium dioxide as a nano-photocatalyst by batch and continuous techniques. Int. J. Chem. Eng. 2019, 2019, 9810728. [Google Scholar] [CrossRef]
- Nadersha, S. Biological treatment of produced water using algae: A proof of concept. Master Thesis, United Arab Emirates University, Al Ain, United Arab Emirates, 2021. [Google Scholar]
- Islam, S. Investigation of Oil Adsorption Capacity of Granular Organoclay Media and the Kinetics of Oil Removal from Oil-in-Water Emulsions. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2006. [Google Scholar]
- Gao, H.; Sun, P.; Zhang, Y.; Zeng, X.; Wang, D.; Zhang, Y.; Wang, W.; Wu, J. A two-step hydrophobic fabrication of melamine sponge for oil absorption and oil/water separation. Surf. Coat. Technol. 2018, 339, 147–154. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, J.; Yan, H.; Xiao, J.; Wang, J. Reversible wettability switching of melamine sponges for oil/water separation. Mater. Chem. Phys. 2021, 257, 123772. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, Z.; Ge, W.; Lu, Y.; Liu, T.; Yang, W.; Dai, J. Robust, fluorine-free and superhydrophobic composite melamine sponge modified with dual silanized SiO2 microspheres for oil–water separation. Chin. J. Chem. Eng. 2020, 23, 50–60. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, D.; Shao, L.; Xia, Y.; Zeng, T.; Wang, Y. Cost-effective one-pot surface modified method to engineer a green superhydrophobic sponge for efficient oil/water mixtures as well as emulsions separation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 576, 43–54. [Google Scholar] [CrossRef]
- Xie, A.; Chen, Y.; Cui, J.; Lang, J.; Li, C.; Yan, Y.; Dai, J. Facile and green fabrication of superhydrophobic sponge for continuous oil/water separation from harsh environments. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 120–129. [Google Scholar] [CrossRef]
- Lei, Z.; Zheng, P.; Niu, L.; Yang, Y.; Shen, J.; Zhang, W.; Wang, C. Ultralight, robustly compressible and super-hydrophobic biomass-decorated carbonaceous melamine sponge for oil/water separation with high oil retention. Appl. Surf. Sci. 2019, 489, 922–929. [Google Scholar] [CrossRef]
- Nguyen-Dinh, M.-T.; Bui, T.S.; Lee, B.-K.; Masoumi, Z. Superhydrophobic MS@CuO@SA sponge for oil/water separation with excellent durability and reusability. Chemosphere 2022, 292, 133328. [Google Scholar] [CrossRef]
- Shayesteh, H.; Khosrowshahi, M.S.; Mashhadimoslem, H.; Maleki, F.; Rabbani, Y.; Emrooz, H.B.M. Durable superhydrophobic/superoleophilic melamine foam based on biomass-derived porous carbon and multi-walled carbon nanotube for oil/water separation. Nature Sci. Rep. 2023, 13, 4515. [Google Scholar] [CrossRef]
- Dano, J.; Abdelrahman, S.; Ali, M. Simulation Study on Polymer Flooding for Enhanced Oil Recovery: A Case Study. Mater. Today Proc. 2019, 19, 1507–1513. [Google Scholar] [CrossRef]
- Bhushan, B. Bioinspired oil–water separation approaches for oil spill clean-up and water purification. Philsophical Trans. A 2019, 377, 20190120. [Google Scholar] [CrossRef]
- Sun, Y.; Ke, Z.; Shen, C.; Sun, R.; Wei, Q.; Yin, Z.; Yang, W. Fabrication of Carbon Aerogels Derived from Metal-Organic Frameworks/Carbon Nanotubes/Cotton Composites as an Efficient Sorbent for Sustainable Oil–Water Separation. Appl. Sci. 2022, 12, 7285. [Google Scholar] [CrossRef]
- Noamani, S.; Niroomand, S.; Rastgar, M.; Sadrzadeh, M. Carbon-based polymer nanocomposite membranes for oily wastewater treatment. Clean Water 2019, 2, 20. [Google Scholar] [CrossRef]
- Elhenawy, S.; Khraisheh, M.; AlMomani, F.; Hassan, M.K.; Al-Ghouti, M.A.; Selvaraj, R. Recent Developments and Advancements in Graphene-Based Technologies for Oil Spill Cleanup and Oil–Water Separation Processes. Nanomaterials 2022, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Puasa, S.; Ismail, K.; Musman, M.; Sulong, N. Enhanced Oily Sludge Dewatering using Plant-Based Surfactant Technology. Mater. Today Proc. 2019, 19, 1159–1165. [Google Scholar] [CrossRef]
- Hussein, S.N.C.M.; Othman, N.H.; Dollah, A.; Rahim, A.N.C.A.; Japperi, N.S.; Ramakrishnan, N.S.M.A. Study of Acid Treated Mixed Sawdust as Natural Oil Sorbent for Oil Spill. Mater. Today Proc. 2019, 19, 1382–1389. [Google Scholar] [CrossRef]
- Aljadiri, A.S.A. Oily Water Treatments for Southern Iraqi Oil Fields. Master Thesis, College of Engineering of Nahrain University, Bagdad, Iraq, 2014. [Google Scholar]
- Kong, W.; Pan, Y.; Bhushan, B.; Zhao, X. Superhydrophilic Al2O3 Particle Layer for Efficient Separation of Oil-in-Water (O/W) and Water-in-Oil (W/O) Emulsions. Langmuir 2020, 36, 13285–13291. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, Y.; Shariaty-Niassar, M.; Ebrahimi, S.S. The effect of superhydrophobicity of prickly shape carbonyl iron particles on the oil-water adsorption. Ceram. Int. 2021, 47, 28400–28410. [Google Scholar] [CrossRef]
- Xu, L.; Han, M.; AlGhunaimi, F.; Bataweel, M. Salinity and Temperature Effects on Oily Produced Water Treatment Using Polyaluminium Chloride. In Proceedings of the Congress Middle East Oil, Gas and Geosciences Show, Manama, Bahrain, 19–21 February 2023. [Google Scholar] [CrossRef]
- Nollet, L.M.L.; Thiele, B. Handbook of Water Analysis; CRC Press: Boca Ratón, FL, USA; Taylor & Francis Group: Abingdon, UK, 2007. [Google Scholar]
- American Chemical Society. CAS SCIFinder. Available online: https://www.cas.org/solutions/cas-scifinder-discovery-platform/cas-scifinder-n (accessed on 15 December 2024).
- Charles, M. Hansen. Hansen Solubility Parameters. A User’s Handbook; CRC Press: Boca Ratón, FL, USA; Taylor & Francis Group: Abingdon, UK, 2007. [Google Scholar]
- Bailón-Moreno, R.; Bailón-Ruiz, M.A.; Aljadiri, A.S.A. Free Surface Energy and Hansen Solubility Parameter Vector Field. Interface Thickness. Appl. Sci. 2024, 14, 5834. [Google Scholar] [CrossRef]
- Joonaki, E.; Buckman, J.; Burgass, R.; Tohidi, B. Water versus Asphaltenes; Liquid–Liquid and Solid–Liquid Molecular Interactions Unravel the Mechanisms behind an Improved Oil Recovery Methodology. Sci. Rep. 2019, 9, 11369. [Google Scholar] [CrossRef] [PubMed]
- SciFinder. Available online: https://scifinder-n.cas.org/ (accessed on 20 January 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).