Muscle Activity of Superimposed Vibration in Suspended Kneeling Rollout
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
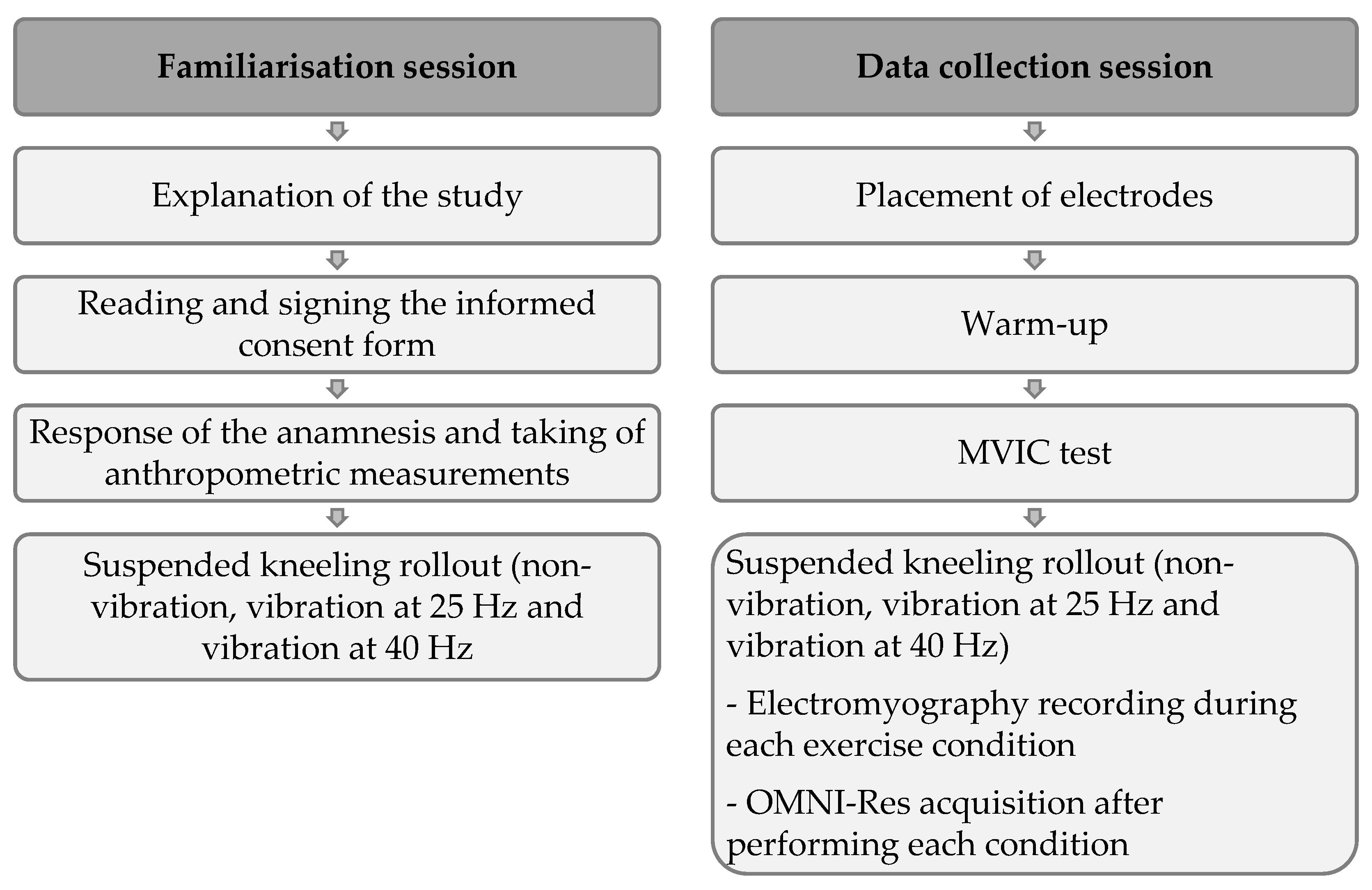
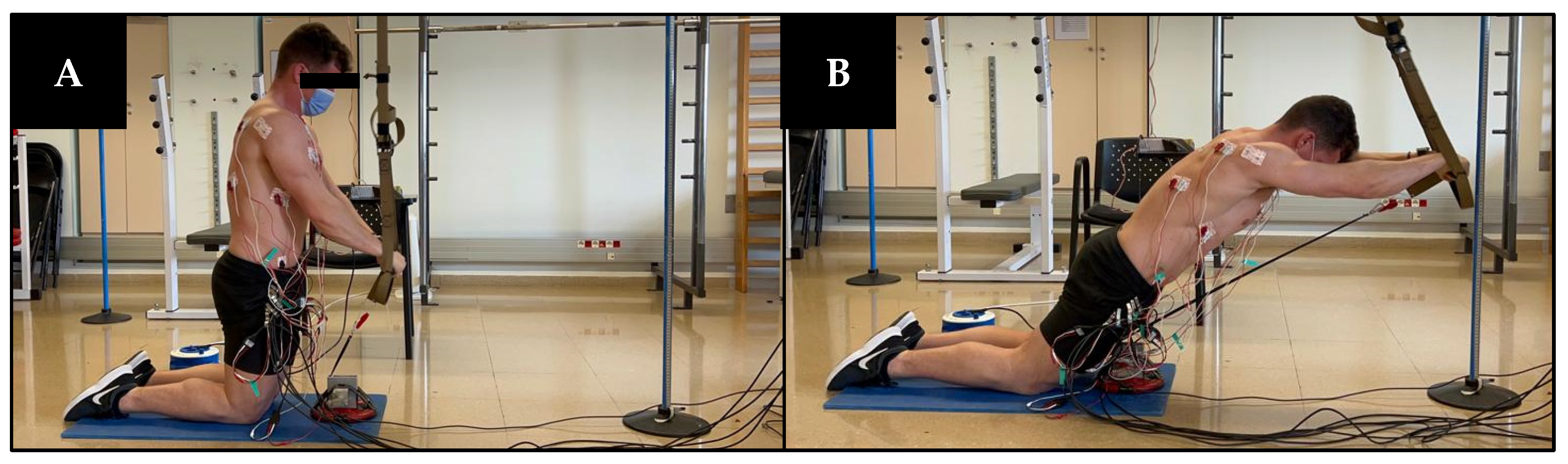
2.3. Procedures
2.4. MVIC Trials
2.5. sEMG Assessment
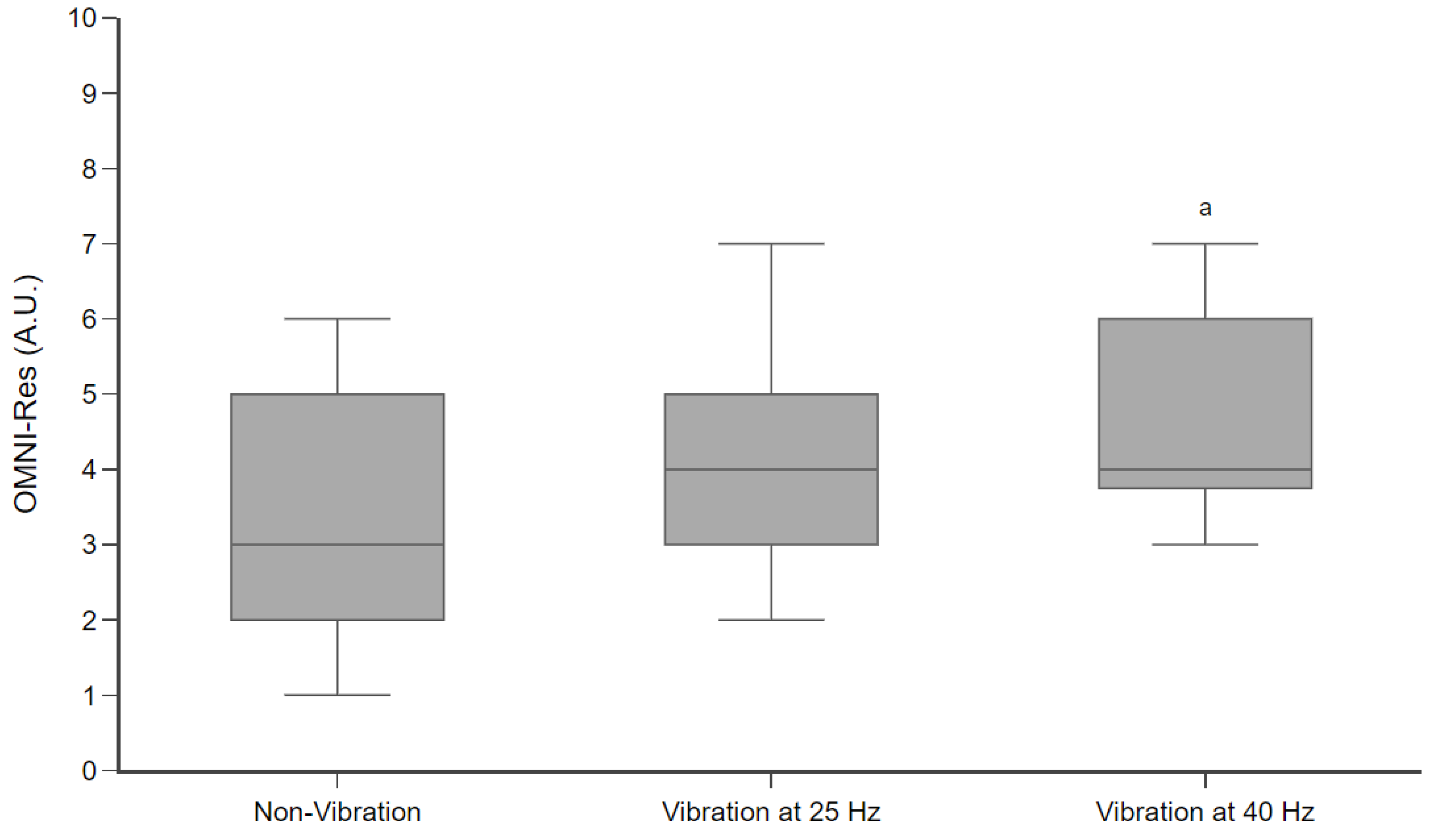
2.6. OMNI-Res
2.7. Data Analysis
2.8. Stadistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tomljanović, M.; Spasić, M.; Gabrilo, G.; Uljević, O.; Foretić, N. Effects of Five Weeks of Functional vs. Traditional Resistance Training on Anthropometric and Motor Performance Variables. Kinesiology 2011, 43, 145–154. [Google Scholar]
- Behm, D.G.; Muehlbauer, T.; Kibele, A.; Granacher, U. Effects of Strength Training Using Unstable Surfaces on Strength, Power and Balance Performance across the Lifespan: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 1645–1669. [Google Scholar] [CrossRef] [PubMed]
- Behm, D.G.; Colado, J.C. The Effectiveness of Resistance Training Using Unstable Surfaces and Devices for Rehabilitation. Int. J. Sports Phys. Ther. 2012, 7, 226–241. [Google Scholar] [PubMed]
- Moreno, F.J.; Barbado, D.; Caballero, C.; Urbán, T.; Sabido, R. Variations Induced by the Use of Unstable Surface Do Not Facilitate Motor Adaptation to a Throwing Skill. PeerJ 2023, 11, e14434. [Google Scholar] [CrossRef]
- Dawes, J. Complete Guide to TRX Suspension Training, 1st ed.; Klug, J., Earle, R., Pulliam, L., Gindes, A.C., Eds.; Human Kinetics: Champaign, IL, USA, 2017; ISBN 978-1-4925-3558-4. [Google Scholar]
- Aguilera-Castells, J.; Buscà, B.; Fort-Vanmeerhaeghe, A.; Montalvo, A.M.; Peña, J. Muscle Activation in Suspension Training: A Systematic Review. Sports Biomech. 2020, 19, 55–75. [Google Scholar] [CrossRef]
- Calatayud, J.; Borreani, S.; Colado, J.C.; Martín, F.; Rogers, M.E. Muscle Activity Levels in Upper-Body Push Exercises with Different Loads and Stability Conditions. Physician Sportsmed. 2014, 42, 106–119. [Google Scholar] [CrossRef]
- Calatayud, J.; Borreani, S.; Colado, J.C.; Martin, F.; Rogers, M.E.; Behm, D.G.; Andersen, L.L. Muscle Activation During Push-Ups with Different Suspension Training Systems. J. Sports Sci. Med. 2014, 13, 502–510. [Google Scholar]
- Marín, P.J.; Cochrane, D.J. The Effects of Whole-Body Vibration on EMG Activity of the Lower Body Muscles in Supine Static Bridge Position. J. Musculoskelet. Neuronal Interact. 2021, 21, 59–67. [Google Scholar]
- Alam, M.M.; Khan, A.A.; Farooq, M. Effect of Whole-Body Vibration on Neuromuscular Performance: A Literature Review. Work 2018, 59, 571–583. [Google Scholar] [CrossRef]
- Cardinale, M.; Bosco, C. The Use of Vibration as an Exercise Intervention. Exerc. Sport Sci. Rev. 2003, 31, 3–7. [Google Scholar] [CrossRef]
- Issurin, V.B. Vibrations and Their Applications in Sport: A Review. J. Sports Med. Phys. Fit. 2005, 45, 324–336. [Google Scholar]
- Ashnagar, Z.; Shadmehr, A.; Hadian, M.; Talebian, S.; Jalaei, S. The Effects of Whole Body Vibration on EMG Activity of the Upper Extremity Muscles in Static Modified Push up Position. J. Back Musculoskelet. Rehabil. 2016, 29, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.J.; Hawkes, D.H.; McMahon, J.; Horsley, I.; Khaiyat, O.A. Vibration as an Adjunct to Exercise: Its Impact on Shoulder Muscle Activation. Eur. J. Appl. Physiol. 2019, 119, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Tankisheva, E.; Boonen, S.; Delecluse, C.; Druyts, H.L.; Verschueren, S.M.P. Vibration Training for Upper Body: Transmission of Platform Vibrations through Cables. J. Strength Cond. Res. 2014, 28, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Moras, G.; Rodríguez-Jiménez, S.; Tous-Fajardo, J.; Ranz, D.; Mujika, I. A Vibratory Bar for Upper Body: Feasibilityand Acute Effects on EMGrms Activity. J. Strength Cond. Res. 2010, 24, 2132–2142. [Google Scholar] [CrossRef]
- Ni, C.-H.; Lu, Y.-H.; Chou, L.-W.; Kuo, S.-F.; Lin, C.-H.; Chiang, S.-L.; Lu, L.-H.; Wang, X.-M.; Chang, J.-L.; Lin, C.-H. Analysis of Vibration Frequency and Direction for Facilitating Upper-Limb Muscle Activity. Biology 2023, 12, 48. [Google Scholar] [CrossRef]
- Marín, P.J.; Hazell, T.J. Effects of Whole-Body Vibration with an Unstable Surface on Muscle Activation. J. Musculoskelet. Neuronal Interact. 2014, 14, 213–219. [Google Scholar]
- Aguilera-Castells, J.; Buscà, B.; Morales, J.; Solana-Tramunt, M.; Fort-Vanmeerhaeghe, A.; Rey-Abella, F.; Bantulà, J.; Peña, J. Muscle Activity of Bulgarian Squat. Effects of Additional Vibration, Suspension and Unstable Surface. PLoS ONE 2019, 14, e0221710. [Google Scholar] [CrossRef]
- Aguilera-Castells, J.; Buscà, B.; Arboix-Alió, J.; Miró, A.; Fort-Vanmeerhaeghe, A.; Peña, J. SEMG Activity in Superimposed Vibration on Suspended Supine Bridge and Hamstring Curl. Front. Physiol. 2021, 12, 712471. [Google Scholar] [CrossRef]
- Buscà, B.; Aguilera-Castells, J.; Arboix-Alió, J.; Miró, A.; Fort-Vanmeerhaeghe, A.; Huertas, P.; Peña, J. Superimposed Vibration on Suspended Push-Ups. PeerJ 2022, 10, e14435. [Google Scholar] [CrossRef]
- Dawes, J. Core Exercises. In Complete Guide to TRX Suspension Training; Human Kinetics: Champaign, IL, USA, 2017; pp. 137–160. ISBN 9781492535584. [Google Scholar]
- Oliva-Lozano, J.M.; Muyor, J.M. Core Muscle Activity During Physical Fitness Exercises: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 4306. [Google Scholar] [CrossRef] [PubMed]
- Martuscello, J.M.; Nuzzo, J.L.; Ashley, C.S.; Campbell, B.I.; Orriola, J.J.; Mayer, J.M. Systematic Review of Core Muscle Activity During Physical Fitness Exercises. J. Strength Cond. Res. 2013, 27, 1684–1698. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.H.; Schoenfeld, B.J.; da Silva, J.J.; Guiselini, M.A.; de Freitas, F.S.; Pecoraro, S.L.; Gomes, W.A.; Lopes, C.R. Muscle Activation Pattern During Isometric Ab Wheel Rollout Exercise in Different Shoulder Angle-Positions. Med. Express 2015, 2, 15–17. [Google Scholar] [CrossRef]
- Hildenbrand, K.; Noble, L. Abdominal Muscle Activity While Performing Trunk-Flexion Exercises Using the Ab Roller, ABslide, FitBall, and Conventionally Performed Trunk Curls. J. Athl. Train. 2004, 39, 37–43. [Google Scholar]
- Escamilla, R.F.; Babb, E.; DeWitt, R.; Jew, P.; Kelleher, P.; Burnham, T.; Busch, J.; D’Anna, K.; Mowbray, R.; Imamura, R.T. Electromyographic Analysis of Traditional and Nontraditional Abdominal Exercises: Implications for Rehabilitation and Training. Phys. Ther. 2006, 86, 656–671. [Google Scholar] [CrossRef]
- Duncan, M. Muscle Activity of the Upper and Lower Rectus Abdominis During Exercises Performed on and off a Swiss Ball. J. Bodyw. Mov. Ther. 2009, 13, 364–367. [Google Scholar] [CrossRef]
- Escamilla, R.F.; Lewis, C.; Bell, D.; Bramblet, G.; Daffron, J.; Lambert, S.; Pecson, A.; Imamura, R.; Paulos, L.; Dreanws, J.R. Core Muscle Activation During Swiss Ball and Traditional Abdominal Exercises. J. Orthop. Sports Phys. Ther. 2010, 40, 265–276. [Google Scholar] [CrossRef]
- Cugliari, G.; Boccia, G. Core Muscle Activation in Suspension Training Exercises. J. Hum. Kinet. 2017, 56, 61–71. [Google Scholar] [CrossRef]
- Calatayud, J.; Casaña, J.; Martín, F.; Jakobsen, M.D.; Colado, J.C.; Andersen, L.L. Progression of Core Stability Exercises Based on the Extent of Muscle Activity. Am. J. Phys. Med. Rehabil. 2017, 96, 694–699. [Google Scholar] [CrossRef]
- Robertson, R.J.; Goss, F.L.; Rutkowski, J.; Lenz, B.; Dixon, C.; Timmer, J.; Frazee, K.; Dube, J.; Andreacci, J. Concurrent Validation of the OMNI Perceived Exertion Scale for Resistance Exercise. Med. Sci. Sports Exerc. 2003, 35, 333–341. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-001512-8. [Google Scholar]
- Cram, J.R.; Kasman, G.S.; Holtz, J. Introduction to Surface Electromyography; Aspen Publishers: Gaithersburg, Maryland, 1998; ISBN 9780763732745. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Ritzmann, R.; Gollhofer, A.; Kramer, A. The Influence of Vibration Type, Frequency, Body Position and Additional Load on the Neuromuscular Activity During Whole Body Vibration. Eur. J. Appl. Physiol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hazell, T.J.; Jakobi, J.M.; Kenno, K.A. The Effects of Whole-Body Vibration on Upper- and Lower-Body EMG During Static and Dynamic Contractions. Appl. Physiol. Nutr. Metab. 2007, 32, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, M.D.; Sundstrup, E.; Andersen, C.H.; Aagaard, P.; Andersen, L.L. Muscle Activity During Leg Strengthening Exercise Using Free Weights and Elastic Resistance: Effects of Ballistic vs Controlled Contractions. Hum. Mov. Sci. 2013, 32, 65–78. [Google Scholar] [CrossRef]
- Konrad, P. The ABC of EMG: A Practical Introduction of Kinesiological Electromyography, 1st ed.; Noraxon INC: Scottsdale, AZ, USA, 2006; ISBN 0-9771622-1-4. [Google Scholar]
- Borges, D.T.; de Macedo, L.B.; de Lins, C.A.; de Sousa, C.O.; Brasileiro, J.S. Effects of Whole Body Vibration on the Neuromuscular Amplitude of Vastus Lateralis Muscle. J. Sport. Sci. Med. 2017, 16, 414–420. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 1st ed.; Lawrence Erlbaum: Hilldale, NJ, USA, 1988. [Google Scholar]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive Statistics for Studies in Sports Medicine and Exercise Science. Med. Sci. Sports Exerc. 2009, 41, 3–12. [Google Scholar] [CrossRef]
- Eisinga, R.; Heskes, T.; Pelzer, B.; Te Grotenhuis, M. Exact P-Values for Pairwise Comparison of Friedman Rank Sums, with Application to Comparing Classifiers. BMC Bioinform. 2017, 18, 68. [Google Scholar] [CrossRef]
- Cliff, N. Dominance Statistics: Ordinal Analyses to Answer Ordinal Questions. Psychol. Bull. 1993, 114, 494–509. [Google Scholar] [CrossRef]
- Rittweger, J. Vibration as an Exercise Modality: How It May Work, and What Its Potential Might Be. Eur. J. Appl. Physiol. 2010, 108, 877–904. [Google Scholar] [CrossRef]
- Tankisheva, E.; Jonkers, I.; Boonen, S.; Delecluse, C.; Van Lenthe, G.H.; Druyts, H.L.; Spaepen, P.; Verschueren, S.M.P. Transmission of Whole-Body Vibration and Its Effect on Muscle Activation. J. Strength Cond. Res. 2013, 27, 2533–2541. [Google Scholar] [CrossRef]
- Kapandji, A.I. El Hombro. In Fisiología Articular. Tomo 1; Editorial Medica Panamericana: Madrid, Spain, 2006; pp. 2–75. ISBN 84-9835-002-6. [Google Scholar]
- Tsuruike, M.; Ellenbecker, T.S. Serratus Anterior and Lower Trapezius Muscle Activities During Multi-Joint Isotonic Scapular Exercises and Isometric Contractions. J. Athl. Train. 2015, 50, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Tsuruike, M.; Ellenbecker, T.S.; Lauffenburger, C. The Application of Double Elastic Band Exercise in the 90/90 Arm Position for Overhead Athletes. Sports Health 2020, 12, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Tsuruike, M.; Ellenbecker, T.S.; Kagaya, Y.; Lemings, L. Analysis of Scapular Muscle EMG Activity During Elastic Resistance Oscillation Exercises from the Perspective of Different Arm Positions. Sports Health 2020, 20, 395–400. [Google Scholar] [CrossRef]
- Escamilla, R.F.; Yamashiro, K.; Paulos, L.; Andrews, J.R. Shoulder Muscle Activity and Function in Common Shoulder Rehabilitation Exercises. Sports Med. 2009, 39, 663–685. [Google Scholar] [CrossRef]
- Reinold, M.M.; Wilk, K.E.; Fleisig, G.S.; Zheng, N.; Barrentine, S.W.; Chmielewski, T.; Cody, R.C.; Jameson, G.G.; Andrews, J.R. Electromyographic Analysis of the Rotator Cuff and Deltoid Musculature During Common Shoulder External Rotation Exercises. J. Orthop. Sports Phys. Ther. 2004, 34, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Marín, P.J.; Santos-Lozano, A.; Santin-Medeiros, F.; Robertson, R.J.; Garatachea, N. Reliability and Validity of the OMNI-Vibration Exercise Scale of Perceived Exertion. J. Sports Sci. Med. 2012, 11, 438–443. [Google Scholar]


| Muscle Group | Suspended Kneeling Rollout | ||
|---|---|---|---|
| Non-Vibration | Vibration at 25 Hz | Vibration at 40 Hz | |
| Mean ± SD | Mean ± SD | Mean ± SD | |
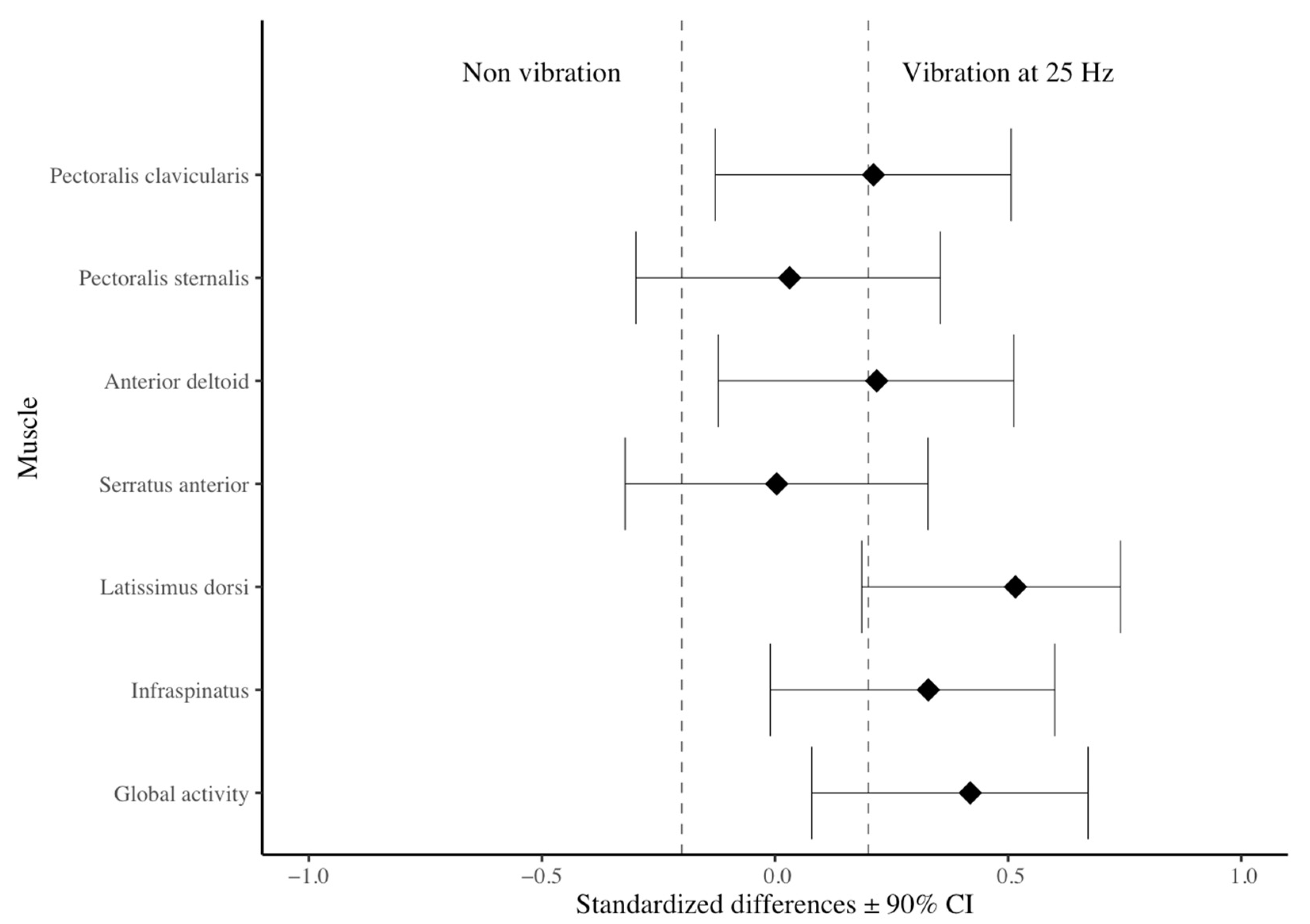
| Pectoralis clavicularis | 23.5 ± 16.6 | 30.3 ± 20.2 | 30.1 ± 18.9 |
| Pectoralis sternalis | 25.5 ± 14.7 | 27.4 ± 17.7 | 30.7 ± 19.7 a |
| Anterior deltoid | 3.3 ± 2.6 | 4.2 ± 3.8 a | 4.8 ± 4.5 a |
| Serratus anterior | 24.9 ± 13.2 | 26.7 ± 16.6 | 28.5 ± 17.2 |
| Latissimus dorsi | 20.7 ± 10.1 | 31.2 ± 13.0 a | 28.5 ± 12.3 a |
| Infraspinatus | 26.0 ± 11.3 | 34.3 ± 15.1 a | 32.5 ± 16.8 a |
| Global activity | 20.6 ± 6.5 | 25.7 ± 7.7 a | 25.9 ± 8.6 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huertas, P.; Buscà, B.; Arboix-Alió, J.; Miró, A.; Esquerrà, L.H.; Peña, J.; Vicens-Bordas, J.; Aguilera-Castells, J. Muscle Activity of Superimposed Vibration in Suspended Kneeling Rollout. Appl. Sci. 2025, 15, 1637. https://doi.org/10.3390/app15031637
Huertas P, Buscà B, Arboix-Alió J, Miró A, Esquerrà LH, Peña J, Vicens-Bordas J, Aguilera-Castells J. Muscle Activity of Superimposed Vibration in Suspended Kneeling Rollout. Applied Sciences. 2025; 15(3):1637. https://doi.org/10.3390/app15031637
Chicago/Turabian StyleHuertas, Pol, Bernat Buscà, Jordi Arboix-Alió, Adrià Miró, Laia H. Esquerrà, Javier Peña, Jordi Vicens-Bordas, and Joan Aguilera-Castells. 2025. "Muscle Activity of Superimposed Vibration in Suspended Kneeling Rollout" Applied Sciences 15, no. 3: 1637. https://doi.org/10.3390/app15031637
APA StyleHuertas, P., Buscà, B., Arboix-Alió, J., Miró, A., Esquerrà, L. H., Peña, J., Vicens-Bordas, J., & Aguilera-Castells, J. (2025). Muscle Activity of Superimposed Vibration in Suspended Kneeling Rollout. Applied Sciences, 15(3), 1637. https://doi.org/10.3390/app15031637










