Co-Hydrothermal Carbonization of Sawdust and Sewage Sludge: Assessing the Potential of the Hydrochar as an Adsorbent and the Ecotoxicity of the Process Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrochar Production
2.3. Adsorption of Methylene Blue
2.3.1. pH Effect
2.3.2. Adsorption Isotherms
2.3.3. Adsorption Kinetics
2.3.4. Adsorption Thermodynamics
2.3.5. Hydrochar Regeneration
2.3.6. Methylene Blue Quantification
2.4. Process Water (PW)
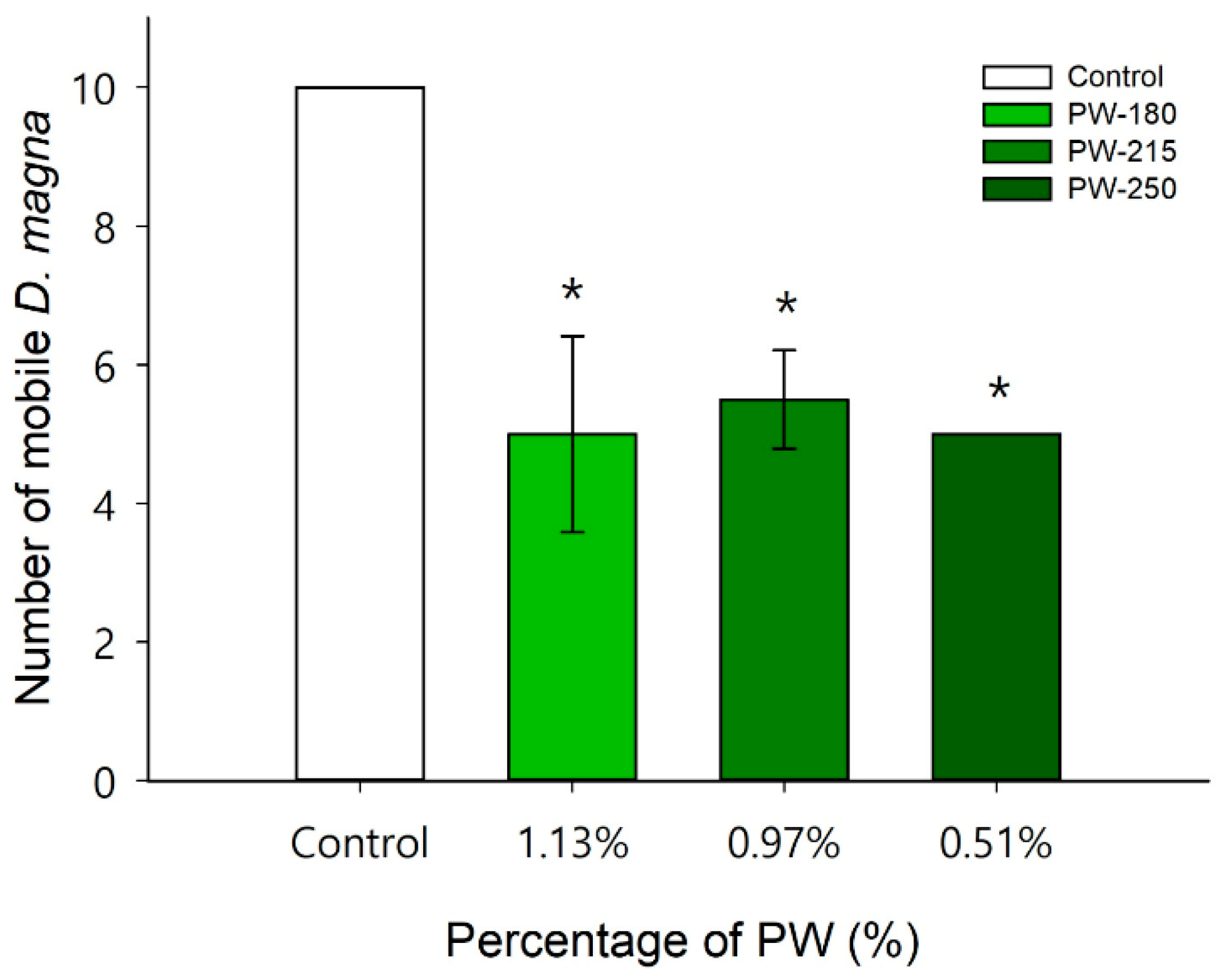
2.4.1. Ecotoxicity of PW
2.4.2. Characterization of PW
3. Results and Discussion
3.1. Hydrochar Production
3.2. Methylene Blue Adsorption
3.2.1. pH Effect
3.2.2. Adsorption Isotherms
| Raw Material | Hydrochar Activation | qmax (mg·g−1) | Reference |
|---|---|---|---|
| Sawdust and sewage sludge | No | 70 | This study |
| Citrus waste | No | 31–66 | [42] |
| Winery waste | No | 2–37 | |
| Sewage sludge | No | 38–71 | [43] |
| Corn straw | Yes | 20–60 | [47] |
| Pomegranate peels | Yes | 50 | [48] |
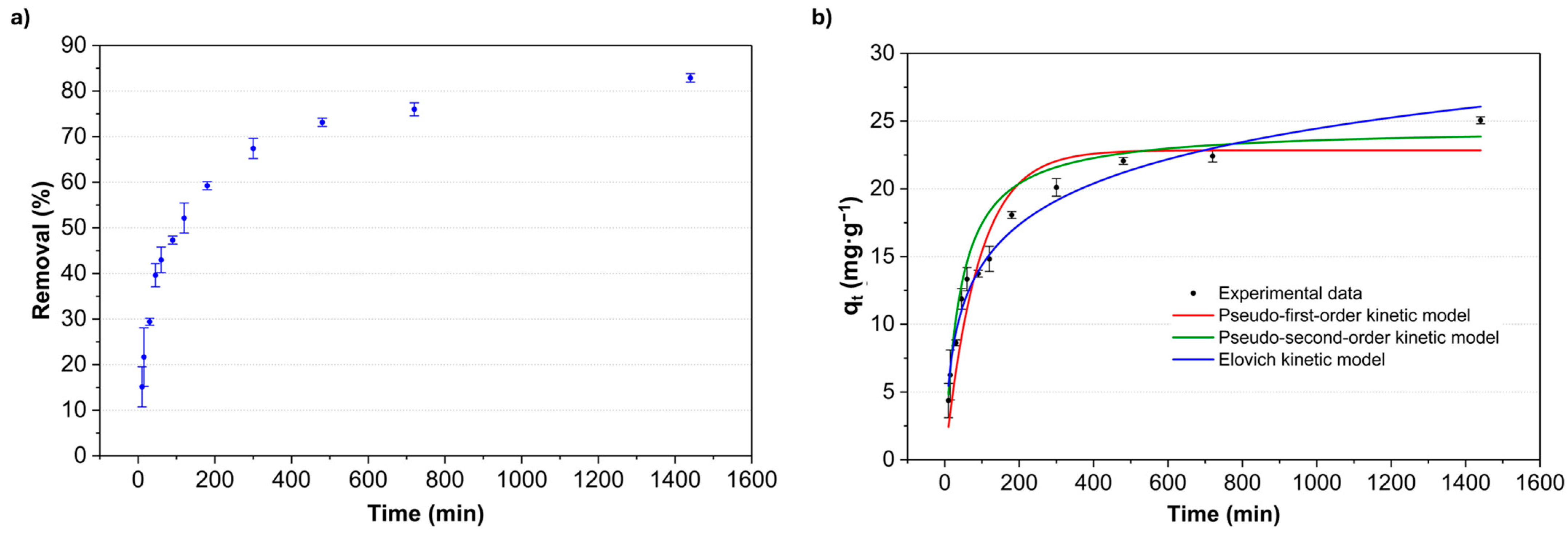
3.2.3. Adsorption Kinetics
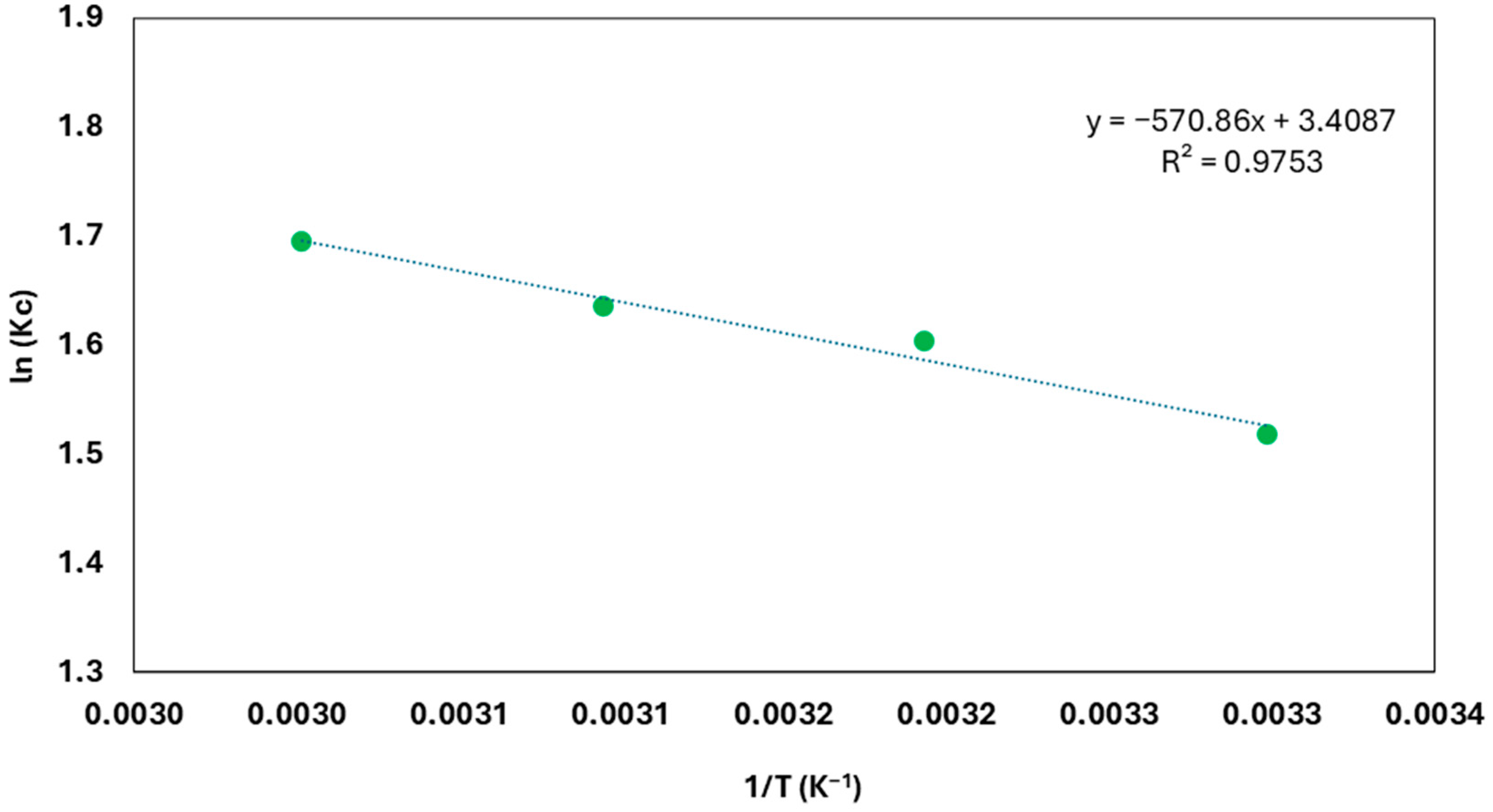
3.2.4. Adsorption Thermodynamics
3.2.5. Hydrochar Regeneration
3.3. Process Water (PW)—Ecotoxicity and Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, C.; Chen, R.; Li, J.J.; Li, J.J.; Drahansky, M.; Paridah, M.; Moradbak, A.; Mohamed, A.Z.; Owolabi, F.H.; Abdulwahab, T.; et al. Pyrolysis: A Sustainable Way to Generate Energy from Waste; Intech: Vienna, Austria, 2012; p. 13. [Google Scholar]
- You, S.; Sik, Y.; Chen, S.S.; Tsang, D.C.W.; Kwon, E.E.; Lee, J.; Wang, C. A Critical Review on Sustainable Biochar System through Gasification: Energy and Environmental Applications. Bioresour. Technol. 2017, 246, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Cavali, M.; Libardi Junior, N.; de Sena, J.D.; Woiciechowski, A.L.; Soccol, C.R.; Belli Filho, P.; Bayard, R.; Benbelkacem, H.; de Castilhos Junior, A.B. A Review on Hydrothermal Carbonization of Potential Biomass Wastes, Characterization and Environmental Applications of Hydrochar, and Biorefinery Perspectives of the Process. Sci. Total Environ. 2023, 857, 159627. [Google Scholar] [CrossRef] [PubMed]
- Prochnow, F.D.; Cavali, M.; Dresch, A.P.; Belli, I.M.; Libardi, N.; de Castilhos, A.B. Biochar: From Laboratory to Industry Scale—An Overview of Scientific and Industrial Advances, Opportunities in the Brazilian Context, and Contributions to Sustainable Development. Processes 2024, 12, 1006. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Chowdhury, S.; Balasubramanian, R. Hydrothermal Conversion of Urban Food Waste to Chars for Removal of Textile Dyes from Contaminated Waters. Bioresour. Technol. 2014, 161, 310–319. [Google Scholar] [CrossRef]
- Zhao, K.; Li, Y.; Zhou, Y.; Guo, W.; Jiang, H.; Xu, Q. Characterization of Hydrothermal Carbonization Products (Hydrochars and Spent Liquor) and Their Biomethane Production Performance. Bioresour. Technol. 2018, 267, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Langone, M.; Basso, D. Process Waters from Hydrothermal Carbonization of Sludge: Characteristics and Possible Valorization Pathways. Int. J. Environ. Res. Public. Health 2020, 17, 6618. [Google Scholar] [CrossRef] [PubMed]
- Kambo, H.S.; Dutta, A. A Comparative Review of Biochar and Hydrochar in Terms of Production, Physico-Chemical Properties and Applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, S.; Cui, D.; Zhou, H.; Wu, D.; Pan, S.; Xu, F.; Wang, Z. Co-Hydrothermal Carbonization of Organic Solid Wastes to Hydrochar as Potential Fuel: A Review. Sci. Total Environ. 2022, 850, 158034. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.; Li, W.; Bao, S.; Li, Y.; Tan, W. Co-Hydrothermal Carbonization of Agricultural Waste and Sewage Sludge for Product Quality Improvement: Fuel Properties of Hydrochar and Fertilizer Quality of Aqueous Phase. J. Environ. Manage 2023, 326, 116781. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Z.; Zhang, Y.N.; Guo, J.Z.; Lv, J.Q.; Huan, W.W.; Li, B. Preparation of Hydrochar with High Adsorption Performance for Methylene Blue by Co-Hydrothermal Carbonization of Polyvinyl Chloride and Bamboo. Bioresour. Technol. 2021, 337, 125442. [Google Scholar] [CrossRef]
- Cavali, M.; Benbelkacem, H.; Kim, B.; Bayard, R.; Libardi Junior, N.; Gonzaga Domingos, D.; Woiciechowski, A.L.; de Castilhos Junior, A.B. Co-Hydrothermal Carbonization of Pine Residual Sawdust and Non-Dewatered Sewage Sludge—Effect of Reaction Conditions on Hydrochar Characteristics. J. Environ. Manage 2023, 340, 117994. [Google Scholar] [CrossRef]
- Masoumi, S.; Borugadda, V.B.; Nanda, S.; Dalai, A.K. Hydrochar: A Review on Its Production Technologies and Applications. Catalysts 2021, 11, 939. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Dutta, S.; Adhikary, S.; Bhattacharya, S.; Roy, D.; Chatterjee, S.; Chakraborty, A.; Banerjee, D.; Ganguly, A.; Nanda, S.; Rajak, P. Contamination of Textile Dyes in Aquatic Environment: Adverse Impacts on Aquatic Ecosystem and Human Health, and Its Management Using Bioremediation. J. Environ. Manage 2024, 353, 120103. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Merzari, F.; Langone, M.; Andreottola, G.; Fiori, L. Methane Production from Process Water of Sewage Sludge Hydrothermal Carbonization. A Review. Valorising Sludge through Hydrothermal Carbonization. Crit. Rev. Environ. Sci. Technol. 2019, 49, 947–988. [Google Scholar] [CrossRef]
- Wirth, B.; Reza, T.; Mumme, J. Influence of Digestion Temperature and Organic Loading Rate on the Continuous Anaerobic Treatment of Process Liquor from Hydrothermal Carbonization of Sewage Sludge. Bioresour. Technol. 2015, 198, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Tian, L.; Liu, Z.; He, J.; Fu, H.; Huang, Q.; Xue, H.; Huang, Z. Distribution and Toxicity of Polycyclic Aromatic Hydrocarbons during CaO-Assisted Hydrothermal Carbonization of Sewage Sludge. Waste Manag. 2021, 120, 616–625. [Google Scholar] [CrossRef]
- Fregolente, L.G.; Miguel, T.B.A.R.; de Castro Miguel, E.; de Almeida Melo, C.; Moreira, A.B.; Ferreira, O.P.; Bisinoti, M.C. Toxicity Evaluation of Process Water from Hydrothermal Carbonization of Sugarcane Industry By-Products. Environ. Sci. Pollut. Res. 2019, 26, 27579–27589. [Google Scholar] [CrossRef] [PubMed]
- Petrovič, A.; Cenčič Predikaka, T.; Škodič, L.; Vohl, S.; Čuček, L. Hydrothermal Co-Carbonization of Sewage Sludge and Whey: Enhancement of Product Properties and Potential Application in Agriculture. Fuel 2023, 350, 128807. [Google Scholar] [CrossRef]
- Kusuma, H.S.; Christa Jaya, D.E.; Illiyanasafa, N.; Ikawati, K.L.; Kurniasari, E.; Darmokoesoemo, H.; Amenaghawon, A.N. A Critical Review and Bibliometric Analysis of Methylene Blue Adsorption Using Leaves. Chemosphere 2024, 356, 141867. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene Blue Dye: Toxicity and Potential Elimination Technology from Wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Cavali, M.; Kim, B.; Tedoldi, D.; Benbelkacem, H.; Bayard, R.; Garnier, V.; Libardi, N.; Woiciechowski, A.L.; Borges de Castilhos, A. Hydrochar from Sawdust and Sewage Sludge—A Potential Media for Retaining Heavy Metals in Sustainable Drainage Systems (SuDS). Environ. Technol. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Velinov, N.; Najdanović, S.; Vučić, R.; Mitrović, J.; Kostić, M.; Bojić, D.; Bojić, A. Biosorption of loperamide by lignocellulosic-Al2O3 hybrid: Optimization, kinetics, isothermal and thermodynamic studies. Cellulose Chem. Technol. 2019, 53, 175–189. [Google Scholar] [CrossRef]
- Filipović, K.; Petrović, M.; Najdanović, S.; Velinov, N.; Hurt, A.; Bojić, A.; Kostić, M. Highly Efficient Nano Sorbent as a Superior Material for the Purification of Wastewater Contaminated with Anthraquinone Dye RB19. J. Water Process Eng. 2024, 67, 106118. [Google Scholar] [CrossRef]
- Isquierdo, E.P.; Caldeira, D.S.A.; Siqueira, V.C.; Martins, E.A.S.; Quequeto, W.D. Fittings of Adsorption Isotherm Models and Thermodynamic Properties of Urunday Seeds. Eng. Agric. 2020, 40, 374–380. [Google Scholar] [CrossRef]
- Velinov, N.; Radović Vučić, M.; Petrović, M.; Najdanović, S.; Kostić, M.; Mitrović, J.; Bojić, A. The Influence of Various Solvents’ Polarity in the Synthesis of Wood Biowaste Sorbent: Evaluation of Dye Sorption. Biomass Convers. Biorefin 2023, 13, 8139–8150. [Google Scholar] [CrossRef]
- Chambers, C.; Saha, S.; Grimes, S.; Calhoun, J.; Reza, M.T. Physical and Morphological Alteration of Sargassum-Derived Ultraporous Superactivated Hydrochar with Remarkable Cationic Dye Adsorption. Biomass Convers. Biorefin 2023, 14, 29131–29144. [Google Scholar] [CrossRef]
- Aktas, K.; Liu, H.; Eskicioglu, C. Treatment of Aqueous Phase from Hydrothermal Liquefaction of Municipal Sludge by Adsorption: Comparison of Biochar, Hydrochar, and Granular Activated Carbon. J. Environ. Manage 2024, 356, 120619. [Google Scholar] [CrossRef] [PubMed]
- Alhawtali, S.; El-Harbawi, M.; Al-Awadi, A.S.; El Blidi, L.; Alrashed, M.M.; Yin, C.Y. Enhanced Adsorption of Methylene Blue Using Phosphoric Acid-Activated Hydrothermal Carbon Microspheres Synthesized from a Variety of Palm-Based Biowastes. Coatings 2023, 13, 1287. [Google Scholar] [CrossRef]
- Genli, N.; Kutluay, S.; Baytar, O.; Şahin, Ö. Preparation and Characterization of Activated Carbon from Hydrochar by Hydrothermal Carbonization of Chickpea Stem: An Application in Methylene Blue Removal by RSM Optimization. Int. J. Phytoremediat. 2022, 24, 88–100. [Google Scholar] [CrossRef]
- Md. Munjur, H.; Hasan, M.N.; Awual, M.R.; Islam, M.M.; Shenashen, M.A.; Iqbal, J. Biodegradable Natural Carbohydrate Polymeric Sustainable Adsorbents for Efficient Toxic Dye Removal from Wastewater. J. Mol. Liq. 2020, 319, 114356. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Baird, R.B., Eaton, A.D., Rice, E.W., Eds.; American Public Health Association: Washington, DC, USA, 2017; ISBN 9780875532875. [Google Scholar]
- Atallah, E.; Kwapinski, W.; Ahmad, M.N.; Leahy, J.J.; Al-Muhtaseb, A.H.; Zeaiter, J. Hydrothermal Carbonization of Olive Mill Wastewater: Liquid Phase Product Analysis. J. Environ. Chem. Eng. 2019, 7, 102833. [Google Scholar] [CrossRef]
- Zhou, F.; Li, K.; Hang, F.; Zhang, Z.; Chen, P.; Wei, L.; Xie, C. Efficient Removal of Methylene Blue by Activated Hydrochar Prepared by Hydrothermal Carbonization and NaOH Activation of Sugarcane Bagasse and Phosphoric Acid. RSC Adv. 2022, 12, 1885–1896. [Google Scholar] [CrossRef] [PubMed]
- Khoshbouy, R.; Takahashi, F.; Yoshikawa, K. Preparation of High Surface Area Sludge-Based Activated Hydrochar via Hydrothermal Carbonization and Application in the Removal of Basic Dye. Environ. Res. 2019, 175, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Dhaouadi, F.; Sellaoui, L.; Hernández-Hernández, L.E.; Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E.; González-Ponce, H.A.; Taamalli, S.; Louis, F.; Lamine, A. Ben Preparation of an Avocado Seed Hydrochar and Its Application as Heavy Metal Adsorbent: Properties and Advanced Statistical Physics Modeling. Chem. Eng. J. 2021, 419, 129472. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, H.; Feng, Q.; Yao, D.; Chen, H.; Wang, H.; Zhou, Z.; Li, H.; Tian, Y.; Lu, X. Effect of Phosphoric Acid on the Surface Properties and Pb(II) Adsorption Mechanisms of Hydrochars Prepared from Fresh Banana Peels. J. Clean. Prod. 2017, 165, 221–230. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen Peroxide Modification Enhances the Ability of Biochar (Hydrochar) Produced from Hydrothermal Carbonization of Peanut Hull to Remove Aqueous Heavy Metals: Batch and Column Tests. Chem. Eng. J. 2012, 200–202, 673–680. [Google Scholar] [CrossRef]
- Saha, N.; Volpe, M.; Fiori, L.; Volpe, R.; Messineo, A.; Reza, M.T. Cationic Dye Adsorption on Hydrochars of Winery and Citrus Juice Industries Residues: Performance, Mechanism, and Thermodynamics. Energies 2020, 13, 4686. [Google Scholar] [CrossRef]
- Ferrentino, R.; Ceccato, R.; Marchetti, V.; Andreottola, G.; Fiori, L. Sewage Sludge Hydrochar: An Option for Removal of Methylene Blue from Wastewater. Appl. Sci. 2020, 10, 3445. [Google Scholar] [CrossRef]
- El Ouadrhiri, F.; Elyemni, M.; Lahkimi, A.; Lhassani, A.; Chaouch, M.; Taleb, M. Mesoporous Carbon from Optimized Date Stone Hydrochar by Catalytic Hydrothermal Carbonization Using Response Surface Methodology: Application to Dyes Adsorption. Int. J. Chem. Eng. 2021, 2021, 5555406. [Google Scholar] [CrossRef]
- El Ouadrhiri, F.; Abdu Musad Saleh, E.; Husain, K.; Adachi, A.; Hmamou, A.; Hassan, I.; Mostafa Moharam, M.; Lahkimi, A. Acid Assisted-Hydrothermal Carbonization of Solid Waste from Essential Oils Industry: Optimization Using I-Optimal Experimental Design and Removal Dye Application. Arab. J. Chem. 2023, 16, 104872. [Google Scholar] [CrossRef]
- Zhou, S.; Hu, A.; Jiang, J.; Tang, J.; Zhou, G.; Zhu, L.; Wang, S. Low-Temperature Synthesized Hierarchical Porous Carbon from Waste Hydrochar with Super Capacity for Dye Adsorption. Biomass Bioenergy 2023, 177, 106938. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, R.; Tan, S.; Zhang, K.; Yin, Q.; Zhao, Z.; Gao, P. Nitrogen-Doped Hydrochar Prepared by Biomass and Nitrogen-Containing Wastewater for Dye Adsorption: Effect of Nitrogen Source in Wastewater on the Adsorption Performance of Hydrochar. J. Environ. Manage 2023, 334, 117503. [Google Scholar] [CrossRef] [PubMed]
- Hessien, M. Methylene Blue Dye Adsorption on Iron Oxide-Hydrochar Composite Synthesized via a Facile Microwave-Assisted Hydrothermal Carbonization of Pomegranate Peels’ Waste. Molecules 2023, 28, 4526. [Google Scholar] [CrossRef] [PubMed]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Review on Recent Advances of Carbon Based Adsorbent for Methylene Blue Removal from Waste Water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Mozaffari Majd, M.; Kordzadeh-Kermani, V.; Ghalandari, V.; Askari, A.; Sillanpää, M. Adsorption Isotherm Models: A Comprehensive and Systematic Review (2010−2020). Sci. Total Environ. 2022, 812, 151334. [Google Scholar] [CrossRef] [PubMed]
- de Vargas Brião, G.; Hashim, M.A.; Chu, K.H. The Sips Isotherm Equation: Often Used and Sometimes Misused. Sep. Sci. Technol. 2023, 58, 884–892. [Google Scholar] [CrossRef]
- Liu, J.L.; Qian, W.C.; Guo, J.Z.; Shen, Y.; Li, B. Selective Removal of Anionic and Cationic Dyes by Magnetic Fe3O4-Loaded Amine-Modified Hydrochar. Bioresour. Technol. 2021, 320, 124374. [Google Scholar] [CrossRef]
- Akbari, A.; Peighambardoust, S.J.; Lotfi, M. Hydrochar Derived from Liquorice Root Pulp Utilizing Catalytic/Non-Catalytic Hydrothermal Carbonization: RSM Optimization and Cationic Dye Adsorption Assessment. J. Water Process Eng. 2023, 55, 104099. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Emik, S.; Öngen, A.; Kurtulus Özcan, H.; Aydın, S. Modelling of Adsorption Kinetic Processes-Errors, Theory and Application. In Advanced Sorption Process Applications; Edebali, S., Ed.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Wang, J.; Guo, X. Adsorption Kinetic Models: Physical Meanings, Applications, and Solving Methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Characteristics of Elovich Equation Used for the Analysis of Adsorption Kinetics in Dye-Chitosan Systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Parmar, K.R.; Ross, A.B. Integration of Hydrothermal Carbonisation with Anaerobic Digestion—Opportunities for Valorisation of Digestate. Energies 2019, 12, 1586. [Google Scholar] [CrossRef]
- Murillo, H.A.; Pagés-Díaz, J.; Díaz-Robles, L.A.; Vallejo, F.; Huiliñir, C. Valorization of Oat Husk by Hydrothermal Carbonization: Optimization of Process Parameters and Anaerobic Digestion of Spent Liquors. Bioresour. Technol. 2022, 343, 126112. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.E.; Adams, J.M.M.; Grasham, O.R.; Camargo-Valero, M.A.; Ross, A.B. An Assessment of Different Integration Strategies of Hydrothermal Carbonisation and Anaerobic Digestion of Water Hyacinth. Energies 2020, 13, 5983. [Google Scholar] [CrossRef]
- Farru, G.; Cappai, G.; Carucci, A.; De Gioannis, G.; Asunis, F.; Milia, S.; Muntoni, A.; Perra, M.; Serpe, A. A Cascade Biorefinery for Grape Marc: Recovery of Materials and Energy through Thermochemical and Biochemical Processes. Sci. Total Environ. 2022, 846, 157464. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Briceño, C.; Ross, A.B.; Camargo-Valero, M.A. Evaluation and Comparison of Product Yields and Bio-Methane Potential in Sewage Digestate Following Hydrothermal Treatment. Appl. Energy 2017, 208, 1357–1369. [Google Scholar] [CrossRef]
- Bamba, D.; Coulibaly, M.; Robert, D. Nitrogen-Containing Organic Compounds: Origins, Toxicity and Conditions of Their Photocatalytic Mineralization over TiO2. Sci. Total Environ. 2017, 580, 1489–1504. [Google Scholar] [CrossRef] [PubMed]
- Farru, G.; Dang, C.H.; Schultze, M.; Kern, J.; Cappai, G.; Libra, J.A. Benefits and Limitations of Using Hydrochars from Organic Residues as Replacement for Peat on Growing Media. Horticulturae 2022, 8, 325. [Google Scholar] [CrossRef]
- Petrovič, A.; Cenčič Predikaka, T.; Parlov Vuković, J.; Jednačak, T.; Hribernik, S.; Vohl, S.; Urbancl, D.; Tišma, M.; Čuček, L. Sustainable Hydrothermal Co-Carbonization of Residues from the Vegetable Oil Industry and Sewage Sludge: Hydrochar Production and Liquid Fraction Valorisation. Energy 2024, 307, 132760. [Google Scholar] [CrossRef]
- Mantovani, M.; Collina, E.; Marazzi, F.; Lasagni, M.; Mezzanotte, V. Microalgal Treatment of the Effluent from the Hydrothermal Carbonization of Microalgal Biomass. J. Water Process Eng. 2022, 49, 102976. [Google Scholar] [CrossRef]
- Czerwińska, K.; Marszałek, A.; Kudlek, E.; Śliz, M.; Dudziak, M.; Wilk, M. The Treatment of Post-Processing Liquid from the Hydrothermal Carbonization of Sewage Sludge. Sci. Total Environ. 2023, 885, 163858. [Google Scholar] [CrossRef]
- Cavali, M.; Ricardo Soccol, C.; Tavares, D.; Alberto Zevallos Torres, L.; Oliveira de Andrade Tanobe, V.; Zandoná Filho, A.; Lorenci Woiciechowski, A. Effect of Sequential Acid-Alkaline Treatment on Physical and Chemical Characteristics of Lignin and Cellulose from Pine (Pinus spp.) Residual Sawdust. Bioresour. Technol. 2020, 316, 123884. [Google Scholar] [CrossRef] [PubMed]
- Cavali, M.; Soccol, C.R.; Tavares, D.; Zevallos Torres, L.A.; Oliveira de Andrade Tanobe, V.; Zandoná Filho, A.; Woiciechowski, A.L. Valorization of Lignin from Pine (Pinus spp.) Residual Sawdust: Antioxidant Activity and Application in the Green Synthesis of Silver Nanoparticles for Antibacterial Purpose. Biomass Convers. Biorefin 2021, 13, 10051–10063. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Pan, X. Efficient Sugar Production from Plant Biomass: Current Status, Challenges, and Future Directions. Renew. Sustain. Energy Rev. 2022, 164, 112583. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Cui, Q.; Feng, Y.; Xuan, J. Composition of Lignocellulose Hydrolysate in Different Biorefinery Strategies: Nutrients and Inhibitors. Molecules 2024, 29, 2275. [Google Scholar] [CrossRef] [PubMed]
- Ischia, G.; Fiori, L. Hydrothermal Carbonization of Organic Waste and Biomass: A Review on Process, Reactor, and Plant Modeling. Waste Biomass Valorization 2021, 12, 2797–2824. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology Development for the Production of Biobased Products from Biorefinery Carbohydrates—The US Department of Energy’s “Top 10” Revisited. Green Chem. 2010, 12, 539–555. [Google Scholar] [CrossRef]
- Faure, E.; Falentin-Daudré, C.; Jérôme, C.; Lyskawa, J.; Fournier, D.; Woisel, P.; Detrembleur, C. Catechols as Versatile Platforms in Polymer Chemistry. Prog. Polym. Sci. 2013, 38, 236–270. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A Renewable and Versatile Platform Molecule for the Synthesis of Chemicals and Fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Fache, M.; Darroman, E.; Besse, V.; Auvergne, R.; Caillol, S.; Boutevin, B. Vanillin, a Promising Biobased Building-Block for Monomer Synthesis. Green Chem. 2014, 16, 1987–1998. [Google Scholar] [CrossRef]




| Hydrochar | Yield (%) | Volatile Matter (%) * | Ashes (%) * | Fixed Carbon (%) * | O/C | H/C | pH | BET Surface Area (m2·g−1) |
|---|---|---|---|---|---|---|---|---|
| H-180 | 87.3 | 78.5 | 10.4 | 11.1 | 0.5 | 1.3 | 5.4 | 14.8 |
| H-215 | 72.7 | 74.4 | 8.0 | 17.6 | 0.4 | 1.2 | 5.2 | 9.6 |
| H-250 | 64.1 | 59.3 | 18.4 | 22.3 | 0.2 | 0.9 | 5.8 | 22.7 |
| Isotherm Model | Parameter | H-180 | H-215 |
|---|---|---|---|
| Langmuir | qmax (mg·g−1) | 69.01± 2.93 (56.40–81.61) * | 67.78 ± 2.83 (55.60–79.96) * |
| KL | 0.048 ± 0.012 | 0.068 ± 0.018 | |
| R2 | 0.9669 | 0.9636 | |
| MRD (%) | 11.56 | 9.89 | |
| Freundlich | KF | 13.92 ± 3.08 | 14.87 ± 3.09 |
| n | 3.79 ± 0.57 | 3.92 ± 0.58 | |
| R2 | 0.9258 | 0.9289 | |
| MRD (%) | 25.54 | 27.68 | |
| Sips | qmax (mg·g−1) | 80.35 ± 7.18 (49.45–111.25) * | 79.96 ± 7.23 (48.85–111.07) * |
| KS | 0.090 ± 0.020 | 0.112 ± 0.022 | |
| n | 0.672 ± 0.100 | 0.647 ± 0.099 | |
| R2 | 0.9852 | 0.9850 | |
| MRD (%) | 9.26 | 11.96 |
| Kinetic Model | Parameter | H-180 |
|---|---|---|
| Pseudo-First-Order | K1 | 0.011 ± 0.001 |
| qe (mg·g−1) | 22.85 ± 0.85 | |
| R2 | 0.9124 | |
| MRD (%) | 5.57 | |
| Pseudo-Second-Order | K2 | 0.001 ± 0.0002 |
| qe (mg·g−1) | 24.54 ± 1.11 | |
| R2 | 0.8909 | |
| MRD (%) | 7.09 | |
| Elovich | β | 0.225 ± 0.012 |
| α | 1.08 ± 0.19 | |
| R2 | 0.9779 | |
| MRD (%) | 2.81 |
| ∆G (J·mol−1) | ∆S (J·mol−1·K−1) | ∆H (J·mol−1) |
|---|---|---|
| −3845.12 (30 °C) | 28.34 | 4746.13 |
| −4128.52 (40 °C) | ||
| −4411.92 (50 °C) | ||
| −4695.32 (60 °C) |
| Waste | Hydrothermal Carbonization Condition * | Chemical Oxygen Demand (g·L−1) | Total Nitrogen (g·L−1) | Reference |
|---|---|---|---|---|
| Agricultural residue digestate | T: 200; S/L: 1/5; t: 1 | 42.2 | 1.9 | [57] |
| T: 250; S/L: 1/5; t: 1 | 46.3 | 2.2 | ||
| Municipal solid waste digestate | T: 200; S/L: 1/5; t: 1 | 18.1 | 2.4 | |
| T: 250; S/L: 1/5; t: 1 | 16.4 | 1.7 | ||
| Sewage sludge digestate | T: 200; S/L: 1/5; t: 1 | 38.9 | 4.5 | |
| T: 250; S/L: 1/5; t: 1 | 43.6 | 4.7 | ||
| Oat husk | T: 219.2; S/L: 1/12.5; t: 0.5 | 13.2 | 1.8 | [58] |
| Water hyacinth | T: 150; S/L: 1/10; t: 1 | 19.0 | - | [59] |
| T: 200; S/L: 1/10; t: 1 | 27.5 | - | ||
| T: 250; S/L: 1/10; t: 1 | 31.4 | - | ||
| Grape Marc | T: 220; S/L: 1/10; t: 1 | 33.3 | [60] | |
| Grape Marc extracted | 31.1 | - | ||
| Sewage sludge digestate | T: 160; S/L: 1/1; t: 0.5 | 12.6 | - | [61] |
| T: 220; S/L: 1/1; t: 0.5 | 12.9 | - | ||
| T: 250; S/L: 1/1; t: 0.5 | 12.2 | - |
| Ion | PW-180 | PW-215 | PW-250 |
|---|---|---|---|
| Acetate (mg·L−1) | 3333.67 ± 18.21 | 3600.67 ± 76.20 | 4584.00 ± 27.31 |
| Chloride (mg·L−1) | 118.94 ± 1.10 | 120.70 ± 1.44 | 124.70 ± 0.64 |
| Nitrite (mg·L−1) | 7.62 ± 0.00 | 1.99 ± 0.03 | nd |
| Nitrate (mg·L−1) | 13.80 ± 0.31 | 6.60 ± 0.14 | nd |
| Sulfate (mg·L−1) | 90.72 ± 0.39 | 74.87 ± 0.90 | 85.45 ± 14.32 |
| Phosphate (mg·L−1) | 441.93 ± 0.99 | 203.47 ± 0.53 | 194.32 ± 1.54 |
| Process Water | Retention Time (min.) | Peak Area (%) | Compound | CAS Number |
|---|---|---|---|---|
| PW-180 | 3.722 | 12.00 | Isobutyl acetate | 000110-19-0 |
| 5.188 | 87.99 | Furfural | 000098-01-1 | |
| 6.120 | 2.32 | Furfural | 000098-01-1 | |
| 26.810 | 1.33 | Cyclohexasiloxane, dodecamethyl- | 000540-97-6 | |
| PW-215 | 3.657 | 4.15 | Isobutyl acetate | 000110-19-0 |
| 5.135 | 5.60 | Furfural | 000098-01-1 | |
| 7.574 | 0.93 | 2-Cyclopenten-1-one, 2-methyl- | 001120-73-6 | |
| 7.806 | 1.05 | Ethanone, 1-(2-furanyl)- | 001192-62-7 | |
| 10.049 | 2.33 | 2-Furancarboxaldehyde, 5-methyl- | 000620-02-0 | |
| 12.661 | 7.74 | 1,2-Cyclopentanedione, 3-methyl- | 000765-70-8 | |
| 15.510 | 14.07 | Phenol, 2-methoxy- | 000090-05-1 | |
| 17.071 | 3.05 | 2-Cyclopenten-1-one, 3-ethyl-2-hydroxy- | 021835-01-8 | |
| 24.371 | 3.54 | (Z)-4-Methyl-5-(2-oxopropylidene)-5H-furan-2-one | 026474-45-3 | |
| 27.564 | 7.93 | Phenol, 2,6-dimethoxy- | 000091-10-1 | |
| 29.885 | 3.02 | Vanillin | 000121-33-5 | |
| 35.049 | 5.98 | Homovanillyl alcohol | 002380-78-1 | |
| PW-250 | 3.716 | 1.50 | Isobutyl acetate | 000110-19-0 |
| 5.028 | 0.42 | Pyrazine, methyl- | 000109-08-0 | |
| 5.223 | 1.21 | 2-Cyclopenten-1-one | 000930-30-3 | |
| 7.580 | 4.67 | 2-Cyclopenten-1-one, 2-methyl- | 001120-73-6 | |
| 10.144 | 1.26 | 2-Cyclopenten-1-one, 3-methyl- | 002758-18-1 | |
| 10.981 | 1.27 | Phenol | 000108-95-2 | |
| 11.467 | 1.99 | 2-Cyclopenten-1-one, 2,3-dimethyl- | 001121-05-7 | |
| 12.773 | 6.22 | 1,2-Cyclopentanedione, 3-methyl- | 000765-70-8 | |
| 13.248 | 1.39 | 2-Cyclopenten-1-one, 2,3-dimethyl- | 001121-05-7 | |
| 15.551 | 21.46 | Phenol, 2-methoxy- | 000090-05-1 | |
| 20.240 | 0.42 | Furan, 2,5-dihydro-2,5-dimethyl- | 059242-27-2 | |
| 20.560 | 0.80 | Creosol | 000093-51-6 | |
| 21.724 | 5.04 | Catechol | 000120-80-9 | |
| 23.890 | 1.86 | 1,2-Benzenediol, 3-methoxy- | 000934-00-9 | |
| 24.478 | 3.57 | Phenol, 4-ethyl-2-methoxy- | 002785-89-9 | |
| 26.804 | 1.31 | Cyclohexasiloxane, dodecamethyl- | 000540-97-6 | |
| 27.712 | 8.91 | Phenol, 2,6-dimethoxy- | 000091-10-1 | |
| 30.223 | 1.10 | 4-Hydroxy-2-methoxybenaldehyde | 018278-34-7 | |
| 33.434 | 0.55 | 5-Hepten-3-yn-2-ol, 6-methyl-5-(1-methylethyl)- | 063922-41-8 | |
| 33.933 | 0.51 | Cycloheptasiloxane, tetradecamethyl- | 000107-50-6 | |
| 35.102 | 3.86 | Homovanillyl alcohol | 002380-78-1 | |
| 39.589 | 1.12 | Homovanillic acid | 000306-08-1 | |
| 39.725 | 2.30 | Homovanillic acid | 000306-08-1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavali, M.; Hennig, T.B.; Libardi Junior, N.; Kim, B.; Garnier, V.; Benbelkacem, H.; Bayard, R.; Woiciechowski, A.L.; Matias, W.G.; de Castilhos Junior, A.B. Co-Hydrothermal Carbonization of Sawdust and Sewage Sludge: Assessing the Potential of the Hydrochar as an Adsorbent and the Ecotoxicity of the Process Water. Appl. Sci. 2025, 15, 1052. https://doi.org/10.3390/app15031052
Cavali M, Hennig TB, Libardi Junior N, Kim B, Garnier V, Benbelkacem H, Bayard R, Woiciechowski AL, Matias WG, de Castilhos Junior AB. Co-Hydrothermal Carbonization of Sawdust and Sewage Sludge: Assessing the Potential of the Hydrochar as an Adsorbent and the Ecotoxicity of the Process Water. Applied Sciences. 2025; 15(3):1052. https://doi.org/10.3390/app15031052
Chicago/Turabian StyleCavali, Matheus, Thuanne Braúlio Hennig, Nelson Libardi Junior, Boram Kim, Vincent Garnier, Hassen Benbelkacem, Rémy Bayard, Adenise Lorenci Woiciechowski, William Gerson Matias, and Armando Borges de Castilhos Junior. 2025. "Co-Hydrothermal Carbonization of Sawdust and Sewage Sludge: Assessing the Potential of the Hydrochar as an Adsorbent and the Ecotoxicity of the Process Water" Applied Sciences 15, no. 3: 1052. https://doi.org/10.3390/app15031052
APA StyleCavali, M., Hennig, T. B., Libardi Junior, N., Kim, B., Garnier, V., Benbelkacem, H., Bayard, R., Woiciechowski, A. L., Matias, W. G., & de Castilhos Junior, A. B. (2025). Co-Hydrothermal Carbonization of Sawdust and Sewage Sludge: Assessing the Potential of the Hydrochar as an Adsorbent and the Ecotoxicity of the Process Water. Applied Sciences, 15(3), 1052. https://doi.org/10.3390/app15031052








