In Vitro Effect of Sequential Compressive Loading and Thermocycling on Marginal Microleakage of Digitally Fabricated Overlay Restorations Made from Five Materials
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Sample Selection and Storage
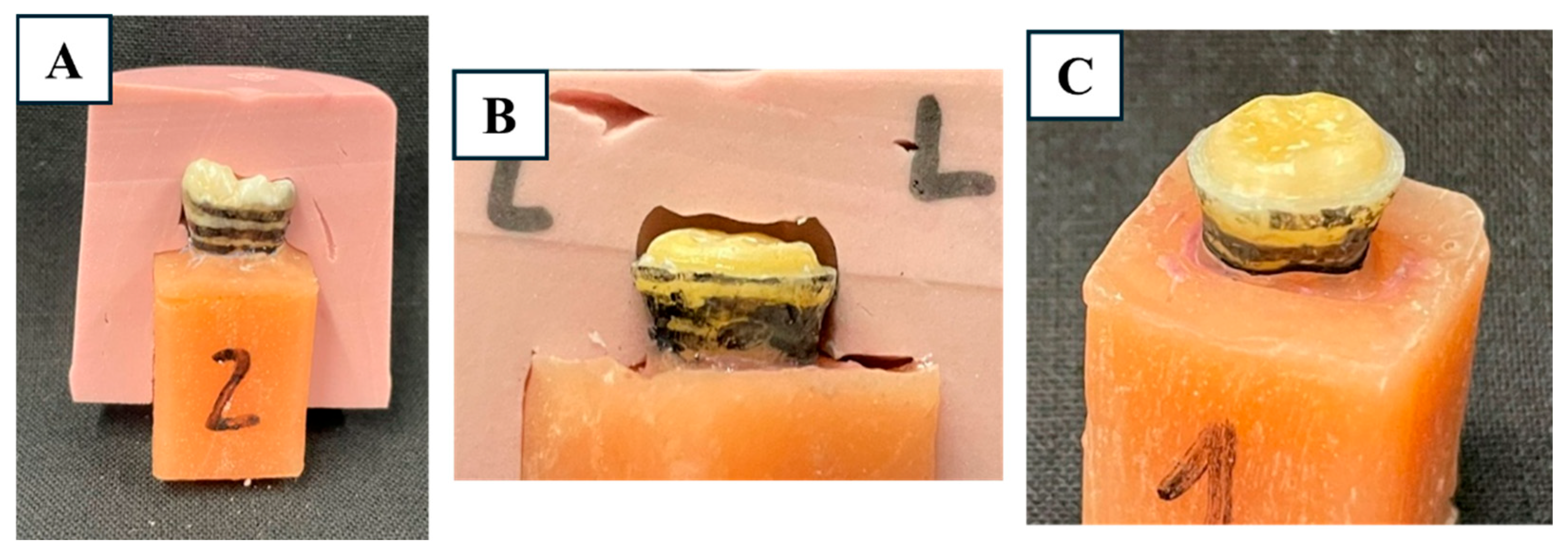
2.3. Periodontal Ligament Simulation and Mounting
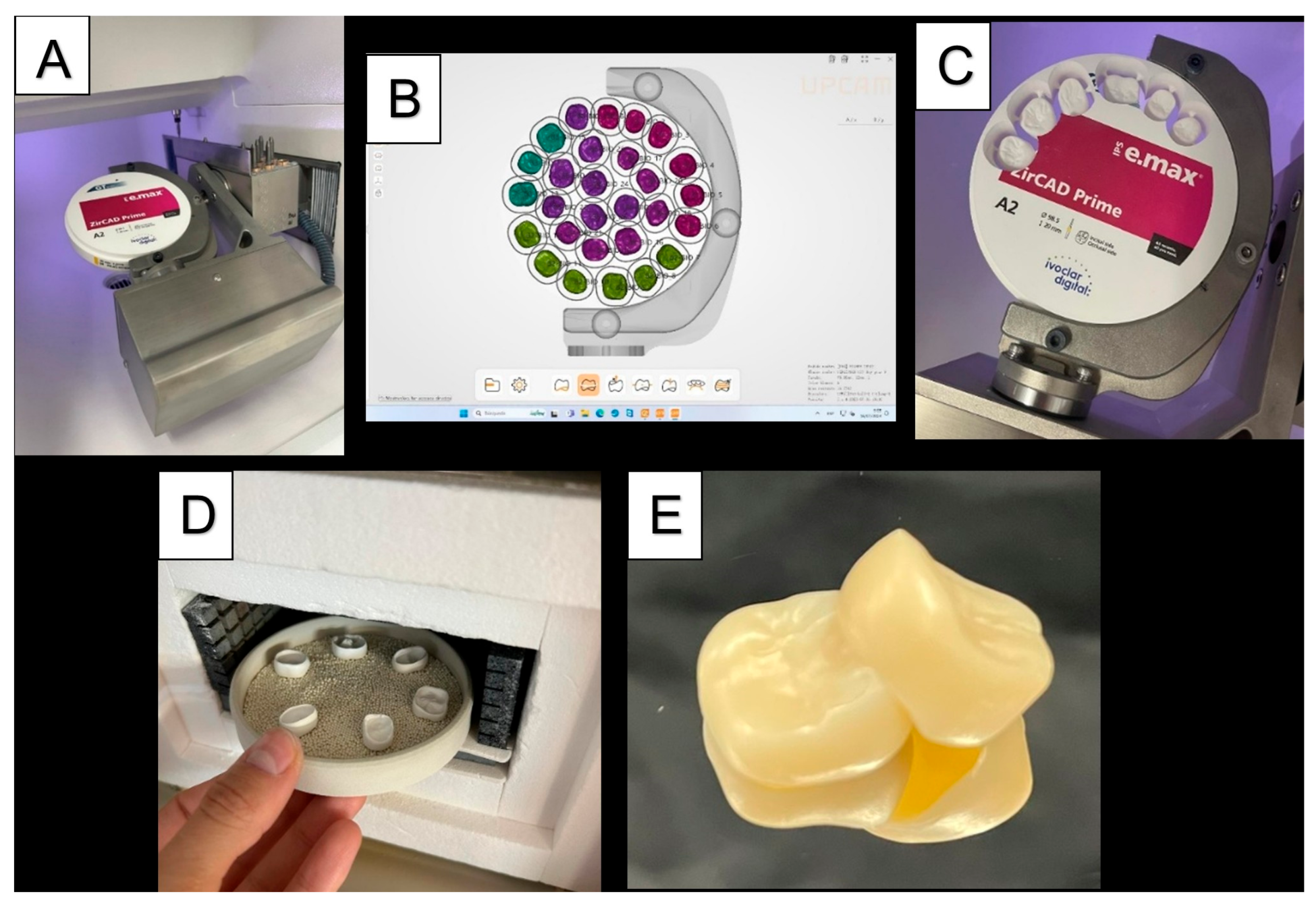
2.4. Cavity Preparation and Scanning
2.5. Restoration Fabrication
2.6. Adhesive Cementation
2.7. Experimental Groups and Loading Protocols
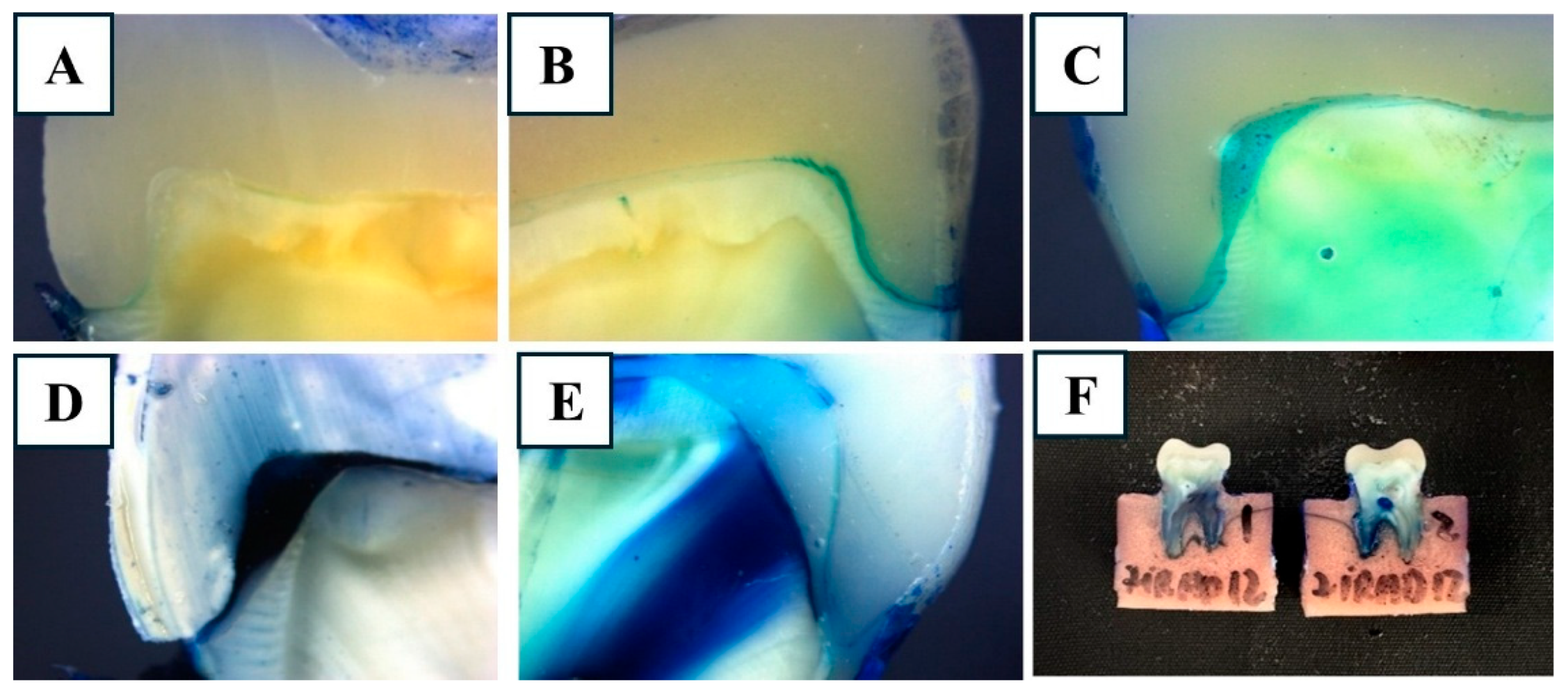
2.8. Microleakage Evaluation
2.9. Statistical Analysis
3. Results
3.1. Inter-Examiner Agreement
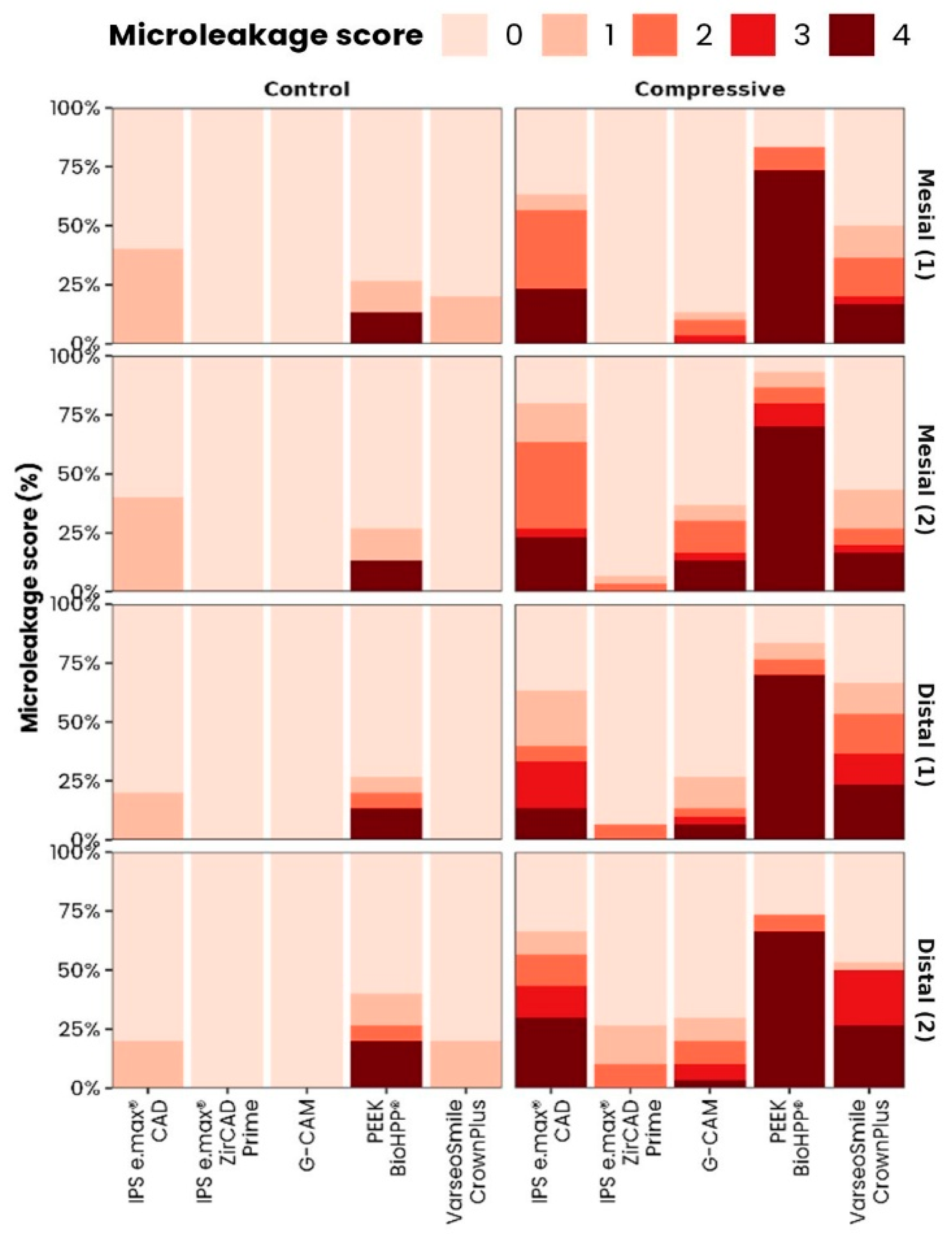
3.2. Effect of Loading and Ageing on Marginal Microleakage
3.3. Multiple Comparisons and Representative Images
4. Discussion
4.1. Material-Dependent Differences
4.2. Functional and Mechanical Interpretation
4.3. Influence of Cementation
4.4. Clinical Relevance
4.5. Limitations and Future Directions
5. Conclusions
- IPS e.max® ZirCAD Prime and G-CAM exhibited the lowest marginal microleakage values under all experimental conditions, indicating superior marginal sealing compared with the other tested materials.
- VarseoSmile CrownPlus and IPS e.max® CAD showed intermediate performance, while BioHPP® presented the highest microleakage, particularly after mechanical loading and thermal aging.
- These findings suggest that zirconia-based and graphene-reinforced CAD/CAM materials provide greater marginal stability under functional stress, making them potentially more suitable for patients with high occlusal loads or parafunctional habits.
- Although this in vitro protocol simulated thermomechanical fatigue, clinical studies are necessary to confirm the long-term behavior of these restorative materials under real intraoral conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| Al2O3 | Aluminum oxide |
| CAD/CAM | Computer-aided design/computer-aided manufacturing |
| CEJ | Cementoenamel junction |
| DC | Dual-curing |
| DM1/DM2 | Distal microleakage evaluation 1/2 |
| HF | Hydrofluoric acid |
| κ | Kappa coefficient |
| M | Mesial |
| MM1/MM2 | Mesial microleakage evaluation 1/2 |
| N | Newton |
| PEEK | Polyetheretherketone |
| SD | Standard deviation |
| SEM | Scanning electron microscopy |
| SR | Significant result |
| °C | Degrees Celsius |
| µm | Micrometer |
| mm | Millimeter |
References
- Schmid-Schwap, M.; Graf, A.; Preinerstorfer, A.; Watts, D.C.; Piehslinger, E.; Schedle, A. Microleakage after thermocycling of cemented crowns—A meta-analysis. Dent. Mater. 2011, 27, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.J.M.C.; Freitas, M.C.; Azevedo, L.M.; Santos, G.C.; Navarro, M.F.; Francischone, C.E.; Mondelli, R.F. Clinical evaluation of ceramic inlays and onlays fabricated with two systems: 12-year follow-up. Clin. Oral Investig. 2016, 20, 1683–1690. [Google Scholar] [CrossRef]
- Abduo, J.; Sambrook, R.J. Longevity of ceramic onlays: A systematic review. J. Esthet. Restor. Dent. 2018, 30, 193–215. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R. Ceramic inlays, onlays, and overlays have a high survival rate and a low rate of complications. J. Am. Dent. Assoc. 2017, 148, e3. [Google Scholar] [CrossRef]
- Vagropoulou, G.I.; Klifopoulou, G.L.; Vlahou, S.G.; Hirayama, H.; Michalakis, K. Complications and survival rates of inlays and onlays vs complete coverage restorations: A systematic review and analysis of studies. J. Oral Rehabil. 2018, 45, 903–920. [Google Scholar] [CrossRef]
- Opdam, N.J.M.; Bronkhorst, E.M.; Roeters, J.M.; Loomans, B.A.C. A retrospective clinical study on longevity of posterior composite and amalgam restorations. Dent. Mater. 2007, 23, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Smales, R.J.; Etemadi, S. Survival of ceramic onlays placed with and without metal reinforcement. J. Prosthet. Dent. 2004, 91, 548–553. [Google Scholar] [CrossRef]
- Ferracane, J.L. Materials in Dentistry. Principles and Applications; J.B. Lippincott Company: Philadelphia, PA, USA, 1995. [Google Scholar]
- AlHabdan, A. Review of microleakage evaluation tools. J. Int. Oral Health 2017, 9, 141–145. [Google Scholar] [CrossRef]
- Saini, S.; Chauhan, A.; Butail, A.; Rana, S. Evaluation of Marginal Microleakage and Depth of Penetration of Different Materials Used as Pit and Fissure Sealants: An In Vitro Study. Int. J. Clin. Pediatr. Dent. 2020, 13, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Gerdolle, D.A.; Mortier, E.; Loos-Ayav, C.; Jacquot, B.; Panighi, M.M. In vitro evaluation of microleakage of indirect composite inlays cemented with four luting agents. J. Prosthet. Dent. 2005, 93, 563–570. [Google Scholar] [CrossRef]
- Youngson, C.C.; Jones, G.; Manogue, M.; Smith, I.S. In vitro dentinal penetration by tracers used in microleakage studies. Int. Endod. J. 1998, 31, 90–99. [Google Scholar] [CrossRef]
- Juloski, J.; Köken, S.; Ferrari, M. No correlation between two methodological approaches applied to evaluate cervical margin relocation. Dent. Mater. J. 2020, 39, 624–632. [Google Scholar] [CrossRef]
- Şeker, E.; Ozcelik, T.B.; Rathi, N.; Yilmaz, B. Evaluation of marginal fit of CAD/CAM restorations fabricated through cone beam computerized tomography and laboratory scanner data. J. Prosthet. Dent. 2016, 115, 47–51. [Google Scholar] [CrossRef]
- Grzebieluch, W.; Kowalewski, P.; Grygier, D.; Rutkowska-Gorczyca, M.; Kozakiewicz, M.; Jurczyszyn, K. Printable and machinable dental restorative composites for cad/cam application—Comparison of mechanical properties, fractographic, texture and fractal dimension analysis. Materials 2021, 14, 4919. [Google Scholar] [CrossRef]
- Rosentritt, M.; Haas, L.; Rauch, A.; Schmidt, M. Influence of Fabrication Settings on the In Vitro Performance of Subtractively Manufactured Resin-Based Molar Crowns. Int. J. Prosthodont. 2024, 37, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Naert, I.; VAN DER Donck, A.; Beckers, L. Precision of fit and clinical evaluation of all-ceramic full restorations followed between 0·5 and 5 years. J. Oral Rehabil. 2005, 32, 51–57. [Google Scholar] [CrossRef]
- Syu, J.Z.; Byrne, G.; Laub, L.W.; Land, M.F. Influence of finish-line geometry on the fit of crowns. Int. J. Prosthodont. 1993, 6, 25–30. [Google Scholar] [PubMed]
- Prechtel, A.; Stawarczyk, B.; Hickel, R.; Edelhoff, D.; Reymus, M. Fracture load of 3D printed PEEK inlays compared with milled ones, direct resin composite fillings, and sound teeth. Clin. Oral Investig. 2020, 24, 3457–3466. [Google Scholar] [CrossRef]
- Costa-Palau, S.; Torrents-Nicolas, J.; Barberà, M.B.-D.; Cabratosa-Termes, J. Use of polyetheretherketone in the fabrication of a maxillary obturator prosthesis: A clinical report. J. Prosthet. Dent. 2014, 112, 680–682. [Google Scholar] [CrossRef]
- Monteiro, L.C.; Pecorari, V.G.A.; Gontijo, I.G.; Marchi, G.M.; Lima, D.A.N.L.; Aguiar, F.H.B. PEEK and fiberglass intra-radicular posts: Influence of resin cement and mechanical cycling on push-out bond strength. Clin. Oral Investig. 2022, 26, 6907–6916. [Google Scholar] [CrossRef] [PubMed]
- Dejak, B.; Młotkowski, A. A comparison of mvM stress of inlays, onlays and endocrowns made from various materials and their bonding with molars in a computer simulation of mastication—FEA. Dent. Mater. 2020, 36, 854–864. [Google Scholar] [CrossRef]
- Nakamura, T.; Imanishi, A.; Kashima, H.; Ohyama, T.; Ishigaki, S. Stress analysis of metal-free polymer crowns using the three-dimensional finite element method. Int. J. Prosthodont. 2001, 14, 401–405. [Google Scholar]
- Al Fodeh, R.S.; Al-Johi, O.S.; Alibrahim, A.N.; Al-Dwairi, Z.N.; Husain, N.A.-H.; Özcan, M. Fracture strength of endocrown maxillary restorations using different preparation designs and materials. J. Mech. Behav. Biomed. Mater. 2023, 148, 106184. [Google Scholar] [CrossRef]
- Soares, C.J.; Martins, L.R.M.; Fonseca, R.B.; Correr-Sobrinho, L.; Neto, A.J.F. Influence of cavity preparation design on fracture resistance of posterior Leucite-reinforced ceramic restorations. J. Prosthet. Dent. 2006, 95, 421–429. [Google Scholar] [CrossRef]
- von Fraunhofer, J.A.; Adachi, E.I.; Barnes, D.M.; Romberg, E. The effect of tooth preparation on microleakage behavior. Oper. Dent. 2000, 25, 526–533. [Google Scholar]
- Crim, G.A.; Garcia-Godoy, F. Microleakage: The effect of storage and cycling duration. J. Prosthet. Dent. 1987, 57, 574–576. [Google Scholar] [CrossRef]
- Mosharrafian, S.; Farahmand, N.; Poorzandpoush, K.; Hosseinipour, Z.S.; Kahforushan, M. In vitro microleakage at the enamel and dentin margins of class II cavities of primary molars restored with a bulk-fill and a conventional composite. Clin. Exp. Dent. Res. 2023, 9, 512–517. [Google Scholar] [CrossRef]
- Metin, D.S.; Schmidt, F.; Beuer, F.; Prause, E.; Ashurko, I.; Sarmadi, B.S.; Unkovskiy, A. Accuracy of the intaglio surface of 3D-printed hybrid resin-ceramic crowns, veneers and table-tops: An in vitro study. J. Dent. 2024, 144, 104960. [Google Scholar] [CrossRef]
- Kassem, A.S.; Atta, O.; El-Mowafy, O. Fatigue Resistance and Microleakage of CAD/CAM Ceramic and Composite Molar Crowns. J. Prosthodont. 2012, 21, 28–32. [Google Scholar] [CrossRef]
- Hatirli, H.; Yasa, B.; Yasa, E. Microleakage and penetration depth of different fissure sealant materials after cyclic thermo-mechanic and brushing simulation. Dent. Mater. J. 2018, 37, 15–23. [Google Scholar] [CrossRef]
- Juntavee, A.; Juntavee, N.; Chaisuntitrakoon, A.; Millstein, P.L.; Abedian, B. Microleakage and penetration capability of various pit and fissure sealants upon different sealant application techniques. J. Clin. Exp. Dent. 2023, 15, e810–e820. [Google Scholar] [CrossRef]
- Ben-Amar, A.; Pilo, R.; Shapinko, E.; Lewinstein, I. A microleakage study of single-bottle adhesives applied to enamel and cementum and aged by both occlusal loading and thermocycling. J. Prosthet. Dent. 2005, 94, 292. [Google Scholar] [CrossRef]
- Keshvad, A.; Hooshmand, T.; Asefzadeh, F.; Khalilinejad, F.; Alihemmati, M.; Van Noort, R. Marginal Gap, Internal Fit, and Fracture Load of Leucite-Reinforced Ceramic Inlays Fabricated by CEREC inLab and Hot-Pressed Techniques. J. Prosthodont. 2011, 20, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Abduo, J.; Laskey, D. Effect of preparation type on the accuracy of different intraoral scanners: An in vitro study at different levels of accuracy evaluation. J. Esthet. Restor. Dent. 2022, 34, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Güleç, C.; Sarıkaya, I. The influence of aging on the fracture load of milled monolithic crowns. BMC Oral Health 2022, 22, 516. [Google Scholar] [CrossRef]
- Kim, S.-H.; Choi, Y.-S.; Kang, K.-H.; Att, W. Effects of thermal and mechanical cycling on the mechanical strength and surface properties of dental CAD-CAM restorative materials. J. Prosthet. Dent. 2022, 128, 79–88. [Google Scholar] [CrossRef]
- Rosentritt, M.; Behr, M.; Gebhard, R.; Handel, G. Influence of stress simulation parameters on the fracture strength of all-ceramic fixed-partial dentures. Dent. Mater. 2006, 22, 176–182. [Google Scholar] [CrossRef]
- Nelson, S.J.; Ash, M.M. Wheeler’s Dental Anatomy, Physiology, and Occlusion; Saunders: Philadelphia, PA, USA, 2015. [Google Scholar]
- Ebrahim, N.Y.; Wassel, M.O.; Hamdy, D. Effect of Laser Etching Versus Self-Etching Adhesive and Conventional Acid-Etching Techniques on Shear Bond Strength, Microleakage and Penetration Depth of a Resin Based Pit and Fissure Sealant (An In Vitro Study). Egypt. Dent. J. 2023, 69, 2523–2538. [Google Scholar] [CrossRef]
- Mao, Z.; Beuer, F.; Wu, D.; Zhu, Q.; Yassine, J.; Schwitalla, A.; Schmidt, F. Microleakage along the implant–abutment interface: A systematic review and meta-analysis of in vitro studies. Int. J. Implant. Dent. 2023, 9, 34. [Google Scholar] [CrossRef]
- Costa, J.F.; Siqueira, W.L.; Loguercio, A.D.; Reis, A.; de Oliveira, E.D.; Alves, C.M.C.; Bauer, J.R.d.O.; Grande, R.H.M. Characterization of aqueous silver nitrate solutions for leakage tests. J. Appl. Oral Sci. 2011, 19, 254–259. [Google Scholar] [CrossRef]
- Heintze, S.; Forjanic, M.; Cavalleri, A. Microleakage of Class II restorations with different tracers--comparison with SEM quantitative analysis. J. Adhes. Dent. 2008, 10, 259–267. [Google Scholar]
- Scotti, N.; Comba, A.; Gambino, A.; Paolino, D.S.; Alovisi, M.; Pasqualini, D.; Berutti, E. Microleakage at enamel and dentin margins with a bulk fills flowable resin. Eur. J. Dent. 2014, 8, 001–008. [Google Scholar] [CrossRef]
- Bartaria, P.; Sharma, P.; Sogi, H.P.-S.; Jain, M.; Shahi, P.; Kapoor, R. Comparative evaluation of microleakage and penetration depth of ACP containing pit and fissure sealant and flowable composite—An in-vitro study. J. Clin. Exp. Dent. 2024, 16, e1027–e1032. [Google Scholar] [CrossRef]
- Sun, L.; Yan, Z.; Duan, Y.; Zhang, J.; Liu, B. Improvement of the mechanical, tribological and antibacterial properties of glass ionomer cements by fluorinated graphene. Dent. Mater. 2018, 34, e115–e127. [Google Scholar] [CrossRef]
- Gupta, S.; Abdulmajeed, A.; Donovan, T.; Boushell, L.; Bencharit, S.; Sulaiman, T.A. Monolithic Zirconia Partial Coverage Restorations: An In Vitro Mastication Simulation Study. J. Prosthodont. 2021, 30, 76–82. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Keul, C.; Eichberger, M.; Figge, D.; Edelhoff, D.; Lumkemann, N. Three generations of zirconia: From veneered to monolithic. Part I. Quintessence Int. 2017, 48, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Camposilvan, E.; Leone, R.; Gremillard, L.; Sorrentino, R.; Zarone, F.; Ferrari, M.; Chevalier, J. Aging resistance, mechanical properties and translucency of different yttria-stabilized zirconia ceramics for monolithic dental crown applications. Dent. Mater. 2018, 34, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Kaizer, M.R.; Kolakarnprasert, N.; Rodrigues, C.; Chai, H.; Zhang, Y. Probing the interfacial strength of novel multi-layer zirconias. Dent. Mater. 2020, 36, 60–67. [Google Scholar] [CrossRef]
- Mousavinasab, S.M.; Atai, M.; Alavi, B. To compare the microleakage among experimental adhesives containing nanoclay fillers after the storages of 24 hours and 6 months. Open Dent. J. 2011, 05, 52–57. [Google Scholar] [CrossRef]
- Soares, C.J.; Martins, L.R.M.; Pfeifer, J.M.; Giannini, M. Fracture resistance of teeth restored with indirect-composite and ceramic inlay systems. Quintessence Int. 2004, 35, 281–286. [Google Scholar] [PubMed]
- Magne, P.; Knezevic, A. Simulated fatigue resistance of composite resin versus porcelain CAD/CAM overlay restorations on endodontically treated molars. Quintessence Int. 2009, 40, 125–133. [Google Scholar]
- Heck, K.; Paterno, H.; Lederer, A.; Litzenburger, F.; Hickel, R.; Kunzelmann, K.H. Fatigue resistance of ultrathin CAD/CAM ceramic and nanoceramic composite occlusal veneers. Dent. Mater. 2019, 35, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.I.; Hafez, M.E. An in vitro study measuring marginal gaps of inlay restorations fabricated from different CAD-CAM materials after thermocycling. BMC Oral Health 2023, 23, 974. [Google Scholar] [CrossRef]
- Çakmak, G.; Rusa, A.M.; Donmez, M.B.; Akay, C.; Kahveci, Ç.; Schimmel, M.; Yilmaz, B. Trueness of crowns fabricated by using additively and subtractively manufactured resin-based CAD-CAM materials. J. Prosthet. Dent. 2024, 131, 951–958. [Google Scholar] [CrossRef]
- Ge, Z.; Yang, L.; Xiao, F.; Wu, Y.; Yu, T.; Chen, J.; Lin, J.; Zhang, Y. Graphene Family Nanomaterials: Properties and Potential Applications in Dentistry. Int. J. Biomater. 2018, 2018, 1539678. [Google Scholar] [CrossRef] [PubMed]
- Gungordu Er, S.; Edirisinghe, M.; Tabish, T.A. Graphene-Based Nanocomposites as Antibacterial, Antiviral and Antifungal Agents. Adv. Health Mater. 2023, 12, e2201523. [Google Scholar] [CrossRef]
- Nagi, N.; Fouda, A.M.; Bourauel, C. Comparative evaluation of internal fit and marginal gap of endocrowns using lithium disilicate and polyether ether ketone materials—An in vitro study. BMC Oral Health 2023, 23, 207. [Google Scholar] [CrossRef]
- Chappuis-Chocano, A.P.; Venante, H.S.; da Costa, R.M.B.; Pordeus, M.D.; Marcillo-Toala, O.O.; Junior, J.F.S.; Porto, V.C. A systematic review and meta-analysis of the clinical performance of implant-supported overdentures retained by CAD-CAM bars. J. Appl. Oral Sci. 2023, 31, e20230054. [Google Scholar] [CrossRef]
- Ahmad, F.; Nimonkar, S.; Belkhode, V.; Nimonkar, P. Role of Polyetheretherketone in Prosthodontics: A Literature Review. Cureus 2024, 16, e60552. [Google Scholar] [CrossRef] [PubMed]




| Material | Abrasion Parameters | Chemical Pretreatment | Adhesive & Curing Protocol |
|---|---|---|---|
| BioHPP® | 110 µm Al2O3, 2 bar, 10 s, 10 mm distance | — | Visio.link (Bredent); light-cured 90 s @ 1000 mW/cm2 |
| G-CAM | 50 µm Al2O3, 2 bar, 15 s, 10 mm distance | — | Scotchbond (3M); light-cured 20 s @ 1000 mW/cm2 |
| IPS e.max® ZirCAD Prime | 30 µm Al2O3, 1 bar, 15 s, 10 mm distance | Ivoclean (60 s), rinse; Monobond® Plus (60 s) | — |
| VarseoSmile CrownPlus | 50 µm glass beads, 2 bar, 15 s, 10 mm distance | 5% HF (20 s), rinse 2 min, dry with oil-free air | Scotchbond (3M); light-cured 10 s @ 1000 mW/cm2 |
| IPS e.max® CAD | — | 5% HF (20 s), rinse 2 min; Monobond® Plus (60 s) | Scotchbond (3M); light-cured 10 s @ 1000 mW/cm2 |
| Material | Resin Cement Used | Cementation Protocol | Polishing System Used |
|---|---|---|---|
| BioHPP® | Variolink® Esthetic DC | Preheated cement; 1 kg load × 10 min; light-cured 20 s/surface | Polish & Go (Bredent) |
| G-CAM | RelyX™ Unicem 2 | 1 kg load × 10 min; light-cured 20 s/surface | KemicPolitur (Aidite) |
| IPS e.max® ZirCAD Prime | SpeedCEM® 100 | 1 kg load × 10 min; light-cured 20 s/surface | OptraGloss® (Ivoclar Vivadent) |
| VarseoSmile CrownPlus | Variolink® Esthetic DC | 1 kg load × 10 min; light-cured 20 s/surface | VarseoSmile Polishing Set (BEGO) |
| IPS e.max® CAD | Variolink® Esthetic DC | 1 kg load × 10 min; light-cured 20 s/surface | OptraGloss® (Ivoclar Vivadent) |
| Group | Aging Procedure | Mechanical Loading | Specimens Per Material | Total Specimens per Group |
|---|---|---|---|---|
| Group 1 (Control) | None | None | 5 | 25 |
| Group 2 (Loading) | None | 3 × 500 N compressive loads | 10 | 50 |
| Group 3 (Aging + Loading) | 6000 thermocycles (5–55 °C) | 3 × 500 N compressive loads | 10 | 50 |
| Score | Description |
|---|---|
| 0 | No dye penetration observed |
| 1 | Dye penetration up to half the chamfer margin (≤0.5 mm) |
| 2 | Dye penetration across the entire marginal interface (≥1 mm) |
| 3 | Dye penetration along the margin and into the ascending wall of the cavity |
| 4 | Dye penetration along the margin and throughout the entire restoration–cavity interface |
| Variable | Evaluation Type | Kappa | Z-Value |
|---|---|---|---|
| Microleakage M1 | GLOBAL | 0.59 | 18.74 |
| Microleakage D1 | GLOBAL | 0.58 | 19.25 |
| Microleakage M2 | GLOBAL | 0.61 | 20.61 |
| Microleakage D2 | GLOBAL | 0.61 | 20.15 |
| Variable | Microleakage Degree | G-CAM | IPS e.max® CAD | IPS e.max® ZirCAD Prime | BioHPP® | VarseoSmile CrownPlus | p-Value | SR |
|---|---|---|---|---|---|---|---|---|
| MM 1 | 0 | 41.7% | 4.48% | 38.81% | 7.4% | 7.46% | <0.001 | * |
| MM 1 | 1 | 14.29% | 14.29% | 14.29% | 0% | 57.14% | ||
| MM 1 | 2 | 0% | 42.86% | 0% | 14.2% | 42.86% | ||
| MM 1 | 3 | 0% | 58.33% | 0% | 0% | 41.67% | ||
| MM 1 | 4 | 0% | 25% | 5.56% | 61.1% | 8.33% | ||
| DM 1 | 0 | 45.45% | 0% | 43.64% | 9.1% | 1.82% | <0.001 | * |
| DM 1 | 1 | 16% | 32% | 0% | 8% | 44% | ||
| DM 1 | 2 | 0% | 36.36% | 22.73% | 9.1% | 31.82% | ||
| DM 1 | 3 | 6.67% | 60% | 0% | 0% | 33.33% | ||
| DM 1 | 4 | 0% | 15.15% | 3.03% | 63.6% | 18.18% | ||
| MM 2 | 0 | 46% | 4% | 32% | 4% | 14% | <0.001 | * |
| MM 2 | 1 | 9.09% | 22.73% | 22.73% | 9.1% | 36.36% | ||
| MM 2 | 2 | 12.5% | 31.25% | 12.5% | 12.5% | 31.25% | ||
| MM 2 | 3 | 11.54% | 42.31% | 23.08% | 11.5% | 11.54% | ||
| MM 2 | 4 | 0% | 19.44% | 2.78% | 58.33% | 19.44% | ||
| DM 2 | 0 | 39.34% | 4.92% | 22.95% | 13.1% | 19.67% | <0.001 | * |
| DM 2 | 1 | 18.75% | 12.5% | 12.5% | 0% | 56.25% | ||
| DM 2 | 2 | 0% | 45.45% | 22.73% | 9.1% | 22.73% | ||
| DM 2 | 3 | 17.65% | 41.18% | 41.18% | 0% | 0% | ||
| DM 2 | 4 | 0% | 23.53% | 5.88% | 58.8% | 11.76% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Ruiz, X.; Cano-Batalla, J.; Figueras-Álvarez, Ò.; Real-Voltas, F.; Núñez-Bielsa, E.; Cabratosa-Termes, J. In Vitro Effect of Sequential Compressive Loading and Thermocycling on Marginal Microleakage of Digitally Fabricated Overlay Restorations Made from Five Materials. Appl. Sci. 2025, 15, 12532. https://doi.org/10.3390/app152312532
Gutiérrez-Ruiz X, Cano-Batalla J, Figueras-Álvarez Ò, Real-Voltas F, Núñez-Bielsa E, Cabratosa-Termes J. In Vitro Effect of Sequential Compressive Loading and Thermocycling on Marginal Microleakage of Digitally Fabricated Overlay Restorations Made from Five Materials. Applied Sciences. 2025; 15(23):12532. https://doi.org/10.3390/app152312532
Chicago/Turabian StyleGutiérrez-Ruiz, Xavier, Jordi Cano-Batalla, Òscar Figueras-Álvarez, Francisco Real-Voltas, Elena Núñez-Bielsa, and Josep Cabratosa-Termes. 2025. "In Vitro Effect of Sequential Compressive Loading and Thermocycling on Marginal Microleakage of Digitally Fabricated Overlay Restorations Made from Five Materials" Applied Sciences 15, no. 23: 12532. https://doi.org/10.3390/app152312532
APA StyleGutiérrez-Ruiz, X., Cano-Batalla, J., Figueras-Álvarez, Ò., Real-Voltas, F., Núñez-Bielsa, E., & Cabratosa-Termes, J. (2025). In Vitro Effect of Sequential Compressive Loading and Thermocycling on Marginal Microleakage of Digitally Fabricated Overlay Restorations Made from Five Materials. Applied Sciences, 15(23), 12532. https://doi.org/10.3390/app152312532






