Natural Strategies for Improving the Antioxidant Status and Health of Rabbits: The Role of Biochar and Tribulus terrestris
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Ethical Approval
2.3. Blood Analysis
2.4. Antioxidant Status
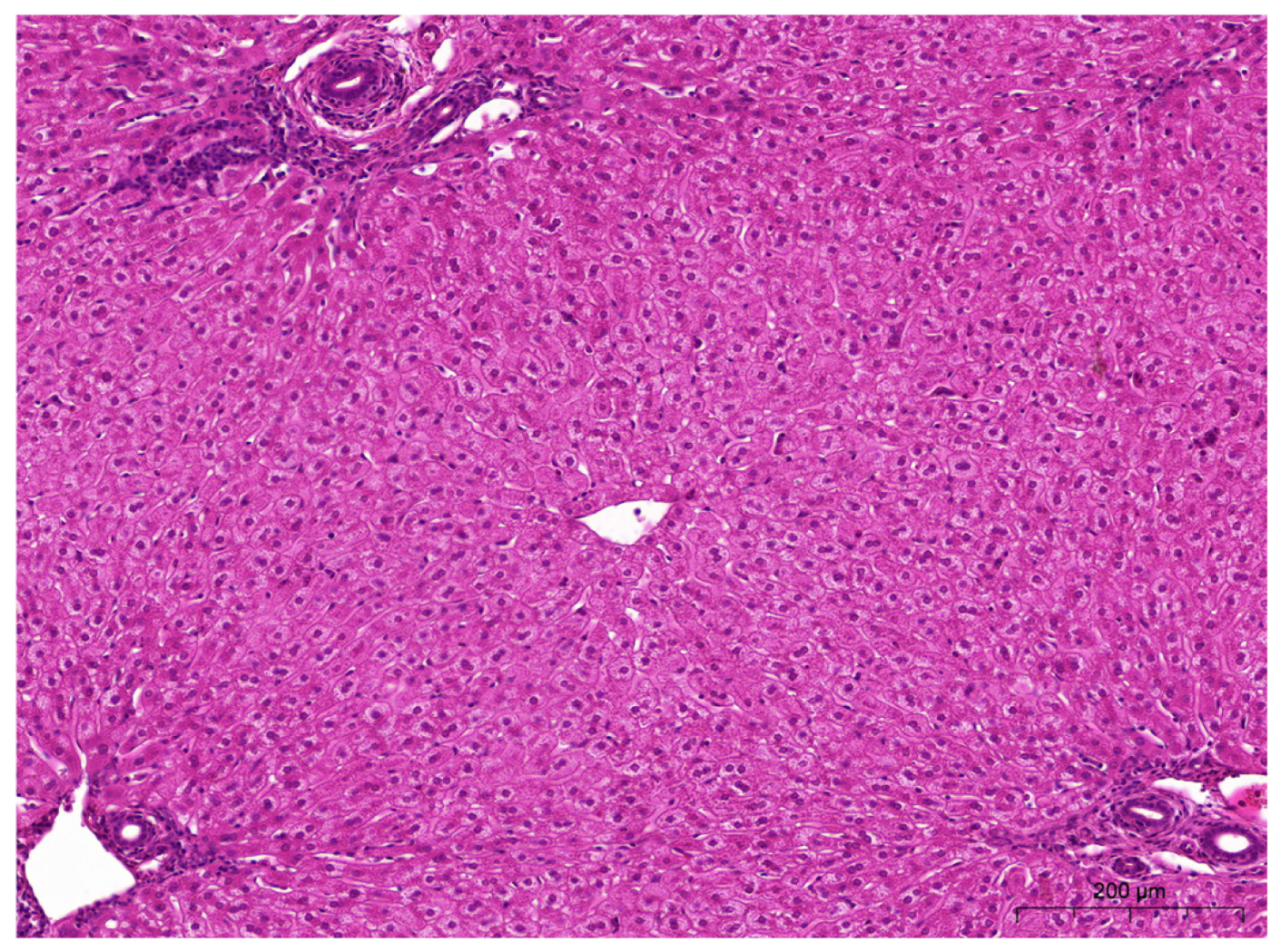
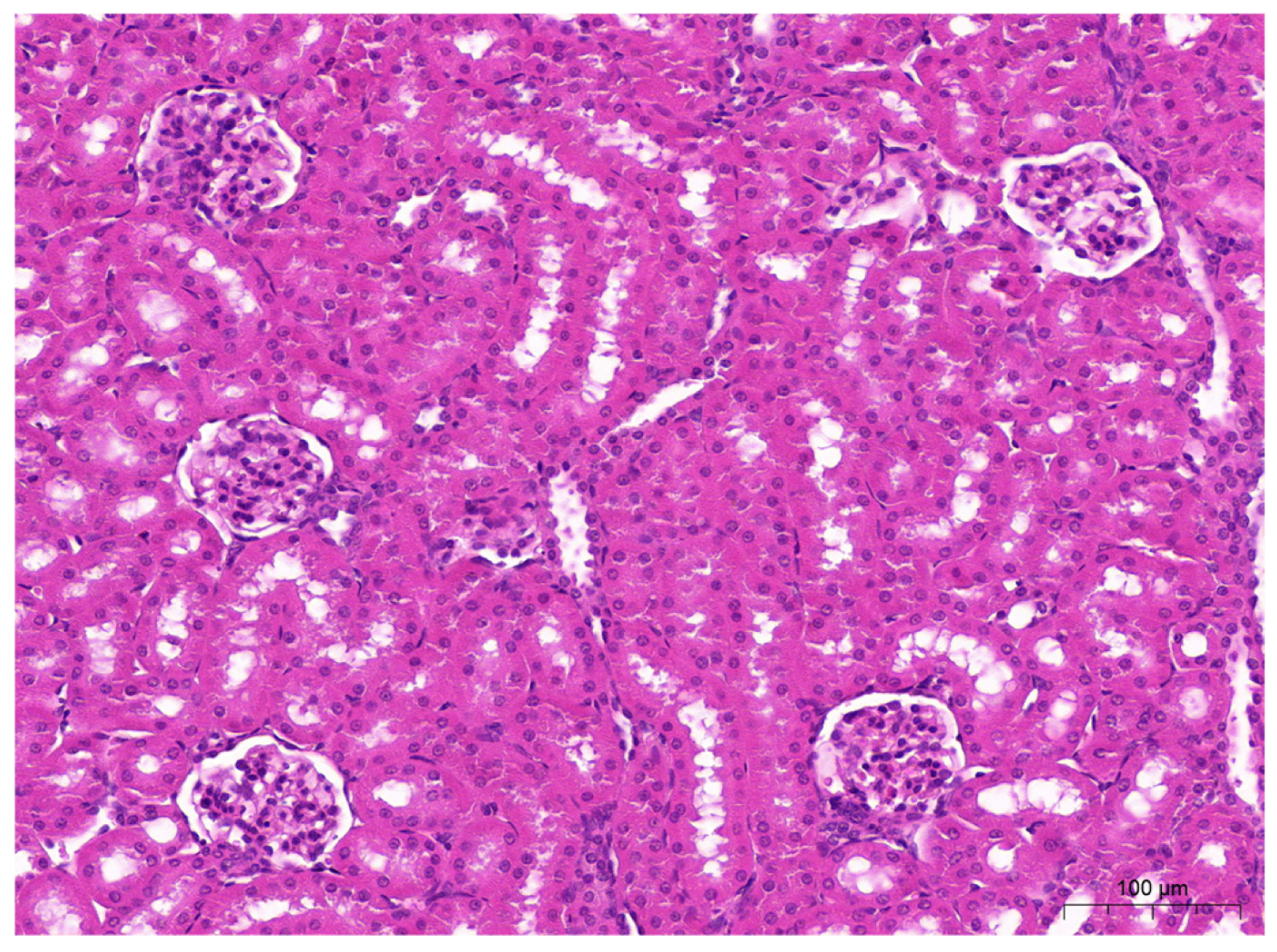
2.5. Histopathological Examinations of the Liver and Kidneys
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TT | Tribulus terrestris |
| NZW | New Zealand White |
| RBC | red blood cells |
| HGB | hemoglobin |
| MCV | mean corpuscular volume |
| RDW-CV | coefficient of variation in red cell distribution width |
| RDW-SD | red cell distribution width—standard deviation |
| PLT | platelet count |
| PDW | platelet distribution width |
| PCT | plateletcrit |
| WBC | white blood cells |
| EOS | eosinophils |
| NEU | neutrophils |
| LYM | lymphocytes |
| MON | monocytes |
| CK | creatine kinase |
| GGTP | gamma-glutamyl transpeptidase |
| MDA | malondialdehyde |
| FRAP | ferric reducing ability of plasma |
| CAT | catalase |
| SOD | superoxide dismutase |
| HCT | hematocrit |
| ALT | alanine aminotransferase |
| AP | alkaline phosphatase |
| AST | aspartate aminotransferase |
| GSH | glutathione concentration |
References
- Siddiqui, S.A.; Gerini, F.; Ikram, A.; Saeed, F.; Feng, X.; Chen, Y. Rabbit meat—Production, consumption and consumers’ attitudes and behavior. Sustainability 2023, 15, 2008. [Google Scholar] [CrossRef]
- Molenda, J.; Drabik, J. Study of the energetic properties of biochars from waste plant biomass using differential scanning calorimetry. Przem. Chem. 2024, 103, 525–528. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Ćwieląg-Piasecka, I. Effect of biochar application on heavy metal mobility in soils impacted by copper smelting processes. Pol. J. Environ. Stud. 2020, 29, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Qomariyah, N.; Ella, A.; Ahmad, S.N.; Yusriani, Y.; Sholikin, M.M.; Prihambodo, T.R.; Retnani, Y.; Jayanegara, A.; Wina, E.; Permana, I.G. Dietary biochar as a feed additive for increasing livestock performance: A meta-analysis of in vitro and in vivo experiment. Czech J. Anim. Sci. 2023, 68, 2. [Google Scholar] [CrossRef]
- Ansah, K.O.; Osafo, E.L.K.; Osman, A.; Opoku, G.C.; Ansah, A.S. Dose-dependent effects of biochar inclusion on growth, hematological, manure and carcass characteristics of rabbits. Ghana J. Anim. Sci. 2024, 15, 60–76. [Google Scholar] [CrossRef]
- Graves, C.; Kolar, P.; Shah, S.; Grimes, J.; Sharara, M. Can biochar improve the sustainability of animal production? Appl. Sci. 2022, 12, 5042. [Google Scholar] [CrossRef]
- Prasai, T.P.; Walsh, K.B.; Bhattarai, S.P.; Midmore, D.J.; Van, T.T.; Moore, R.J.; Stanley, D. Biochar, bentonite and zeolite supplemented feeding of layer chickens alters intestinal microbiota and reduces Campylobacter load. PLoS ONE 2016, 11, e0154061. [Google Scholar] [CrossRef]
- Placha, I.; Gai, F.; Pogány Simonová, M. Natural feed additives in animal nutrition—Their potential as functional feed. Front. Vet. Sci. 2022, 9, 1062724. [Google Scholar] [CrossRef]
- Abdelsalam, M.; Fathi, M. Improving productivity in rabbits by using some natural feed additives under hot environmental conditions—A review. Anim. Biosci. 2023, 36, 540. [Google Scholar] [CrossRef]
- Belhassen, T. Feed additives in rabbit nutrition to ensure sustainability. In Sustainable Use of Feed Additives in Livestock: Novel Ways for Animal Production; Springer International Publishing: Cham, Switzerland, 2023; pp. 859–871. [Google Scholar] [CrossRef]
- Saeed, M.; Munawar, M.; Bi, J.B.; Ahmed, S.; Ahmad, M.Z.; Kamboh, A.A.; Chen, H. Promising phytopharmacology, nutritional potential, health benefits, and traditional usage of Tribulus terrestris L. herb. Heliyon 2024, 10, 4. [Google Scholar] [CrossRef]
- Lee, B.; Jung, J.M.; Song, J.G.; Gwon, H.; Shin, H.; Tsang, Y.F.; Kim, H.W.; Kwon, E.E. Hazardous potential evaluation of biochar exposure on mice through analyses of gut-microbiome and fatty acids in brain. Chem. Eng. J. 2023, 461, 142006. [Google Scholar] [CrossRef]
- Reggi, S.; Frazzini, S.; Fusi, E.; Guagliano, M.; Cristiani, C.; Onelli, E.; Moscatelli, A.; Rossi, L. Biochar’s adsorption of Escherichia coli and probiotics Lactiplantibacillus plantarum and Limosilactobacillus reuteri and its impact on bacterial growth post in vitro digestion. Appl. Sci. 2025, 15, 5090. [Google Scholar] [CrossRef]
- Tuncer, M.A.; Yaymaci, B.; Sati, L.; Cayli, S.; Acar, G.; Altug, T.; Demir, R. Influence of Tribulus terrestris extract on lipid profile and endothelial structure in developing atherosclerotic lesions in the aorta of rabbits on a high-cholesterol diet. Acta Histochem. 2009, 111, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Abadjieva, D.; Abadjiev, M.; Ribarski, S.; Penchev, P. Dose-dependent effect of Tribulus terrestris dry extract on reproductive organs of growing male rabbits. Maced. J. Anim. Sci. 2019, 9, 19–23. [Google Scholar] [CrossRef]
- Gugołek, A. (Ed.) Nutritional Recommendations and Feed Value for Fur Animals; Polish Society of Animal Production: Jabłonna, Poland, 2011. [Google Scholar]
- Wlazło, Ł.; Kowalska, D.; Bielański, P.; Chmielowiec-Korzeniowska, A.; Ossowski, M.; Łukaszewicz, M.; Czech, A.; Nowakowicz-Dębek, B. Effect of fermented rapeseed meal on the gastrointestinal microbiota and immune status of rabbit (Oryctolagus cuniculus). Animals 2021, 11, 716. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Zawistowski, S. Histological Techniques, Histology, and Foundations of Histopathology; PZWL Medical Publishing: Warsaw, Poland, 1986. [Google Scholar]
- Winnicka, A. Wartości Referencyjne Podstawowych Badań Laboratoryjnych w Weterynarii, 1st ed.; Wydawnictwo SGGW: Warszawa, Poland, 1997. [Google Scholar]
- Schmidt, H.P.; Hagemann, N.; Draper, K.; Kammann, C. The use of biochar in animal feeding. PeerJ 2019, 7, e7373. [Google Scholar] [CrossRef]
- Ismaiel, S.I.; Farouk, S.M.; El-Ramady, R.A.; Khalil, W.F. Ameliorative impacts of Tribulus terrestris against ivermectin-induced hepato-renal toxicity in rabbit: Pharmacological and histopathological study. Am. J. Anim. Vet. Sci. 2017, 12, 8–16. [Google Scholar] [CrossRef]
- Cui, C.; Yang, W.; Dang, W.; Chen, R.; García-Caparrós, P.; Yang, G.; Huang, L.J. Bamboo biochar and sodium silicate alleviate oxybenzone-induced phytotoxicity via distinct mechanisms for sustainable plant protection. Plants 2025, 14, 2382. [Google Scholar] [CrossRef]
- Khaleel, H.K.; Abdelnour, S.A.; Osailan, R.; El-Kholy, K.H.; El-Haroun, E.; El-Nagar, H.A.; Mehilp, S.T.; El-Raghi, A.A.; Hassan, M.A.; Moustafa, M.; et al. Improving heat resilience in fattening rabbits: Nutritional strategies for mitigation via regulating blood physiology, inflammation and antioxidant pathways. Front. Vet. Sci. 2025, 12, 1677144. [Google Scholar] [CrossRef]
| Component | Biochar | Tribulus terrestris Extract |
|---|---|---|
| Dry matter (%) | 95–96 | 90 |
| Crude protein (%) | - | <1 |
| Crude fiber (%) | 77–80 | 5–8 |
| Crude ash (%) | 25–28 | 1–2 |
| Fat (%) | - | <1 |
| Volatile matter (Vdaf, %) | 5.3 | - |
| Key bioactive compounds | - | Saponins (protodioscin 47%, dioscin 14%) |
| Parameter | Group | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| C | E1 | E2 | E3 | |||
| RBC [106/uL] | 6.66 | 6.23 | 5.80 | 6.14 | 0.122 | 0.085 |
| HGB [g/dL] | 13.63 | 13.55 | 12.00 | 13.25 | 0.185 | 0.090 |
| HCT [%] | 40.63 a | 39.70 ab | 35.00 b | 39.40 ab | 0.599 | 0.001 |
| MCV [fL] | 61.17 | 63.85 | 60.90 | 64.20 | 0.589 | 0.077 |
| MCH [pg] | 20.53 | 21.80 | 20.90 | 21.65 | 0.213 | 0.102 |
| MCHC [g/dL] | 33.60 a | 34.15 ab | 34.25 b | 33.70 ab | 0.092 | 0.015 |
| MPV [um3] | 6.83 | 6.45 | 6.00 | 6.50 | 0.143 | 0.234 |
| RDW-CV [%] | 12.90 a | 13.60 ab | 14.15 b | 13.45 ab | 0.164 | 0.047 |
| RDW-SD [um3] | 31.77 a | 35.05 b | 34.90 b | 34.70 b | 0.353 | <0.001 |
| PLT [106/uL] | 252.00 ab | 364.00 ab | 455.50 a | 227.50 b | 32.372 | 0.035 |
| PDW | 16.07 | 16.00 | 15.80 | 16.05 | 0.040 | 0.056 |
| PCT [%] | 0.16 | 0.24 | 0.28 | 0.16 | 0.021 | 0.142 |
| Parameter | Group | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| C | E1 | E2 | E3 | |||
| WBC | 12.10 a | 8.87 b | 6.14 c | 5.19 c | 0.614 | <0.001 |
| EOS | 0.29 | 0.27 | 0.15 | 0.13 | 0.037 | 0.278 |
| NEU | 4.70 a | 1.85 b | 1.93 b | 1.08 b | 0.341 | <0.001 |
| LYM | 5.36 | 6.28 | 3.40 | 3.28 | 0.288 | 0.199 |
| MON | 1.43 a | 0.62 b | 0.73 b | 0.48 b | 0.100 | <0.001 |
| Parameter | Group | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| C | E1 | E2 | E3 | |||
| Albumins [g/dL] | 5.74 a | 6.60 b | 5.74 a | 6.07 ab | 0.113 | 0.010 |
| ALT [U/L] | 14.04 | 24.12 | 23.71 | 22.28 | 1.140 | 0.339 |
| AP [U/L] | 200.93 | 310.37 | 240.44 | 225.31 | 15.597 | 0.066 |
| AST [U/L] | 15.26 | 23.32 | 18.97 | 16.75 | 1.401 | 0.195 |
| Total protein [g/dL] | 6.02 | 6.13 | 5.65 | 5.54 | 0.083 | 0.263 |
| Total cholesterol [mg/dL] | 34.19 a | 90.28 b | 105.08 b | 89.24 b | 7.732 | 0.001 |
| CK [U/L] | 1246.92 | 1941.47 | 2548.98 | 2045.35 | 172.399 | 0.051 |
| GGTP [U/L] | 5.84 a | 15.54 b | 12.03 b | 11.36 b | 0.838 | <0.001 |
| Glucose [mg/dL] | 113.35 | 123.30 | 111.17 | 121.04 | 1.794 | 0.065 |
| Urea [mg/dL] | 36.00 | 39.28 | 34.01 | 33.18 | 1.065 | 0.181 |
| Triglycerides [mg/dL] | 128.60 ab | 198.67 b | 85.33 a | 60.20 a | 14.052 | <0.001 |
| Phosphorus [mg/dL] | 4.55 a | 6.62 b | 6.82 b | 7.31 b | 0.234 | <0.001 |
| Iron [ug/dL] | 134.15 a | 253.17 ab | 186.65 b | 216.10 b | 10.666 | 0.000 |
| Magnesium [mg/dL] | 2.33 a | 2.47 a | 2.78 b | 2.91 b | 0.061 | 0.000 |
| Calcium [mg/dL] | 15.28 | 15.94 | 15.31 | 15.42 | 0.140 | 0.312 |
| Parameter | Group | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| C | E1 | E2 | E3 | |||
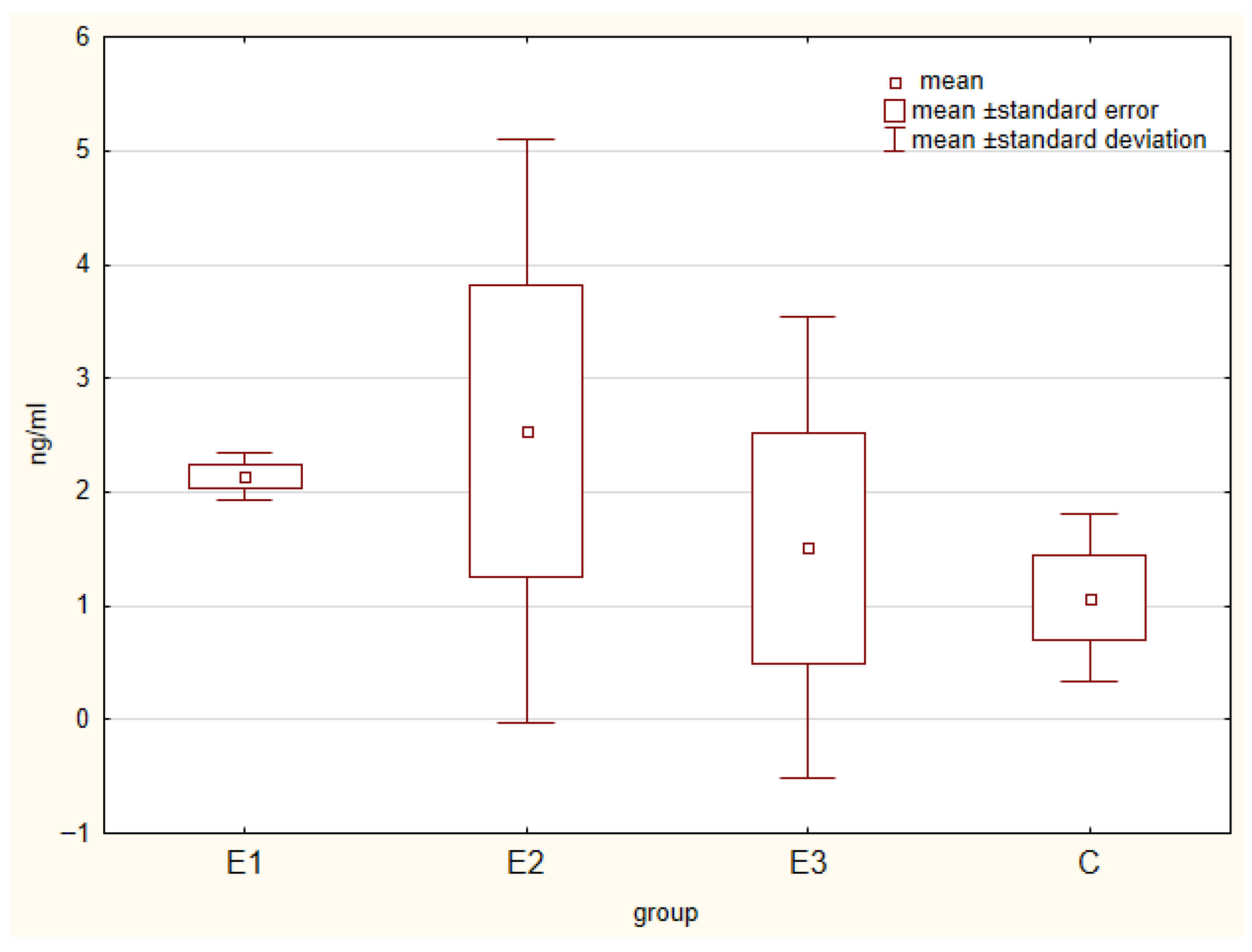
| IgA [ng/mL] | 5.53 | 6.45 | 7.86 | 7.85 | 0.233 | 0.450 |
| IgG [ng/mL] | 31.66 a | 36.73 b | 44.72 bc | 37.82 b | 1.049 | 0.036 |
| IgM [ng/mL] | 12.29 | 16.49 | 13.55 | 13.21 | 0.359 | 0.972 |
| IL-2 [pg/mL] | 8.00 | 6.87 | 6.78 | 7.35 | 0.105 | 0.201 |
| IL-6 [pg/mL] | 117.56 | 84.96 | 97.58 | 86.99 | 2.839 | 0.396 |
| IL-8 [pg/mL] | 22.70 | 33.09 | 25.54 | 27.39 | 0.968 | 0.673 |
| LZM [ng/mL] | 20.59 | 24.96 | 25.85 | 20.74 | 0.699 | 0.771 |
| Parameter | Group | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| C | E1 | E2 | E3 | |||
| MDA [umol/L] | 1.83 bc | 2.02 b | 1.38 a | 1.59 c | 0.068 | <0.001 |
| SOD [U/mL] | 27.29 a | 26.02 b | 25.82 bc | 21.72 c | 0.558 | 0.009 |
| CAT [U/mL] | 20.12 ac | 17.49 a | 16.89 b | 21.19 b | 0.511 | <0.001 |
| GSH [umol/L] | 5.35 ac | 6.34 a | 6.79 b | 4.69 b | 0.230 | <0.001 |
| FRAP [umol/L] | 7.46 a | 8.16 b | 7.97 ab | 5.33 b | 0.310 | <0.001 |
| Oxidative Stress Marker | Experimental Group | IgA | IgG | IgM | IL2 | IL6 | IL8 |
|---|---|---|---|---|---|---|---|
| MDA | C | −0.2511 | 0.4999 | 0.4971 | 0.8576 | −0.6632 | −0.1528 |
| p = 0.631 | p = 0.313 | p = 0.316 | p = 0.029 | p = 0.151 | p = 0.773 | ||
| E1 | 0.6956 | −0.1906 | −0.2568 | 0.4403 | 0.3512 | −0.3272 | |
| p = 0.125 | p = 0.718 | p = 0.623 | p = 0.382 | p = 0.495 | p = 0.527 | ||
| E2 | 0.4346 | 0.7491 | 0.8115 | −0.7104 | −0.4324 | 0.3014 | |
| p = 0.389 | p = 0.087 | p = 0.050 | p = 0.114 | p = 0.392 | p = 0.562 | ||
| E3 | −0.2796 | 0.6306 | 0.8166 | −0.282 | 0.0553 | 0.0855 | |
| p = 0.591 | p = 0.179 | p = 0.047 | p = 0.588 | p = 0.917 | p = 0.872 | ||
| SOD | C | −0.1738 | 0.4667 | 0.6696 | 0.7121 | −0.273 | −0.1925 |
| p = 0.742 | p = 0.351 | p = 0.146 | p = 0.112 | p = 0.601 | p = 0.715 | ||
| E1 | −0.5449 | 0.0179 | −0.4564 | 0.4834 | −0.1728 | 0.449 | |
| p = 0.264 | p = 0.973 | p = 0.363 | p = 0.331 | p = 0.743 | p = 0.372 | ||
| E2 | −0.4864 | −0.2282 | −0.7237 | 0.4881 | 0.5748 | 0.0573 | |
| p = 0.328 | p = 0.664 | p = 0.104 | p = 0.326 | p = 0.233 | p = 0.914 | ||
| E3 | 0.2169 | −0.3725 | −0.7284 | 0.7001 | 0.2741 | 0.0352 | |
| p = 0.680 | p = 0.467 | p = 0.101 | p = 0.121 | p = 0.599 | p = 0.947 | ||
| CAT | C | −0.6161 | −0.0405 | 0.1676 | 0.5012 | −0.9658 | −0.2864 |
| p = 0.193 | p = 0.939 | p = 0.751 | p = 0.311 | p = 0.002 | p = 0.582 | ||
| E1 | 0.4834 | 0.734 | 0.3477 | −0.4329 | 0.1349 | 0.5003 | |
| p = 0.331 | p = 0.097 | p = 0.500 | p = 0.391 | p = 0.799 | p = 0.312 | ||
| E2 | 0.7505 | 0.499 | 0.7532 | −0.3811 | −0.6102 | 0.0992 | |
| p = 0.086 | p = 0.314 | p = 0.084 | p = 0.456 | p = 0.198 | p = 0.852 | ||
| E3 | −0.281 | 0.5726 | 0.8266 | −0.5562 | −0.1173 | 0.1359 | |
| p = 0.590 | p = 0.235 | p = 0.043 | p = 0.252 | p = 0.825 | p = 0.797 | ||
| GSH | C | −0.347 | 0.3044 | 0.2731 | 0.7181 | −0.8255 | −0.2196 |
| p = 0.500 | p = 0.558 | p = 0.601 | p = 0.108 | p = 0.043 | p = 0.676 | ||
| E1 | 0.4564 | 0.7523 | 0.1794 | −0.3816 | 0.1254 | 0.6112 | |
| p = 0.363 | p = 0.084 | p = 0.734 | p = 0.455 | p = 0.813 | p = 0.197 | ||
| E2 | 0.7074 | 0.2507 | 0.6622 | −0.0997 | −0.6906 | 0.0438 | |
| p = 0.116 | p = 0.632 | p = 0.152 | p = 0.851 | p = 0.129 | p = 0.934 | ||
| E3 | 0.7377 | −0.3552 | −0.3108 | 0.409 | 0.6122 | −0.0347 | |
| p = 0.094 | p = 0.490 | p = 0.549 | p = 0.421 | p = 0.196 | p = 0.948 | ||
| FRAP | C | 0.3854 | −0.2507 | −0.4449 | −0.7433 | 0.7164 | 0.4271 |
| p = 0.451 | p = 0.632 | p = 0.377 | p = 0.090 | p = 0.109 | p = 0.398 | ||
| E1 | 0.6467 | 0.65 | 0.3824 | −0.3925 | 0.2066 | 0.3343 | |
| p = 0.165 | p = 0.162 | p = 0.454 | p = 0.441 | p = 0.694 | p = 0.517 | ||
| E2 | −0.3061 | −0.7307 | −0.683 | 0.7548 | 0.2637 | −0.2761 | |
| p = 0.555 | p = 0.099 | p = 0.135 | p = 0.083 | p = 0.614 | p = 0.596 | ||
| E3 | 0.1912 | −0.7091 | −0.9031 | 0.4915 | −0.0626 | −0.3743 | |
| p = 0.717 | p = 0.115 | p = 0.014 | p = 0.322 | p = 0.906 | p = 0.465 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpińska, K.; Nowakowicz-Dębek, B.; Kowalska, D.; Bielański, P.; Wlazło, Ł.; Czech, A. Natural Strategies for Improving the Antioxidant Status and Health of Rabbits: The Role of Biochar and Tribulus terrestris. Appl. Sci. 2025, 15, 12515. https://doi.org/10.3390/app152312515
Karpińska K, Nowakowicz-Dębek B, Kowalska D, Bielański P, Wlazło Ł, Czech A. Natural Strategies for Improving the Antioxidant Status and Health of Rabbits: The Role of Biochar and Tribulus terrestris. Applied Sciences. 2025; 15(23):12515. https://doi.org/10.3390/app152312515
Chicago/Turabian StyleKarpińska, Katarzyna, Bożena Nowakowicz-Dębek, Dorota Kowalska, Paweł Bielański, Łukasz Wlazło, and Anna Czech. 2025. "Natural Strategies for Improving the Antioxidant Status and Health of Rabbits: The Role of Biochar and Tribulus terrestris" Applied Sciences 15, no. 23: 12515. https://doi.org/10.3390/app152312515
APA StyleKarpińska, K., Nowakowicz-Dębek, B., Kowalska, D., Bielański, P., Wlazło, Ł., & Czech, A. (2025). Natural Strategies for Improving the Antioxidant Status and Health of Rabbits: The Role of Biochar and Tribulus terrestris. Applied Sciences, 15(23), 12515. https://doi.org/10.3390/app152312515








