The Bactericidal Effect of Calcium Hydroxide and Triple Antibiotic Paste During Regenerative Endodontic Procedures
Featured Application
Abstract
1. Introduction
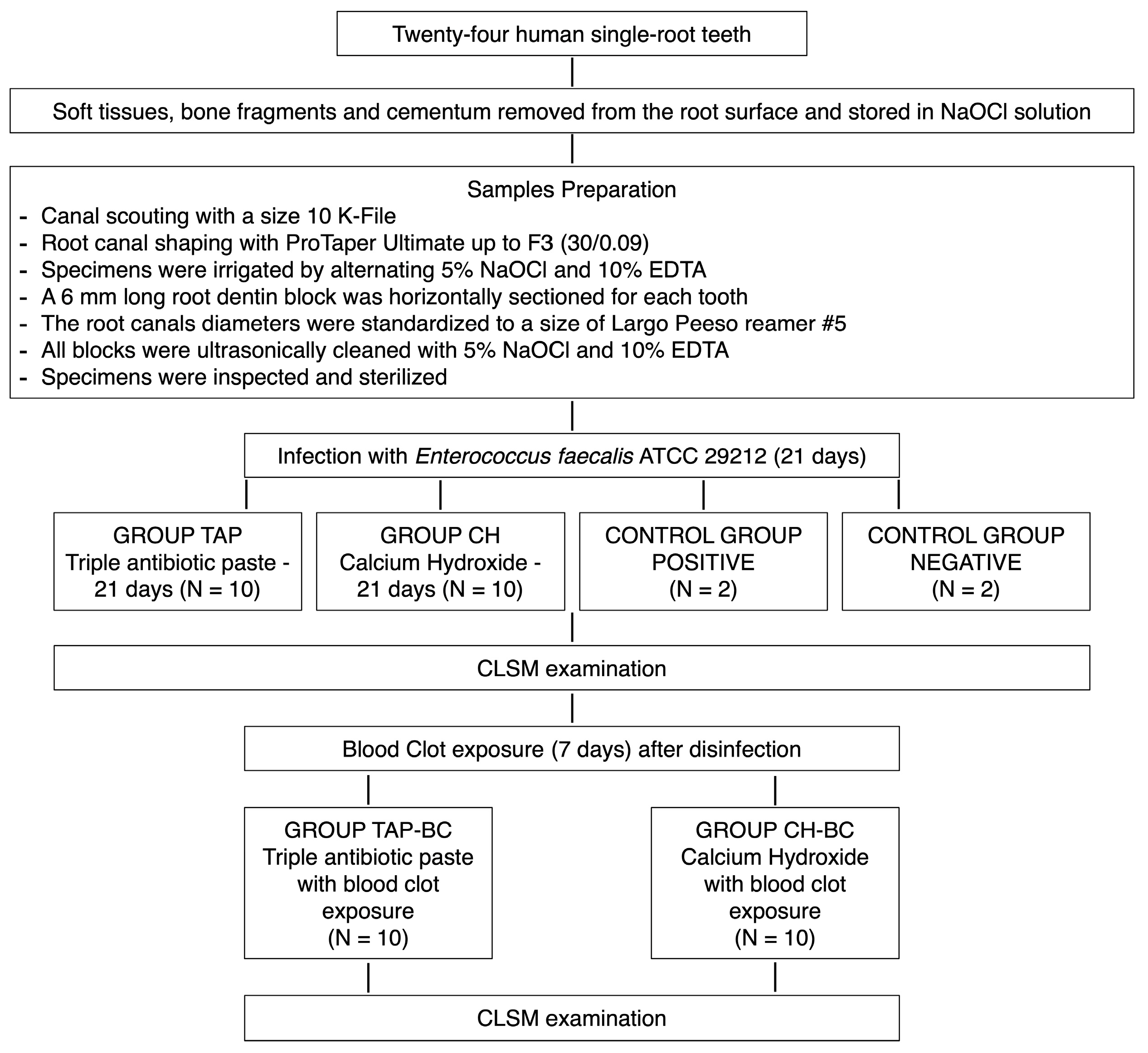
2. Materials and Methods
2.1. Specimen Preparation
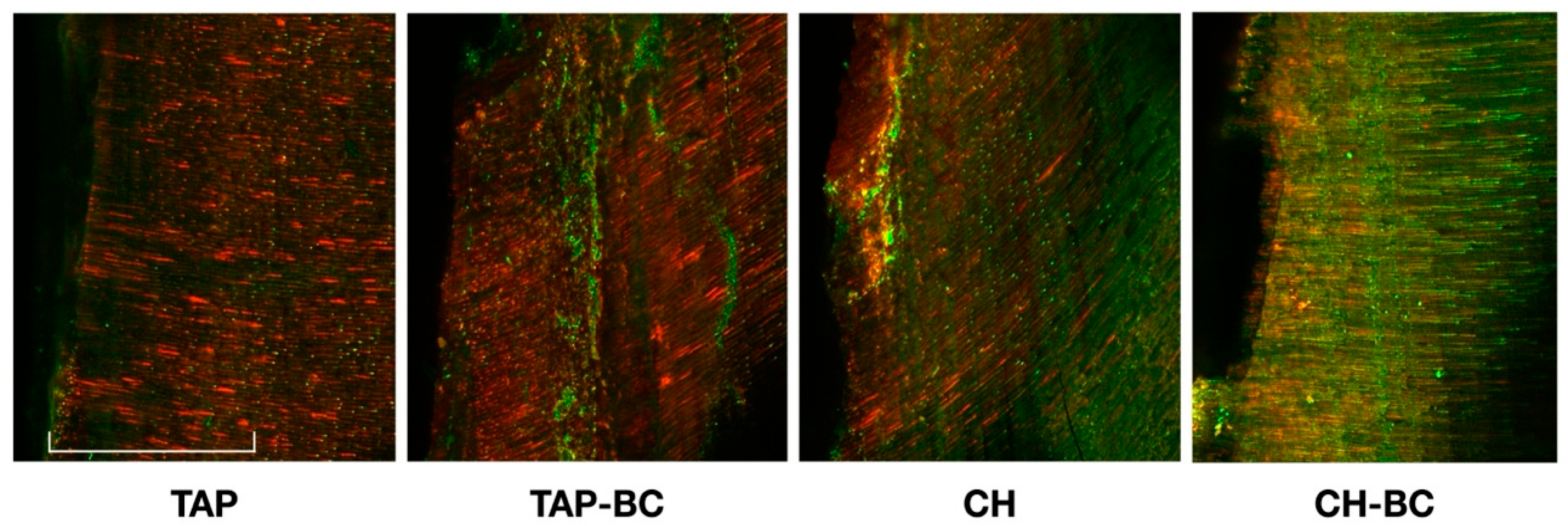
2.2. CLSM Analysis
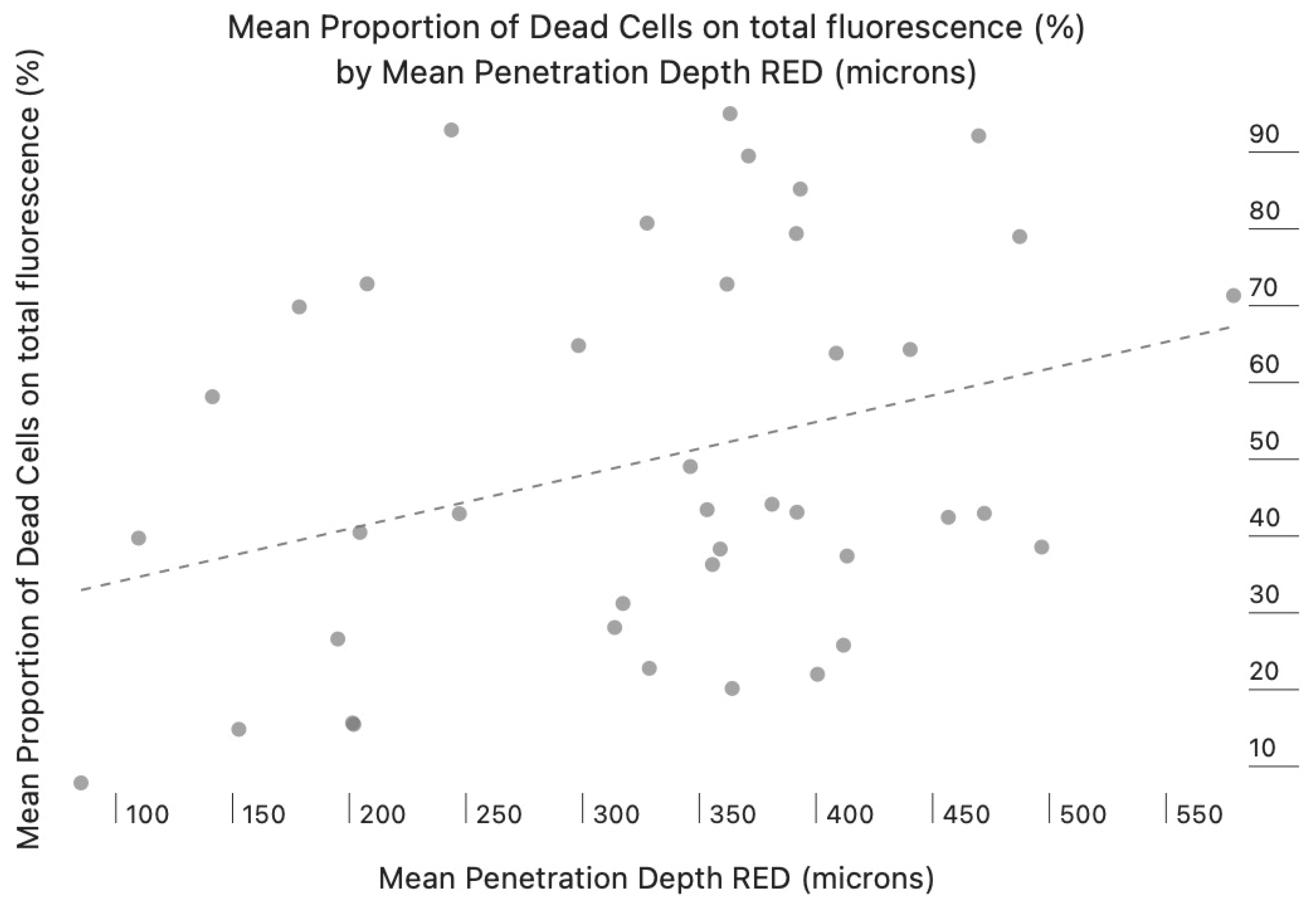
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abbott, P.V.; Yu, C. A clinical classification of the status of the pulp and the root canal system. Aust. Dent. J. 2007, 52 (Suppl. 1), S17–S31. [Google Scholar] [CrossRef]
- Nicoloso, G.F.; Pötter, I.G.; Rocha, R.O.; Montagner, F.; Casagrande, L. A comparative evaluation of endodontic treatments for immature necrotic permanent teeth based on clinical and radiographic outcomes: A systematic review and meta-analysis. Int J Paediatr. Dent. 2017, 27, 217–227. [Google Scholar] [CrossRef]
- Bonte, E.; Beslot, A.; Boukpessi, T.; Lasfargues, J.J. MTA versus Ca(OH)2 in apexification of non-vital immature permanent teeth: A randomized clinical trial comparison. Clin. Oral Investig. 2015, 19, 1381–1388. [Google Scholar] [CrossRef]
- Rafter, M. Apexification: A review. Dent. Traumatol. 2005, 21, 1–8. [Google Scholar] [CrossRef]
- Mente, J.; Leo, M.; Panagidis, D.; Ohle, M.; Schneider, S.; Bermejo, J.L.; Pfefferle, T. Treatment outcome of mineral trioxide aggregate in open apex teeth. J. Endod. 2013, 39, 20–26. [Google Scholar] [CrossRef]
- Pulyodan, M.K.; Paramel Mohan, S.; Valsan, D.; Divakar, N.; Moyin, S.; Thayyil, S. Regenerative Endodontics: A Paradigm Shift in Clinical Endodontics. J. Pharm. Bioallied Sci. 2020, 12 (Suppl. 1), S20–S26. [Google Scholar] [CrossRef] [PubMed]
- American Association of Endodontists. Glossary of Endodontic Terms, 10th ed.; American Association of Endodontists: Chicago, IL, USA, 2020. [Google Scholar]
- Takimoto, K.; Widbiller, M.; Diogenes, A. Expression of Toll-like Receptors in Stem Cells of the Apical Papilla and Its Implication for Regenerative Endodontics. Cells 2023, 12, 2502. [Google Scholar] [CrossRef]
- Galler, K.M.; Krastl, G.; Simon, S.; Van Gorp, G.; Meschi, N.; Vahedi, B.; Lambrechts, P.; Dummer, P.M. European Society of Endodontology position statement: Revitalization procedures. Int. Endod. J. 2016, 49, 717–723. [Google Scholar] [CrossRef] [PubMed]
- American Association of Endodontists (AAE). Clinical Considerations for a Regenerative Procedure. Available online: https://f3f142zs0k2w1kg84k5p9i1o-wpengine.netdna-ssl.com/specialty/wpcontent/uploads/sites/2/2018/06/ConsiderationsForRegEndo_AsOfApril2018.pdf (accessed on 13 April 2018).
- Yoo, Y.-J.; Perinpanayagam, H.; Choi, Y.; Gu, Y.; Chang, S.-W.; Baek, S.-H.; Zhu, Q.; Fouad, A.F.; Kum, K.-Y. Characterization of Histopathology and Microbiota in Contemporary Regenerative Endodontic Procedures: Still Coming up Short. J. Endod. 2021, 47, 1285–1293.e1. [Google Scholar] [CrossRef]
- Verma, R.; Sharma, D.S.; Pathak, A.K. Antibacterial Efficacy of Pastes Against E Faecalis in Primary Root Dentin: A Confocal Microscope Study. J. Clin. Pediatr. Dent. 2015, 39, 247–254. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Shen, Y.; Haapasalo, M. A new noninvasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopy. J. Endod. 2011, 37, 1380–1385. [Google Scholar] [CrossRef]
- Mandras, N.; Alovisi, M.; Roana, J.; Crosasso, P.; Luganini, A.; Pasqualini, D.; Genta, E.; Arpicco, S.; Banche, G.; Cuffini, A.; et al. Evaluation of the Bactericidal Activity of a Hyaluronic Acid-Vehicled Clarithromycin Antibiotic Mixture by Confocal Laser Scanning Microscopy. Appl. Sci. 2020, 10, 761. [Google Scholar] [CrossRef]
- Galler, K.M. Clinical procedures for revitalization: Current knowledge and considerations. Int. Endod. J. 2016, 49, 926–936. [Google Scholar] [CrossRef]
- Almutairi, W.; Yassen, G.H.; Aminoshariae, A.; Williams, K.A.; Mickel, A. Regenerative Endodontics: A Systematic Analysis of the Failed Cases. J. Endod. 2019, 45, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Nosrat, A.; Kim, J.R.; Price, J.B.; Wang, P.; Bair, E.; Xu, H.H.; Fouad, A.F. Effect of Residual Bacteria on the Outcome of Pulp Regeneration In Vivo. J. Dent. Res. 2017, 96, 100–106. [Google Scholar] [CrossRef]
- Vishwanat, L.; Duong, R.; Takimoto, K.; Phillips, L.; Espitia, C.O.; Diogenes, A.; Ruparel, S.B.; Kolodrubetz, D.; Ruparel, N.B. Effect of bacterial biofilm on the osteogenic differentiation of stem cells of apical papilla. J. Endod. 2017, 43, 916–922. [Google Scholar] [CrossRef]
- Van Gorp, G.; Declerck, D. Long-term Outcome of Endodontically Treated Traumatized Immature Upper Incisors. J. Endod. 2023, 49, 1106–1119. [Google Scholar] [CrossRef]
- Tagelsir, A.; Yassen, G.H.; Gomez, G.F.; Gregory, R.L. Effect of Antimicrobials Used in Regenerative Endodontic Procedures on 3-week-old Enterococcus faecalis Biofilm. J. Endod. 2016, 42, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Panchal, K.G.; Virani, K.; Patel, V.; Khan, A.A.; Pettiwala, A.; Puranik, S.S.; Joshi, S. Triple Antibiotic Paste: A Game Changer in Endodontics. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. 3), S1913–S1915. [Google Scholar] [CrossRef] [PubMed]
- Montero-Miralles, P.; Martín-González, J.; Alonso-Ezpeleta, O.; Jiménez-Sánchez, M.D.C.; Velasco-Ortega, E.; Segura-Egea, J.J. Effectiveness and clinical implications of the use of topical antibiotics in regenerative endodontic procedures: A review. Int. Endod. J. 2018, 51, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Yassen, G.H.; Vail, M.M.; Chu, T.G.; Platt, J.A. The effect of medicaments used in endodontic regeneration on root fracture and microhardness of radicular dentine. Int. Endod. J. 2013, 46, 688–695. [Google Scholar] [CrossRef]
- Ragab, R.A.; El Lattif, A.E.A.; Dokky, N.A.E.W.E. Comparative Study between Revitalization of Necrotic Immature Permanent Anterior Teeth with and without Platelet Rich Fibrin: A Randomized Controlled Trial. J. Clin. Pediatr. Dent. 2019, 43, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Latham, J.; Fong, H.; Jewett, A.; Johnson, J.D.; Paranjpe, A. Disinfection Efficacy of Current Regenerative Endodontic Protocols in Simulated Necrotic Immature Permanent Teeth. J. Endod. 2016, 42, 1218–1225. [Google Scholar] [CrossRef]
- Mandras, N.; Roana, J.; Allizond, V.; Pasqualini, D.; Crosasso, P.; Burlando, M.; Banche, G.; Denisova, T.; Berutti, E.; Cuffini, A. Antibacterial efficacy and drug-induced tooth discolouration of antibiotic combinations for endodontic regenerative procedures. Int. J. Immunopathol. Pharmacol. 2013, 26, 557–563. [Google Scholar] [CrossRef]
- Amsden, G.W. Advanced-generation macrolides: Tissue-directed antibiotics. Int. J. Antimicrob. Agents 2001, 18 (Suppl. 1), S11–S15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Du, J.; Peng, Z. Correlation between Enterococcus faecalis and Persistent Intraradicular Infection Compared with Primary Intraradicular Infection: A Systematic Review. J. Endod. 2015, 41, 1207–1213. [Google Scholar] [CrossRef]
- Kishen, A.; Shrestha, A.; Del Carpio-Perochena, A. Validation of Biofilm Assays to Assess Antibiofilm Efficacy in Instrumented Root Canals after Syringe Irrigation and Sonic Agitation. J. Endod. 2018, 44, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Hage, W.; Karam Sarkis, D.; Kallassy, M.; Mallah, M.; Zogheib, C. In vitro evaluation of enterococcus faecalis growth in different conditions on dentinal substrate. Biomater. Investig. Dent. 2023, 10, 2287668. [Google Scholar] [CrossRef]
- de Araújo, L.; Goulart, T.S.; Gil, A.C.K.; Schuldt, D.P.V.; Coelho, B.S.; Figueiredo, D.d.R.; Garcia, L.d.F.R.; de Almeida, J. Do alternative scaffolds used in regenerative endodontics promote better root development than that achieved with blood clots? Braz. Dent. J. 2022, 33, 22–32. [Google Scholar] [CrossRef]
- Altaii, M.; Richards, L.; Rossi-Fedele, G. Histological assessment of regenerative endodontic treatment in animal studies with different scaffolds: A systematic review. Dent. Traumatol. 2017, 33, 235–244. [Google Scholar] [CrossRef]
- Ulusoy, A.T.; Turedi, I.; Cimen, M.; Cehreli, Z.C. Evaluation of Blood Clot, Platelet-rich Plasma, Platelet-rich Fibrin, and Platelet Pellet as Scaffolds in Regenerative Endodontic Treatment: A Prospective Randomized Trial. J. Endod. 2019, 45, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Said, M.S.; Tirthani, E.; Lesho, E. Enterococcus Infections. 12 February 2024. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
| Groups | Mean Depth of Action (µm) | Mean Proportion of Dead Cells Volume (Red Fluorescence Ratio) |
|---|---|---|
| CH | 402.5 ± 58.2 a | 0.41 ± 0.08 a |
| TAP | 372.8 ± 41.6 a | 0.81 ± 0.06 b |
| CH-BC | 313.7 ± 37.4 b | 0.23 ± 0.11 c |
| TAP-BC | 355.63 ± 32.6 a | 0.45 ± 0.12 d |
| Positive controls | 0.5 ± 0 | 0.01 ± 0 |
| Negative controls | Nd | Nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandras, N.; Pancini, N.; Roana, J.; Gai, M.; Pasqualini, D.; Fenoglio, V.; Allizond, V.; Banche, G.; Scotti, N.; Alovisi, M. The Bactericidal Effect of Calcium Hydroxide and Triple Antibiotic Paste During Regenerative Endodontic Procedures. Appl. Sci. 2025, 15, 12478. https://doi.org/10.3390/app152312478
Mandras N, Pancini N, Roana J, Gai M, Pasqualini D, Fenoglio V, Allizond V, Banche G, Scotti N, Alovisi M. The Bactericidal Effect of Calcium Hydroxide and Triple Antibiotic Paste During Regenerative Endodontic Procedures. Applied Sciences. 2025; 15(23):12478. https://doi.org/10.3390/app152312478
Chicago/Turabian StyleMandras, Narcisa, Nicolò Pancini, Janira Roana, Marta Gai, Damiano Pasqualini, Vittorio Fenoglio, Valeria Allizond, Giuliana Banche, Nicola Scotti, and Mario Alovisi. 2025. "The Bactericidal Effect of Calcium Hydroxide and Triple Antibiotic Paste During Regenerative Endodontic Procedures" Applied Sciences 15, no. 23: 12478. https://doi.org/10.3390/app152312478
APA StyleMandras, N., Pancini, N., Roana, J., Gai, M., Pasqualini, D., Fenoglio, V., Allizond, V., Banche, G., Scotti, N., & Alovisi, M. (2025). The Bactericidal Effect of Calcium Hydroxide and Triple Antibiotic Paste During Regenerative Endodontic Procedures. Applied Sciences, 15(23), 12478. https://doi.org/10.3390/app152312478











