Color Stability of a Composite Containing Hydroxyapatite, Fluorine, and Silver Fillers After Artificial Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Fabrication and Preparation
2.2. Color Measurements
2.3. Aging Processes
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
- -
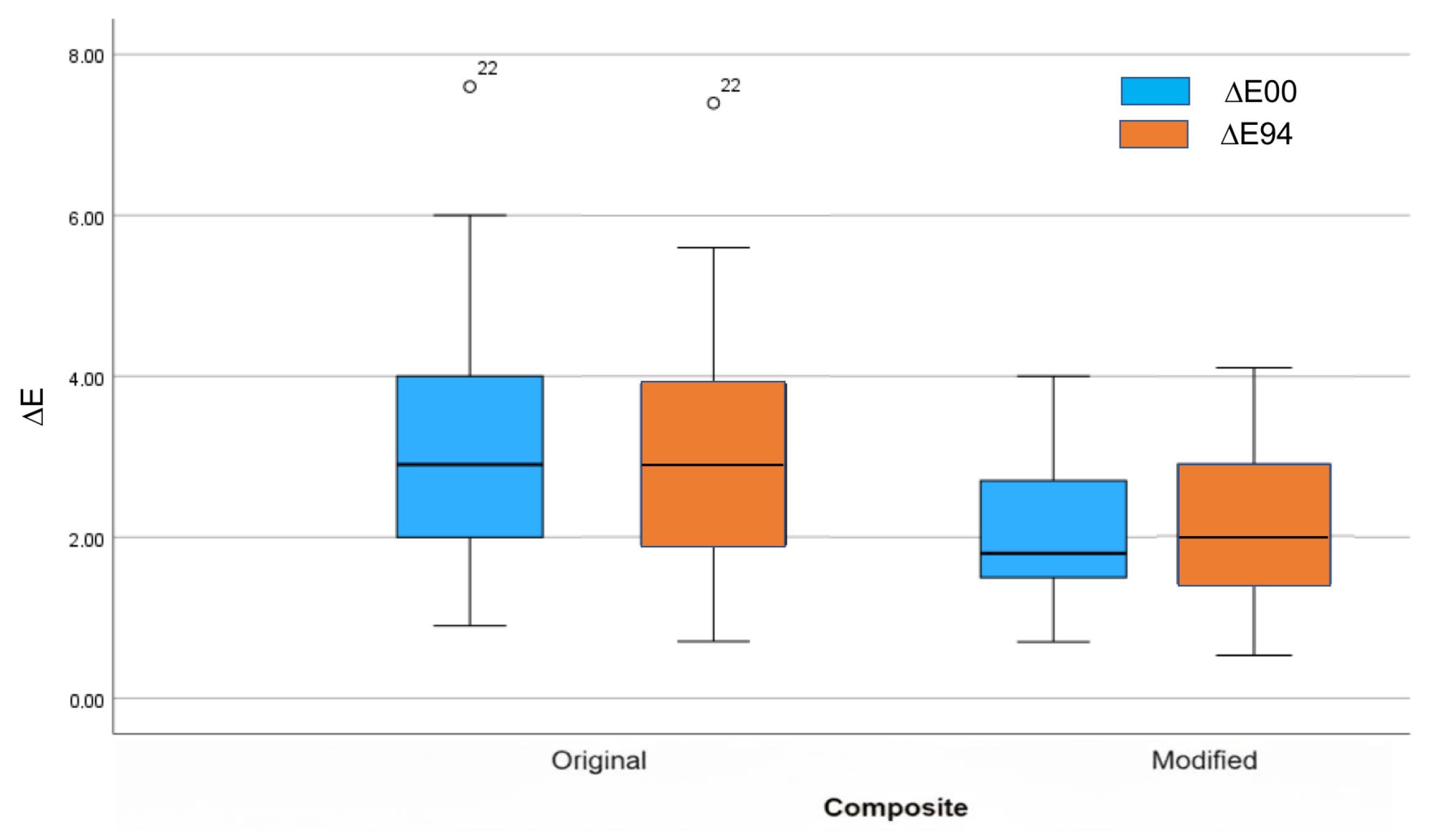
- The modified composite showed reduced color differences compared to the original one.
- -
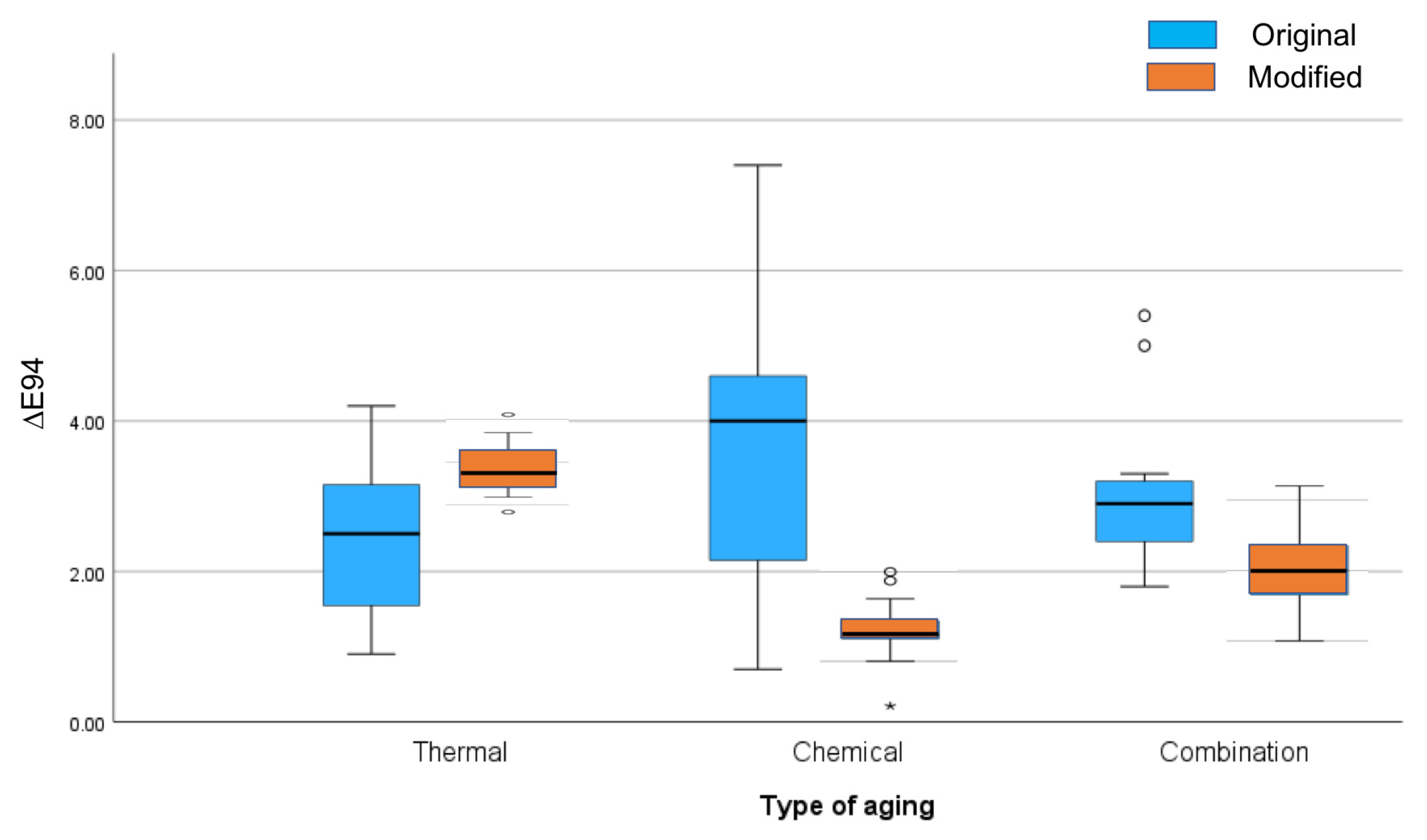
- Differences were observed between aging processes for the modified composite, but not for the original composite.
- -
- The incorporation of hydroxyapatite results in an increase in lightness in the composite material.
- -
- A modified composite with hydroxyapatite, silver, and fluorine could better withstand color changes when submitted to cumulative thermal and saliva aging processes.
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newton, J.T.; Subramanian, S.S.; Westland, S.; Gupta, A.K.; Luo, W.; Joiner, A. The impact of tooth colour on the perceptions of age and social judgements. J. Dent. 2021, 112, 103771. [Google Scholar] [CrossRef]
- Dellepiane, E.; Pera, F.; Zunino, P.; Mugno, M.G.; Pesce, P.; Menini, M. Oral Health-Related Quality of Life and Full-Arch Immediate Loading Rehabilitation: An Evaluation of Preoperative, Intermediate, and Posttreatment Assessments of Patients Using a Modification of the OHIP Questionnaire. J. Oral Implantol. 2020, 46, 540–549. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, Y.; Weir, M.D.; Xu, H.H.K.; Bai, Y.; Melo, M.A.S. Current Insights into the Modulation of Oral Bacterial Degradation of Dental Polymeric Restorative Materials. Materials 2017, 10, 507. [Google Scholar] [CrossRef]
- Hajdu, A.I.; Dumitrescu, R.; Balean, O.; Lalescu, D.V.; Buzatu, B.L.R.; Bolchis, V.; Floare, L.; Utu, D.; Jumanca, D.; Galuscan, A. Enhancing Esthetics in Direct Dental Resin Composite: Investigating Surface Roughness and Color Stability. J. Funct. Biomater. 2024, 15, 208. [Google Scholar] [CrossRef] [PubMed]
- Schulze, K.A.; Marshall, S.J.; A Gansky, S.; Marshall, G.W. Color stability and hardness in dental composites after accelerated aging. Dent. Mater. 2003, 19, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Burrow, M.F.; Tyas, M. Influence of food simulating solutions and surface finish on susceptibility to staining of aesthetic restorative materials. J. Dent. 2005, 33, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Poggio, C.; Vialba, L.; Berardengo, A.; Federico, R.; Colombo, M.; Beltrami, R.; Scribante, A. Color Stability of New Esthetic Restorative Materials: A Spectrophotometric Analysis. J. Funct. Biomater. 2017, 8, 26. [Google Scholar] [CrossRef]
- Dimitrova, M.; Chuchulska, B.; Zlatev, S.; Kazakova, R. Colour Stability of 3D-Printed and Prefabricated Denture Teeth After Immersion in Different Colouring Agents—An In Vitro Study. Polymers 2022, 14, 3125. [Google Scholar] [CrossRef]
- Alkhadim, Y.K.; Hulbah, M.J.; Nassar, H.M. Color Shift, Color Stability, and Post-Polishing Surface Roughness of Esthetic Resin Composites. Materials 2020, 13, 1376. [Google Scholar] [CrossRef]
- Bahbishi, N.; Mzain, W.; Badeeb, B.; Nassar, H.M. Color Stability and Micro-Hardness of Bulk-Fill Composite Materials After Exposure to Common Beverages. Materials 2020, 13, 787. [Google Scholar] [CrossRef]
- Ilie, N.; Ionescu, A.C.; Huth, K.C.; Moldovan, M. Light transmission characteristics and cytotoxicity within a dental composite color palette. Materials 2023, 16, 3773. [Google Scholar] [CrossRef]
- Paolone, G.; Mazzitelli, C.; Boggio, F.; Breschi, L.; Vichi, A.; Gherlone, E.; Cantatore, G. Effect of Different Artificial Staining Procedures on the Color Stability and Translucency of a Nano-Hybrid Resin-Based Composite. Materials 2023, 16, 2336. [Google Scholar] [CrossRef]
- Paulraj, J.; Maiti, S.; Shanmugam, R. Comparative analysis of color stability and its impact on artificial aging: An in vitro study of bioactive chitosan, titanium, zirconia, and hydroxyapatite nanoparticle-reinforced glass ionomer cement compared with conventional glass ionomer cement. Cureus 2024, 16, e54517. [Google Scholar] [CrossRef]
- Hajdu, A.I.; Dumitrescu, R.; Balean, O.; Jumanca, D.; Sava-Rosianu, R.; Floare, L.; Bolchis, V.; Vlase, T.; Galuscan, A. Microscopic and Color Changes in Direct Dental Restorative Composite Resins upon Immersion in Beverages: Characterization by Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS). Biomedicines 2024, 12, 1740. [Google Scholar] [CrossRef]
- Habib, E.; Wang, R.; Wang, Y.; Zhu, M.; Zhu, X.X. Inorganic Fillers for Dental Resin Composites: Present and Future. ACS Biomater. Sci. Eng. 2016, 2, 1–11. [Google Scholar]
- Mansoor, A.; Khurshid, Z.; Khan, M.T.; Mansoor, E.; Butt, F.A.; Jamal, A.; Palma, P.J. Medical and Dental Applications of Titania Nanoparticles: An Overview. Nanomaterials 2022, 12, 3670. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, M.; Dudea, D.; Cuc, S.; Sarosi, C.; Prodan, D.; Petean, I.; Furtos, G.; Ionescu, A.; Ilie, N. Chemical and Structural Assessment of New Dental Composites with Graphene Exposed to Staining Agents. J. Funct. Biomater. 2023, 14, 163. [Google Scholar] [CrossRef]
- Raorane, D.V.; Chaughule, R.S.; Pednekar, S.R.; Lokur, A. Experimental synthesis of size-controlled TiO2 nanofillers and their possible use as composites in restorative dentistry. Saudi Dent. J. 2019, 31, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zafar, M.; Qasim, S.; Shahab, S.; Naseem, M.; AbuReqaiba, A. Advances in nanotechnology for restorative dentistry. Materials 2015, 8, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Samiei, M.; Janani, M.; Asl-Aminabadi, N.; Ghasemi, N.; Divband, B.; Shirazi, S.; Kafili, K. Effect of the Titanium dioxide (TiO2) nanoparticles on the selected physical properties of mineral trioxide aggregate. J. Clin. Exp. Dent. 2017, 9, 191–195. [Google Scholar]
- Gu, X.; Yang, L.; Yang, D.; Gao, Y.; Duan, X.; Zhu, X.; Yuan, H.; Li, J. Esthetic Improvements of Postorthodontic White-Spot Lesions Treated with Resin Infiltration and Microabrasion: A Split-Mouth, Randomized Clinical Trial. Angle Orthod. 2019, 89, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Campus, G.; Cocco, F.; Wierichs, R.J.; Wolf, T.G.; Salerno, C.; Arghittu, A.; Dettori, M.; Cagetti, M.G. Effects of Hydroxyapatite-Containing Toothpastes on Some Caries-Related Variables: A Randomised Clinical Trial. Int. Dent. J. 2024, 74, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Novozhilova, N.; Mun, A.; Polyakova, M.; Mikheikina, A.; Zaytsev, A.; Babina, K. Color Change and Color Stability of White Spot Lesions Treated with Resin Infiltration, Microabrasion, or Nano-Hydroxyapatite Remineralization: An In Vitro Study. Dent. J. 2025, 13, 112. [Google Scholar] [CrossRef]
- Yadav, R.; Lee, H.; Lee, J.H.; Singh, J.K.; Lee, H.H. A comprehensive review: Physical, mechanical, and tribological characterization of dental resin composite materials. Tribol. Int. 2023, 179, 108102. [Google Scholar] [CrossRef]
- Awdaljan, M.W.; Roque, J.C.; Choi, J.; Rondón, L.F. Introducing a novel approach to dental color reproduction using AI technology. J. Esthet. Restor. Dent. 2024, 36, 1623–1637. [Google Scholar] [CrossRef]
- Menini, M.; Rivolta, L.; Manauta, J.; Nuvina, M.; Kovacs-Vajna, Z.M.; Pesce, P. Dental Color-Matching Ability: Comparison between Visual Determination and Technology. Dent. J. 2024, 12, 284. [Google Scholar] [CrossRef]
- Fonseca, V.; Neves, C.B.; Portugal, J.; Anes, V.; Chasqueira, F.; Roque, J.C. Color Evaluation of Pre-Shaded Monolithic Zirconia Restorations on Different Substrates and Resin Cements. Appl. Sci. 2025, 15, 4160. [Google Scholar] [CrossRef]
- Rizzi, A.; Bonanomi, C.; Brazzoli, S.; Cerutti, A.; Kovacs-Vajna, Z.M. Assessing appearance in human dental colour space. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2018, 6, 59–67. [Google Scholar]
- Paravina, R.D.; Perez, M.M.; Ghinea, R. Acceptability and perceptibility thresholds in dentistry: A comprehensive review of clinical and research applications. J. Esthet. Restor. Dent. 2019, 31, 103–112. [Google Scholar] [CrossRef]
- Douglas, R.D.; Steinhauer, T.J.; Wee, A.G. Intraoral determination of the tolerance of dentists for perceptibility and acceptability of shade mismatch. J. Prosthet. Dent. 2007, 97, 200–208. [Google Scholar] [CrossRef]
- Gale, M.S.; Darvell, B.W. Thermal cycling procedures for laboratory testing of dental restorations. J. Dent. 1999, 27, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Bettencourt, A.; Madeira, A.; Nepomuceno, L.; Portugal, J.; Neves, C.B. Surface properties after Chemical Aging of chlorhexidine delivery systems based on acrylic resin. Rev. Port. Estomatol. Med. Dent. Cir. Maxilofac. 2019, 60, 155–162. [Google Scholar] [CrossRef]
- Preetha, A.; Banerjee, R. Comparison of artificial saliva substitutes. Trends Biomater. Artif. Organs 2005, 18, 178–186. [Google Scholar]
- Ghinea, R.; Pérez, M.M.; Herrera, L.J.; Rivas, M.J.; Yebra, A.; Paravina, R.D. Color difference thresholds in dental ceramics. J. Dent. 2010, 38 (Suppl. 2), e57–e64. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.D.; Pop, L.C.; Benea, H.-R.-C.; Tomoaia, G.; Racz, C.-P.; Mocanu, A.; Dobrota, C.-T.; Balint, R.; Soritau, O.; Tomoaia-Cotisel, M. Remineralization Induced by Biomimetic Hydroxyapatite Toothpastes on Human Enamel. Biomimetics 2023, 8, 450. [Google Scholar] [CrossRef]
- O’Hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The use of hydroxyapatite toothpaste to prevent dental caries. Odontology 2022, 110, 223–230. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Iafisco, M. Characterization of a toothpaste containing bioactive hydroxyapatites and In Vitro evaluation of its efficacy to remineralize enamel and to occlude dentinal tubules. Materials 2020, 13, 2928. [Google Scholar] [CrossRef]
- Amaechi, B.T.; AbdulAzees, P.A.; Alshareif, D.O.; Shehata, M.A.; Lima, P.P.C.S.; Abdollahi, A.; Kalkhorani, P.S.; Evans, V. Comparative efficacy of a hydroxyapatite and a fluoride toothpaste for prevention and remineralization of dental caries in children. BDJ Open 2019, 5, 18. [Google Scholar] [CrossRef]
- Limeback, H.; Meyer, F.; Enax, J. Tooth whitening with hydroxyapatite: A systematic review. Dent. J. 2023, 11, 50. [Google Scholar] [CrossRef]
- Sarembe, S.; Enax, J.; Morawietz, M.; Kiesow, A.; Meyer, F. In vitro whitening effect of a hydroxyapatite-based oral care gel. Eur. J. Dent. 2020, 14, 335–341. [Google Scholar] [CrossRef]
- Epple, M.; Meyer, F.; Enax, J. A critical review of modern concepts for teeth whitening. Dent. J. 2019, 7, 79. [Google Scholar] [CrossRef]
- Hojabri, N.; Kaisarly, D.; Kunzelmann, K.H. Adhesion and whitening effects of P11-4 self-assembling peptide and HAP suspension on bovine enamel. Clin. Oral Investig. 2021, 25, 3237–3247. [Google Scholar] [CrossRef] [PubMed]
- Garibay-Alvarado, J.A.; Garcia-Zamarron, D.J.; Silva-Holguín, P.N.; Donohue-Cornejo, A.; Cuevas-González, J.C.; Espinosa-Cristóbal, L.F.; Ruíz-Baltazar, Á.d.J.; Reyes-López, S.Y. Polymer-Based Hydroxyapatite–Silver Composite Resin with Enhanced Antibacterial Activity for Dental Applications. Polymers 2024, 16, 2017. [Google Scholar] [CrossRef]
- Hashem, R.; Mohsen, C.A.; Abu-Eittah, M.R. Effect of silver nanoparticles and silver hydroxyapatite nanoparticles on color and fracture strength of dental ceramic. Mater. Sci. Med. 2015, 61, 2–7. [Google Scholar]
- Moheet, I.A.; Luddin, N.; Ab Rahman, I.; Masudi, S.A.; Kannan, T.P.; Abd Ghani, N.R. Evaluation of fluoride ion release and color stability of nano-hydroxyapatite-silica added glass ionomer cement for dental application. Fluoride 2020, 53, 100–111. [Google Scholar]
- Aldhuwayhi, S.D. Evaluation of fracture toughness, color stability, and sorption solubility of a fabricated novel glass ionomer nano zirconia-silica-hydroxyapatite hybrid composite material. Int. J. Polym. Sci. 2021, 1, 6626712. [Google Scholar] [CrossRef]
- Kula, Z.; Neves, C.B.; Dąbrowska, K.; Roque, J.C.; Klimek, L. Evaluation of the Impact of Various Functional Fillers on Key Properties of Dental Composites. Appl. Sci. 2025, 15, 4961. [Google Scholar] [CrossRef]
- Yago, R.; Kawamoto, C.; Islam, R.; Kaneko, H.; Yamauti, M.; Otsuki, M.; Sano, H.; Tomokiyo, A. Prevention of Tooth Discoloration Using Fluoride Varnish Immediately After Bleaching. J. Funct. Biomater. 2025, 16, 245. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Y.; Bai, Y.; Zhang, Z.; Wu, Q. Study on the mechanical and aging properties of an antibacterial composite resin loaded with fluoride-doped nano-zirconia fillers. Front. Bioeng. Biotechnol. 2024, 12, 1397459. [Google Scholar] [CrossRef]
- Fernandez, C.C.; Sokolonski, A.R.; Fonseca, M.S.; Stanisic, D.; Araújo, D.B.; Azevedo, V.; Portela, R.D.; Tasic, L. Applications of Silver Nanoparticles in Dentistry: Advances and Technological Innovation. Int. J. Mol. Sci. 2021, 22, 2485. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pennell, K.G.; Hurt, R.H. Kinetics and Mechanisms of Nanosilver Oxysulfidation. Environ. Sci. Technol. 2011, 45, 7345–7353. [Google Scholar] [CrossRef]
- Xu, G.Y.; Yin, I.X.; Zhao, I.S.; Lung, C.Y.; Lo, E.C.; Chu, C.H. Minimizing tooth discoloration caused by topical silver diamine fluoride application: A systematic review. J. Dent. 2024, 150, 105353. [Google Scholar] [CrossRef] [PubMed]
- Abuljadayel, R.; Mushayt, A.; Al Mutairi, T.; Sajini, S.; Almutairi, T. Evaluation of Bioactive Restorative Materials’ Color Stability: Effect of Immersion Media and Thermocycling. Cureus 2023, 15, e43038. [Google Scholar] [CrossRef] [PubMed]
- ISO/TR 28642:2016; Dentistry—Guidance on Colour Measurement. International Organization for Standardization: Geneva, Switzerland, 2016.
- Ruiz-López, J.; Melgosa, M.; Ghinea, R.; Tejada-Casado, M.; Pop-Ciutrila, I.-S.; Pérez, M.M. Effect of White Light-Emitting Diode Illuminants Recommended by the CIE on Colors of Dental Ceramic Materials. Appl. Sci. 2023, 13, 1518. [Google Scholar] [CrossRef]
- Salas, M.; Lucena, C.; Herrera, L.J.; Yebra, A.; Della Bona, A.; Pérez, M.M. Translucency thresholds for dental materials. Dent. Mater. 2018, 34, 1168–1174. [Google Scholar] [CrossRef]
- Fidan, M. Accelerated Aging Effects on Color Change, Translucency Parameter, and Surface Hardness of Resin Composites. BioMed Res. Int. 2022, 2022, 6468281. [Google Scholar] [CrossRef]
- Ardu, S.; Gutemberg, D.; Krejci, I.; Feilzer, A.J.; Di Bella, E.; Dietschi, D. Influence of Water Sorption on Resin Composite Color and Color Variation Amongst Various Composite Brands with Identical Shade Code: An In Vitro Evaluation. J. Dent. 2011, 39 (Suppl. 1), e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Alrefaie, T.; Abdou, A.; Almasabi, W.; Qi, F.; Nakamoto, A.; Nakajima, M.; Otsuki, M.; Shimada, Y. Effect of Water Storage and Bleaching on Light Transmission Properties and Translucency of Nanofilled Flowable Composite. Materials 2023, 16, 10. [Google Scholar] [CrossRef]
- ElEmbaby, A.E.; Nassar, A.E.; Elawsya, M.E. Impact of silica nanoparticles incorporation on the properties of resin infiltration: An in vitro study. BMC Oral Health 2024, 24, 1484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalachandra, S. Influence of fillers on the water sorption of composites. Dent Mater. 1989, 5, 283–288. [Google Scholar] [CrossRef]
- Santos, C.; Ferracane, J.L. Water absorption characteristics of dental composites: Influence of filler type and silanization. J. Biomed. Mater. Res. 2002, 60, 620–626. [Google Scholar] [CrossRef]
- Berger, S.B.; Costa, R.M. Characterization of water sorption, solubility and filler content in resin composites. Braz. Dent. J. 2009, 20, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Iazzetti, G.; Burgess, J.O.; Gardiner, D.; Ripps, A. Color stability of fluoride-containing restorative materials. Oper. Dent. 2000, 25, 520–525. [Google Scholar] [PubMed]
- Salles de Oliveira, D.C.R.; Ayres, A.P.A.; Ferracane, J.L.; Sinhoreti, M.A.C.; Rocha, M.G.; Giannini, M.; Rontani, R.M.P. Effect of Different In Vitro Aging Methods on Color Stability of a Dental Resin-Based Composite Using CIELAB and CIEDE2000 Color-Difference Formulas. J. Esthet. Restor. Dent. 2015, 27, 322–330. [Google Scholar] [CrossRef]


| Sample Group | Composite Type | Resin Type | Filler Content | Aging Processes | ||
|---|---|---|---|---|---|---|
| HAp [wt%] | F [wt%] | Ag [wt%] | ||||
| 1 | ArconaFlow original | Bis-GMA | - | - | - | Thermal (T) + Chemical (C) + Combination (TC) |
| 2 | ArconaFlow modified | Bis-GMA | 2 | 0.2 | 1 | Thermal (T) + Chemical (C) + Combination (TC) |
| After Aging Test Values | Thermal (T) Mean (SD) Median (IR) | Chemical (C) Mean (SD) Median (IR) | Thermal & Chemical (T + C) Mean (SD) Median (IR) | |||
|---|---|---|---|---|---|---|
| Group | ΔE00 | ΔE94 | ΔE00 | ΔE94 | ΔE00 | ΔE94 |
| 1-Arkona Original No Fillers | 2.6 (1.14) 2.4 (1.50) | 2.4 (1.04) 2.5 (1.80) | 3.7 (1.91) 1.5 (4.00) | 3.6 (1.91) 4.0 (3.30) | 2.9 (0.93) 2.8 (1.10) | 3.0 (1.00) 2.9 (0.90) |
| 2-Arkona Modified With Fillers | 2.8 (0.56) 2.7 (0.70) | 3.0 (0.58) 2.9 (0.67) | 1.5 (0.32) 1.5 (0.60) | 1.4 (0.36) 1.3 (0.30) | 1.9 (0.62) 1.8 (0.80) | 2.1 (0.67) 2.0 (0.90) |
| After | Thermal (T) | Chemical (C) | Thermal + Chemical (T + C) | |||
|---|---|---|---|---|---|---|
| Aging Test | ||||||
| Values | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Group | ΔE00 | ΔE00 | ΔE00 | |||
| 1-Arkona Original | 2.6 (1.14) | 3.7 (1.91) | 2.9 (0.93) | |||
| No Fillers | ||||||
| 2-Arkona Modified | 2.8 (0.56) | 1.5 (0.32) | 1.9 (0.62) | |||
| With Fillers | ||||||
| in vitro | ΔE00 ≈ 1 | ΔE00 ≈ 2 | ΔE00 ≈ 1 | ΔE00 ≈ 2 | ΔE00 ≈ 1 | ΔE00 ≈ 2 |
| in vivo | ΔE00 ≈ 1.2 | ΔE00 ≈ 2.7 | ΔE00 ≈ 1.2 | ΔE00 ≈ 2.7 | ΔE00 ≈ 1.2 | ΔE00 ≈ 2.7 |
| Tresholds | perceptability | acceptability | perceptability | acceptability | perceptability | acceptability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kula, Z.; Neves, C.B.; Bettencourt, A.; Oliveira, S.; Roque, J.C. Color Stability of a Composite Containing Hydroxyapatite, Fluorine, and Silver Fillers After Artificial Aging. Appl. Sci. 2025, 15, 12426. https://doi.org/10.3390/app152312426
Kula Z, Neves CB, Bettencourt A, Oliveira S, Roque JC. Color Stability of a Composite Containing Hydroxyapatite, Fluorine, and Silver Fillers After Artificial Aging. Applied Sciences. 2025; 15(23):12426. https://doi.org/10.3390/app152312426
Chicago/Turabian StyleKula, Zofia, Cristina Bettencourt Neves, Ana Bettencourt, Sara Oliveira, and João Carlos Roque. 2025. "Color Stability of a Composite Containing Hydroxyapatite, Fluorine, and Silver Fillers After Artificial Aging" Applied Sciences 15, no. 23: 12426. https://doi.org/10.3390/app152312426
APA StyleKula, Z., Neves, C. B., Bettencourt, A., Oliveira, S., & Roque, J. C. (2025). Color Stability of a Composite Containing Hydroxyapatite, Fluorine, and Silver Fillers After Artificial Aging. Applied Sciences, 15(23), 12426. https://doi.org/10.3390/app152312426





