L-Tryptophan Adsorbed on Au and Ag Nanostructured Substrates: A SERS Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Ag and Au Colloids
2.3. Instrumentation
2.4. RS Experiments
2.5. Computational Method
3. Results and Discussion
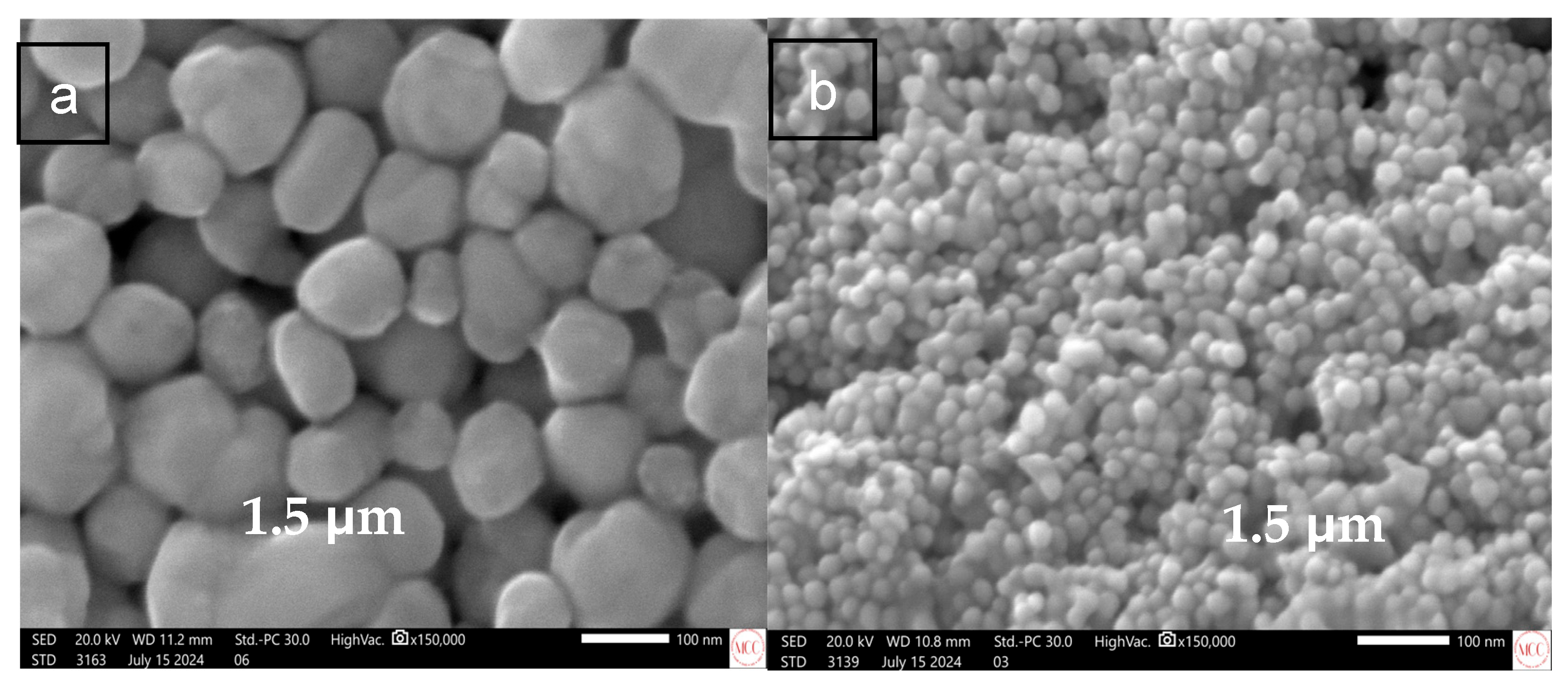
3.1. Nanoparticles Characterization
- τ is the mean size of the ordered (crystalline) domains, which may be smaller or equal to the grain size.
- Κ is a dimensionless shape factor, with a value close to unity. The shape factor has a typical value of about 0.9, but varies with the actual shape of the crystallite.
- λ is the X-ray wavelength.
- β is the line broadening at half the maximum intensity (FWHM), after subtracting the instrumental line broadening, in radians. This quantity is also sometimes denoted as Δ(2θ).
- θ = is the Bragg angle.
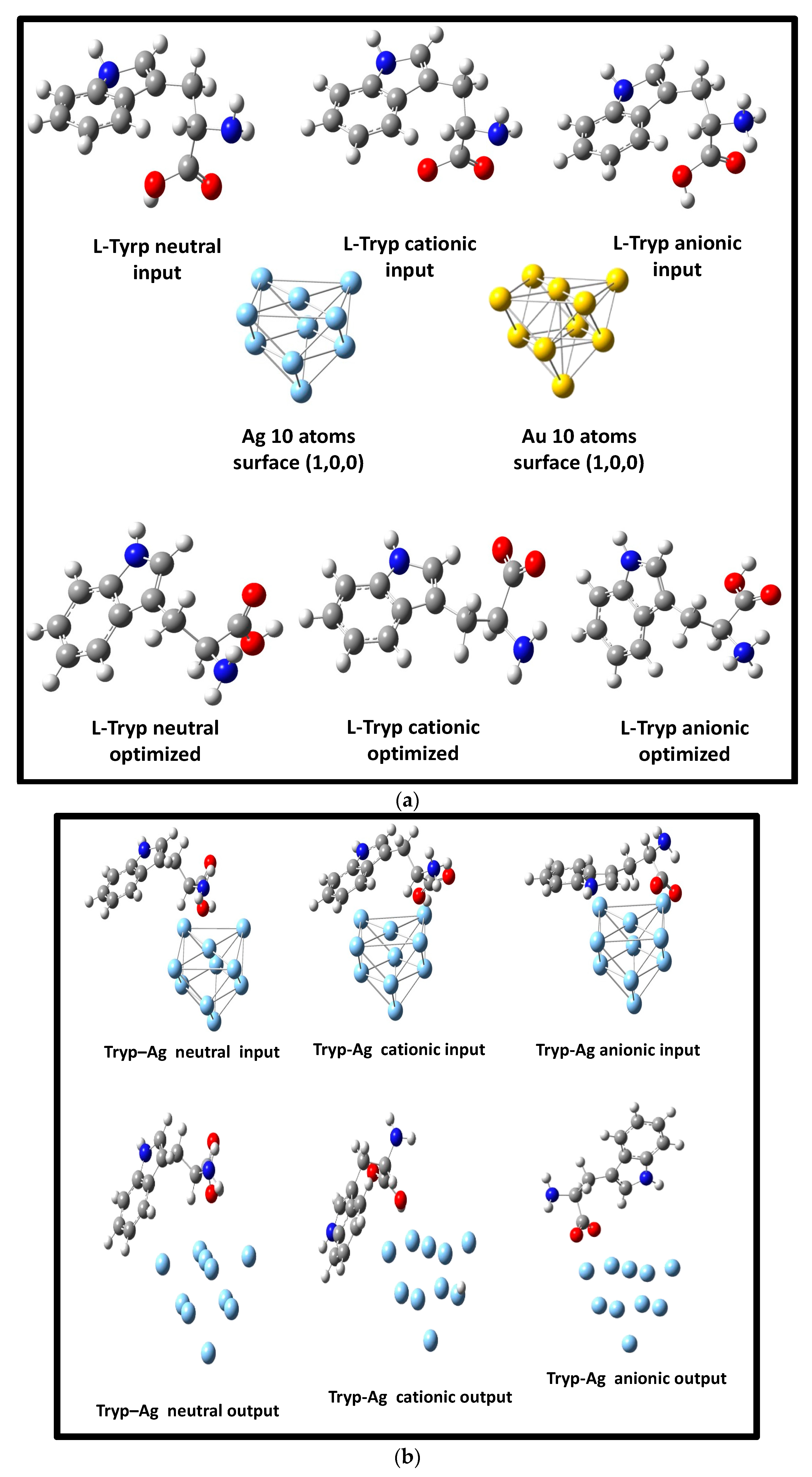
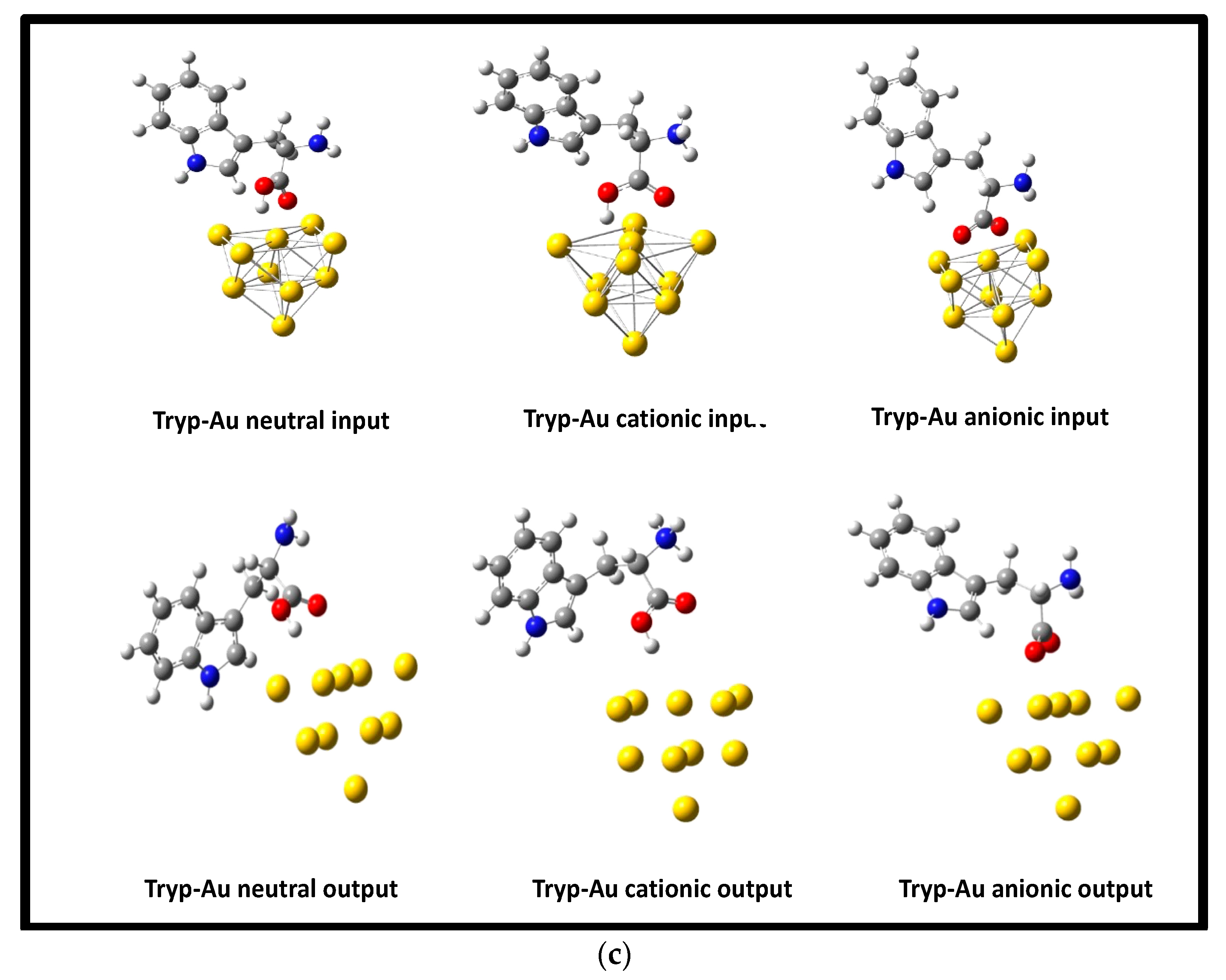
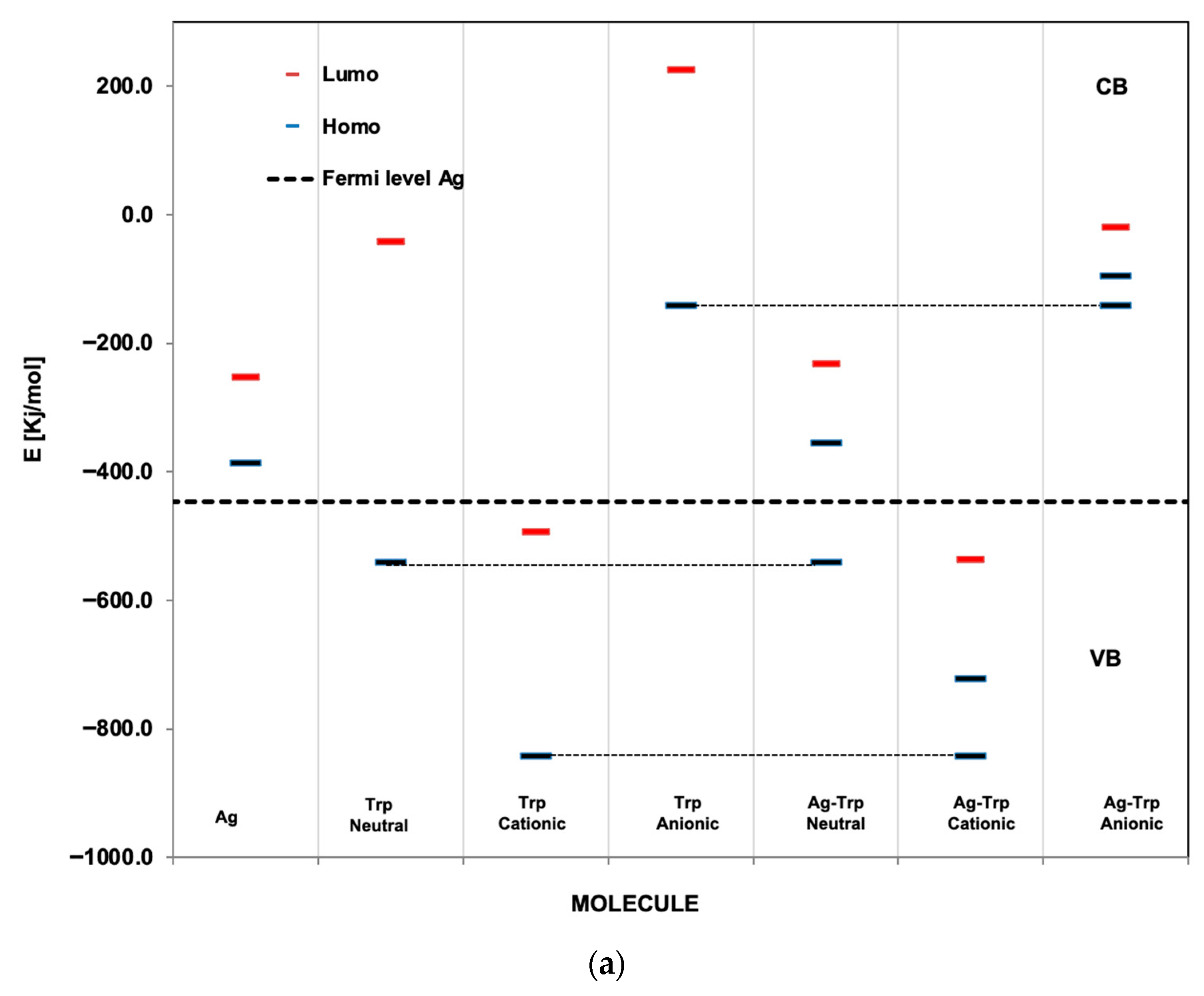
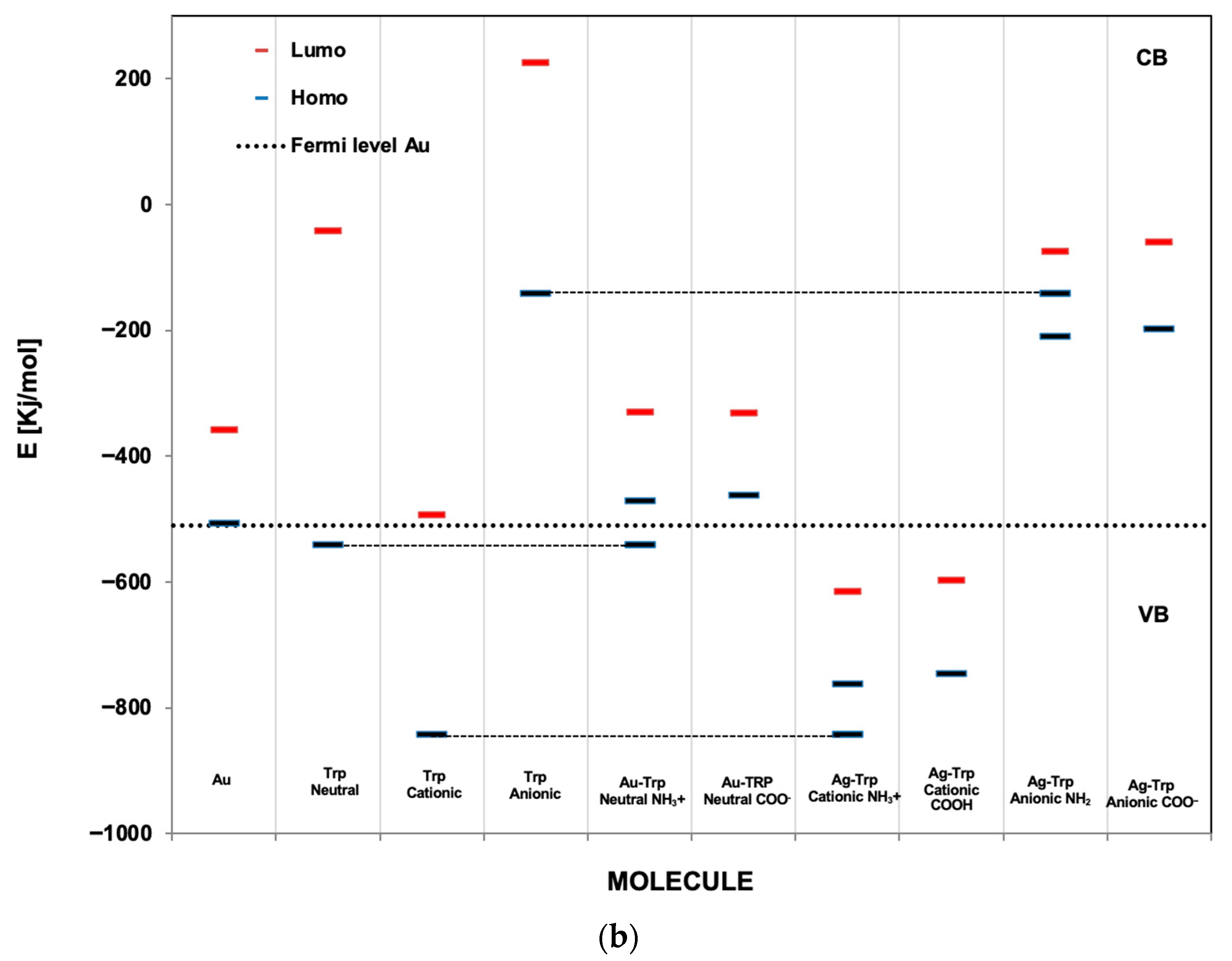
3.2. Optimization of Geometries of L-Tryp on NP Substrate Models
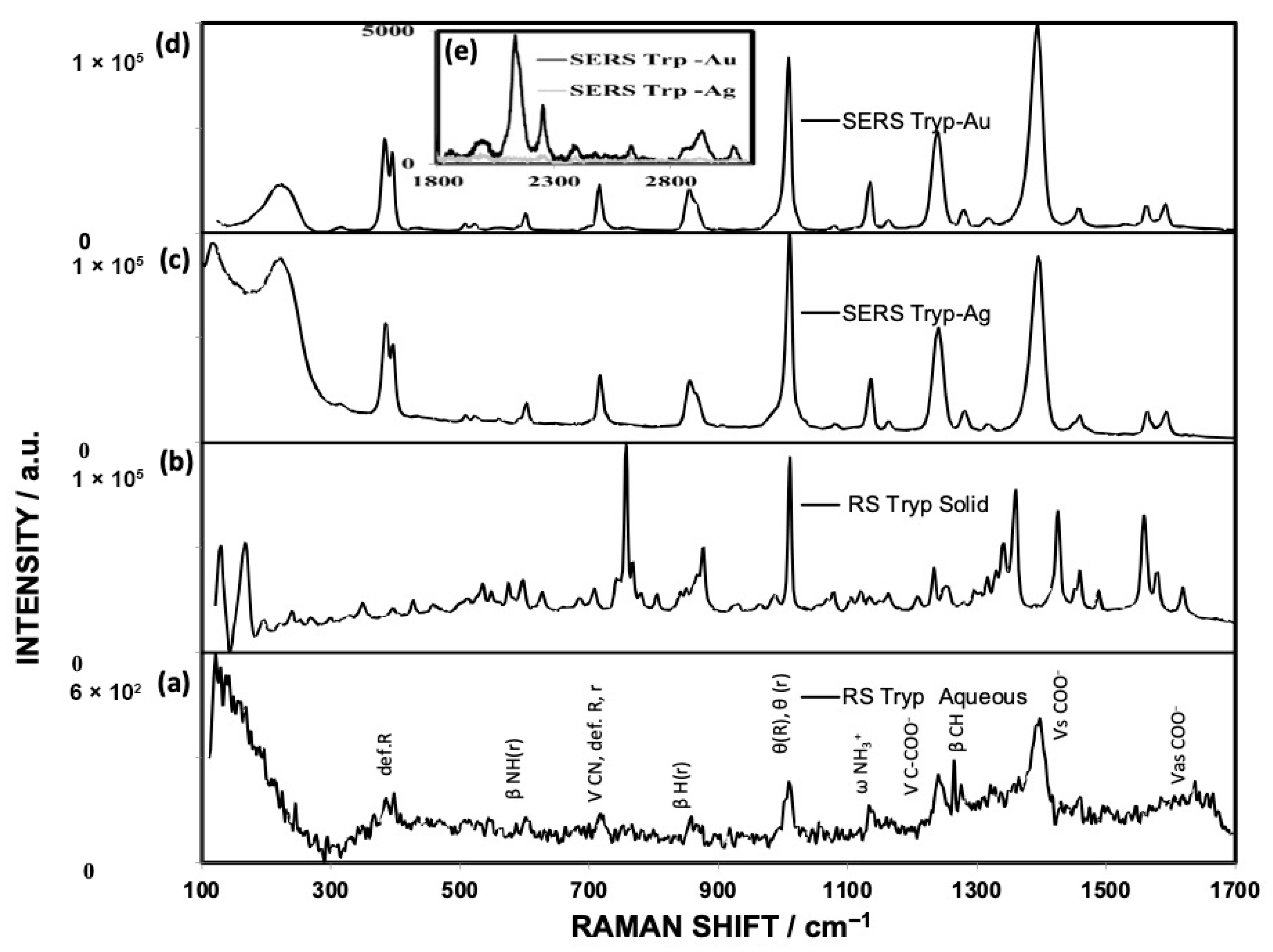
3.3. RS Spectra
3.4. Theoretical Discussion
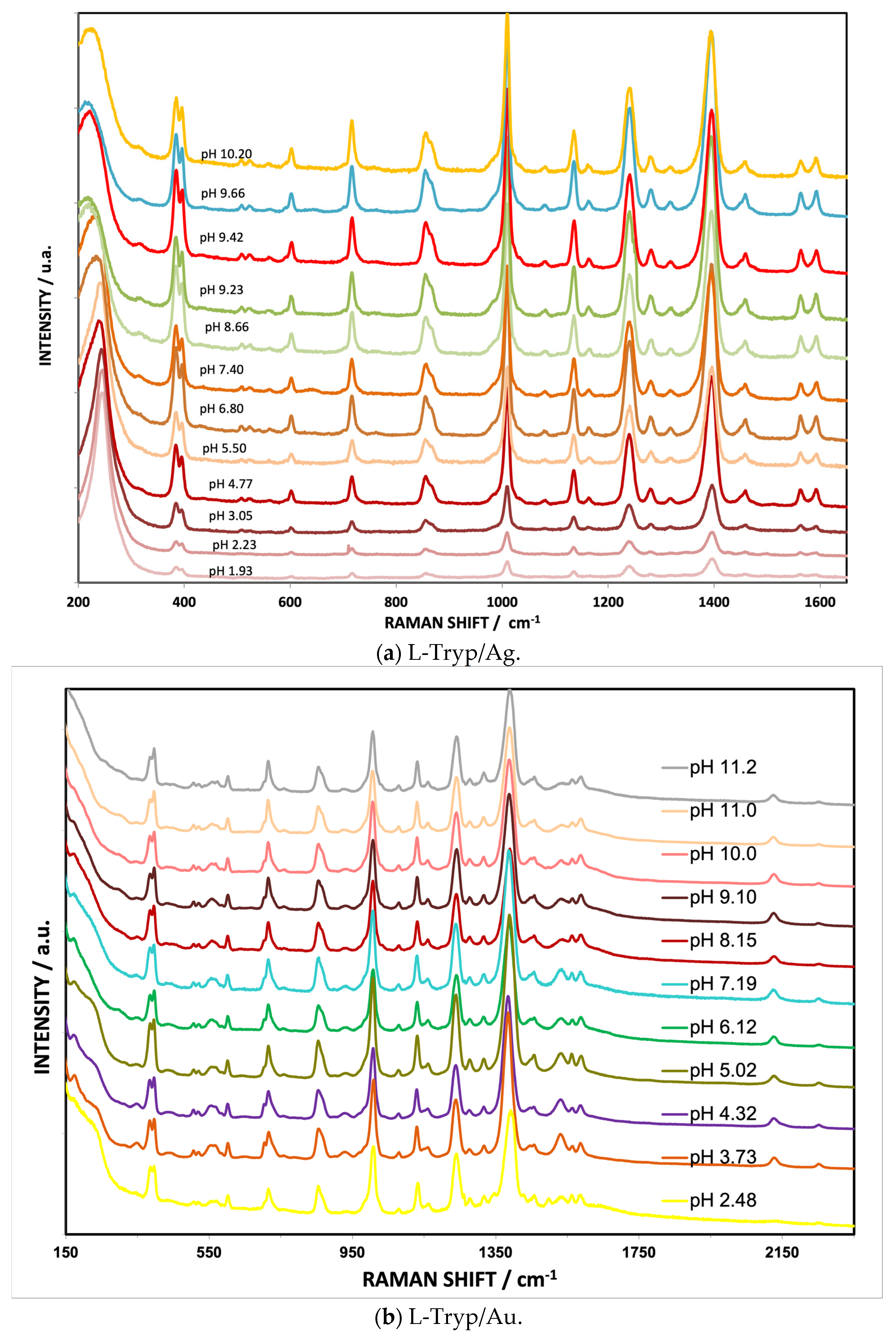
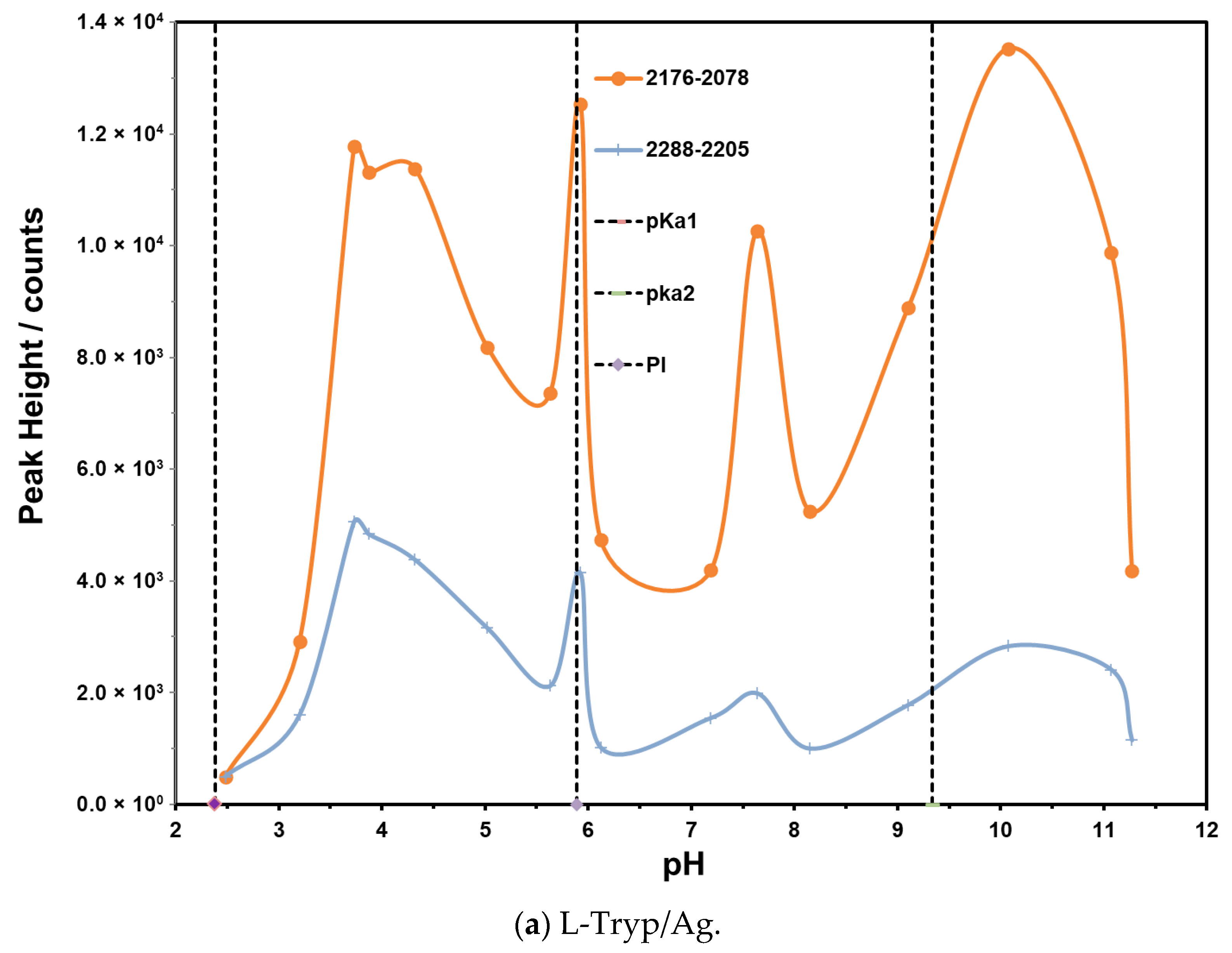
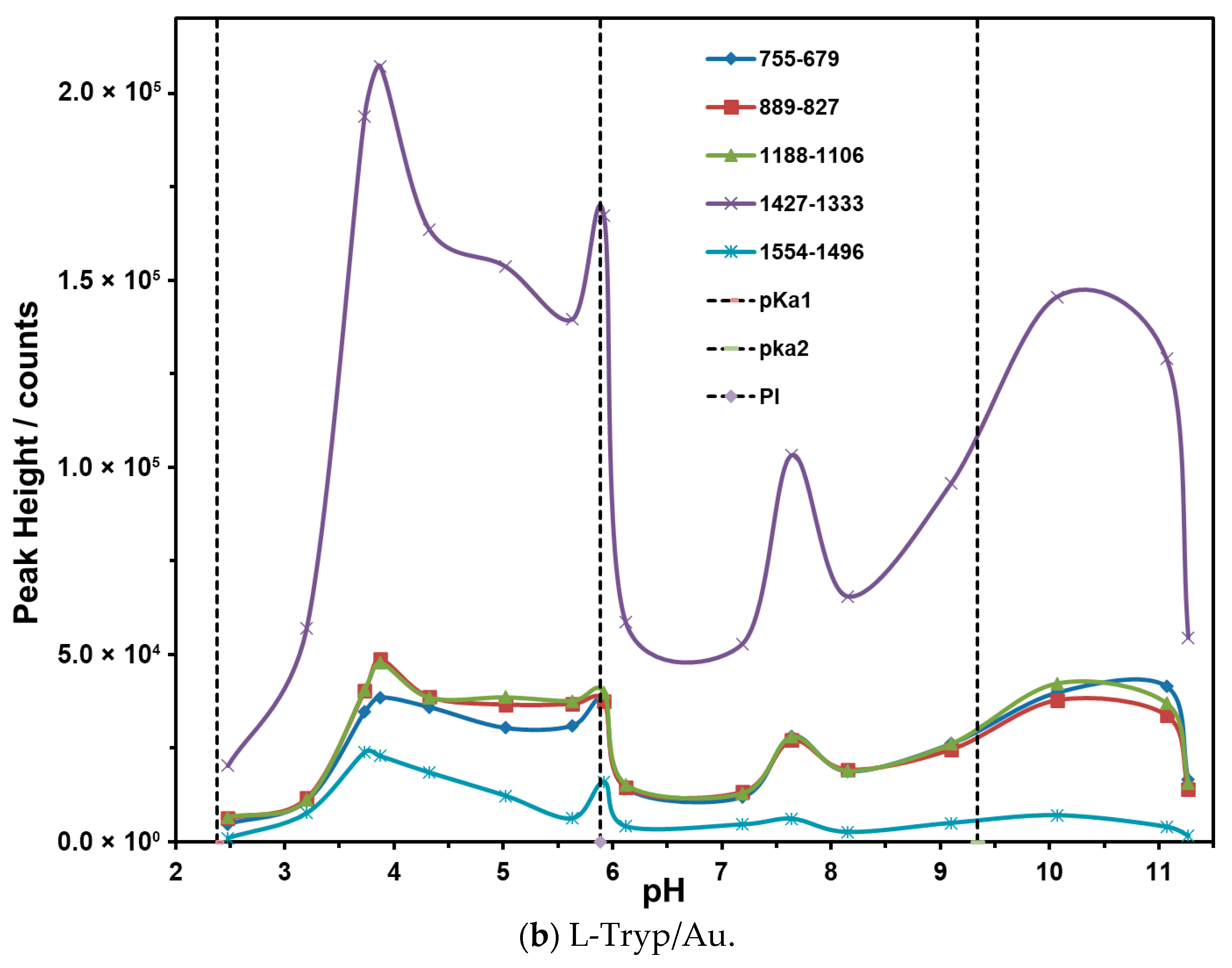
3.5. Effect of pH on Vibrational Bands of L-Tryp on Ag and Au Colloids
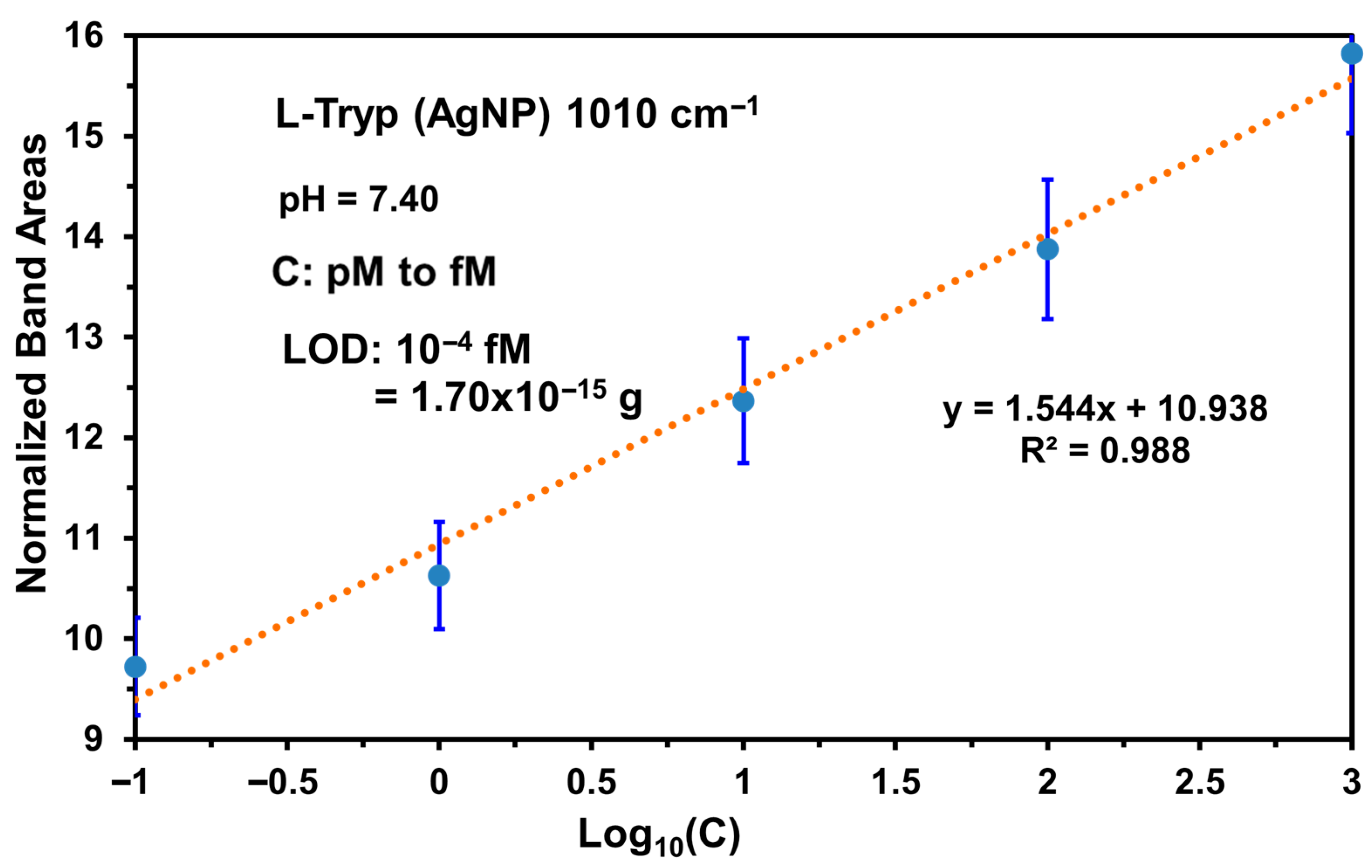
3.6. Concentration Profile: L-Tryp on AgNP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| L-Tryp | L-Tryptophan. |
| RS | Raman Scattering and Normal Raman Scattering. |
| SERS | Surface-Enhanced Raman Scattering. |
| Ag | Silver. |
| NP | Nanoparticles. |
| AgNP | Silver Nanoparticles. |
| Au | Gold. |
| AuNP | Gold Nanoparticles. |
| DFT | Density Functional Theory. |
| UV-Vis | Ultraviolet–visible. |
References
- Aliaga, A.E.; Osorio, I.; Leyton, P.; Garrido, C.; Caniulefa, C.; Diaz, G.; Celis, F.; Diaz, G.; Clavijo, E.; Gomez, S.; et al. Surface-enhanced Raman scattering study of L-tryptophan. Raman Spectrosc. 2009, 40, 164–169. [Google Scholar] [CrossRef]
- Kandakkathara, A.; Utkin, I.; Fedosejevs, R. Surface-enhanced raman scattering (SERS) detection of low concentrations of tryptophan amino acid in silver colloid. App. Spec. 2011, 65, 507–513. [Google Scholar] [CrossRef]
- Dechan, M.F.; Lu, D.; You, R.; Chem, C.; Lu, Y.; Wu, Y.; Huiying, S.; Feng, S. Highly sensitive detection of tryptophan (Trp) in serum based on diazo-reaction coupling with Surface-Enhanced Raman Scattering and colorimetric assay. Anal. Chim. Acta 2020, 1119, 52–59. [Google Scholar]
- Zaheer, Z.; Ahmad-Malika, M.; Al-Nowaiser, F.M.; Khana, Z. Preparation of silver nanoparticles using tryptophan and its formation mechanism. Colloids Surf. B Biointerfaces 2010, 81, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Section 3.1, Proteins Are Built from a Repertoire of 20 Amino Acids. In Biochemistry, 5th ed.; W.H. Freeman: New York, NY, USA, 2002. Available online: http://www.ncbi.nlm.nih.gov/books/NBK22379/ (accessed on 1 August 2025).
- Fukuwatari, T.; Shibata, K. Nutritional Aspect of Tryptophan Metabolism. Int. J. Tryptophan Res. 2013, 21, 3–8. [Google Scholar] [CrossRef]
- Badawy, A.; Inter, J. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Tryp Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Effects and Side Effects Associated with the Non-Nutritional Use of Tryptophan by Humans. J. Nutr. 2012, 142, 2236S–2244S. [Google Scholar] [CrossRef] [PubMed]
- Contreiras, I.B.; Toscano, A.E.; Cabral, D.; Barreta, M.S.; Manhaes, R.; Bonfim, T.C.; Barreto, J.M. L-tryptophan administration and increase in cerebral serotonin levels: Systematic review. Eur. J. Pharmacol. 2018, 836, 129–135. [Google Scholar] [CrossRef]
- Powers, R.; Cilp-Hill, R.; Ludwig, M.; Smith, K.; Waugh, K.; Minter, R.; Tuttle, K.; Lewis, H.; Rachbinki, A.; Gransrath, R.; et al. Trisomy 21 activates the kynurenine pathway via increased dosage of interferon receptors. Nature Commu. 2019, 10, 4766. [Google Scholar] [CrossRef] [PubMed]
- Floc’h, N.; Otten, W.; Merlot, E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acid 2011, 41, 195–205. [Google Scholar] [CrossRef]
- Chung, K.T.; Gadupudi, G.S. Possible roles of excess tryptophan metabolites in cancer. Environ. Mol. Mutagen. 2011, 52, 81–104. [Google Scholar] [CrossRef]
- Ramirez, M.L.; Pacheco, L.C.; Barreto, M.A.; Hernández-Rivera, S.P. Enhanced Raman Detection using Spray-On Nanoparticles/Remote Sensed Raman Spectroscopy. In Nanoscience and Nanotechnology for Chemical and Biological Defense; Nagarajan, R., Zukas, W., Hatton, T.A., Lee, S., Eds.; ACS Symposium Series # 1016; Oxford University Press: New York, NY, USA, 2009; Chapter 10; pp. 131–140. [Google Scholar]
- Hernández-Rivera, S.P.; Briano, J.G.; de la Cruz-Montoya, E.; Pérez-Acosta, G.A.; Jeréz-Rozo, J.I. Enhanced Raman Scattering of Nitroexplosives on Metal Oxides and Nanoparticles of Ag/TiO2. In Nanoscience and Nanotechnology for Chemical and Biological Defense; Nagarajan, R., Zukas, W., Hatton, T.A., Lee, S., Eds.; ACS Symposium Series # 1016; Oxford University Press: New York, NY, USA, 2009; Chapter 16; pp. 205–216. [Google Scholar]
- Primera-Pedrozo, O.M.; Rodríguez, G.D.; Castellanos, J.; Felix-Rivera, H.; Resto, O.; Hernández-Rivera, S.P. Increasing surface-enhanced Raman spectroscopy effect of RNA and DNA components by changing the pH of silver colloidal suspensions. Spectrochim. Acta Part A 2012, 87, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Félix-Rivera, H.; González, R.; Rodríguez GDPrimera-Pedrozo, O.M.; Ríos-Velázquez, C.; Hernández-Rivera, S.P. Improving SERS Detection of Bacillus thuringiensis using Silver Nanoparticles Reduced with Hydroxylamine and with Citrate Capped Borohydride. Int. J. Spectrosc. 2011, 2011, 989504. [Google Scholar] [CrossRef]
- Oliveira, M.M.; Ugarte, D.; Zanchet, D.; Zarbin, A.J.G.J. Influence of synthetic parameters on the size, structure, and stability of dodecanethiol-stabilized silver nanoparticles. Colloid Interface Sci. 2005, 292, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Hugh, C.; Chena, Y. Raman scattering of L-tryptophan enhanced by surface plasmon of silver nanoparticles: Vibrational assignment and structural determination. J. Raman Spectrosc. 2009, 40, 150–156. [Google Scholar]
- Guiherme, B.; Sodre, L. Sant’Ana. Spectroquim. Acta P. A Mol. Biomol. Spectrosc. 2017, 190, 383–391. [Google Scholar]
- Ramanauskaite, L.; Snitka, V. Surface enhanced Raman spectroscopy of L-alanyl-L-tryptophan dipeptide adsorbed on Si substrate decorated with triangular silver nanoplates. Chem. Phys. Lett. 2015, 623, 46–50. [Google Scholar] [CrossRef]
- Chamoun-Emanuelli, A.M.; Primera-Pedrozo, O.M.; Barreto-Cabán, M.A.; Jerez-Rozo, J.I.; Hernández-Rivera, S.P. Enhanced Raman Scattering of TNT on Nanoparticles Substrates: Ag, Au, and Bimetallic Au/Ag Colloidal Suspensions. In Nanoscience and Nanotechnology for Chemical and Biological Defense; Nagarajan, R., Zukas, W., Hatton, T.A., Lee, S., Eds.; ACS Symposium Series # 1016; Oxford University Press: New York, NY, USA, 2009; Chapter 17; pp. 217–232. [Google Scholar]
- Pacheco-Londoño, L.C.; Aparicio-Bolaños, J.; Primera-Pedrozo, O.M.; Hernandez-Rivera, S.P. Growth of Ag, Au, Cu, and Pt Nanostructures on Surfaces by Micropatterned Laser Image Formations. Appl. Opt. 2011, 50, 4161–4169. [Google Scholar] [CrossRef]
- Primera-Pedrozo, O.M.; Chamoun-Emanuelli, A.N.; Medina-Ramos, W.; Hernández-Rivera, S.P. Surface Enhanced Raman Spectroscopy Gold Nanorods Substrates for Detection of 2,4,6-Trinitrotoluene and 3,5-Dinitro-4-methylbenzoic Acid Explosives. U.S. Patent 8,932,384 B1, 13 January 2015. [Google Scholar]
- Alvarez, R.; Arceo, E.; Goulet, P.; Garrido, J.; Aroca, R.J. Role of Nanoparticle Surface Charge in Surface-Enhanced Raman Scattering. Phys. Chem. B 2005, 109, 3787–3792. [Google Scholar] [CrossRef]
- Fierro Mercado, P.M.; Hernández-Rivera, S.P. Highly Sensitive Filter Paper Substrate for SERS Trace Explosives Detection. Int. J. Spec. 2012, 2012, 716527. [Google Scholar] [CrossRef]
- Fierro Mercado, P.M.; Rentería-Beleño, B.; Hernández-Rivera, S.P. Preparation of SERS-Active Substrates Using Thermal Inkjet Technology. Chem. Phys. Lett. 2012, 552, 108–113. [Google Scholar] [CrossRef]
- Murphy, C.J.; Gole, A.M.; Stone, J.W.; Sisco, P.N.; Alkilany, A.M.; Goldsmith, E.C.; Baxter, S.C. Gold Nanoparticles in Biology: Beyond Toxicity to Cellular Imaging. Acc. Chem. Res. 2008, 41, 1721–1730. [Google Scholar] [CrossRef]
- Fleger, Y.; Mastai, Y.; Rosenbluha, M.; Dressler, D.H. SERS as a probe for adsorbate orientation on silver nanoclusters. J. Raman Spectrosc. 2009, 40, 1572–1577. [Google Scholar] [CrossRef]
- Ahern, M.; Garrell, R.L. Surface-enhanced Raman spectroscopy of peptides: Preferential N-terminal adsorption on colloidal silver. J. Am. Chem. Soc. 1991, 113, 846. [Google Scholar]
- Lee, H.I.; Suh, S.W.; Kim, M.S. Raman spectroscopy of L-tryptophan-containing peptides adsorbed on a silver surface. J. Raman Spectrosc. 1988, 19, 491–495. [Google Scholar]
- Kim, S.K.; Kim, M.S.; Suh, S.W. Surface-enhanced Raman scattering (SERS) of aromatic amino acids and their glycyl dipeptides in silver sol. J. Raman Spectrosc. 1987, 18, 171. [Google Scholar]
- Kazanci, M.; Schulte, J.P.; Douglas, C.; Fratzl, P.; Pink, D.; Smith-Palmer, T. Tuning the Surface-Enhanced Raman Scattering Effect to Different Molecular Groups by Switching the Silver Colloid Solution pH. Appl. Spec. 2008, 63, 214–223. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 88, 3391. [Google Scholar] [CrossRef]
- Lisiecki, I.; Billoudet, F.; Pileni, M.P. Control of the Shape and the Size of Copper Metallic Particles. J. Phys. Chem. 1996, 100, 4160–4166. [Google Scholar] [CrossRef]
- Chamoun-Emanuelli, A.M.; Primera-Pedrozo, O.M.; Barreto-Caban, M.I.; Jerez-Rozo, J.; Hernandez-Rivera, S.P. Nanoscience and Nanotechnology for Chemical and Biological Defense; American Chemical Society: Washington, DC, USA, 2009; pp. 217–232. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian™ 16.0 and GaussView™ 5.0; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Suh, J.S.; Moskovits, M. Surface-enhanced Raman spectroscopy of amino acids and nucleotide bases adsorbed on silver. J. Am. Chem. Soc. 1986, 108, 4711. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Prentice-Hall Inc.: Hoboken, NJ, USA, 2001; pp. 96–102. ISBN 0-201-61091-4. [Google Scholar]
- Jenkins, R.; Snyder, R.L. Introduction to X-Ray Powder Diffractometry; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1996; pp. 89–91. ISBN 0-471-51339-3. [Google Scholar]
- Klug, H.P.; Alexander, L.E. X-Ray Diffraction Procedures, 2nd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1974; pp. 687–703. ISBN 978-0-471-49369-3. [Google Scholar]
- Warren, B.E. X-Ray Diffraction; Addison-Wesley Publishing Co.: Boston, MA, USA, 1969; pp. 251–254. ISBN 0-201-08524-0. [Google Scholar]
- Cao, X.; Fisher, G. Infrared Spectral, Structural, and Conformational Studies of Zwitterionic l-Tryptophan. J. Phys. Chem. B 1999, 103, 9995–10003. [Google Scholar] [CrossRef]
- Madzharova, F.; Heiner, Z.; Kneipp, J. Surface-Enhanced Hyper Raman Spectra of Aromatic Thiols on Gold and Silver Nanoparticles. J. Phys. Chem. C 2016, 124, 6233–6241. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.J.; Lee, C.; Choi, I.S.; Hwang, C.S.; Gong, M.S.; Kim, K.; Joo, S.W. Adsorption of 4-Biphenylisocyanide on Gold and Silver Nanoparticle Surfaces: Surface-Enhanced Raman Scattering Study. J. Phys. Chem. B 2002, 106, 7076. [Google Scholar] [CrossRef]
- Ru, E.; Blackie, E.; Meyer, M.; Etchegoin, P. Surface enhanced Raman scattering enhancement factors: A comprehensive study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Nibbering, E.T.; Elsaesser, T. Ultrafast Vibrational Dynamics of Hydrogen Bonds in the Condensed Phase. Chem. Rev. 2004, 104, 10. [Google Scholar] [CrossRef]
- Yadav, R.A.; Yogesha, M.; Yadav, B.; Sabthosh, C. Conformational and vibrational investigations of L-Tryptophan zwitterion. J. Molec. Struc. 2018, 1171, 867–879. [Google Scholar] [CrossRef]
- Tadjiki, S.; Montaño, M.D.; Assemi, S.; Barber, A.; Ranville, J.; Beckett, R. Measurement of the density of engineered silver nanoparticles using centrifugal FFF-TEM and single particle ICP-MS. Anal. Chem. 2017, 89, 6056–6064. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.; Aveyard, J.; Ferning, D. Determination of size and concentration of gold nanoparticles from UV−Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley: New York, NY, USA, 1984. [Google Scholar]
- Markovits, A.; Garcia-Hernandez, M.; Ricart, J.M.; Illas, F. Theoretical Study of Bonding of Carbon Trioxide and Carbonate on Pt(111): Relevance to the Interpretation of “in Situ” Vibrational Spectroscopy. J. Phys. Chem. B 1999, 103, 509. [Google Scholar]
- Suh, J.S.; Kim, J. Three distinct geometries of surface-adsorbed carboxylate groups. J. Raman Spectrosc. 1998, 29, 143. [Google Scholar] [CrossRef]
- Hernández, B.; Pflüger, F.; Adenier, A.; Kruglik, S.G.; Ghomi, M. Vibrational Analysis of Amino Acids and Short Peptides in Hydrated Media. VIII. Amino Acids with Aromatic Side Chains: L-Phenylalanine, L-Tyrosine, and L-Tryptophan. J. Phys. Chem. B 2010, 114, 15319–15330. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, T.; Ashida, T.; Sasada, Y.; Kakudo, M. The Crystal Structures of l-Tryptophan Hydrochloride and Hydrobromide. Chem. Soc. Japan 1966, 39, 2369–2378. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.S.; Van Duyne, R.P. Molecular generality of surface-enhanced Raman spectroscopy (SERS). A detailed investigation of the hexacyanoruthenate ion adsorbed on silver and copper electrodes. J. Am. Chem. Soc. 1981, 103, 7497. [Google Scholar] [CrossRef]
- Roy, D.; Furtak, T.E. Characterization of surface complexes in enhanced Raman scattering. J. Chem. Phys. 1984, 81, 4168. [Google Scholar] [CrossRef]
- Adamson, A.W. Physical Chemistry of Surfaces; John Wiley and Sons: New York, NY, USA, 1990. [Google Scholar]
- Bhattacharya, S.; Vyas, N.; Ojha, A.K.; Dasgupta, S.; Roya, A. Surface-enhanced Raman measurements and DFT calculations for L-tryptophan of varying pH in silver sol. J. Raman Spectrosc. 2012, 43, 718–723. [Google Scholar] [CrossRef]

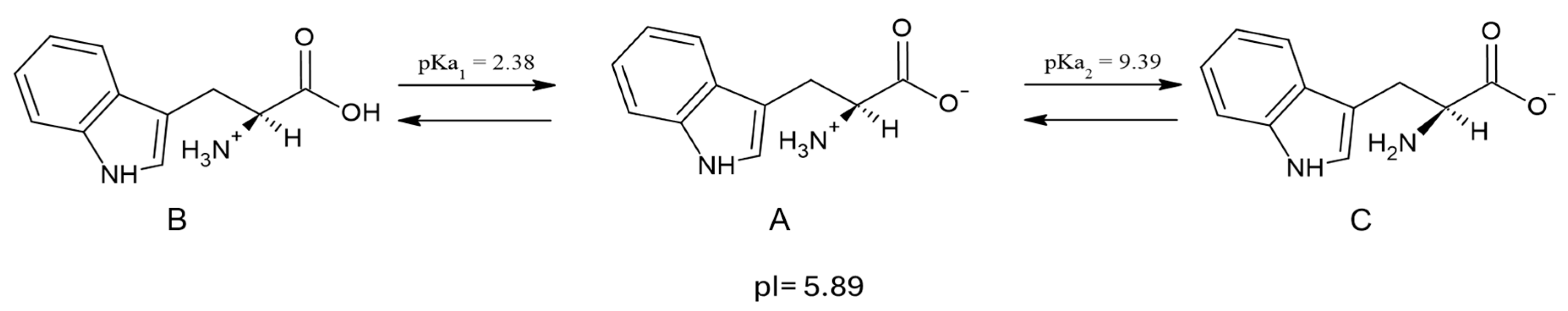
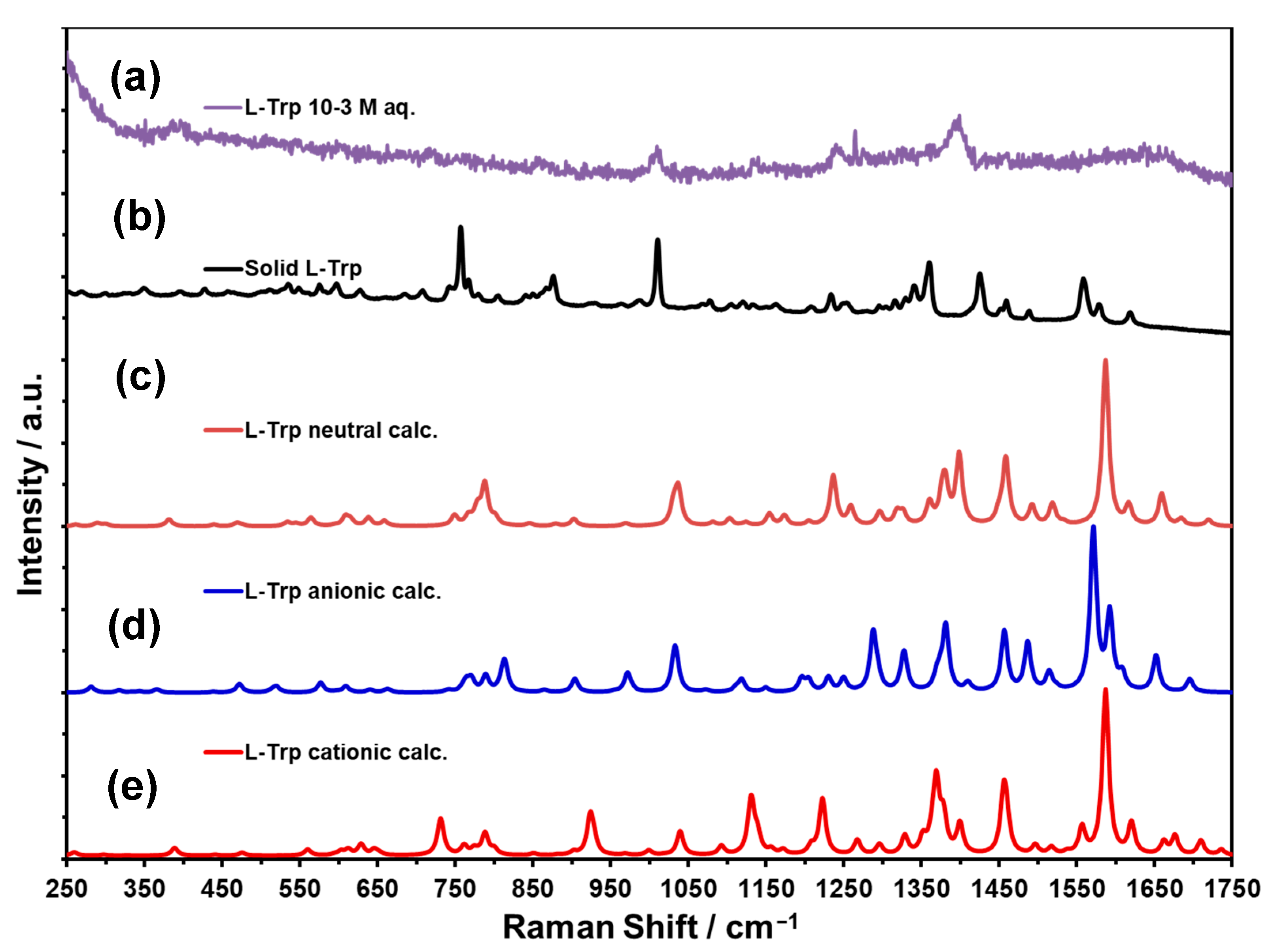
| RS L-Tryp Neutral DFT Calculations | RS L-Tryp Solid | RS L-Tryp Aqueous Solution | SERS DFT Calculations CCSDE | SERS Experimental CCSDE | Assignment (cm−1) DFT Calculations LANL2DZ CCSDE, and Compared with Refs. [2,43] | |||
|---|---|---|---|---|---|---|---|---|
| Ag | Au | Ag | Au | Ag | Au | |||
| 260 | 243 | 245 | 232 | nAg-O | ||||
| 359 | 352 | 380 | 323 | 329 | 359 | ω-NH2 | ||
| 408 | 395 | 385 | 385 | 386 | def. R. | |||
| 430 | 412 | 396 | 398 | def. R, r | ||||
| 476 | 472 | 467 | β-CC-R, r, γ-CH2 | |||||
| 526 | 514 | 579 | 523 | 520 | β-N-H (r) ν R, r | |||
| 537 | 533 | 590 | 529 | 536 | δ(r), β i.p. | |||
| 595 | 598 | 551 | 599 | 574 | 574 | b (R)oop; γ-CH2 (high intensities in SERS) | ||
| 630 | 607 | 620 | 640 | 604 | 607 | γ-CH2; α-NH2; β C-O | ||
| 689 | 679 | ν R, r | ||||||
| 710 | 722 | 718 | 711 | Def. R, r, n-CN | ||||
| 748 | 748 | 749 | 724 | 744 | 740 | 721 | ω-H(R); γ-CH2; β-COO1− | |
| 771 | 757 | 759 | 771 | 758 | θ (R), θ (r) | |||
| 778 | 778 | 768 | 793 | 775 | Def. R, r, α-COO− | |||
| 787 | 788 | 789 | 790 | β (R)-CC-; β (r) | ||||
| 808 | γ-CH2, β-COO1− | |||||||
| 845 | 845 | 846 | 869 | 843 | 824 | β-CH(r) | ||
| 858 | 859 | 858 | 864 | δ-H(R), β-CH(-NH) (r) | ||||
| 878 | 878 | 877 | 891 | 889 | 868 | β-H(R), α -H(r) | ||
| 902 | 902 | 933 | 929 | 893 | 942 | n-CC-(r); n-CNC-, (r) | ||
| 959 | 974 | 975 | 980 | δ-CH(r); γ-CH2; β-CH; γ-NH2 | ||||
| 969 | 969 | 993 | 976 | 984 | δ-CH2; ν-CN | |||
| 1001 | 1010 | 1011 | 1010 | 1015 | 1011 | 1009 | θ (R), θ (r); β H(R) | |
| 1031 | 1031 | 1028 | 1024 | γ-NH3+, β H(C) | ||||
| 1037 | 1066 | 1060 | 1036 | α(R)s in plane, β-H (R) | ||||
| 1081 | 1079 | 1065 | 1085 | 1090 | 1083 | αH(r) | ||
| 1103 | 1103 | 1112 | 1125 | 1100 | ω-NH3 +, β-H(C) | |||
| 1124 | 1109 | 1137 | 1132 | ω-NH3 +, β-H(C) | ||||
| 1154 | 1154 | 1143 | 1140 | 1148 | 1153 | α-H(R), ω-NH3 +, β-CH | ||
| 1174 | 1174 | 1167 | 1170 | 1169 | 1174 | 1170 | 1168 | γ-CH2, α-H(R); |
| 1204 | 1213 | 1206 | υ-(r), υ-C-COO − | |||||
| 1236 | 1236 | 1235 | 1243 | 1237 | 1241 | 1242 | 1243 | α-H(R), γ-H(r) |
| 1259 | 1264 | 1264 | 1267 | 1250 | 1251 | γ-H(R), γ-H(r), β-CH | ||
| 1296 | 1296 | 1300 | 1276 | 1277 | 1284 | 1289 | β-H(-CH2) | |
| 1318 | 1318 | 1318 | 1319 | 1291 | 1296 | υ (R), υ (r) | ||
| 1326 | 1326 | 1337 | 1324 | 1316 | 1331 | 1334 | ν-CN, β-CH; ω-CH2 | |
| 1342 | 1345 | 1343 | β-CH, β-H (-CH2) | |||||
| 1360 | 1360 | 1361 | 1361 | 1365 | ω-CH2, β-CH | |||
| 1376 | 1376 | 1374 | 1375 | 1374 | υ (r), υ (R) | |||
| 1380 | 1380 | 1391 | 1398 | γ-CH(R); n (r); β-CH (-NH); δ-CH2 | ||||
| 1398 | 1398 | 1427 | 1399 | 1404 | 1405 | 1396 | 1387 | υs -COO1− |
| 1450 | 1450 | 1457 | 1416 | 1416 | 1463 | 1457 | α-CH2, δ-NH2 | |
| 1459 | 1459 | 1461 | 1461 | 1453 | 1441 | 1464 | υ (r), υ (R), ns-NH3 + | |
| 1492 | 1492 | 1490 | 1489 | 1479 | β -CH(R) | |||
| 1517 | 1517 | 1504 | 1513 | 1506 | δ-CH2; SERS: n(R)-CC-, β-CH, β-CH (r) (-NH) | |||
| 1560 | 1557 | 1541 | 1560 | 1541 | n(r), n(R) | |||
| 1587 | 1587 | 1581 | 1603 | 1598 | 1595 | 1578 | α-NH3+ | |
| 1616 | 1616 | 1628 | 1638 | 1618 | 1618 | 1594 | Φ (ringst)-CC-st, β-CC-bend, β -CHalk; β-CH (r) (-NH), δ-CH2 | |
| 1653 | nas-COO−, α-CCO | |||||||
| 1659 | 1659 | 1666 | 1661 | 1667 | n (R)-CC-, β-CC-, β CHalk; β-CH (r) (-NH), α-NH3+ | |||
| 2136 | nas-CN | |||||||
| 2254 | ns-CN | |||||||
| 2952 | 2950 | 2942 | nas-H(R) | |||||
| 3065 | 3059 | 3083 | ns-NH3+ | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Félix-Massa, T.; Padilla-Jiménez, A.C.; Vega-Reyes, T.P.; Colón-González, F.M.; Pacheco-Londoño, L.C.; Galán-Freyle, N.J.; Castro-Suárez, J.R.; Ortega-Zúñiga, C.A.; González-Arvelo, E.L.; Lebrón-Ramírez, E.S.; et al. L-Tryptophan Adsorbed on Au and Ag Nanostructured Substrates: A SERS Study. Appl. Sci. 2025, 15, 12273. https://doi.org/10.3390/app152212273
Félix-Massa T, Padilla-Jiménez AC, Vega-Reyes TP, Colón-González FM, Pacheco-Londoño LC, Galán-Freyle NJ, Castro-Suárez JR, Ortega-Zúñiga CA, González-Arvelo EL, Lebrón-Ramírez ES, et al. L-Tryptophan Adsorbed on Au and Ag Nanostructured Substrates: A SERS Study. Applied Sciences. 2025; 15(22):12273. https://doi.org/10.3390/app152212273
Chicago/Turabian StyleFélix-Massa, Tamara, Amira C. Padilla-Jiménez, Tatiana P. Vega-Reyes, Francheska M. Colón-González, Leonardo C. Pacheco-Londoño, Nataly J. Galán-Freyle, John R. Castro-Suárez, Carlos A. Ortega-Zúñiga, Edgardo L. González-Arvelo, Elvin S. Lebrón-Ramírez, and et al. 2025. "L-Tryptophan Adsorbed on Au and Ag Nanostructured Substrates: A SERS Study" Applied Sciences 15, no. 22: 12273. https://doi.org/10.3390/app152212273
APA StyleFélix-Massa, T., Padilla-Jiménez, A. C., Vega-Reyes, T. P., Colón-González, F. M., Pacheco-Londoño, L. C., Galán-Freyle, N. J., Castro-Suárez, J. R., Ortega-Zúñiga, C. A., González-Arvelo, E. L., Lebrón-Ramírez, E. S., Centeno-Ortiz, J. A., & Hernández-Rivera, S. P. (2025). L-Tryptophan Adsorbed on Au and Ag Nanostructured Substrates: A SERS Study. Applied Sciences, 15(22), 12273. https://doi.org/10.3390/app152212273








