Non-Invasive Study of Gold Nanoparticles in Famille rose and Ruby-Back Qing Porcelain by Luminescence, Low-Wavenumber Raman Scattering and pXRF
Abstract
1. Introduction
2. Materials and Methods
2.1. Optical Microscopy
2.2. Portable X-Ray Fluorescence Spectroscopy (pXRF)
2.3. Raman Microspectroscopy
2.4. Artifacts
3. Results and Discussion
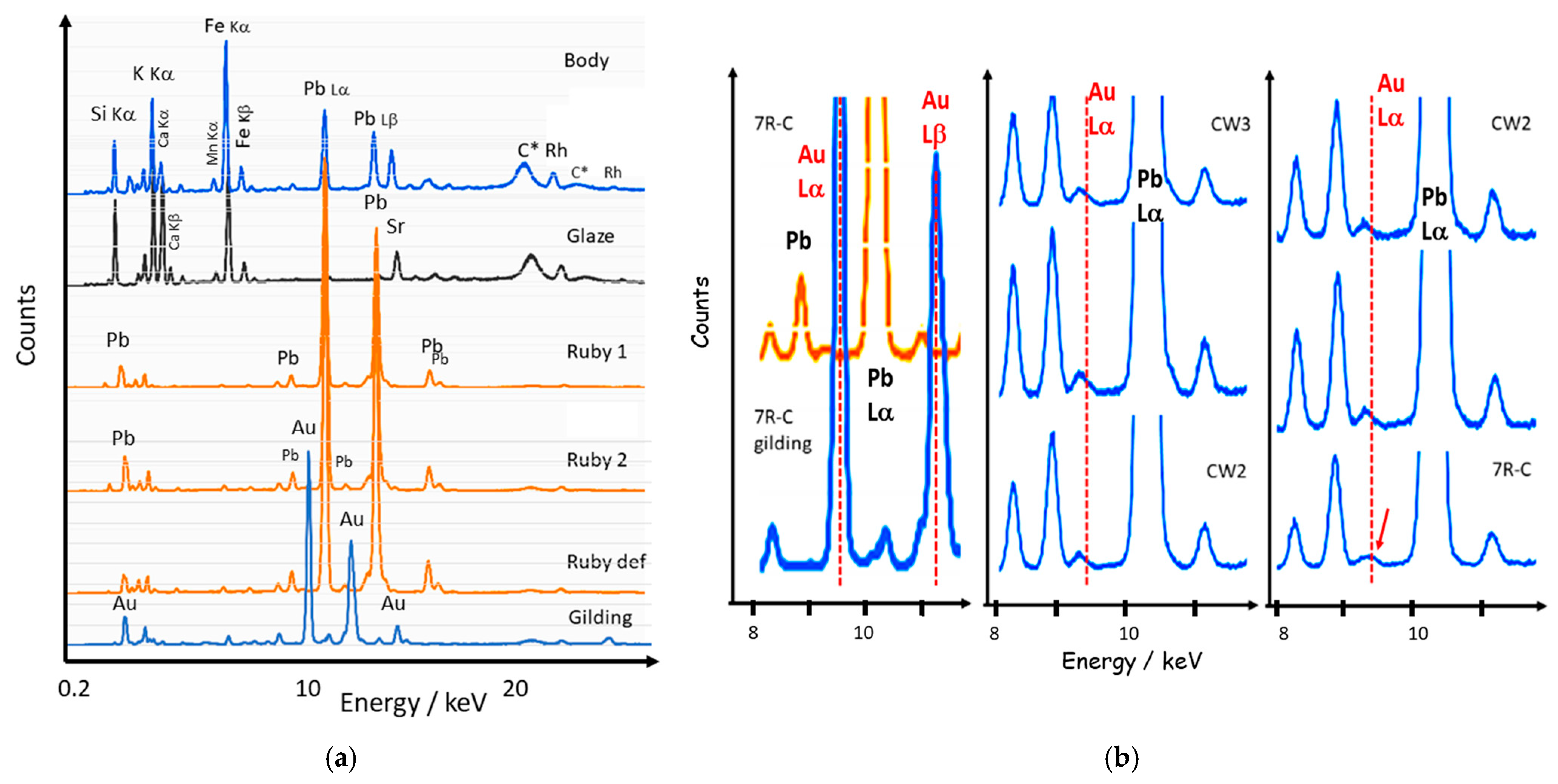
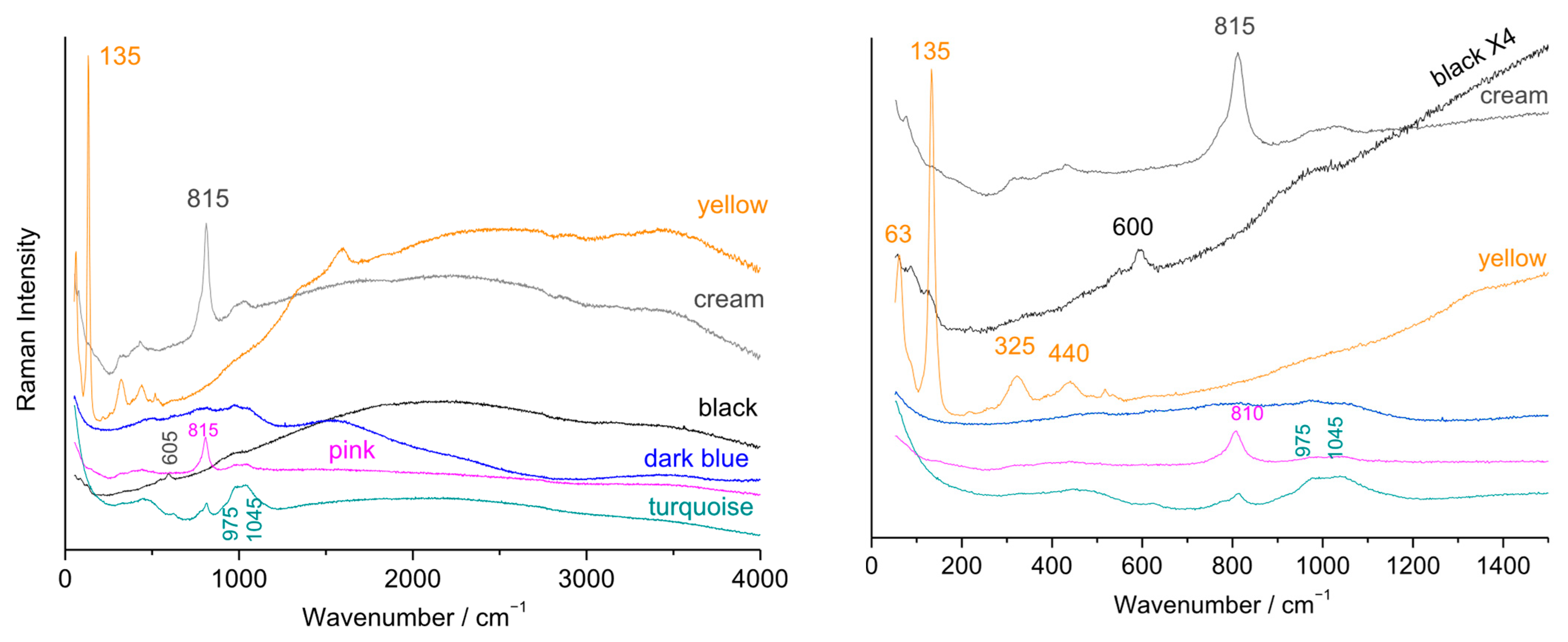
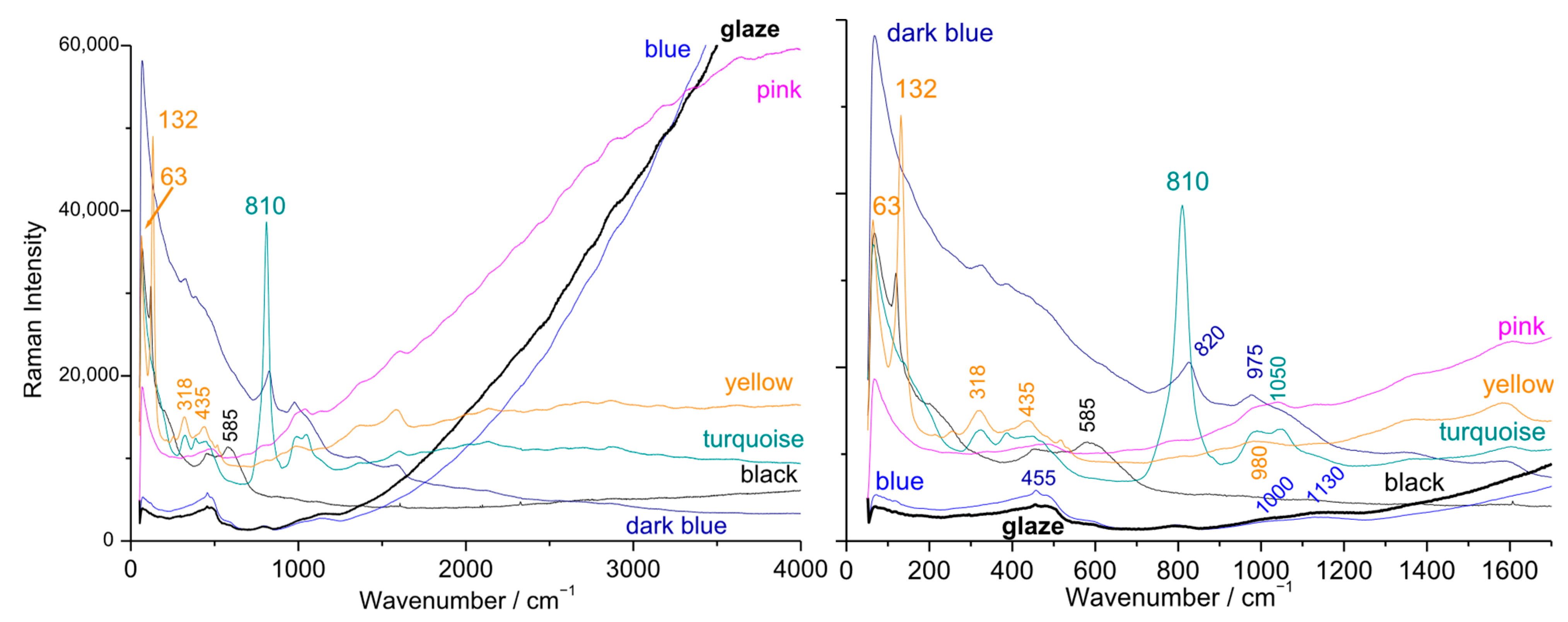
3.1. Fluorescence and Raman Signatures of Eggshell Plates
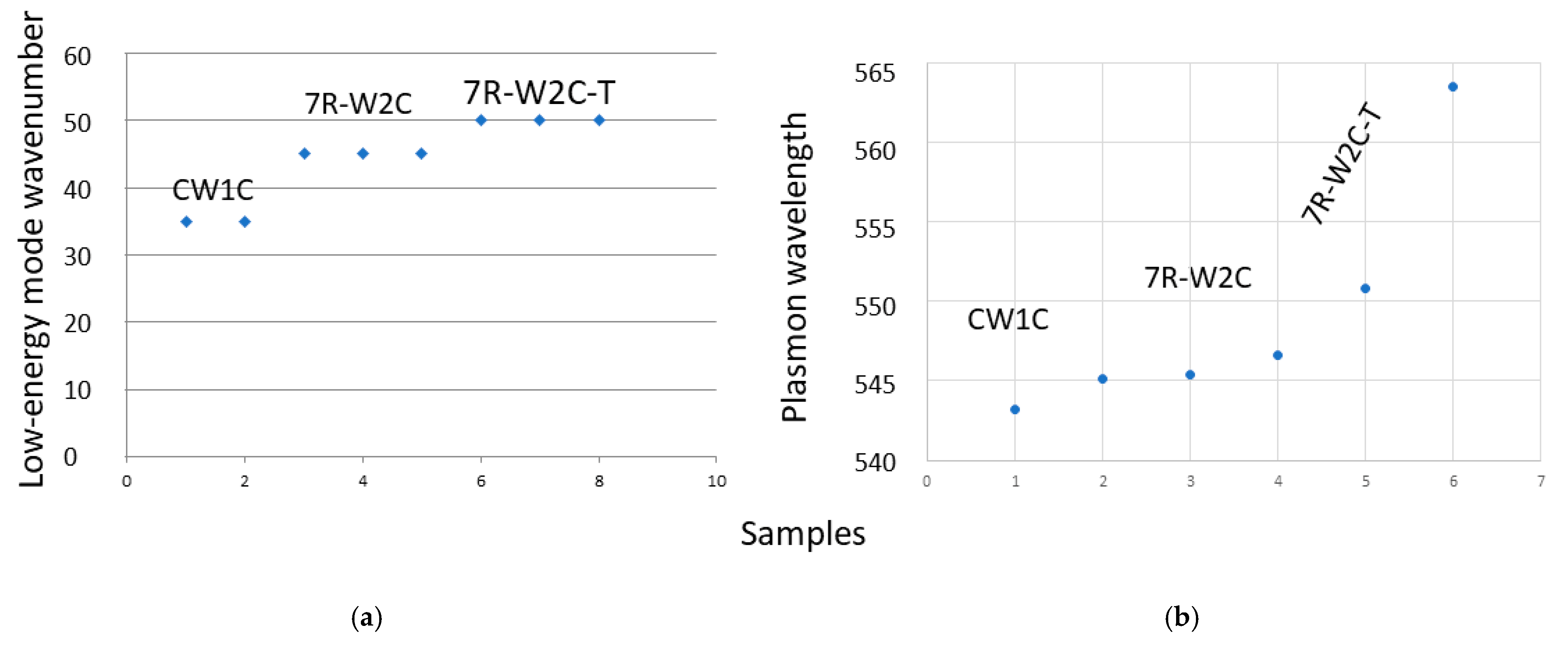
3.2. Boson Peak and/or Lamb’s Mode?
3.3. Variability
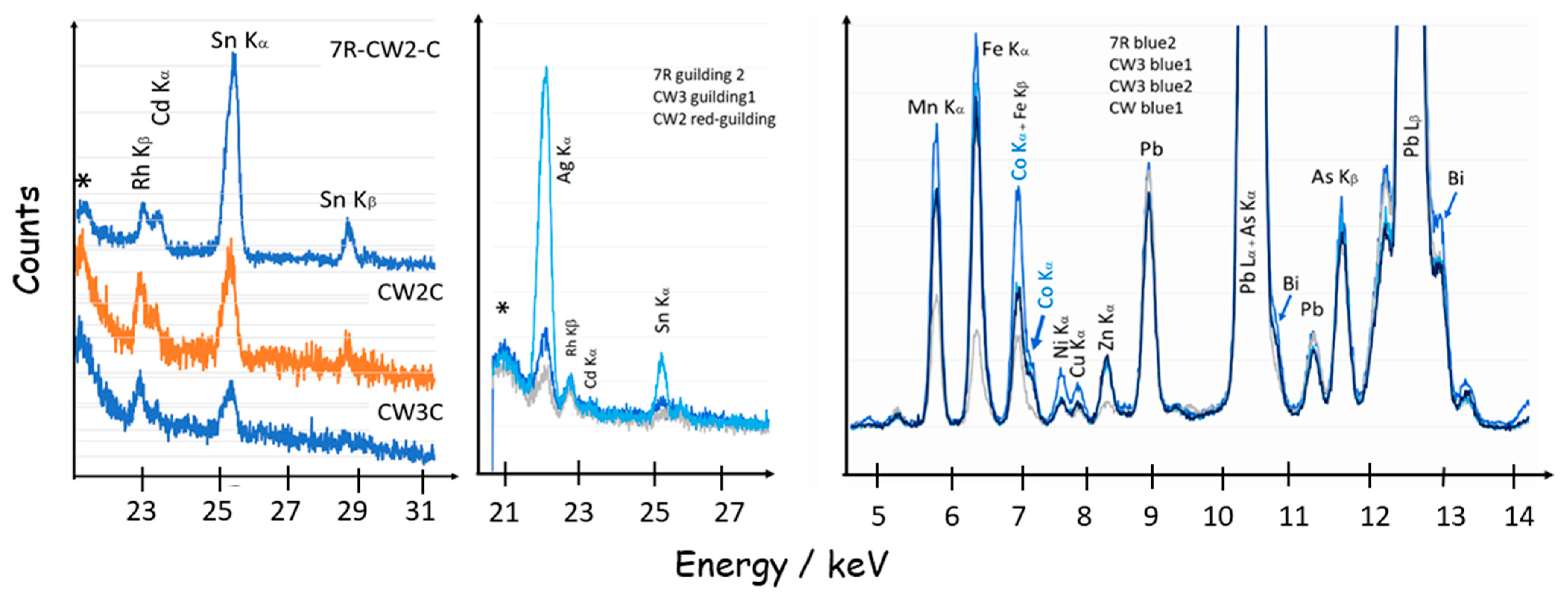
3.4. Detection of Au° NPs Content by pXRF
3.5. Gilding, Blue, and Other Colors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landry-Deron, I. Les Mathématiciens envoyés en Chine par Louis XIV en 1685. Arch. Hist. Exact Sci. 2001, 55, 423–463. [Google Scholar] [CrossRef]
- Shih, C.-F. The Arrival of a New Colour Palette in Eighteenth-century Jingdezhen. In Le Secret des Couleurs, Céramiques de Chine et d’Europe du XVIIIe Siècle à nos Jours; d’Abrigeon, P., Ed.; Fondation Baur-Musée des Arts d’Extrême Orient -5 Continents: Genève, Switzerland, 2022; pp. 21–59. [Google Scholar]
- Bellemare, J. A New Palette: Reassessing the Development of Enamel Colors in Early-Eighteenth-Century China. J. Glass Stud. 2022, 64, 147–167. Available online: https://www.jstor.org/stable/48703407 (accessed on 3 March 2025).
- Curtis, E.B. A Plan of the Emperor’s Glassworks. Arts Asiat. 2001, 56, 81–90. [Google Scholar] [CrossRef]
- Kırmızı, B.; Colomban, P.; Quette, B. On-site analysis of Chinese Cloisonné enamels from fifteenth to nineteenth centuries. J. Raman Spectrosc. 2010, 41, 780–790. [Google Scholar] [CrossRef]
- Quette, B. Cloisonné: Chinese Enamels from the Yuan, Ming and Qing Dynasties; Yale University Press: New York, NY, USA, 2011. [Google Scholar]
- Colomban, P. The Quest for the Western Colors in China Under the Qing Emperors. Journal18 2024, 17, 7219. Available online: https://www.journal18.org/7219 (accessed on 15 September 2025).
- Colomban, P. Tracer l’utilisation de recettes et/ou d’ingrédients européens dans les objets émaillés: Stratégie et premiers résultats. Artefact. Tech. Hist. Sci. Hum. 2023, 18, 161–193. [Google Scholar] [CrossRef]
- Colomban, P.; Gironda, M.; Simsek Franci, G.; d’Abrigeon, P. Distinguishing genuine imperial Qing dynasty porcelain from ancient replicas by on-site non-invasive XRF and Raman spectroscopy. Materials 2022, 15, 5747. [Google Scholar] [CrossRef]
- Sciau, P.; Noé, L.; Colomban, P. Metal nanoparticles in contemporary potters’ master pieces: Lustre and red “pigeon blood” potteries as models to understand the ancient pottery. Ceram. Int. 2016, 42, 15349–15357. [Google Scholar] [CrossRef]
- Colomban, P. The use of metal nanoparticles to produce yellow, red and iridescent colour, from bronze age to present times in lustre pottery and glass: Solid state chemistry, spectroscopy and nanostructure. J. Nano Res. 2009, 8, 109–132. [Google Scholar] [CrossRef]
- Jacquemart, A. Histoire Artistique, Industrielle et Commerciale de la Porcelaine: Sujets et Emblèmes Qui La Décorent, Marques et Inscriptions; Qui Font Reconnaître les Fabriques D’où elle Sort, les Variations de Prix Qu’ont Obtenus les Principaux Objets Connus & Les Collections où Ils Sont Conservés Aujourd’hui; J. Techener: Paris, France, 1862. [Google Scholar]
- Cardinal, C. Les Montres et Horloges de Table du Musée du Louvre; RMN: Paris, France, 2000. [Google Scholar]
- Colomban, P.; Kırmızı, B.; Gougeon, C.; Gironda, M.; Cardinal, C. Pigments and glassy matrix of the 17th–18th century enamelled French watches: A non-invasive on-site Raman and pXRF study. J. Cul. Herit. 2020, 44, 1–14. [Google Scholar] [CrossRef]
- Geyssant, J. (Ed.) Bernard Perrot (1640–1709), Secrets et Chefs d’œuvre des Verreries Royales d’Orléans; Catalogue, Musée des Beaux-Arts d’Orléans—SOMOGY Editions d’Arts: Paris, France, 2013. [Google Scholar]
- Colomban, P.; Kırmızı, B. Non-invasive on-site Raman study of polychrome and white enamelled glass artefacts in imitation of porcelain assigned to Bernard Perrot and his follower. J. Raman Spectrosc. 2020, 51, 133–146. [Google Scholar] [CrossRef]
- Ricciardi, P.; Colomban, P.; Tournié, A.; Macchiarola, M.; Ayed, N. A non-invasive study of Roman Age mosaic glass tesserae by means of Raman spectroscopy. J. Archaeol. Sci. 2009, 36, 2551–2559. [Google Scholar] [CrossRef]
- Spiegl, W.; Johann Kunckel und die Erfindung des Goldrubins. Weltkunst 1988. Available online: https://www.uni-wittenberg.de/wp-content/uploads/application/pdf/Spiegl-1988_Kunckel-und-die-Erfindung-des-Goldrubins.pdf (accessed on 5 September 2025).
- Heimann, R.R. Semidiaphanam Tremuli Narcissuli Ideam Lacteam: Ehrenfried Walther von Tschirnhaus (1651–1708) and His Determined Search for the Porcelain Principle. Archaeometry, 2025; in press. [Google Scholar] [CrossRef]
- Casadio, F.; Bezur, A.; Domoney, K.; Eremin, K.; Lee, L.; Mass, J.L.; Shortland, A.; Zumbulyadis, N. X-ray fluorescence applied to overglaze enamel decoration on eighteenth-and nineteenth-century porcelain from central Europe. Stud. Conserv. 2012, 57 (Suppl. S1), S61–S72. [Google Scholar] [CrossRef]
- Maggetti, M.; D’Albis, A. Phase and compositional analysis of a Sèvres soft paste porcelain plate from 1781, with a review of early porcelain techniques. Eur. J. Mineral. 2017, 29, 347–367. [Google Scholar] [CrossRef]
- Colomban, P.; Simsek Franci, G.; Gerken, M.; Gironda, M.; Mesqui, V. Non-Invasive On-Site XRF and Raman classification and dating of ancient ceramics: Application to 18th and 19th Century Meissen Porcelain (Saxony) and Comparison with Chinese Porcelain. Ceramics 2023, 6, 2178–2212. [Google Scholar] [CrossRef]
- Hunt, L.B. Gold in the pottery industry: The history and technology of gilding processes. Gold Bull. 1979, 12, 116–127. [Google Scholar] [CrossRef]
- Domoney, K.; Shortland, A.J.; Kuhn, S. Characterization of 18th-century Meissen porcelain using SEM–EDS. Archaeometry 2012, 54, 454–474. [Google Scholar] [CrossRef]
- Colomban, P.; Ngo, A.-T.; Fournery, N. Non-invasive Raman analysis of 18th Century Chinese export/armorial overglazed porcelain: Identification of the different enameling techniques. Heritage 2022, 5, 233–259. [Google Scholar] [CrossRef]
- Williamson, G.C. The Book of Famille Rose; Methuen and Co.: London, UK, 1927. [Google Scholar]
- Garner, H. The Origins of Famillie Rose. Trans. Orient. Ceram. Soc. 1967–1969, 37, 1–16. [Google Scholar]
- Kerr, R. What were the Origins of Chinese famille rose? Orientations 2000, 31, 53–60. [Google Scholar]
- Guo, X. The Origin and Early Features of Canton Enamels. In Sparkle and Charm: Canton Enamels of the Qing Dynasty; Xu, X., Ed.; The Chinese University of Hong Kong: Hong Kong, China, 2023; pp. 41–112. [Google Scholar]
- von Freymann, G.; Kitaev, V.; Lotsch, B.V.; Ozin, G.A. Bottom-up assembly of photonic crystals. Chem. Soc. Rev. 2013, 42, 2528–2554. [Google Scholar] [CrossRef]
- Bandaru, S.; Arora, D.; Ganesh, K.M.; Umrao, S.; Thomas, S.; Bhaskar, S.; Chakrabortty, S. Recent advances in research from nanoparticle to nano-assembly: A review. Nanomaterials 2024, 14, 1387. [Google Scholar] [CrossRef]
- Parviz, B.A.; Ryan, D.; Whitesides, G.M. Using self-assembly for the fabrication of nano-scale electronic and photonic devices. IEEE Trans. Adv. Packag. 2003, 26, 233–241. [Google Scholar] [CrossRef]
- Cai, Z.; Li, Z.; Ravaine, S.; He, M.; Song, Y.; Yin, Y.; Zheng, H.; Teng, J.; Zhang, A.O. From colloidal particles to photonic crystals: Advances in self-assembly and their emerging applications. Chem. Soc. Rev. 2021, 50, 5898–5951. [Google Scholar] [CrossRef]
- Pileni, M.P. Self-assembly of inorganic nanocrystals: Fabrication and collective intrinsic properties. Acc. Chem. Res. 2007, 40, 685–693. [Google Scholar] [CrossRef]
- Blanco-Formoso, M.; Pazos-Perez, N.; Alvarez-Puebla, R.A. Fabrication of plasmonic supercrystals and their SERS enhancing properties. ACS Omega 2020, 5, 25485–25492. [Google Scholar] [CrossRef]
- Courty, A.; Lisiecki; Pileni, M.P. Vibration of self-organized silver nanocrystals. J. Chem. Phys. 2002, 116, 8074–8078. [Google Scholar] [CrossRef]
- Courty, A.; Bayle, M.; Carles, R. Plasmon-enhanced inelastic scattering by 2 D and 3 D superlattices made of silver nanocrystals. J. Raman Spectrosc. 2019, 50, 74–84. [Google Scholar] [CrossRef]
- Colomban, P.; Tournie, A.; Ricciardi, P. Raman spectroscopy of copper nanoparticle-containing glass matrices: Ancient red stained-glass windows. J. Raman Spectrosc. 2009, 40, 1949–1955. [Google Scholar] [CrossRef]
- Li, R.; Pang, C.; Li, Z.; Chen, F. Plasmonic nanoparticles in dielectrics synthesized by ion beams: Optical properties and photonic applications. Adv. Opt. Mater. 2020, 8, 1902087. [Google Scholar] [CrossRef]
- Stepanov, A.L. Synthesis of silver nanoparticles in dielectric matrix by ion implantation: A review. Rev. Adv. Mater. Sci. 2010, 26, 1–29. [Google Scholar]
- Quandt, A.; Ferrari, M.; Righini, G.C. Advancement of glass-ceramic materials for photonic applications. In Sol-Gel Based Nanoceramic Materials: Preparation, Properties and Applications; Springer International Publishing: Cham, Switzerland, 2016; pp. 133–155. [Google Scholar]
- Wei, Y.; Ebendorff-Heidepriem, H.; Zhao, J. Recent advances in hybrid optical materials: Integrating nanoparticles within a glass matrix. Adv. Opt. Mater. 2019, 7, 1900702. [Google Scholar] [CrossRef]
- Colomban, P.; Ambrosi, F.; Ngo, A.T.; Lu, T.A.; Feng, X.L.; Chen, S.; Choi, C.L. Comparative analysis of wucai Chinese porcelains using mobile and fixed Raman microspectrometers. Ceram Int. 2017, 43, 14244–14256. [Google Scholar] [CrossRef]
- Colomban, P.; Simsek Franci, G.; Gallet, X. Non-Invasive Mobile Raman and pXRF Analysis of Armorial Porcelain with the Coat of Arms of Louis XV and Others Enamelled in Canton: Analytical Criteria for Authentication. Heritage 2024, 7, 4881–4913. [Google Scholar] [CrossRef]
- Colomban, P.; Truong, C. Non-destructive Raman study of the glazing technique in lustre potteries and faience (9–14th centuries): Silver ions, nanoclusters, microstructure and processing. J. Raman Spectrosc. 2004, 35, 195–207. [Google Scholar] [CrossRef]
- Colomban, P.; Schreiber, H.D. Raman signature modification induced by copper nanoparticles in silicate glass. J. Raman Spectrosc. 2005, 36, 884–890. [Google Scholar] [CrossRef]
- Norris, D.; Braekmans, D.; Domoney, K.; Shortland, A. The composition and technology of polychrome enamels on Chinese ruby-backed plates identified through nondestructive micro-X-ray fluorescence. X-Ray Spectrom. 2020, 49, 502–510. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, X.; Kang, B.; Wang, G.; Qu, L.; Lei, Y. Non-destructive analysis and deterioration study of a decorated Famille Rose porcelain bowl of Qianlong Reign from the Forbidden City. Stud. Conserv. 2019, 64, 311–322. [Google Scholar] [CrossRef]
- Kingery, W.D.; Vandiver, P.B. The eighteenth-century change in technology and style from the famille-verte palette to the famille-rose palette. In Proceedings of the Technology and Style: Proceedings of a Symposium on Ceramic History and Archaeology at the 87th Annual Meeting of the American Ceramic Society, Cincinnati, OH, USA, 6 May 1985; The American Ceramic Society: Westerville, OH, USA, 1985; Volume 2, pp. 363–381. [Google Scholar]
- Bruker. Available online: https://xrfcheck.bruker.com/InfoDepth (accessed on 9 August 2025).
- Slitine, F. C’est un vrai Samson! Sèvres. Rev. Société Amis Musée Natl. Céramique 1998, 7, 66–80. [Google Scholar] [CrossRef]
- Albis, A.D. Saint-Cloud ou King-Te-Tchen? Sèvres. Rev. Société Amis Musée Natl. Céramique 2007, 16, 46–50. [Google Scholar] [CrossRef]
- Antikeo. Available online: https://www.antikeo.com/magazine/la-manufacture-samson-une-histoire-de-famille/ (accessed on 9 July 2024).
- Bushell, S.W. Chinese Eggshell Porcelain with’ Marks,’ from the Collection of the Late Hon. Sir Robert Meade, GCB Part II (Conclusion). Burlingt. Mag. Connoiss. 1906, 9, 393–395. [Google Scholar]
- Ming, C.; Yang, Y.; Zhu, J.; Guan, L.; Fan, C.; Xu, C.; Yao, Z.; Kenoyer, J.M.; Song, G.; Wang, C. Archaeometric investigation of the relationship between ancient egg-white glazed porcelain (Luanbai) and bluish white glazed porcelain (Qingbai) from Hutian Kiln, Jingdezhen, China. J. Archaeol. Sci. 2014, 47, 78–84. [Google Scholar] [CrossRef]
- Fang, L. Ceramics of the Qing Dynasty. In The History of Chinese Ceramics; Springer Nature Singapore: Singapore, 2023; pp. 807–1072. [Google Scholar]
- Yan, C. Elegance of Porcelain from the Yongzheng Reign. In A Brief History of Chinese Imperial Porcelain: From Song Dynasty to Qing Dynasty; Springer Nature Singapore: Singapore, 2024; pp. 291–316. [Google Scholar]
- Li, Y.; Zhu, J.; Ji, L.; Shan, Y.; Jiang, S.; Chen, G.; Sciau, P.; Wang, W.; Wang, C. Study of arsenic in Famille rose porcelain from the Imperial Palace of Qing Dynasty, Beijing, China. Ceram. Int. 2018, 44, 1627–1632. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef]
- Mustafa, D.E.; Yang, T.; Xuan, Z.; Chen, S.; Tu, H.; Zhang, A. Surface plasmon coupling effect of gold nanoparticles with different shape and size on conventional surface plasmon resonance signal. Plasmonics 2010, 5, 221–231. [Google Scholar] [CrossRef]
- Peng, S.; McMahon, J.M.; Schatz, G.C.; Gray, S.K.; Sun, Y. Reversing the size-dependence of surface plasmon resonances. PNAS 2010, 107, 14530–14534. [Google Scholar] [CrossRef] [PubMed]
- Susman, M.D.; Feldman, Y.; Bendikov, T.A.; Vaskevich, A.; Rubinstein, I. Real-time plasmon spectroscopy study of the solid-state oxidation and Kirkendall void formation in copper nanoparticles. Nanoscale 2017, 9, 12573–12589. [Google Scholar] [CrossRef]
- Colomban, P.; Arberet, L.; Kırmızı, B. On-site Raman analysis of 17th and 18th century Limoges enamels: Implications on the European cobalt sources and the technological relationship between Limoges and Chinese enamels. Ceram. Int. 2017, 43, 10158–10165. [Google Scholar] [CrossRef]
- Colomban, P.; Kırmızı, B.; Simsek Franci, G. Cobalt and Associated Impurities in Blue (and Green) Glass, Glaze and Enamel: Relationships between Raw Materials, Processing, Composition, Phases and International Trade. Minerals 2021, 11, 633. [Google Scholar] [CrossRef]
- Colomban, P.; Simsek Franci, G.; Gironda, M.; d’Abrigeon, P.; Schumacher, A.-C. pXRF Data Evaluation Methodology for On-Site Analysis of Precious Artifacts: Cobalt Used in the Blue Decoration of Qing Dynasty Overglazed Porcelain Enameled at Customs District (Guangzhou), Jingdezhen and Zaobanchu (Beijing) Workshops. Heritage 2022, 5, 1752–1778. [Google Scholar] [CrossRef]
- Colomban, P.; Simsek Franci, G.; Burlot, J.; Gallet, X.; Zhao, B.; Clais, J.-B. Non-Invasive On-Site pXRF Analysis of Coloring Agents, Marks and Enamels of Qing Imperial and Non-Imperial Porcelain. Ceramics 2023, 6, 447–474. [Google Scholar] [CrossRef]
- Kissin, S.A. Five-element (Ni-Co-As-Ag-Bi) veins. Geosci. Can. 1992, 19, 113–124. Available online: https://journals.lib.unb.ca/index.php/gc/article/view/3768/4282 (accessed on 4 September 2025).
- Van Pevenage, J.; Lauwers, D.; Herremans, D.; Verhaeven, E.; Vekemans, B.; De Clercq, W.; Vincze, L.; Moens, L.; Vandenabeele, P. A combined spectroscopic study on Chinese porcelain containing Ruan-Cai colors. Anal. Methods 2014, 6, 387–394. [Google Scholar] [CrossRef]
- Giannini, R.; Freestone, I.C.; Shortland, A.J. European cobalt sources identification in the production of Chinese Famille Rose porcelain. J. Archaeol. Sci. 2016, 80, 27–36. [Google Scholar] [CrossRef]
- Colomban, P.; Simsek Franci, G. Timurid, Ottoman, Safavid and Qajar ceramics: Raman and composition classification of the different types of glaze and pigments. Minerals 2023, 13, 977. [Google Scholar] [CrossRef]
- Colomban, P.; Milande, V. On-site Raman analysis of the earliest known Meissen porcelain and stoneware. J. Raman Spectrosc. 2006, 37, 606–613. [Google Scholar] [CrossRef]
- Colomban, P. Polymerization degree and Raman identification of ancient glasses used for jewelry, ceramic enamels and mosaics. J. Non-Crystall. Solids 2003, 323, 180–187. [Google Scholar] [CrossRef]
- Malinovsky, V.K.; Sokolov, A.P. The nature of boson peak in Raman scattering in glasses. Solid State Comm. 1986, 57, 757–761. [Google Scholar] [CrossRef]
- Schroeder, J.; Wu, W.; Apkarian, J.L.; Lee, M.; Hwa, L.G.; Moynihan, C.T. Raman scattering and Boson peaks in glasses: Temperature and pressure effects. J. Non-Crystall. Solids 2004, 349, 88–97. [Google Scholar] [CrossRef]
- Duval, E.; Mermet, A.; Saviot, L. Boson peak and hybridization of acoustic modes with vibrations of nanometric heterogeneities in glasses. Phys. Rev. B 2007, 75, 024201. [Google Scholar] [CrossRef]
- Duval, E.; Boukenter, A.; Champagnon, B. Vibration eigenmodes and size of microcrystallites in glass: Observation by very-low-frequency Raman scattering. Phys. Rev. Lett. 1986, 56, 2052. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.; Lermé, J.; Gehan, H.; Margueritat, J.; Mermet, A. Mechanisms of resonant low frequency Raman scattering from metallic nanoparticle Lamb modes. J. Chem. Phys. 2017, 146, 194201. [Google Scholar] [CrossRef]
- Palpant, B.; Portales, H.; Saviot, L.; Lermé, J.; Prével, B.; Pellarin, M.; Duval, E.; Perez, A.; Broyer, M. Quadrupolar vibrational mode of silver clusters from plasmon-assisted Raman scattering. Phys. Rev. B 1999, 60, 17107. [Google Scholar] [CrossRef]
- Tamura, A.; Higeta, K.; Ichinokawa, T. Lattice vibrations and specific heat of a small particle. J. Phys. C Solid State Phys. 1982, 15, 4975. [Google Scholar] [CrossRef]
- Ivanda, M.; Furić, K.; Musić, S.; Ristić, M.; Gotić, M.; Ristić, D.; Tonejc, A.M.; Djerdj, I.; Mattarelli, M.; Montagna, M.; et al. Low wavenumber Raman scattering of nanoparticles and nanocomposite materials. J. Raman Spectrosc. 2007, 38, 647–659. [Google Scholar] [CrossRef]
- Burlot, J.; Vangu, D.; Bellot-Gurlet, L.; Colomban, P. Raman identification of pigments and opacifiers: Interest and limitation of multivariate analysis by comparison with solid state spectroscopical approach—I. Lead-tin and Naples Yellow. J. Raman Spectrosc. 2024, 55, 161–183. [Google Scholar] [CrossRef]
- Sakellariou, K.; Miliani, C.; Morresi, A.; Ombelli, M. Spectroscopic investigation of yellow majolica glazes. J. Raman Spectrosc. 2004, 35, 61–67. [Google Scholar] [CrossRef]
- Sandalinas, C.; Ruiz-Moreno, S.; Lopez-Gil, A.; Miralles, J. Experimental confirmation by Raman spectroscopy of a Pb-Sn-Sb triple oxide yellow pigment in sixteenth-century Italian pottery. J. Raman Spectrosc. 2006, 37, 1146–1153. [Google Scholar] [CrossRef]
- Rosi, F.; Manuali, V.; Miliani, C.; Brunetti, B.G.; Sgamellotti, A.; Grygar, T.; Hradil, D. Raman scattering features of lead pyroantimonate compounds. Part I: XRD and Raman characterization of Pb2Sb2O7 doped with tin and zinc. J. Raman Spectrosc. 2009, 40, 107–111. [Google Scholar] [CrossRef]
- Pelosi, C.; Agresti, G.; Santamaria, U.; Mattei, E. Artificial yellow pigments: Production and characterization through spectroscopic methods of analysis. E-Preserv. Sci. 2010, 7, 108–115. Available online: http://www.morana-rtd.com/e-preservationscience/2010/Pelosi-10-05-2010.pdf (accessed on 25 May 2025).











| Label | View | Figure | Decor | Period | Diameter (cm) | Weight (g) |
|---|---|---|---|---|---|---|
| 7R-W2C-T |   | 2 | 7 borders, Chinese women with 2 children | Yongzheng (1723–1735) | 21 | 180 |
| 7R-W2C |   | 2 | 7 borders Chinese women with 2 children | Yongzheng (1723–1735) | 21 | 197 |
| CW1C |   | 3 | Chinese women with 1 child | Yongzheng (1723–1735) | 21 | 168 |
| CW2C |   | 3 | Chinese women with 2 children | Yongzheng (1723–1735) | 20.5 | 221 |
| CW3C |   | 2 | Chinese women with 3 children | Yongzheng (1723–1735) | 20 | 207 |
| EW |   | 2 | European women | Yongzheng (1723–1735) | 11 | 44 |
| SM |   | 3 | Bird and flowers | 19th century | 19.7 | 332 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colomban, P.; Tang, H.; Simsek-Franci, G. Non-Invasive Study of Gold Nanoparticles in Famille rose and Ruby-Back Qing Porcelain by Luminescence, Low-Wavenumber Raman Scattering and pXRF. Appl. Sci. 2025, 15, 12265. https://doi.org/10.3390/app152212265
Colomban P, Tang H, Simsek-Franci G. Non-Invasive Study of Gold Nanoparticles in Famille rose and Ruby-Back Qing Porcelain by Luminescence, Low-Wavenumber Raman Scattering and pXRF. Applied Sciences. 2025; 15(22):12265. https://doi.org/10.3390/app152212265
Chicago/Turabian StyleColomban, Philippe, Hui Tang, and Gulsu Simsek-Franci. 2025. "Non-Invasive Study of Gold Nanoparticles in Famille rose and Ruby-Back Qing Porcelain by Luminescence, Low-Wavenumber Raman Scattering and pXRF" Applied Sciences 15, no. 22: 12265. https://doi.org/10.3390/app152212265
APA StyleColomban, P., Tang, H., & Simsek-Franci, G. (2025). Non-Invasive Study of Gold Nanoparticles in Famille rose and Ruby-Back Qing Porcelain by Luminescence, Low-Wavenumber Raman Scattering and pXRF. Applied Sciences, 15(22), 12265. https://doi.org/10.3390/app152212265








