From Light to Insight: Hemodynamic Models for Optical Monitoring of the Brain in Cardiac Arrest
Abstract
1. Introduction
2. Cardiac Arrest
3. Optical Modalities in Cardiac Arrest
3.1. Near-Infrared Spectroscopy (NIRS)
3.2. Laser Doppler Flowmetry (LDF)
3.3. Diffuse Correlation Spectroscopy (DCS)
3.4. Impacts of Extracerebral Tissues on NIRS and DCS Measurements of the Brain
- Combining DCS with high-density time-resolved NIRS (TR-NIRS) or hybrid devices provides joint estimation of absorption, scattering, and flow with better discrimination of cortical versus extracerebral signals [46].
- Proper coupling, minimizing pressure artifacts, and accounting for pigmentation effects [71].
3.5. CBF/CMRO2 Measurements
- Inter-subject or cross-study comparisons, normative ranges, and effect sizes.
- Diagnostics/triage thresholds (e.g., hypoperfusion or metabolic failure).
- Model validation and multimodal integration (e.g., comparing optical estimates to gold-standard PET or microspheres).
- Therapy titration where dose targets rely on absolute levels.
4. Hemodynamic Modeling Frameworks
4.1. Coherent Hemodynamics Spectroscopy Model
4.2. The BrainSignals Model
- (1)
- Physiological specificity—separating overlapping contributions of flow, volume, and metabolism to observed optical signals.
- (2)
- Quantification of hidden variables—estimating parameters like CMRO2 and oxCCO that cannot be measured directly at the bedside.
- (3)
- Adaptability to non-steady-state conditions—extending to large, rapid changes characteristic of cardiac arrest and resuscitation.
4.3. Model Inversion, Identifiability and Parameter Sensitivity Issues
4.4. Common Observability Gaps
- Venous–capillary indistinguishability: [14].
- Superficial contamination [8].
- Metabolic unobservability [17].
4.5. Recommended Minimum Measurement Sets for Stable Inversion
5. Integrated Optical-Modeling Approaches in Cardiac Arrest
6. Clinical Translation, Limitations, Implementation, and Future Directions
7. Discussion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, S.; Gan, Y.; Jiang, N.; Wang, R.; Chen, Y.; Luo, Z.; Zong, Q.; Chen, S.; Lv, C. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: A systematic review and meta-analysis. Crit. Care 2020, 24, 61. [Google Scholar] [CrossRef] [PubMed]
- Callaway, C.W. Cerebral oximetry and cardiopulmonary resuscitation. J. Am. Heart Assoc. 2015, 4, e002373. [Google Scholar] [CrossRef] [PubMed]
- Takegawa, R.; Hayashida, K.; Rolston, D.M. Near-Infrared Spectroscopy Assessments of Regional Cerebral Oxygen Saturation for the Prediction of Clinical Outcomes in Patients With Cardiac Arrest: A Review of Clinical Impact, Evolution, and Future Directions. Front. Med. 2020, 7, 587930. [Google Scholar] [CrossRef] [PubMed]
- Jöbsis, F.F. Noninvasive, Infrared Monitoring of Cerebral and Myocardial Oxygen Sufficiency and Circulatory Parameters. Science (1979) 1977, 198, 1264–1267. [Google Scholar] [CrossRef]
- Cheung, C.; Culver, J.P.; Takahashi, K. In vivocerebrovascular measurement combining diffuse near-infrared absorption and correlation spectroscopies. Phys. Med. Biol. 2001, 46, 2053–2065. [Google Scholar] [CrossRef]
- Culver, J.P.; Durduran, T.; Furuya, D. Diffuse Optical Tomography of Cerebral Blood Flow, Oxygenation, and Metabolism in Rat during Focal Ischemia. J. Cereb. Blood Flow Metab. 2003, 23, 911–924. [Google Scholar] [CrossRef]
- Dirnagl, U.; Kaplan, B.; Jacewicz, M. Continuous Measurement of Cerebral Cortical Blood Flow by Laser-Doppler Flowmetry in a Rat Stroke Model. J. Cereb. Blood Flow Metab. 1989, 9, 589–596. [Google Scholar] [CrossRef]
- Caldwell, M.; Scholkmann, F.; Wolf, U.; Wolf, M.; Elwell, C.; Tachtsidis, I. Modelling confounding effects from extracerebral contamination and systemic factors on functional near-infrared spectroscopy. Neuroimage 2016, 143, 91–105. [Google Scholar] [CrossRef]
- Bale, G.; Elwell, C.E.; Tachtsidis, I. From Jöbsis to the present day: A review of clinical near-infrared spectroscopy measurements of cerebral cytochrome-c-oxidase. J. Biomed. Opt. 2016, 21, 91307. [Google Scholar]
- Lanka, P.; Yang, L.; Orive-Miguel, D. Multi-laboratory performance assessment of diffuse optics instruments: The BitMap exercise. J. Biomed. Opt. 2022, 27, 074716. [Google Scholar] [CrossRef]
- Delpy, D.T.; Cope, M. Quantification in tissue near-infrared spectroscopy. Philos. Trans. R. Soc. Lon. Ser. B Biol. Sci. 1997, 352, 649–659. [Google Scholar] [CrossRef]
- Fantini, S. Dynamic model for the tissue concentration and oxygen saturation of hemoglobin in relation to blood volume, flow velocity, and oxygen consumption: Implications for functional neuroimaging and coherent hemodynamics spectroscopy (CHS). Neuroimage 2014, 85, 202–221. [Google Scholar] [CrossRef]
- Durduran, T.; Yodh, A.G. Diffuse correlation spectroscopy for non-invasive, micro-vascular cerebral blood flow measurement. Neuroimage 2014, 85, 51–63. [Google Scholar] [CrossRef]
- Sassaroli, A.; Kainerstorfer, J.M.; Fantini, S. Nonlinear extension of a hemodynamic linear model for coherent hemodynamics spectroscopy. J. Theor. Biol. 2016, 389, 132–145. [Google Scholar] [CrossRef]
- Caldwell, M.; Hapuarachchi, T.; Highton, D. BrainSignals revisited: Simplifying a computational model of cerebral physiology. PLoS ONE 2015, 10, e0126695. [Google Scholar] [CrossRef] [PubMed]
- Banaji, M.; Mallet, A.; Elwell, C.E. A Model of Brain Circulation and Metabolism: NIRS Signal Changes during Physiological Challenges. PLoS Comput. Biol. 2008, 4, e1000212. [Google Scholar] [CrossRef] [PubMed]
- Russell-Buckland, J.; Barnes, C.P.; Tachtsidis, I. A Bayesian framework for the analysis of systems biology models of the brain. PLoS Comput. Biol. 2019, 15, e1006631. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.S.; Mavroudis, C.D. Non-invasive diffuse optical neuromonitoring during cardiopulmonary resuscitation predicts return of spontaneous circulation. Sci. Rep. 2021, 11, 3828. [Google Scholar] [CrossRef]
- Nosrati, R.; Lin, S.; Ramadeen, A. Cerebral Hemodynamics and Metabolism During Cardiac Arrest and Cardiopulmonary Resuscitation Using Hyperspectral Near Infrared Spectroscopy. Circ. J. 2017, 81, 879–887. [Google Scholar] [CrossRef]
- Nosrati, R.; Lin, S.; Mohindra, R. Study of the Effects of Epinephrine on Cerebral Oxygenation and Metabolism During Cardiac Arrest and Resuscitation by Hyperspectral Near-Infrared Spectroscopy. Crit. Care Med. 2019, 47, 349–357. [Google Scholar] [CrossRef]
- Khalifehsoltani, N.; Rennie, O.; Mohindra, R.; Lin, S.; Toronov, V. Tracking Cerebral Microvascular and Metabolic Parameters during Cardiac Arrest and Cardiopulmonary Resuscitation. Appl. Sci. 2023, 13, 12303. [Google Scholar] [CrossRef]
- Francoeur, C.; Landis, W.P.; Winters, M. Near-infrared spectroscopy during cardiopulmonary resuscitation for pediatric cardiac arrest: A prospective, observational study. Resuscitation 2022, 174, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Raschdorf, K.; Mohseni, A.; Hogle, K. Evaluation of transcutaneous near-infrared spectroscopy for early detection of cardiac arrest in an animal model. Sci. Rep. 2023, 13, 4537. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Wu, W.; Woldemichael, E. Hyperspectral near-infrared spectroscopy assessment of the brain during hypoperfusion. J. Biomed. Opt. 2019, 24, 035007. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, L.N.; Milej, D.; Mistry, J. Using depth-enhanced diffuse correlation spectroscopy and near-infrared spectroscopy to isolate cerebral hemodynamics during transient hypotension. Neurophotonics 2023, 10, 025013. [Google Scholar] [CrossRef]
- Nosrati, R.; Vesely, K.; Schweizer, T.A. Event-related changes of the prefrontal cortex oxygen delivery and metabolism during driving measured by hyperspectral fNIRS. Biomed. Opt. Express 2016, 7, 1323. [Google Scholar] [CrossRef]
- Kovacsova, Z.; Bale, G.; Mitra, S. Absolute quantification of cerebral tissue oxygen saturation with multidistance broadband NIRS in newborn brain. Biomed. Opt. Express 2021, 12, 907. [Google Scholar] [CrossRef]
- Giannoni, L.; Lange, F.; Sajic, M. A Hyperspectral Imaging System for Mapping Haemoglobin and Cytochrome-c-Oxidase Concentration Changes in the Exposed Cerebral Cortex. IEEE J. Sel. Top. Quantum Electron. 2021, 27, 7400411. [Google Scholar] [CrossRef]
- Lange, F.; Peyrin, F.; Montcel, B. Broadband time-resolved multi-channel functional near-infrared spectroscopy system to monitor in vivo physiological changes of human brain activity. Appl. Opt. 2018, 57, 6417. [Google Scholar] [CrossRef]
- Kaynezhad, P.; Mitra, S.; Bale, G. Quantification of the severity of hypoxic-ischemic brain injury in a neonatal preclinical model using measurements of cytochrome-c-oxidase from a miniature broadband-near-infrared spectroscopy system. Neurophotonics 2019, 6, 045009. [Google Scholar] [CrossRef]
- Siddiqui, M.F.; Lloyd-Fox, S.; Kaynezhad, P. Non-invasive measurement of a metabolic marker of infant brain function. Sci. Rep. 2017, 7, 1330. [Google Scholar] [CrossRef]
- Sekhon, M.S.; Smielewski, P.; Bhate, T.D. Using the relationship between brain tissue regional saturation of oxygen and mean arterial pressure to determine the optimal mean arterial pressure in patients following cardiac arrest: A pilot proof-of-concept study. Resuscitation 2016, 106, 120–125. [Google Scholar] [CrossRef]
- Hoiland, R.L.; Sekhon, M.S.; Cardim, D. Lack of agreement between optimal mean arterial pressure determination using pressure reactivity index versus cerebral oximetry index in hypoxic ischemic brain injury after cardiac arrest. Resuscitation 2020, 152, 184–191. [Google Scholar] [CrossRef]
- Rajaram, A.; Milej, D.; Suwalski, M. Optical monitoring of cerebral perfusion and metabolism in adults during cardiac surgery with cardiopulmonary bypass. Biomed. Opt. Express 2020, 11, 5967. [Google Scholar] [CrossRef]
- Soltani, N.; Mohindra, R.; Lin, S.; Toronov, V. Assessing the Relationship Between Cerebral Metabolic Rate of Oxygen and Redox Cytochrome C Oxidase During Cardiac Arrest and Cardiopulmonary Resuscitation. Appl. Sci. 2025, 15, 1542. [Google Scholar] [CrossRef]
- Morse, P.T.; Goebel, D.J.; Wan, J. Cytochrome c oxidase-modulatory near-infrared light penetration into the human brain: Implications for the noninvasive treatment of ischemia/reperfusion injury. IUBMB Life 2021, 73, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Wider, J.M.; Gruley, E.; Morse, P.T. Modulation of mitochondrial function with near-infrared light reduces brain injury in a translational model of cardiac arrest. Crit. Care 2023, 27, 491. [Google Scholar] [CrossRef] [PubMed]
- Morse, P.T.; Tuck, S.; Kerns, M. Non-invasive treatment of ischemia/reperfusion injury: Effective transmission of therapeutic near-infrared light into the human brain through soft skin-conforming silicone waveguides. Bioeng. Transl. Med. 2024, 8, e10496. [Google Scholar] [CrossRef]
- Li, Z.; Baker, W.B.; Parthasarathy, A.B. Calibration of diffuse correlation spectroscopy blood flow index with venous-occlusion diffuse optical spectroscopy in skeletal muscle. J. Biomed. Opt. 2015, 20, 125005. [Google Scholar] [CrossRef]
- Ferradal, S.L.; Yuki, K.; Vyas, R. Non-invasive assessment of cerebral blood flow and oxygen metabolism in neonates during hypothermic cardiopulmonary bypass: Feasibility and clinical implications. Sci. Rep. 2017, 7, srep44117. [Google Scholar] [CrossRef]
- Zavriyev, A.I.; Kaya, K.; Farzam, P. The role of diffuse correlation spectroscopy and frequency-domain near-infrared spectroscopy in monitoring cerebral hemodynamics during hypothermic circulatory arrests. JTCVS Tech. 2021, 7, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.W.; Dar, I.A.; Donohue, K.L. Cerebral Blood Flow Hemispheric Asymmetry in Comatose Adults Receiving Extracorporeal Membrane Oxygenation. Front. Neurosci. 2022, 16, 858404. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.; Mavroudis, C.D.; Ko, T.S. The use of novel diffuse optical spectroscopies for improved neuromonitoring during neonatal cardiac surgery requiring antegrade cerebral perfusion. Front. Pediatr. 2023, 11, 1125985. [Google Scholar] [CrossRef] [PubMed]
- Yücel, M.A.; Selb, J.; Aasted, C.M.; Petkov, M.P.; Becerra, L.; Borsook, D.; Boas, D.A. Short separation regression improves statistical significance and better localizes the hemodynamic response obtained by near-infrared spectroscopy for tasks with differing autonomic responses. Neurophotonics 2015, 2, 35005. [Google Scholar] [CrossRef]
- Blaney, G.; Sassaroli, A.; Pham, T.; Fernandez, C.; Fantini, S. Phase dual-slopes in frequency-domain near-infrared spectroscopy for enhanced sensitivity to brain tissue: First applications to human subjects. J. Biophotonics 2020, 13, 201960018. [Google Scholar] [CrossRef]
- Kamar, F.; Shoemaker, L.N.; Eskandari, R.; Milej, D.; Drosdowech, D.; Murkin, J.M.; Lawrence, K.S.; Chui, J.; Diop, M. Assessing changes in regional cerebral hemodynamics in adults with a high-density full-head coverage time-resolved NIRS device. J. Biomed. Opt. 2024, 29, S33302. [Google Scholar] [CrossRef]
- Cugmas, B.; Bürmen, M.; Bregar, M.; Pernuš, F.; Likar, B. Pressure-induced near infrared spectra response as a valuable source of information for soft tissue classification. J. Biomed. Opt. 2013, 18, 047002. [Google Scholar] [CrossRef]
- Baker, W.B.; Parthasarathy, A.B.; Ko, T.S.; Busch, D.R.; Abramson, K.; Tzeng, S.Y.; Mesquita, R.C.; Durduran, T.; Greenberg, J.H.; Kung, D.K.; et al. Pressure modulation algorithm to separate cerebral hemodynamic signals from extracerebral artifacts. Neurophotonics 2015, 2, 035004. [Google Scholar] [CrossRef]
- Tichauer, K.M.; Elliott, J.T.; Hadway, J.A.; Lee, D.S.; Lee, T.-Y.; Lawrence, K.S. Using near-infrared spectroscopy to measure cerebral metabolic rate of oxygen under multiple levels of arterial oxygenation in piglets. J. Appl. Physiol. 2010, 109, 878–885. [Google Scholar] [CrossRef]
- Verdecchia, K.; Diop, M.; Lee, T.-Y.; Lawrence, K.S. Quantifying the cerebral metabolic rate of oxygen by combining diffuse correlation spectroscopy and time-resolved near-infrared spectroscopy. J. Biomed. Opt. 2013, 18, 27007. [Google Scholar] [CrossRef]
- Milej, D.; Shahid, M.; Abdalmalak, A. Characterizing dynamic cerebral vascular reactivity using a hybrid system combining time-resolved near-infrared and diffuse correlation spectroscopy. Biomed. Opt. Express 2020, 11, 4571. [Google Scholar] [CrossRef]
- Yamashita, O.; Shimokawa, T.; Kosaka, T.; Amita, T.; Inoue, Y.; Sato, M.-A. Hierarchical Bayesian model for diffuse optical tomography of the human brain: Human experimental study. J. Adv. Comput. Intell. Intell. Inform. 2014, 18, 1026–1033. [Google Scholar] [CrossRef]
- Liebert, A.; Wabnitz, H.; Steinbrink, J.; Obrig, H.; Möller, M.; Macdonald, R.; Villringer, A.; Rinneberg, H. Time-resolved multidistance near-infrared spectroscopy of the adult head: Intracerebral and extracerebral absorption changes from moments of distribution of times of flight of photons. Appl. Opt. 2004, 43, 3037–3047. [Google Scholar] [CrossRef] [PubMed]
- Milej, D.; Rajaram, A.; Suwalski, M.; Morrison, L.B.; Shoemaker, L.N.; Lawrence, K.S. Assessing the relationship between the cerebral metabolic rate of oxygen and the oxidation state of cytochrome-c-oxidase. Neurophotonics 2022, 9, 35001. [Google Scholar] [CrossRef]
- Ko, T.S. Attenuation of mitochondrial dysfunction in a ventricular fibrillation swine model of cardiac arrest treated with carbon monoxide. Resuscitation 2024, 213, 110647. [Google Scholar] [CrossRef]
- White, B.R.; Ko, T.S.; Morgan, R.W. Low frequency power in cerebral blood flow is a biomarker of neurologic injury in the acute period after cardiac arrest. Resuscitation 2022, 178, 12–18. [Google Scholar] [CrossRef]
- Lin, H.W.; Gresia, V.L.; Stradecki, H.M. Protein kinase C delta modulates endothelial nitric oxide synthase after cardiac arrest. J. Cereb. Blood Flow Metab. 2014, 34, 613–620. [Google Scholar] [CrossRef]
- Larson, A.C.; Jamrogowicz, J.L.; Kulikowicz, E. Cerebrovascular autoregulation after rewarming from hypothermia in a neonatal swine model of asphyxic brain injury. J. Appl. Physiol. 2013, 115, 1433–1442. [Google Scholar] [CrossRef]
- Candan, T.; Candan, M.; Yildiz, C.E. Comparison of bilateral cerebral and somatic tissue oxygenation with near-infrared spectroscopy in cyanotic and acyanotic pediatric patients receiving cardiac surgery. Arch. Med. Sci. Atheroscler. Dis. 2020, 5, 320–331. [Google Scholar] [CrossRef]
- Cournoyer, A.; Iseppon, M.; Chauny, J.M. Near-infrared Spectroscopy Monitoring During Cardiac Arrest: A Systematic Review and Meta-analysis. Acad. Emerg. Med. 2016, 23, 851–862. [Google Scholar] [CrossRef]
- Nosrati, R.; Ramadeen, A.; Hu, X. Simultaneous measurement of cerebral and muscle tissue parameters during cardiac arrest and cardiopulmonary resuscitation. In Optical Techniques in Neurosurgery, Neurophotonics, and Optogenetics II; Hirschberg, H., Madsen, S.J., Jansen, E.D., Eds.; SPIE: Bellingham, WA, USA, 2015; p. 93051. [Google Scholar]
- Kirschen, M.P.; Majmudar, T.; Beaulieu, F. Deviations from NIRS-derived optimal blood pressure are associated with worse outcomes after pediatric cardiac arrest. Resuscitation 2021, 168, 110–118. [Google Scholar] [CrossRef]
- Mavroudis, C.D.; Ko, T.S.; Morgan, R.W. Epinephrine’s effects on cerebrovascular and systemic hemodynamics during cardiopulmonary resuscitation. Crit. Care 2020, 24, 583. [Google Scholar] [CrossRef]
- Hou, L.; Liu, Y.; Qian, L. Portable Near-Infrared Technologies and Devices for Noninvasive Assessment of Tissue Hemodynamics. J. Healthc. Eng. 2019, 12, 3750495. [Google Scholar] [CrossRef] [PubMed]
- Nabacino, M.; Amendola, C.; Contini, D. Fast Multi-Distance Time-Domain NIRS and DCS System for Clinical Applications. Sensors 2024, 24, 7375. [Google Scholar] [CrossRef] [PubMed]
- Blaney, G.; Fernandez, C.; Sassaroli, A. Dual-slope imaging of cerebral hemodynamics with frequency-domain near-infrared spectroscopy. Neurophotonics 2023, 10, 013508. [Google Scholar] [CrossRef]
- Lange, F.; Tachtsidis, I. Clinical brain monitoring with time domain NIRS: A review and future perspectives. Appl. Sci. 2019, 9, 1612. [Google Scholar] [CrossRef]
- Fallon, P.; Roberts, I.; Kirkham, F.J. Cerebral hemodynamics during cardiopulmonary bypass in children using near-infrared spectroscopy. Ann. Thorac. Surg. 1993, 56, 1473–1477. [Google Scholar] [CrossRef]
- Yeganeh, H.Z.; Toronov, V.; Elliott, J.T. Broadband continuous-wave technique to measure baseline values and changes in the tissue chromophore concentrations. Biomed. Opt. Express 2012, 3, 2761. [Google Scholar] [CrossRef]
- Smith, M. Shedding light on the adult brain: A review of the clinical applications of near-infrared spectroscopy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 4452–4469. [Google Scholar] [CrossRef]
- Tachtsidis, I.; Scholkmann, F. False positives and false negatives in functional near-infrared spectroscopy: Issues, challenges, and the way forward. Neurophotonics 2016, 3, 31405. [Google Scholar] [CrossRef]
- Ayaz, H.; Baker, W.B.; Blaney, G. Optical imaging and spectroscopy for the study of the human brain: Status report. Neurophotonics 2022, 9, S24001. [Google Scholar] [CrossRef]
- Leadley, G.; Austin, T.; Bale, G. Review of measurements and imaging of cytochrome-c-oxidase in humans using near-infrared spectroscopy: An update. Biomed. Opt. Express 2023, 15, 162. [Google Scholar] [CrossRef]
- Denault, A.Y.; Shaaban-Ali, M.; Cournoyer, A. Near-Infrared Spectroscopy. In Neuromonitoring Techniques; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Sandroni, C.; Skrifvars, M.B.; Taccone, F.S. Brain monitoring after cardiac arrest. Curr. Opin. Crit. Care 2023, 29, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Genbrugge, C.; Dens, J.; Meex, I. Regional Cerebral Oximetry during Cardiopulmonary Resuscitation: Useful or Useless? J. Emerg. Med. 2016, 50, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Okuma, Y.; Shinozaki, K.; Yagi, T.; Hayashida, K.; Aoki, T.; Yin, T.; Kiguchi, T.; Iwami, T.; Becker, L.B. Oxyhaemoglobin Level Measured Using Near-Infrared Spectrometer Is Associated with Brain Mitochondrial Dysfunction After Cardiac Arrest in Rats. In Oxygen Transport to Tissue XLIII; Felix, S., Joseph, L., Wolf, U., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 385–390. [Google Scholar]
- Fredriksson, I.; Larsson, M.; Salomonsson, F. Improved calibration procedure for laser Doppler perfusion monitors. In Optical Diagnostics and Sensing XI: Toward Point-of-Care Diagnostics; and Design and Performance Validation of Phantoms Used in Conjunction with Optical Measurement of Tissue III; SPIE: Bellingham, WA, USA, 2011; p. 790602. [Google Scholar]
- Culver, J.P.; Durduran, T.; Cheung, C. Diffuse Optical Measurement of Hemoglobin and Cerebral Blood Flow in Rat Brain During Hypercapnia, Hypoxia and Cardiac Arrest. In Oxygen Transport To Tissue XXIII; Springer: New York, NY, USA, 2003; pp. 293–297. [Google Scholar]
- Buckley, E.M.; Lynch, J.M.; Goff, D.A. Early postoperative changes in cerebral oxygen metabolism following neonatal cardiac surgery: Effects of surgical duration. J. Thorac. Cardiovasc. Surg. 2013, 145, 196–205.e1. [Google Scholar] [CrossRef]
- Setchfield, K.; Gorman, A.; Simpson, A.H.R.W.; Somekh, M.G.; Wright, A.J. Effect of skin color on optical properties and the implications for medical optical technologies: A review. J. Biomed. Opt. 2024, 29, 010901. [Google Scholar] [CrossRef] [PubMed]
- Strangman, G.E.; Zhang, Q.; Li, Z. Scalp and skull influence on near-infrared photon propagation in the Colin27 brain template. NeuroImage 2014, 85, 136–149. [Google Scholar] [CrossRef]
- Carp, S.A.; Robinson, M.B.; Franceschini, M.A. Diffuse correlation spectroscopy: Current status and future outlook. Neurophotonics 2023, 10, 013509. [Google Scholar] [CrossRef]
- James, E.; Munro, P.R.T. Diffuse Correlation Spectroscopy: A Review of Recent Advances in Parallelisation and Depth Discrimination Techniques. Sensors 2023, 23, 9338. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, M.; Kreiss, L.; Samaei, S.; Carp, S.A.; Johansson, J.D.; Zhang, Y.; Wu, M.; Horstmeyer, R.; Diop, M.; et al. A comprehensive overview of diffuse correlation spectroscopy: Theoretical framework, recent advances in hardware, analysis, and applications. NeuroImage 2024, 298, 120793. [Google Scholar] [CrossRef]
- Ozana, N.; Lue, N.; Renna, M.; Robinson, M.B.; Martin, A.; Zavriyev, A.I.; Carr, B.; Mazumder, D.; Blackwell, M.H.; Franceschini, M.A.; et al. Functional Time-Domain Diffuse Correlation Spectroscopy. Front. Neurosci. 2022, 16, 932119. [Google Scholar] [CrossRef] [PubMed]
- Eleveld, N. The Influence of Extracerebral Tissue on Continuous-Wave NIRS. J. Clin. Med. 2023, 12, 2776. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Buckley, E.M. Influence of source–detector separation on diffuse correlation spectroscopy measurements of cerebral blood flow with a multilayered analytical model. Neurophotonics 2022, 9, 035002. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, M.D. Signal Processing in fNIRS: A Review. Front. Hum. Neurosci. 2018, 11, 641. [Google Scholar]
- Meadow, W.; Rudinsky, B.; Raju, T.; John, E.; Fornell, L.; Shankararao, R. Correlation of flow probe determinations of common carotid artery blood flow and internal carotid artery blood flow with microsphere determinations of cerebral blood flow in piglets. Pediatr. Res. 1999, 45, 324–330. [Google Scholar] [CrossRef]
- Powers, K.M.; Schimmel, C.; Glenny, R.W.; Bernards, C. Cerebral blood flow determinations using fluorescent microspheres: Variations on the sedimentation method validated. J. Neurosci. Methods 1999, 87, 159–165. [Google Scholar] [CrossRef]
- Mörtberg, E.; Cumming, P.; Wiklund, L.; Rubertsson, S. Cerebral metabolic rate of oxygen (CMRO2) in pig brain determined by PET after resuscitation from cardiac arrest. Resuscitation 2009, 80, 701–706. [Google Scholar] [CrossRef]
- Toronov, V.; Soltani, N.; Leung, L.; Mohindra, R.; Lin, S. Using Coherent Hemodynamic Spectroscopy Model to Investigate Cardiac Arrest. Algorithms 2025, 18, 128. [Google Scholar] [CrossRef]
- Xu, M.; Zheng, Y.; Chen, X. Dynamic microcirculation PIPE model for functional neuroimaging, non-neuroimaging, and coherent hemodynamics spectroscopy: Blood volume and flow velocity variations, and vascular autoregulation. Biomed. Opt. Express 2020, 11, 4602. [Google Scholar] [CrossRef]
- Fantini, S. A new hemodynamic model shows that temporal perturbations of cerebral blood flow and metabolic rate of oxygen cannot be measured individually using functional near-infrared spectroscopy. Physiol. Meas. 2013, 35, N1–N9. [Google Scholar] [CrossRef]
- Grubb, R.L., Jr.; Raichle, M.E.; Eichling, J.O.; Ter-Pogossian, T.-P. The effect of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke 1974, 5, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Buxton, R.B.; Wong, E.C.; Frank, L.R. Dynamics of blood flow and oxygenation changes during brain activation: The balloon model. Magn. Reson. Med. 1998, 39, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Mechelli, A.; Turner, R.; Price, C. Nonlinear responses in fMRI: The Balloon model, Volterra kernels, and other hemodynamics. NeuroImage 2000, 12, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Stephan, K.E.; Weiskopf, N.; Drysdale, P.M.; Robinson, P.A.; Friston, K.J. Comparing hemodynamic models with DCM. NeuroImage 2007, 38, 387–401. [Google Scholar] [CrossRef]
- Tak, S.; Kempny, A.M.; Friston, K.; Leff, A.; Penny, W. Dynamic causal modelling for functional near-infrared spectroscopy. NeuroImage 2015, 111, 338–349. [Google Scholar] [CrossRef]
- Ciftçi, K.; Sankur, B.; Kahya, Y.P.; Akin, A. Multilevel statistical inference from functional near-infrared spectroscopy data during Stroop interference. IEEE Trans. Biomed. Eng. 2008, 55, 1865–1872. [Google Scholar] [CrossRef]
- Colebank, M.J. Assessing parameter identifiability of a hemodynamics PDE model using spectral surrogates and dimension reduction. PLoS Comput. Biol. 2025, 21, 1013553. [Google Scholar] [CrossRef]
- Hapuarachchi, T.; Scholkmann, F.; Caldwell, M. Simulation of Preterm Neonatal Brain Metabolism During Functional Neuronal Activation Using a Computational Model. In Oxygen Transport to Tissue XXXVII; Springer: New York, NY, USA, 2016; pp. 111–120. [Google Scholar]
- Russell-Buckland, J.; Tachtsidis, I. Developing a Model to Simulate the Effect of Hypothermia on Cerebral Blood Flow and Metabolism. Adv. Exp. Med. Biol. 2020, 1232, 299–306. [Google Scholar]
- Durduran, T.; Choe, R.; Baker, W.B.; Yodh, A.G. Diffuse optics for tissue monitoring and tomography. Rep. Prog. Phys. 2010, 73, 76701. [Google Scholar] [CrossRef]
- Jubran, A. Pulse oximetry. Crit. Care 2015, 19, 272. [Google Scholar] [CrossRef]
- Brigadoi, S.; Cooper, R.J. How short is short? Optimum source–detector distance for short-separation channels in functional near-infrared spectroscopy. Neurophotonics 2015, 2, 25005. [Google Scholar] [CrossRef] [PubMed]
- Zimeo Morais, G.A.; Balardin, J.B.; Sato, J.R. fNIRS Optodes’ Location Decider (fOLD): A toolbox for probe arrangement guided by brain regions-of-interest. Sci. Rep. 2018, 8, 3341. [Google Scholar] [CrossRef] [PubMed]
- Yücel, M.A. Best practices for fNIRS publications. Neurophotonics 2021, 8, 012101. [Google Scholar] [CrossRef] [PubMed]
- Blaney, G.; Sassaroli, A.; Fantini, S. Algorithm for determination of thresholds of significant coherence in time-frequency analysis. Biomed. Signal Process. Control. 2020, 56, 101704. [Google Scholar] [CrossRef]
- Soltani, N.; Toronov, V.; Rennie, O. Investigating cerebral dynamics during cardiac arrest using an optical technique and a hemodynamic model. SPIE Int. Soc. Opt. Eng. 2024, 21, 19. [Google Scholar]
- Shichkova, P.; Coggan, J.S.; Kanari, L. Breakdown and repair of metabolism in the aging brain. Front. Sci. 2025, 3, 1441297. [Google Scholar] [CrossRef]
- Serraino, G.F.; Murphy, G.J. Effects of cerebral near-infrared spectroscopy on the outcome of patients undergoing cardiac surgery: A systematic review of randomised trials. BMJ Open 2017, 7, e016613. [Google Scholar] [CrossRef]
- Zheng, F.; Sheinberg, R.; Yee, M.S. Cerebral near-infrared spectroscopy monitoring and neurologic outcomes in adult cardiac surgery patients: A systematic review. Anesth. Analg. 2013, 116, 663–676. [Google Scholar] [CrossRef]
- Nenna, A.; Barbato, R.; Greco, S.M. Near-infrared spectroscopy in adult cardiac surgery: Between conflicting results and unexpected uses. J. Geriatr. Cardiol. 2017, 14, 659–661. [Google Scholar]
- Sun, Q.; Wu, W. Effect of near-infrared spectroscopy on postoperative delirium in cardiac surgery with cardiopulmonary bypass: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2024, 11, 1404210. [Google Scholar] [CrossRef]
- Israel, H.; Richter, R.R. A guide to understanding meta-analysis. J. Orthop. Sports Phys. Ther. 2011, 41, 496–504. [Google Scholar] [CrossRef]
- Zhou, C.; Eucker, S.A.; Durduran, T. Diffuse optical monitoring of hemodynamic changes in piglet brain with closed head injury. J. Biomed. Opt. 2009, 14, 34015. [Google Scholar] [CrossRef]
- Caldwell, M.; Moroz, T.; Hapuarachchi, T. Modelling Blood Flow and Metabolism in the Preclinical Neonatal Brain during and Following Hypoxic-Ischaemia. PLoS ONE 2015, 10, 140171. [Google Scholar] [CrossRef]
- Eskandari, R. Quantification of oxidized and reduced cytochrome-c-oxidase by combining discrete-wavelength time-resolved and broadband continuous-wave near-infrared spectroscopy. Biomed. Opt. Express 2025, 16, 3797–3812. [Google Scholar] [CrossRef]
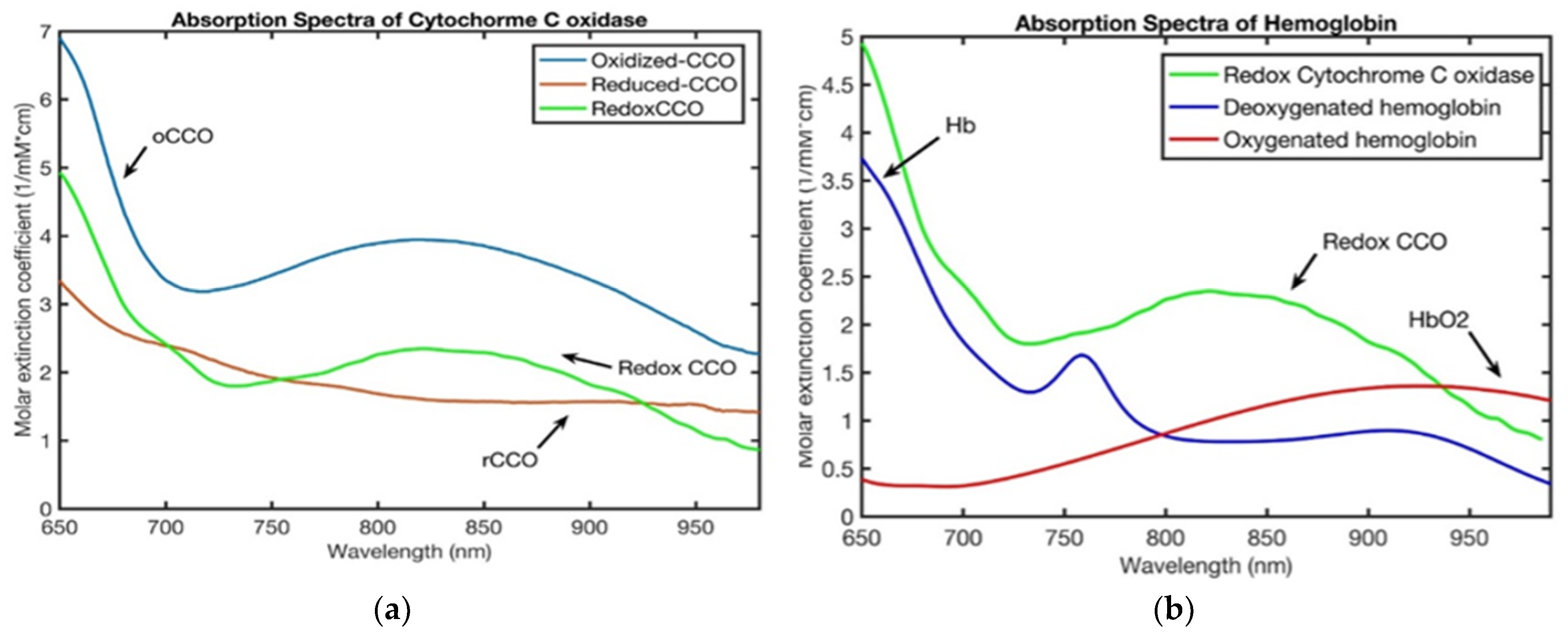
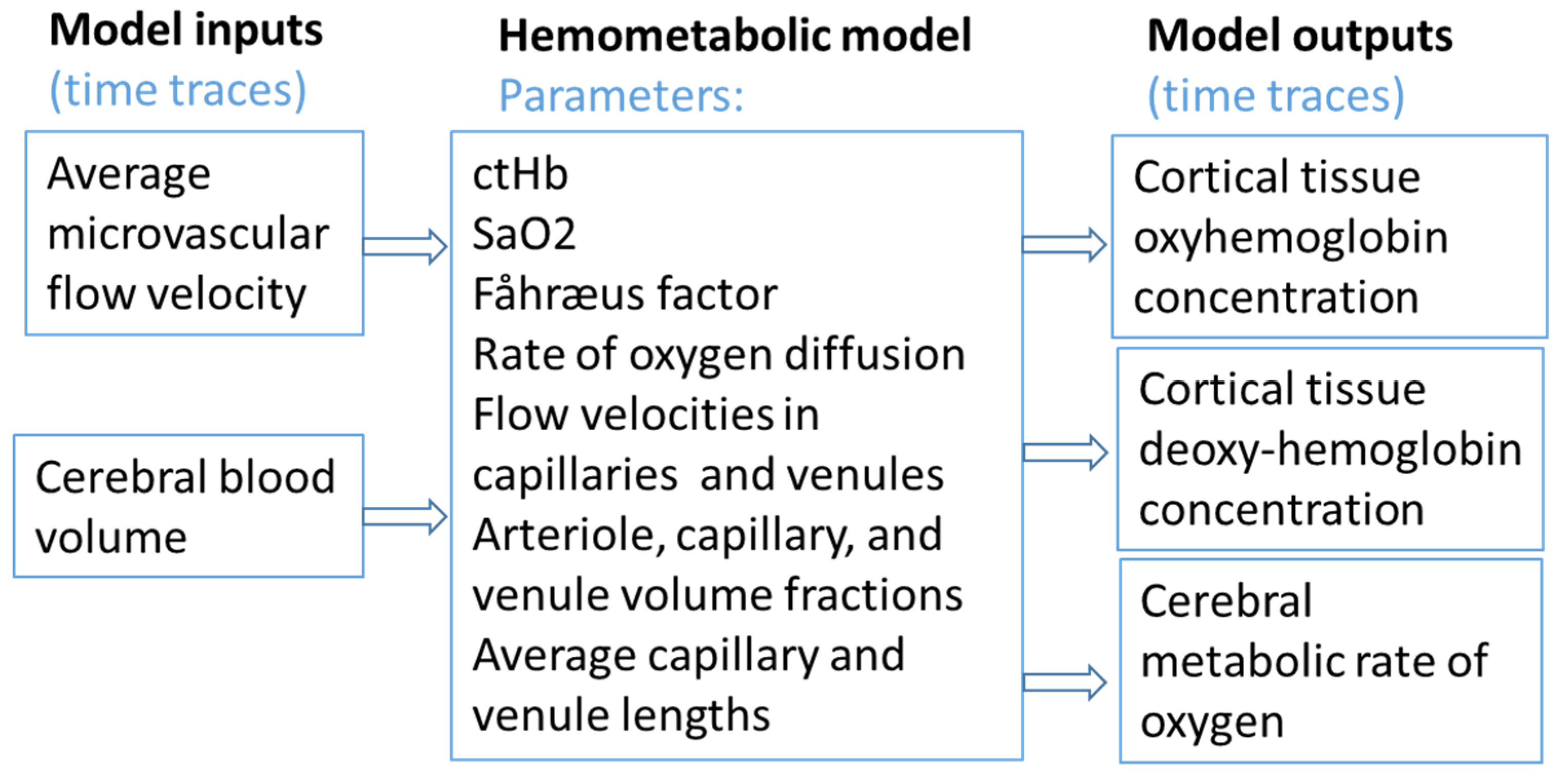
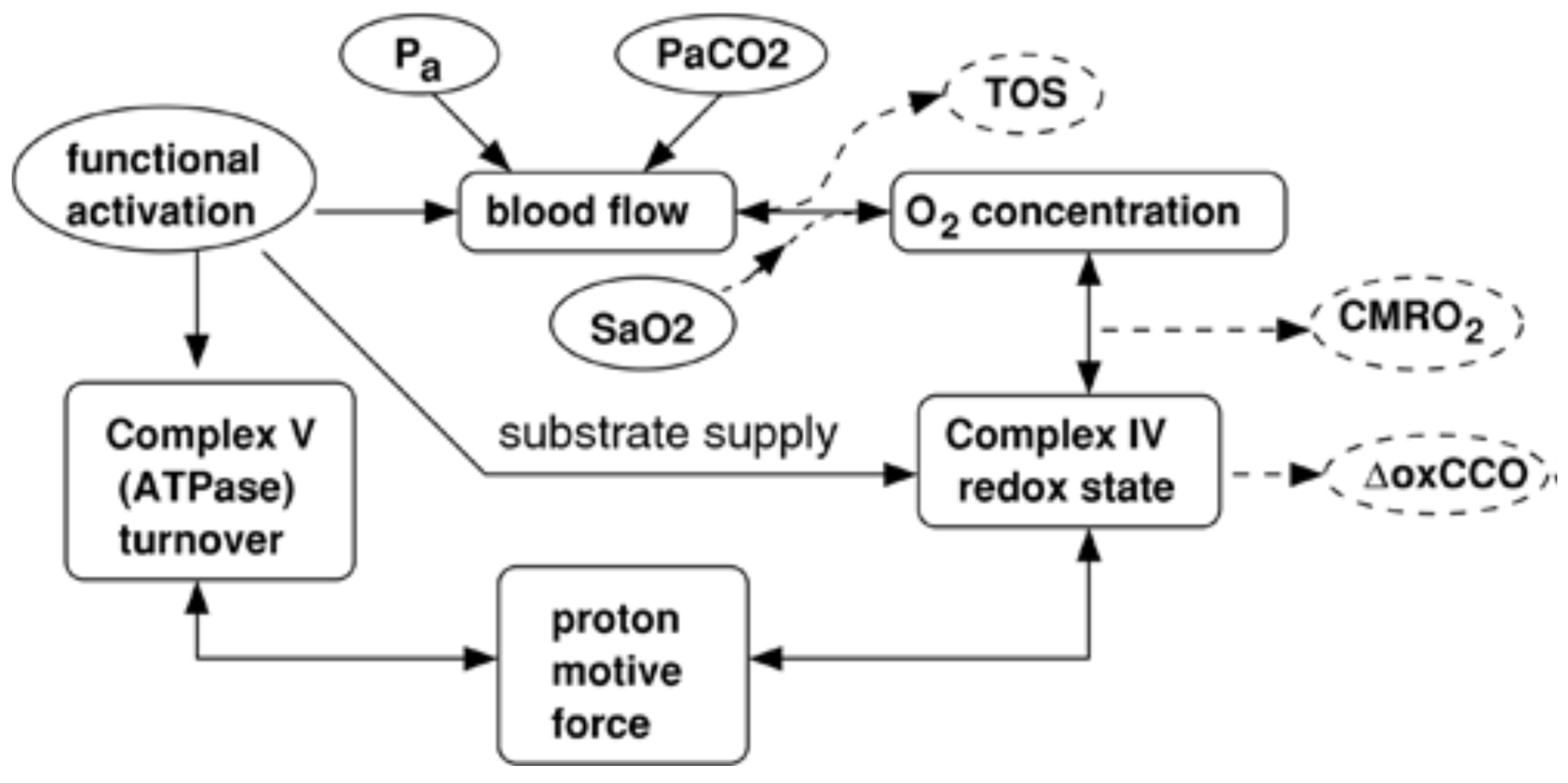
| Ref. # | Species and Sample Size | Device/Manufacturer | NIRS Type and DCS (Y/N) | Primary Endpoints | Key Finding | Quality/Limitations |
|---|---|---|---|---|---|---|
| [18] | Human (CPR) 52 | Imagent ISS Inc., Champaig, IL, USA | FD NIRS | Optical indices vs. ROSC | Optical signals predicted ROSC during CPR. | Exact device and n require full text. |
| [19] | Animal (pigs; CA/CPR) 14 | Custom | hHIRS | O2 delivery, ΔoxCCO, Hb species | hNIRS-tracked cerebral hemo-metabolic shifts during CA/CPR. | Model-based deconvolution; motion in CPR. |
| [20] | Animal (pigs; CA/CPR) 9 | Custom | hNIRS | ΔoxCCO, HbO/HbR, O2 delivery | Epinephrine bolus produced transient metabolic/oxygenation boosts. | Small n; translation uncertain. |
| [21] | Animal (pigs; CA/CPR) 10 | Custom | hNIRS | ΔoxCCO, Hb species; | CHS inversion-tracked microvascular and metabolic parameters during CA/CPR; majority of cases fit adequately. | Model dependence; preclinical instrumentation; modest n. |
| [22] | Human (pediatric CPR) 21 | Equanox 7600; (Nonin Medical, Plymouth, MN, USA) | CW cerebral oximeter | rSO2 during CPR; ROSC/survival links | Higher rSO2 associated with ROSC. | Observational; motion/compression artifacts. |
| Human (pediatric post-arrest) 34 | (Nonin SenSmart Nonin Medical, Inc., Plymouth, MN, USA)) | CW cerebral oximeter | Deviation from MAPopt (NIRS-COx) vs. outcome | Time below MAPopt → worse outcomes. | Retrospective aspects; device-specific index. | |
| [23] | Animal 8 | NX-BF/OF/E; Oxford Optronix, Oxford, UK | Combined tissue pO2 and blood flow monitor | TSI%, pulse detection in barbiturate CA | NIRS detected arrest early. | Anesthetic CA model; small n. |
| [24] | Animal/human (hypoperfusion) 10 | Hyperspectral NIRS, custom | hNIRS | HbO2/HHb/tHb; cortical mapping | Hyperspectral NIRS captured brain changes during hypoperfusion. | Small cohorts; model dependence. |
| [25] | Human (healthy adults) 9 | Custom | Depth-enhanced DCS + TR-NIRS | ΔCBF, rSO2 under hypotension; extracerebral removal | Depth-enhanced DCS improved cerebral specificity. | Experimental hypotension; lab system. |
| [26] | Human (drivers) 16 | Custom | hNIRS | O2 delivery (HbT × SaO2), metabolism indices | Driving task modulated prefrontal delivery/metabolism. | Task-based fNIRS; algorithmic deconvolution. |
| [27] | Human (term infant with HIE) 1 | Custom | Multi-distance hNIRS, TR-NIRS | Absolute StO2 (cerebral) | Demonstrated absolute cerebral StO2 quantification in neonates. | Algorithm/device specific; clinical validation pending. |
| [28] | Phantom, Animal (mice, exposed cortex) 3 | Custom | hNIRS | Hb, oxCCO maps | Mapped Hb and oxCCO changes across cortex. | Exposed cortex; through-skull translation pending. |
| [29] | Human 5 | Custom | TD hNIRS | Functional responses (HbO/HbR) | In vivo monitoring of brain activity with broadband TD-fNIRS. | Research system; small cohorts. |
| [30] | Animal (neonatal pig model) 27 | Custom | hNIRS | ΔoxCCO, Hb changes vs. injury severity | oxCCO tracked hypoxic–ischemic injury severity. | Preclinical model; miniature device. |
| [31] | Human (infants) 33 | Custom | hNIRS | ΔoxCCO as metabolic marker | Detected task-related oxCCO in infants. | Signal-to-noise; motion; developmental variability. |
| [32] | Human (post-CA) 20 | Invos, (Covidien, Dublin, Ireland) | CW cerebral oximeter | MAP-rSO2 reactivity (COx), MAPopt | Derived patient-specific MAPopt post-CA. | Pilot; single-center. |
| [33] | Human (post-CA) 10 | INVOS, (Medtronic, Minneapolis, MN, USA) | CW cerebral oximeter | Agreement of MAPopt (COx vs. Prox) | Poor agreement between COx- and PRx-derived MAPopt. | Physiologic index comparison; modest n. |
| [34] | Human (adults; cardiac surgery) 10 | Custom | hNIRS + DCS | rSO2, rCBF, CMRO2 (indices) | Continuous intra-op perfusion/metabolism tracking feasible. | Single-center; research hardware. |
| [35] | Animal (pigs; CA/CPR) 11 | Custom | hNIRS | CMRO2 index vs. ΔoxCCO during CA/CPR | oxCCO changes paralleled model-derived CMRO2 trends. | Model dependence. |
| [36] | Human cadavers/ex vivo 4 | Custom | Photobiomodulation delivery | NIR penetration to brain; therapeutic feasibility | Transmission to cortex via silicone waveguides context. | Mixed experimental/simulation; translational assumptions. |
| [37] | Animal (pig cardiac arrest model) 30 | Custom | PBM (810–1064 nm) + physiologic monitoring | Neurologic injury markers; survival | PBM reduced brain injury in translational CA model. | Model selection; dosing regimen. |
| [38] | Human cadavers/ex vivo 4 | Custom | Silicone waveguide PBM system | Delivered dose at scalp/skull/CSF | Effective brain-directed PBM transmission demonstrated. | Delivery study; not neuro-outcome trial. |
| [39] | Human (skeletal muscle) 10 | Imagent (ISS Medical, Champaign, IL, USA) | FD NIRS Custom DCS | DCS BFI calibration vs. DOS in muscle | Established calibration between DCS and DOS. | Muscle not brain; still physiologically experimental. |
| Human (neonates with CHD) 36 | Imagent (ISS Medical, Champaign, IL, USA) | FD NIRS + custom DCS | rCBF, rCMRO2, rOEF | Quantified post-op trends in rCBF/rCMRO2/rOEF. | Observational; relative metrics; heterogeneous cases. | |
| [40] | Human neonates 9 | Imagent (ISS Medical, Champaign, IL, USA) | FD NIRS custom DCS | CBF, CMRO2 (indices), rSO2 | CBF and CMRO2 decreased during deep hypothermic CPB; feasibility shown. | Pilot; small n; custom device; motion/cooling confounds. |
| [41] | Human (adults; HCA strategies) 12 | MetaOx (ISS Inc., Champaign, IL, USA) | FD NIRS custom DCS | CBF index, CMRO2 index, rSO2 | DCS showed near-zero CBF in HCA; ACP restored flow. | Indices; perfusion strategy heterogeneity. |
| [42] | Human (comatose adults on VA-ECMO) 13 | Custom | Bilateral DCS | CBF asymmetry index | Frequent hemispheric rCBF asymmetry on ECMO. | Single-center; relative CBF. |
| [43] | Human (neonates; cardiac surgery) ~5 | Custom | FD-NIRS + DCS | CBF index, rSO2 during ACP | Hybrid optics provided value beyond rSO2 alone. | Small series; descriptive. |
| [44] | Human (task fNIRS) 11 | CW6 (TechEn Inc., Milford, MA, USA) | CW NIRS | HbO/HbR HRF significance, localization | Short-separation regression improved stats/localization. | Task fNIRS; device-algorithm specificity. |
| [45] | Human (adults) 4 | Imagent, (ISS Medical, Champaign, IL, USA) | FD dual-slope NIRS | Cortical sensitivity metrics | Enhanced brain sensitivity with phase dual-slope FD-NIRS. | First applications; modest n. |
| [46] | Human (adults) 37 | Custom | TD NIRS | Regional hemodynamics during tasks | Full-head TR-NIRS mapped regional responses. | Complex headgear; lab setup. |
| [47] | Human (soft tissue) 4 | Custom | hNIRS | Pressure-induced spectral response | Pressure modulates spectra; tissue classification aid. | Non-cerebral tissue; lab conditions. |
| [48] | Humans 9 | Imagent (ISS Medical, Champaign, IL, USA | FD-NIRS + custom DCS | Cerebral Hb and CBF changes | Pressure modulation separates cerebral hemodynamic signals from extracerebral artifacts | Pressure needs to be applied to the probe |
| [49] | Animal (newborn piglets) 12 | Custom | TR NIRS + DCS | Absolute CBF, SvO2, CMRO2 | Validated NIRS-based CMRO2 across SaO2 levels. | Catheter references; specialized setup. |
| [50] | Human (adults) 7 | Custom | TD NIRS + DCS | Absolute/calibrated CBF, SvO2, CMRO2 | Enabled calibrated CMRO2 changes; SvO2 validated. | Small cohort; invasive venous ref. |
| [51] | Human (healthy adults) 9 | Custom | TD NIRS + DCS | ΔCBF, ΔHbO2, ΔHHb | Decomposed static/dynamic CVR to CO2. | Healthy cohort; research setup. |
| [52] | Human 1 | FOIRE3000 (Shimadzu Corp., Kyoto, Japan) | High-density CW fNIRS | DOT reconstruction of brain activity | Hierarchical Bayes DOT with human data. | Single subject; computational focus. |
| [53] | Human (adult) 1 | Custom | TD NIRS | ICG-based depth separation (DTOF moments) | Separated intra- vs. extracerebral absorption. | Single subject; ICG required. |
| [54] | Animal (piglets) 6 | Custom | TD NIRS+ DCS | CMRO2 vs. oxCCO relationship | Reported association between CMRO2 changes and oxCCO. | Small n. |
| [55] | Animal (swine VF CA) 11 | Custom | FD-NIRS + DCS | Mitochondrial/vascular outcomes | CO-attenuated mitochondrial dysfunction post-CA. | Translational gap to humans. |
| [56] | Piglets (swine CA) 37 | Imagent (ISS Medical, Champaign, IL, USA) | FD NIRS+ DCS | Low-frequency CBF power vs. injury | Low-frequency CBF power linked to neurologic injury. | Observational; small n. |
| [57] | Animal (post-CA) ~30 | Custom | No NIRS, two-photon laser scanning microscopy , LDF | PKCδ ↔ eNOS modulation after CA | Protein kinase C delta modulates endothelial nitric oxide synthase after cardiac arrest | LDF used to monitor CA and recovery; molecular focus; not NIRS/DCS. |
| [58] | Animal (neonatal swine) 48 | Covidien (Boulder, CO, USA); LDF—Moor Instruments DRT4 (Devon, UK) | CW cerebral oximeter | rSO2, rTHb, COx, HVx, CBF (LDF) | Autoregulation altered post-hypothermia. | Model specificity. |
| [59] | Human (pediatric cardiac surgery) 30 | INVOS (Somanetics, Covidien Mansfield, MA, USA) | CW cerebral oximeter | Bilateral cerebral vs. somatic rSO2 | Compared cyanotic vs. acyanotic patterns. | Observational; specific cohort. |
| Variable | Definition | Unit | CHS | BrainSignals |
|---|---|---|---|---|
| CMRO2 | Cerebral metabolic rate of oxygen | ✗ | ✓ | |
| CBF | Cerebral blood flow | ✗ | ✓ | |
| StO2 | Absolute total oxygen saturation | % | ✓ | ✓ |
| ∆oxCCO | Changes in oxidized state of cytochrome C oxidase | ✗ | ✓ | |
| ∆[tHB] | Changes in total hemoglobin | ✓ | ✓ | |
| ∆[HbO2] | Changes in oxyhemoglobin | ✓ | ✓ | |
| ∆[HHb] | Changes in deoxyhemoglobin | ✓ | ✓ | |
| Oxygen saturation of venous blood | % | ✓ | ✓ | |
| Oxygen saturation of capillary blood | % | ✓ | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soltani, N.; Toronov, V. From Light to Insight: Hemodynamic Models for Optical Monitoring of the Brain in Cardiac Arrest. Appl. Sci. 2025, 15, 12260. https://doi.org/10.3390/app152212260
Soltani N, Toronov V. From Light to Insight: Hemodynamic Models for Optical Monitoring of the Brain in Cardiac Arrest. Applied Sciences. 2025; 15(22):12260. https://doi.org/10.3390/app152212260
Chicago/Turabian StyleSoltani, Nima, and Vladislav Toronov. 2025. "From Light to Insight: Hemodynamic Models for Optical Monitoring of the Brain in Cardiac Arrest" Applied Sciences 15, no. 22: 12260. https://doi.org/10.3390/app152212260
APA StyleSoltani, N., & Toronov, V. (2025). From Light to Insight: Hemodynamic Models for Optical Monitoring of the Brain in Cardiac Arrest. Applied Sciences, 15(22), 12260. https://doi.org/10.3390/app152212260







