Abrupt Change Detection of ECG by Spiking Neural Networks: Policy-Aware Operating Points for Edge-Level MI Screening
Abstract
1. Introduction
2. Related Works
3. Materials and Methods
3.1. Dataset Description
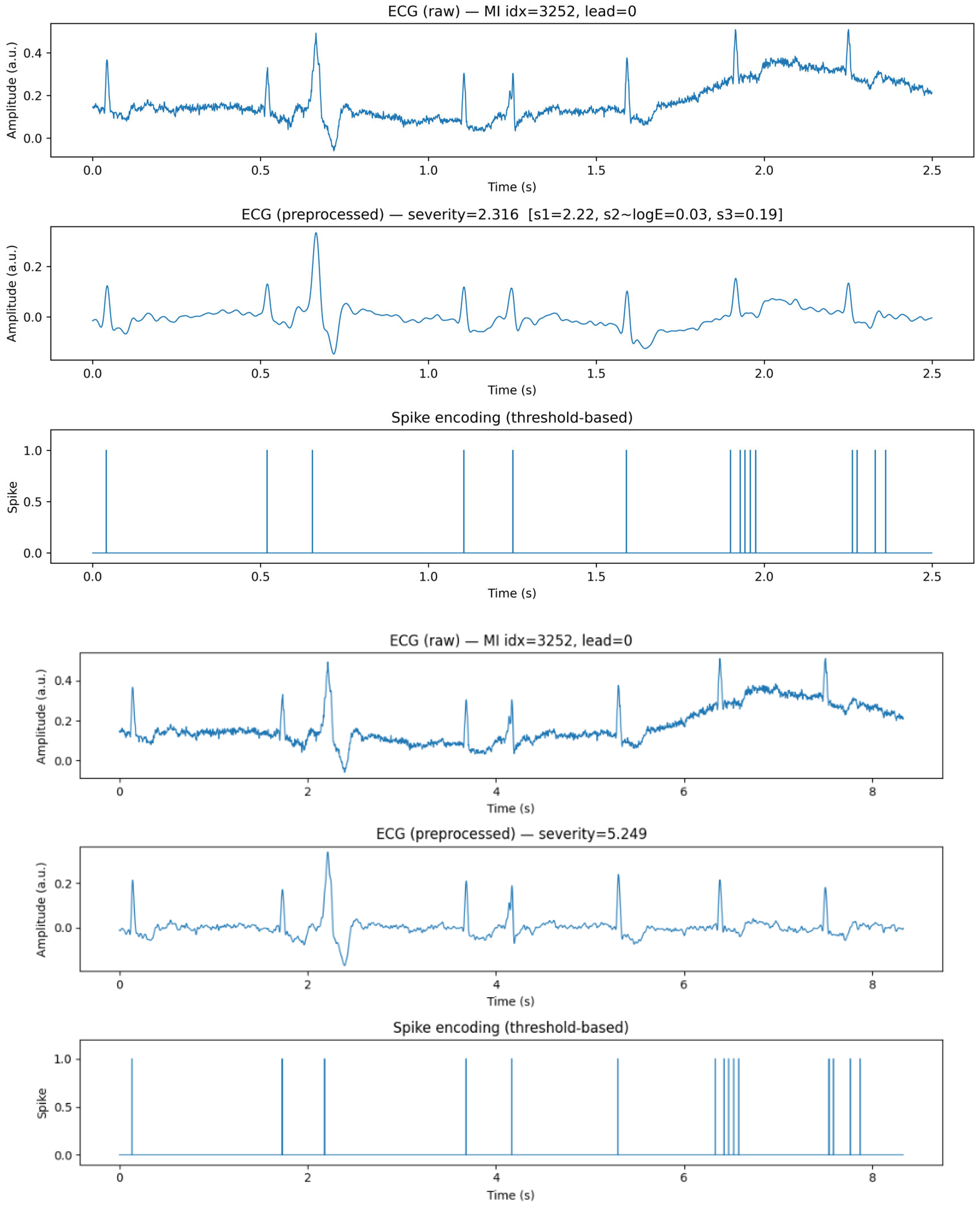
3.2. Signal Preprocessing
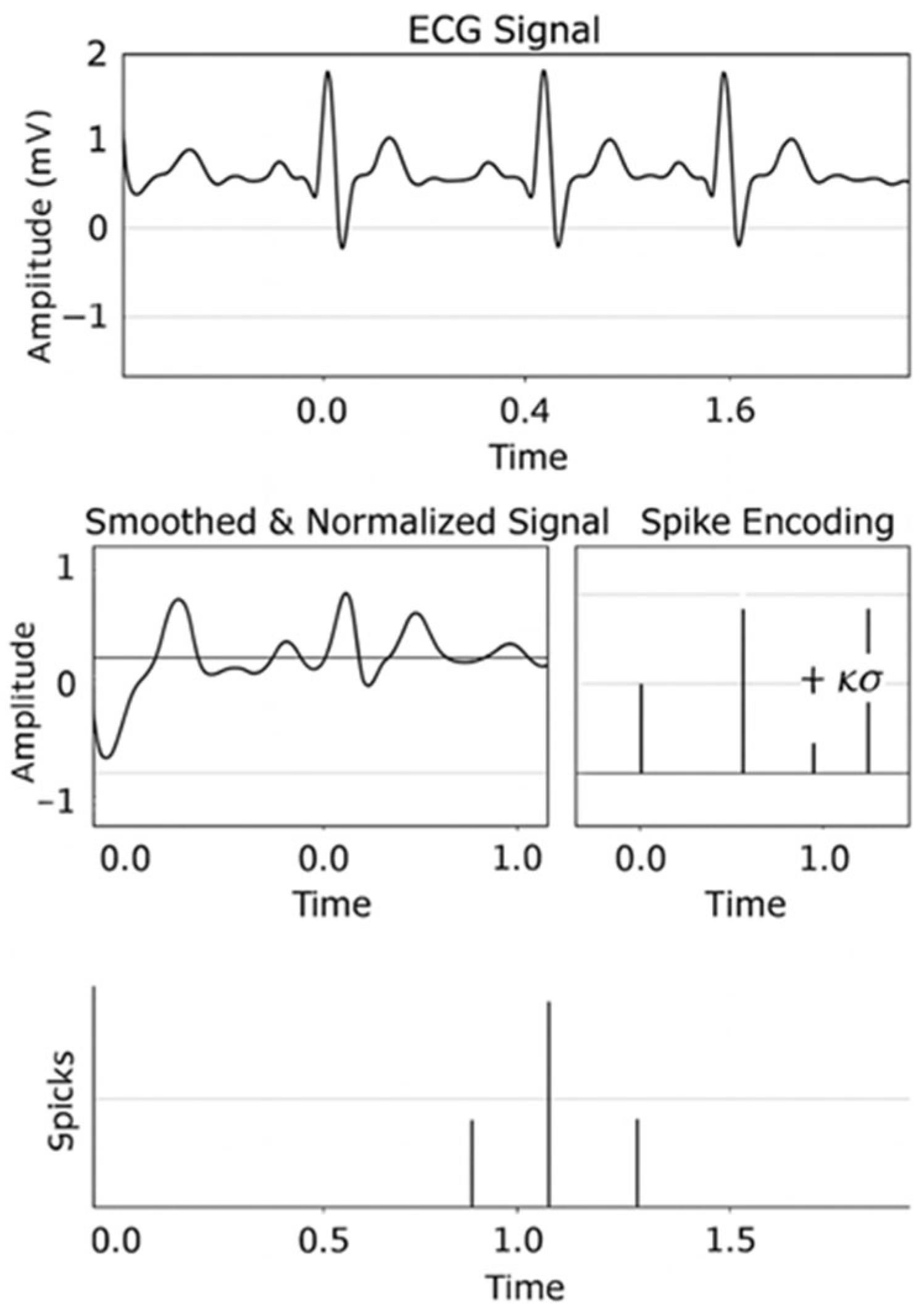
3.3. Spike Encoding
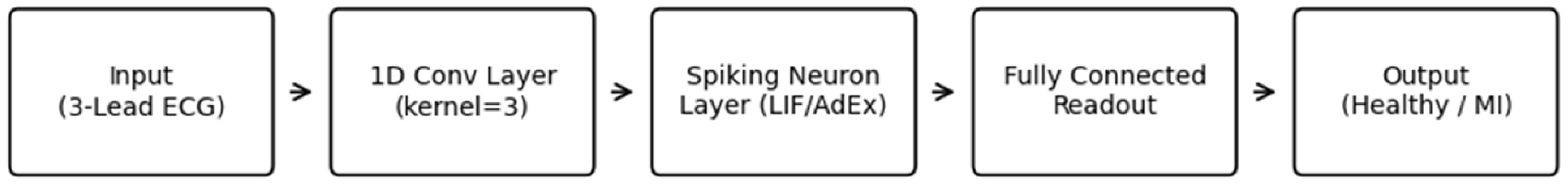
3.4. Spiking Neural Network Architecture
3.5. Training Configuration
3.6. Evaluation Metrics
4. Results
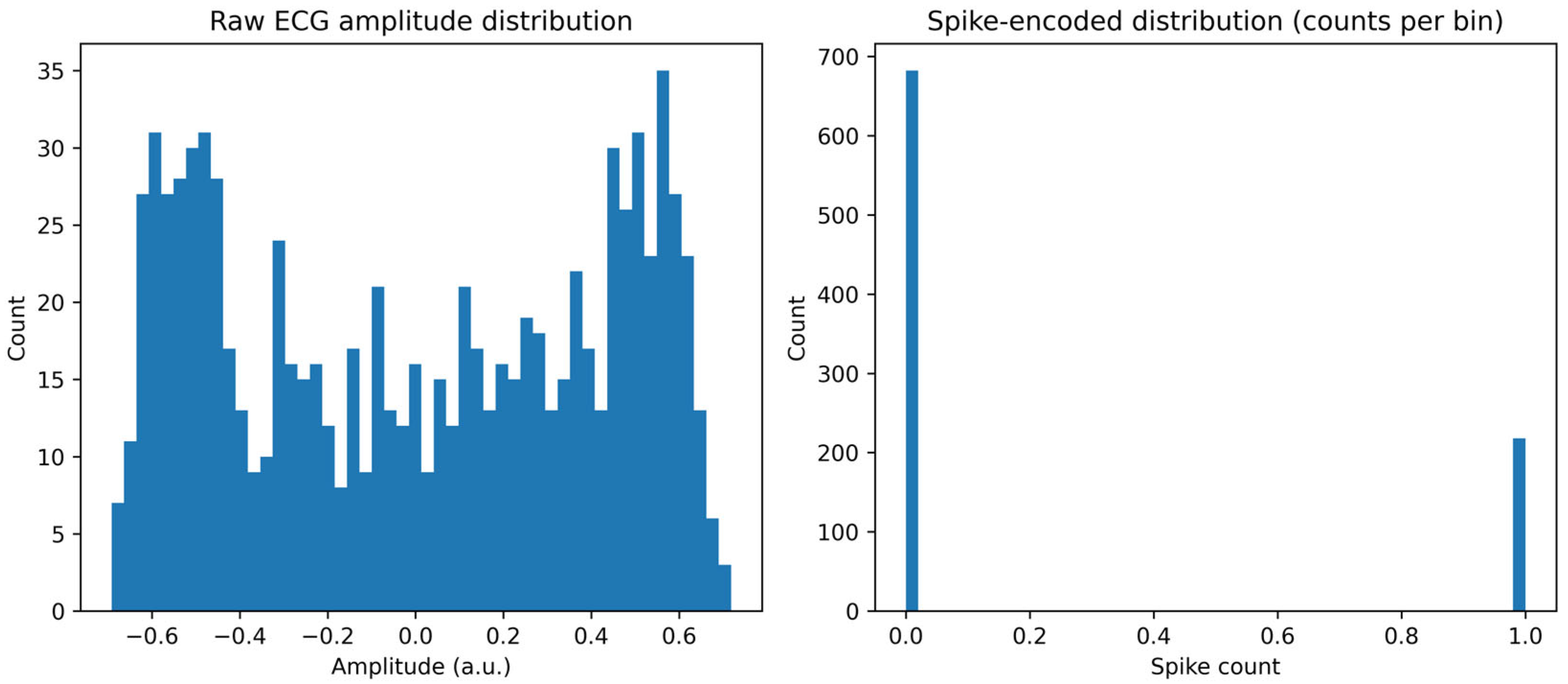
4.1. Dataset Summary and Preprocessing Outcomes
4.2. Spike Encoding Results
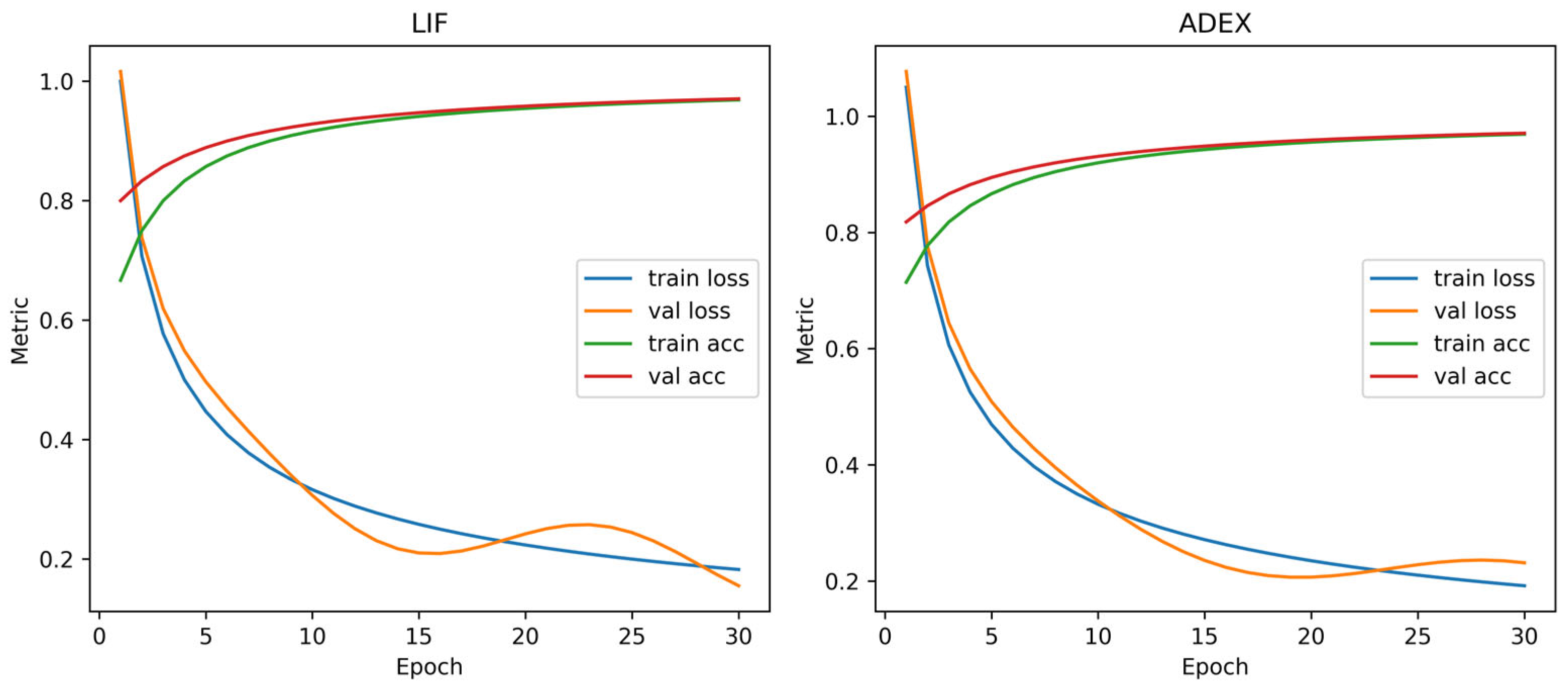
4.3. Model Training and Performance Comparison
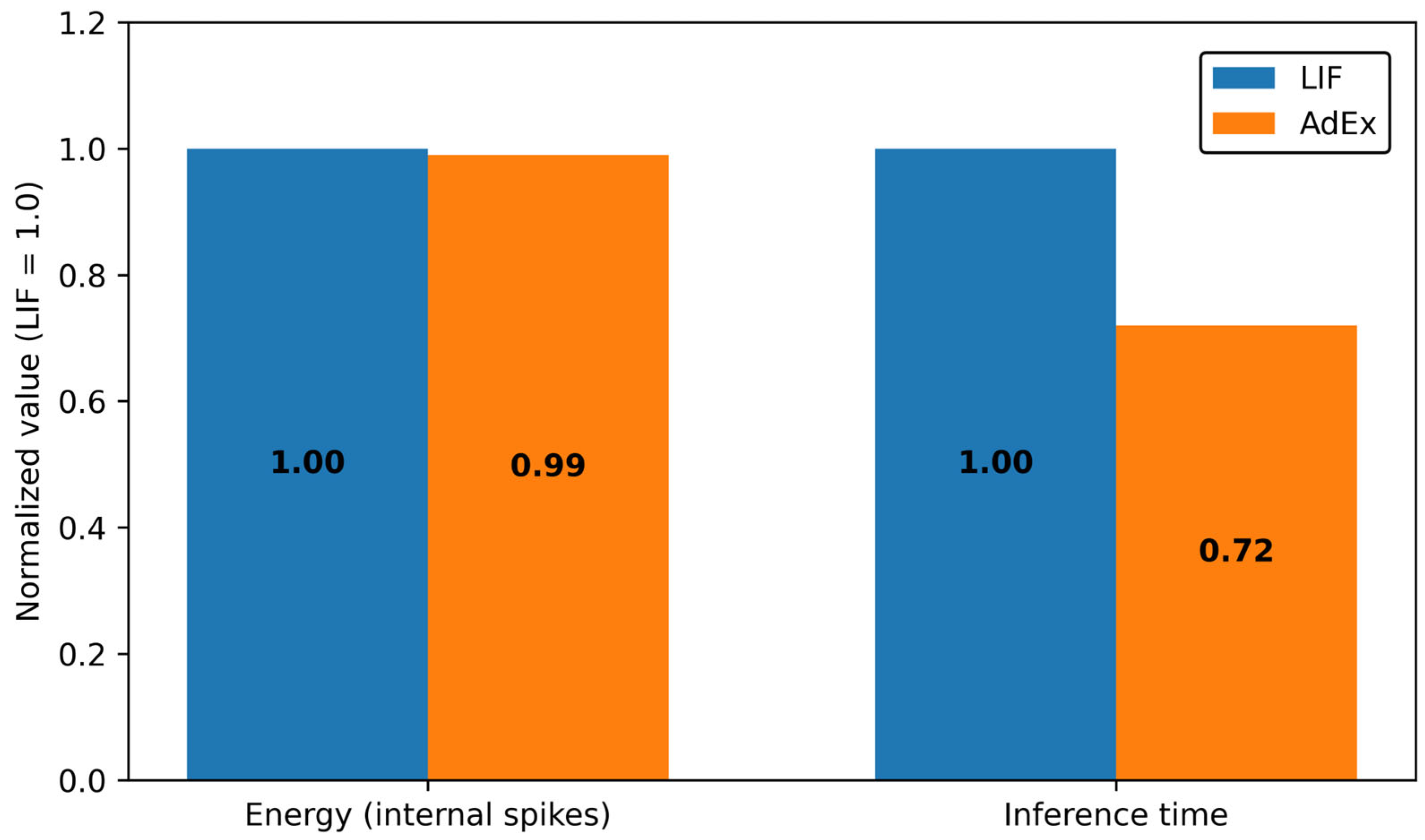
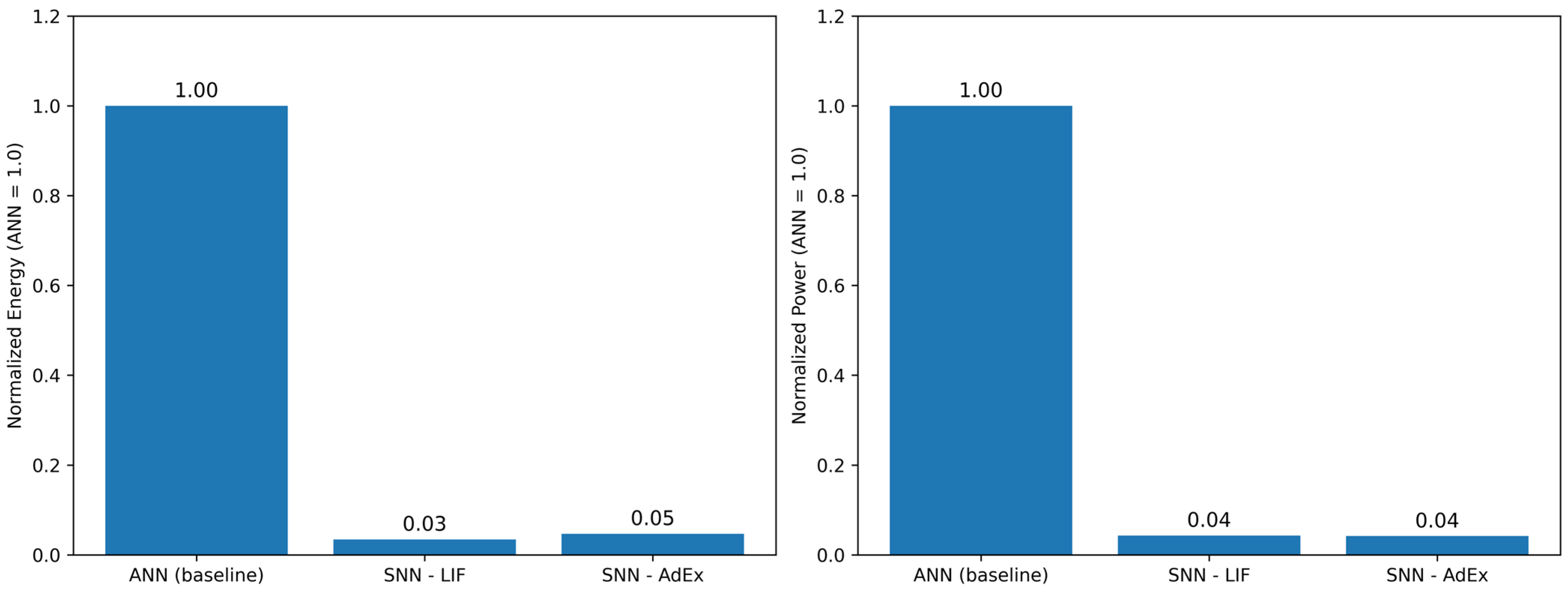
4.4. Power Analysis and Resource Consideration
- Balanced accuracy. Under this symmetric criterion, AdEx (balanced) is preferred, yielding accuracy 0.793 and recall 0.851 at with cost 0.5038. For reference, LIF (balanced) attains accuracy 0.790 and recall 0.810 at (cost 0.5882). Thus, when equal weighting of sensitivity and specificity is desired, AdEx offers a modest performance advantage at its selected threshold.
- Cost minimization. When the objective emphasizes the program’s cost functional, LIF (cost_min) is recommended, achieving accuracy 0.767, recall 0.959, , and the lowest cost 0.3146. The AdEx (cost_min) alternative reaches a higher recall (0.974) but incurs higher cost (0.3402) and lower accuracy (0.711) at , indicating a less favorable cost–performance compromise.
- Conservative sensitivity. For sensitivity-critical regimes prioritizing the reduction in missed alarms, LIF (conservative) is preferred, securing recall 0.959 with accuracy 0.767 at and cost 0.3146. The AdEx (conservative) counterpart achieves recall 0.949 and accuracy 0.754 at (cost 0.3478). Hence, when safety is paramount, LIF provides a slightly stronger sensitivity profile at comparable thresholds and lower cost.
4.5. Summary of Findings
- Across SNN configurations, AdEx attains the highest balanced accuracy (e.g., Acc 0.793, Rec 0.851 at ), whereas LIF achieves the highest sensitivity under a conservative threshold (e.g., Rec 0.959 at ) with competitive accuracy (see Table 5). Note that Figure 7 reports per-split test accuracy at the best epoch, whereas Table 5 reports policy-selected operating points obtained from threshold sweeps.
- In terms of resources, LIF exhibits the lowest normalized energy (Figure 9) and yields cost-efficient operating points, supporting low-power hardware implementation.
- Relative to the dense ANN baseline, the proposed SNNs substantially reduce computational energy, with normalized power at the recommended operating points (Figure 10), underscoring their suitability for real-time, edge-level ECG analytics.
- System implications. LIF is well suited to battery-constrained wearables and sensitivity-critical monitoring, while AdEx is compelling when symmetric operating regimes and top accuracy are prioritized, provided a modest efficiency overhead is acceptable.
- Under balanced accuracy, AdEx (τ = 0.4489) offers a modest performance advantage (Acc 0.793/Rec 0.851; cost 0.5038) and under cost minimization and conservative sensitivity, LIF (τ = 0.3557) is preferred (Acc 0.767/Rec 0.959; cost 0.3146), providing the strongest sensitivity at the lowest cost among the tested operating points.
5. Discussion
5.1. Principal Findings and Interpretation
5.2. Clinical and Engineering Implications
5.3. Relation to Prior Work
5.4. Robustness Considerations
5.5. Limitations
5.6. Future Directions
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECG | Electrocardiogram |
| MI | Myocardial Infarction |
| SNN | Spiking Neural Network |
| LIF | Leaky Integrate-and-Fire |
| AdEx | Adaptive Exponential Integrate-and-Fire |
| ANN | Artificial Neural Network |
| ROC | Receiver Operating Characteristic |
| PR | Precision–Recall |
| AUC | Area Under the Curve |
References
- World Health Organization (WHO). Cardiovascular Diseases (CVDs). 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 9 October 2025).
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Acharya, U.R.; Fujita, H.; Lih, O.S.; Hagiwara, Y.; Tan, J.H.; Adam, M. Automated Detection of Arrhythmias Using Different Intervals of Tachycardia ECG Segments with Convolutional Neural Network. Inf. Sci. 2017, 405, 81–90. [Google Scholar] [CrossRef]
- Yildirim, Ö. A Novel Wavelet Sequence Based on Deep Bidirectional LSTM Network Model for ECG Signal Classification. Comput. Biol. Med. 2018, 96, 189–202. [Google Scholar] [CrossRef]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Bourn, C.; Ng, A.Y. Cardiologist-Level Arrhythmia Detection with Convolutional Neural Networks. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liu, J.; Yang, H.; Liu, Q.; Wang, N.; Zhu, Z.; Chen, Y.; Long, Y.; Chang, L.; Zhou, L.; et al. ULECGNet: An Ultra-Lightweight End-to-End ECG Classification Neural Network. IEEE J. Biomed. Health Inform. 2022, 26, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Ansari, Y.; Mourad, O.; Qaraqe, K.; Serpedin, E. Deep Learning for ECG Arrhythmia Detection and Classification: An Overview of Progress for the Period 2017–2023. Front. Physiol. 2023, 14, 1246746. [Google Scholar] [CrossRef] [PubMed]
- Ahsanuzzaman, M.; Ahmed, S. Low-Cost, Portable ECG Monitoring and Alarming on Mobile Devices. Available online: https://www.semanticscholar.org/paper/Low-Cost%2C-Portable-ECG-Monitoring-and-Alarming-on-Ahsanuzzaman-Ahmed/1bc35520d96a568ed29c9702fbedee4a1372189f (accessed on 13 November 2025).
- Roy, K.; Jaiswal, A.; Panda, P. Towards Spike-Based Machine Intelligence with Neuromorphic Computing. Nature 2019, 575, 607–617. [Google Scholar] [CrossRef]
- Maass, W. Networks of Spiking Neurons: The Third Generation of Neural Network Models. Neural Netw. 1997, 10, 1659–1671. [Google Scholar] [CrossRef]
- Tavanaei, A.; Ghodrati, M.; Kheradpisheh, S.R.; Masquelier, T.; Maida, A.S. Deep Learning in Spiking Neural Networks. Neural Netw. 2019, 111, 47–63. [Google Scholar] [CrossRef]
- Ponulak, F.; Kasinski, A. Introduction to Spiking Neural Networks: Information Processing, Learning and Applications. Acta Neurobiol. Exp. 2011, 71, 409–433. [Google Scholar] [CrossRef]
- Brette, R.; Gerstner, W. Adaptive Exponential Integrate-and-Fire Model as an Effective Description of Neuronal Activity. J. Neurophysiol. 2005, 94, 3637–3642. [Google Scholar] [CrossRef]
- Davies, M.; Srinivasa, N.; Lin, T.H.; Chinya, G.; Cao, Y.; Choday, S.H. Loihi: A Neuromorphic Manycore Processor with On-Chip Learning. IEEE Micro 2018, 38, 82–99. [Google Scholar] [CrossRef]
- Indiveri, G.; Chicca, E.; Douglas, R.J. Artificial Cognitive Systems: From VLSI Networks of Spiking Neurons to Neuromorphic Cognition. Cogn. Comput. 2009, 1, 119–127. [Google Scholar] [CrossRef]
- Choi, H.; Park, J.; Lee, J.; Sim, D. Review on Spiking Neural Network-Based ECG Classification Methods for Low-Power Environments. Biomed. Eng. Lett. 2024, 14, 917–941. [Google Scholar] [CrossRef]
- Scrugli, M.A.; Busia, P.; Leone, G.; Meloni, P. On-FPGA Spiking Neural Networks for Integrated Near-Sensor ECG Analysis. In Proceedings of the 2024 Design, Automation & Test in Europe Conference (DATE 2024), Valencia, Spain, 25–27 March 2024; EDAA: Doha, Qatar, 2024; p. 6, ISBN 979-8-3503-4859-0. [Google Scholar]
- Rana, A.; Kim, K.K. Electrocardiography Classification with Leaky Integrate-and-Fire Neurons in an Artificial Neural Network-Inspired Spiking Neural Network Framework. Sensors 2024, 24, 3426. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.N.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a New Research Resource for Complex Physiologic Signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef] [PubMed]
- Gerstner, W.; Kistler, W.M.; Naud, R.; Paninski, L. Neuronal Dynamics: From Single Neurons to Networks and Models of Cognition; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Tyagi, P.K.; Agrawal, D. Automatic Detection of Sleep Apnea from a Single-Lead ECG Signal Based on Spiking Neural Network Model. Comput. Biol. Med. 2024, 179, 108877. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.; Gemmeke, T. Lossless Sparse Temporal Coding for SNN-Based Classification of Time-Continuous Signals. In Proceedings of the 2023 Design, Automation & Test in Europe Conference (DATE 2023), Antwerp, Belgium, 17–19 April 2023; EDAA: Doha, Qatar, 2023. [Google Scholar]
- Verma, G.; Gocher, H.; Verma, S. Enhanced Arrhythmia Detection Using Spiking Neural Networks: An In-Depth Analysis of ECG Data from the MIMIC-IV Clinical Database. Neural Comput. Appl. 2025, 37, 24365–24384. [Google Scholar] [CrossRef]
- Banjo, O.; Ghoraani, B. QCSNN: A Memory-Efficient Spiking Neural Network for On-Device ECG-Based Arrhythmia Detection. In Proceedings of the 2025 IEEE International Conference on Omni-layer Intelligent Systems (COINS 2025), Madison, WI, USA, 4–6 August 2025; IEEE: Piscataway, NJ, USA, 2025; pp. 1–6. [Google Scholar]
- Xing, Y.; Zhang, L.; Hou, Z.; Li, X.; Shi, Y.; Yuan, Y.; Zhang, F.; Liang, S.; Li, Z.; Yan, L. Accurate ECG Classification Based on Spiking Neural Network and Attentional Mechanism for Real-Time Implementation on Personal Portable Devices. Electronics 2022, 11, 1889. [Google Scholar] [CrossRef]
- Kasabov, N. Evolving Spiking Neural Networks and Neurogenetic Systems for Spatio- and Spectro-Temporal Data Modelling and Pattern Recognition. In Advances in Computational Intelligence; Liu, J., Alippi, C., Bouchon-Meunier, B., Greenwood, G.W., Abbass, H.A., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2012; Volume 7311, pp. 161–174. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Wang, L.; Yang, Z. QuantNAS: Quantization-Aware Neural Architecture Search For Efficient Deployment On Mobile Device. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Seattle, WA, USA, 16–22 June 2024. [Google Scholar] [CrossRef]
- Wang, X. Advances in Neural Architecture Search. Neural Sci. Rev. 2024, 11, 123–145. [Google Scholar] [CrossRef]
- Speckhard, D.T.; Misiunas, K.; Perel, S.; Zhu, T.; Carlile, S.; Slaney, M. Neural Architecture Search for Energy-Efficient Always-On Audio Machine Learning. Neural Comput. Appl. 2023, 35, 12133–12144. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, G.; Lin, Z.; Long, J.; Zhou, T.; Sheng, B.; Yang, X. Semi-Supervised Privacy-Preserving EEG-Based Motor Imagery Classification via Self and Adversarial Training. IEEE Trans. Autom. Sci. Eng. 2025, 22, 20679–20690. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, G.; Hu, Q.; Wang, B.; Zhou, T.; Qin, J. Dual Contrastive Training and Transferability-Aware Adaptation for Multisource Privacy-Preserving Motor Imagery Classification. IEEE Trans. Instrum. Meas. 2024, 73, 2500213. [Google Scholar] [CrossRef]
- Zacchigna, S.; Russo, V.; Fiorentini, S. Flexible Quantization for Efficient Convolutional Neural Networks. Electronics 2024, 13, 1923. [Google Scholar] [CrossRef]
- Sun, S.F.; Bai, J.Y.; Chen, H.T. Model Quantization for Computing-In-Memory: A Survey. Sci. China Inf. Sci. 2025, 68, 211401. [Google Scholar] [CrossRef]



| Study (Year) | Task/Dataset | Encoding | Neuron Model | Pipeline Matched | Metrics | Energy/Power Evidence | H/W Validation |
|---|---|---|---|---|---|---|---|
| [16] (2024) | ECG classification/ PhyioNet | rate/temporal (reviewed) | mixed (survey) | – | accuracy (varies) | survey-level | survey |
| [17] (2024) | 5-class arrhythmia/ Single lead | delta modulation | (impl.-specific) | N/A | accuracy | μJ/inference reported | FPGA |
| [18] (2024) | ECG classification/MIT-BIH | rate(+attention) | LIF | No | Accuracy, F1 | not direct (software) | No |
| This work | MI screening (abrupt change)/PTB | adaptive μ + kσ | LIF & AdEx | Yes (identical pipeline) | precision, F1, AUPRC + CI | spike-count proxy (not HW) | Plan outlined |
| Layer | Stride | Padding | Params |
|---|---|---|---|
| Conv1d | 1 | 2 | 512 |
| BatchNorm1d | - | - | 64 |
| AvgPool1d | 2 | 0 | 0 |
| Spiking (Leaky) [beta = 0.9] | - | - | 0 |
| Linear | - | - | 66 |
| Class | Samples | Proportion (%) |
|---|---|---|
| Healthy | 976 | 19.9 |
| Myocardial Infarction (MI) | 3934 | 80.1 |
| Total | 4910 | 100.0 |
| SNN Model | Precision | Recall | F1-Score |
|---|---|---|---|
| LIF | 0.794 | 0.826 | 0.810 |
| AdEx | 0.759 | 0.841 | 0.798 |
| Decision Criterion | Recommended Combo | Acc/Recall | Tau (Threshold) | Cost per Sample |
|---|---|---|---|---|
| Balanced accuracy | AdEx (balanced) | 0.793/0.851 | 0.4489 | 0.5038 |
| Cost minimization | LIF (cost_min) | 0.767/0.959 | 0.3557 | 0.3146 |
| Conservative sensitivity | LIF (conservative) | 0.767/0.959 | 0.3557 | 0.3146 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y. Abrupt Change Detection of ECG by Spiking Neural Networks: Policy-Aware Operating Points for Edge-Level MI Screening. Appl. Sci. 2025, 15, 12210. https://doi.org/10.3390/app152212210
Lee Y. Abrupt Change Detection of ECG by Spiking Neural Networks: Policy-Aware Operating Points for Edge-Level MI Screening. Applied Sciences. 2025; 15(22):12210. https://doi.org/10.3390/app152212210
Chicago/Turabian StyleLee, Youngseok. 2025. "Abrupt Change Detection of ECG by Spiking Neural Networks: Policy-Aware Operating Points for Edge-Level MI Screening" Applied Sciences 15, no. 22: 12210. https://doi.org/10.3390/app152212210
APA StyleLee, Y. (2025). Abrupt Change Detection of ECG by Spiking Neural Networks: Policy-Aware Operating Points for Edge-Level MI Screening. Applied Sciences, 15(22), 12210. https://doi.org/10.3390/app152212210






