Glass Property Predictions and the Design and Characterization of Leucite Glass-Ceramics
Abstract
1. Introduction
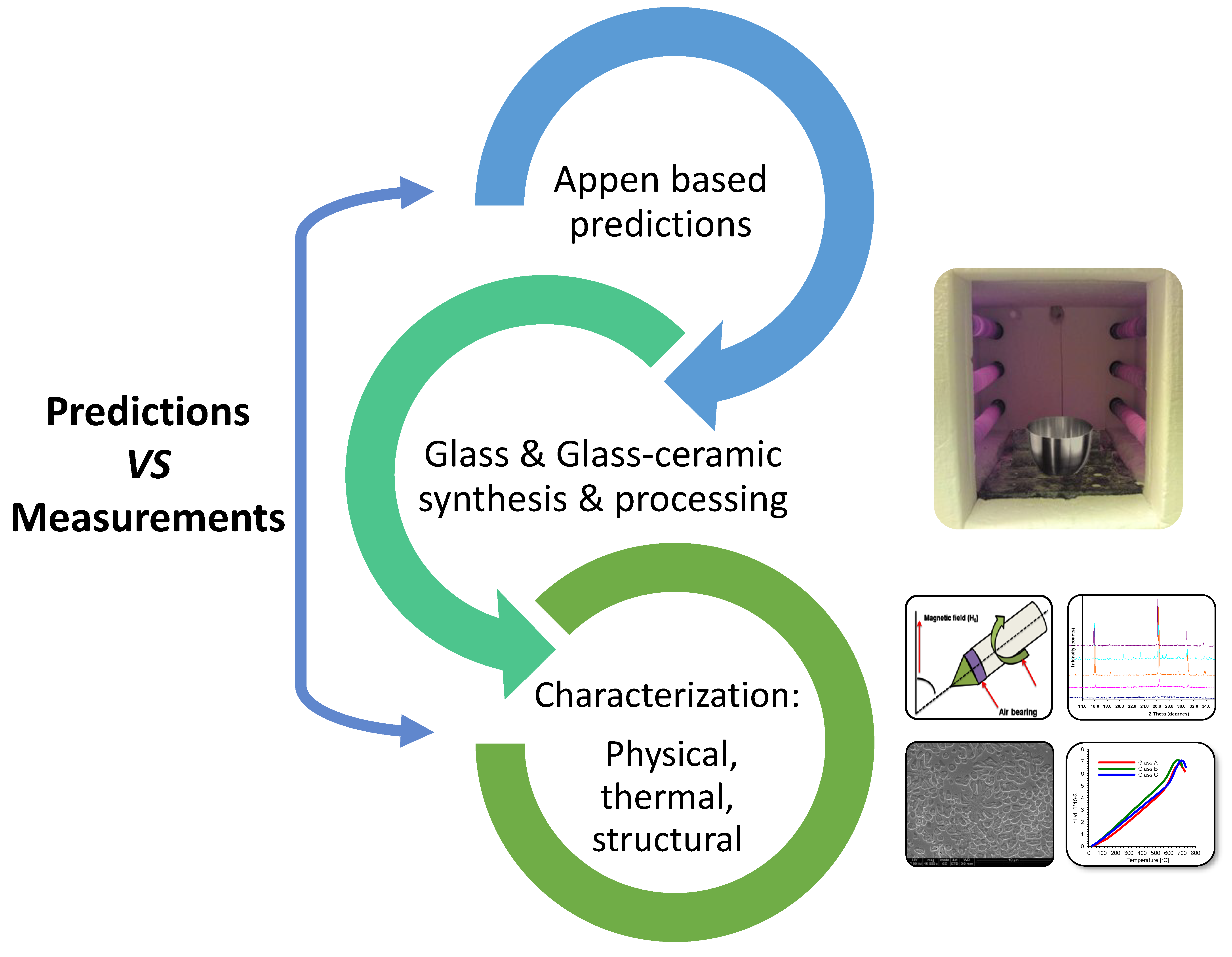
2. Materials and Methods
2.1. Glass Design
2.2. Glass Synthesis and Processing
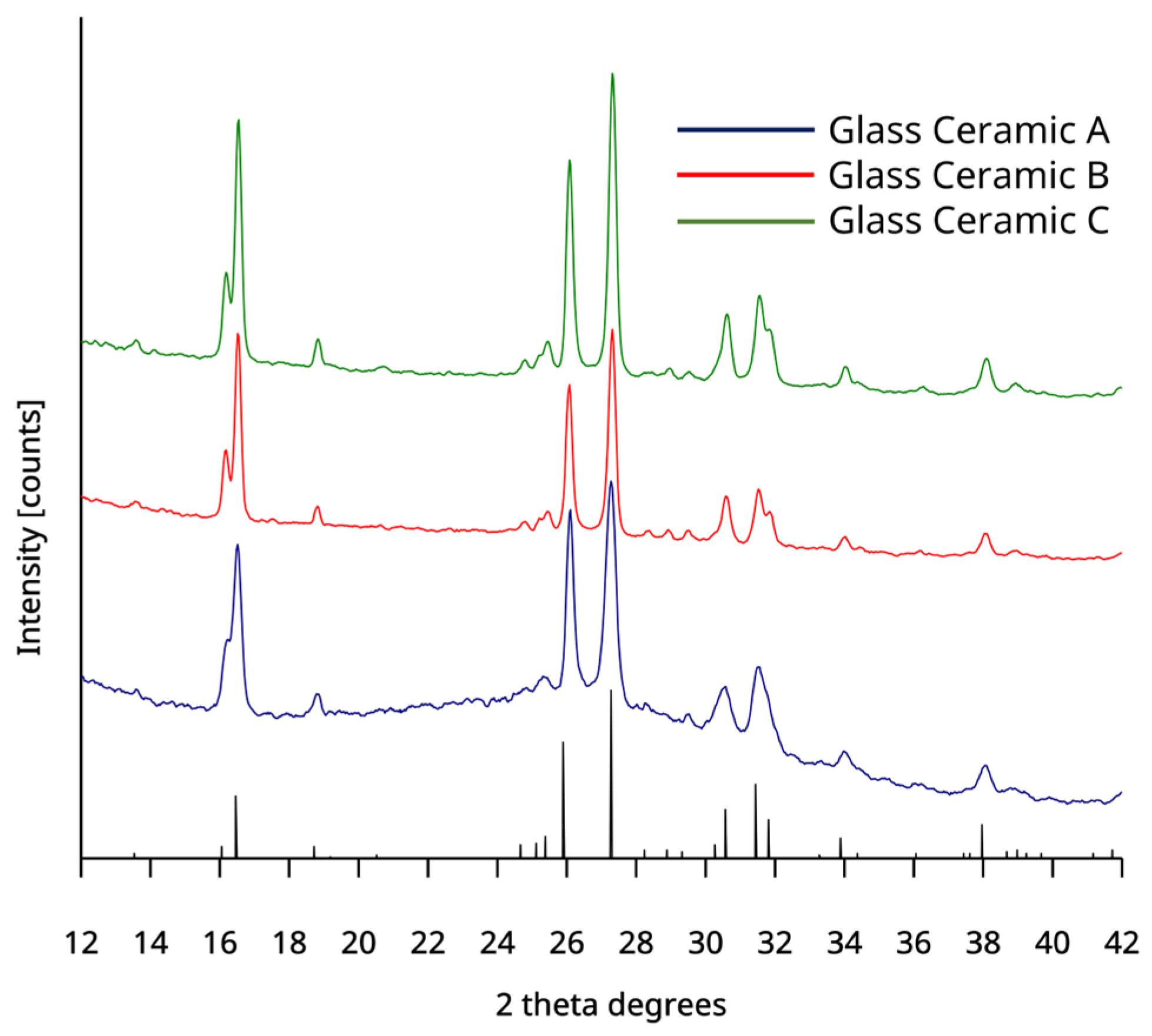
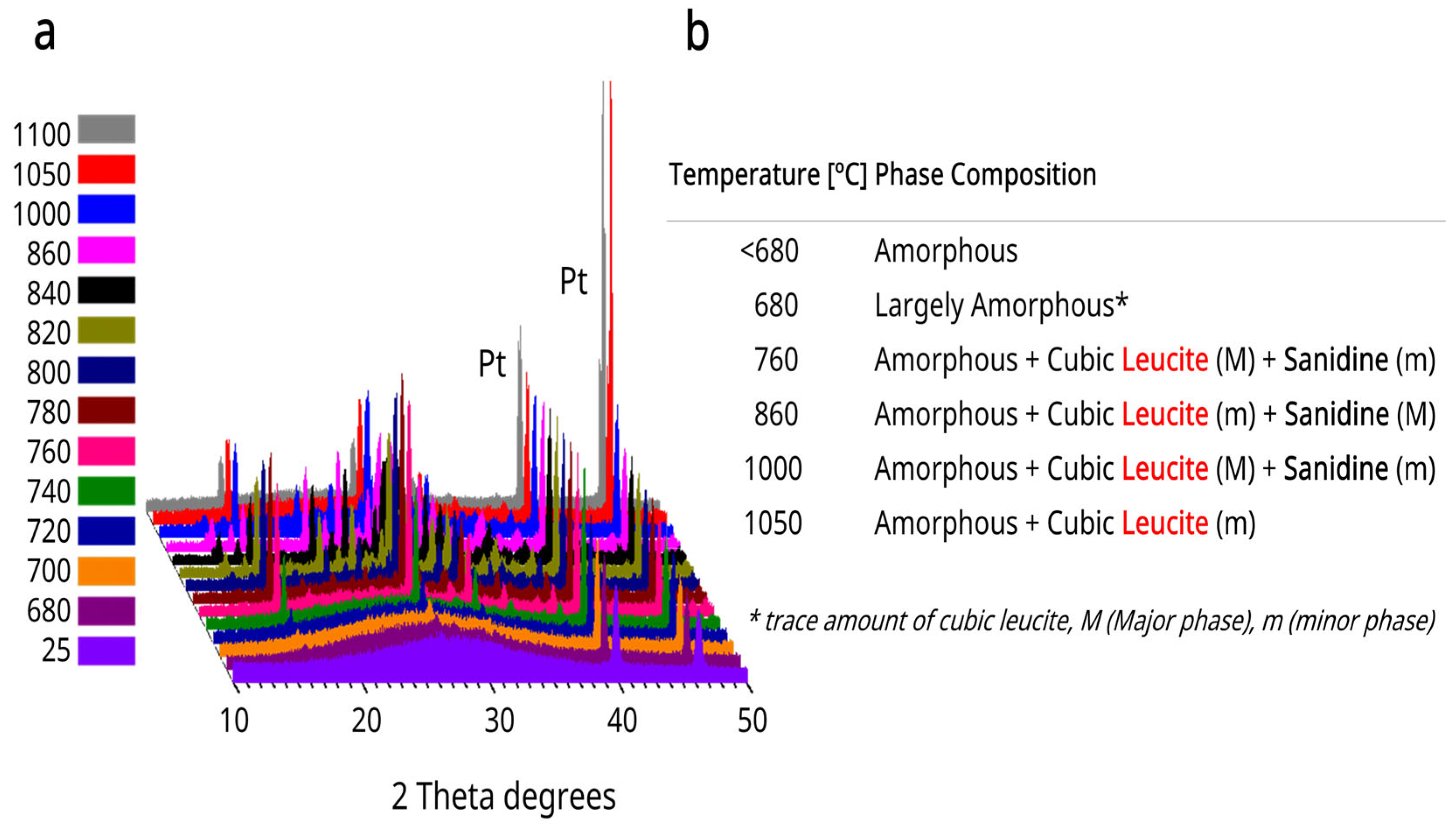
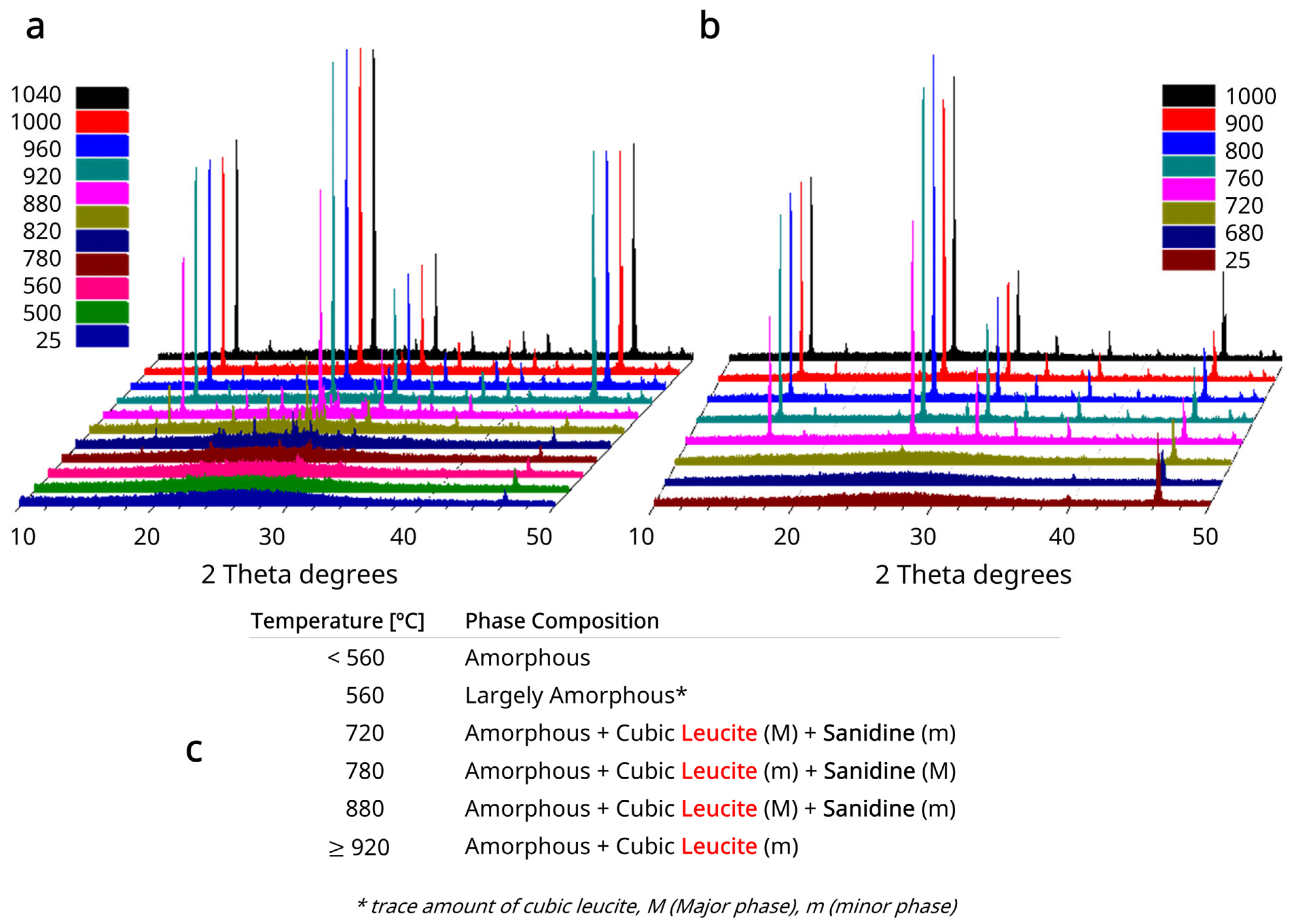
2.3. High-Temperature X-Ray Diffraction
2.4. Glass-Ceramic Powder Production
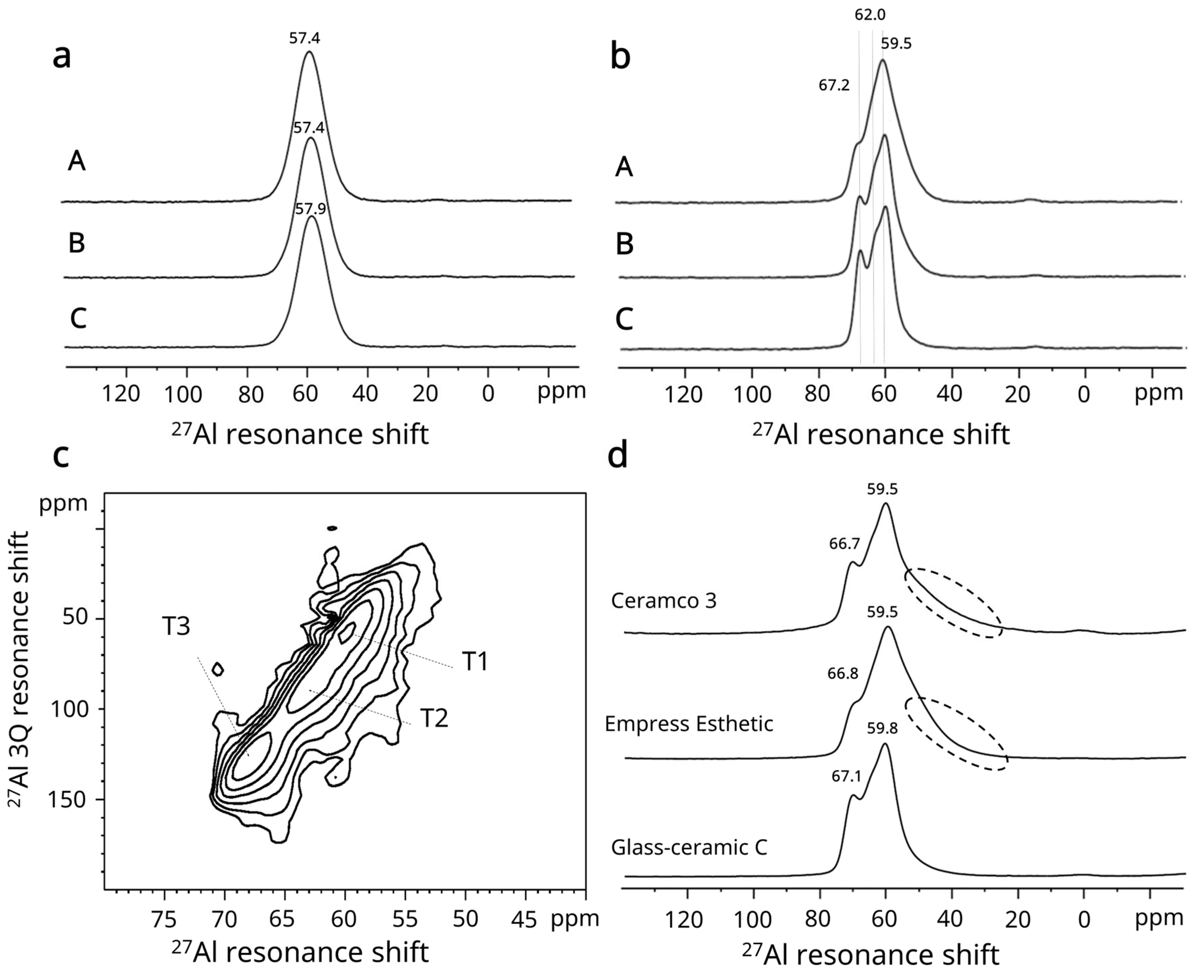
2.5. Solid State MAS-NMR
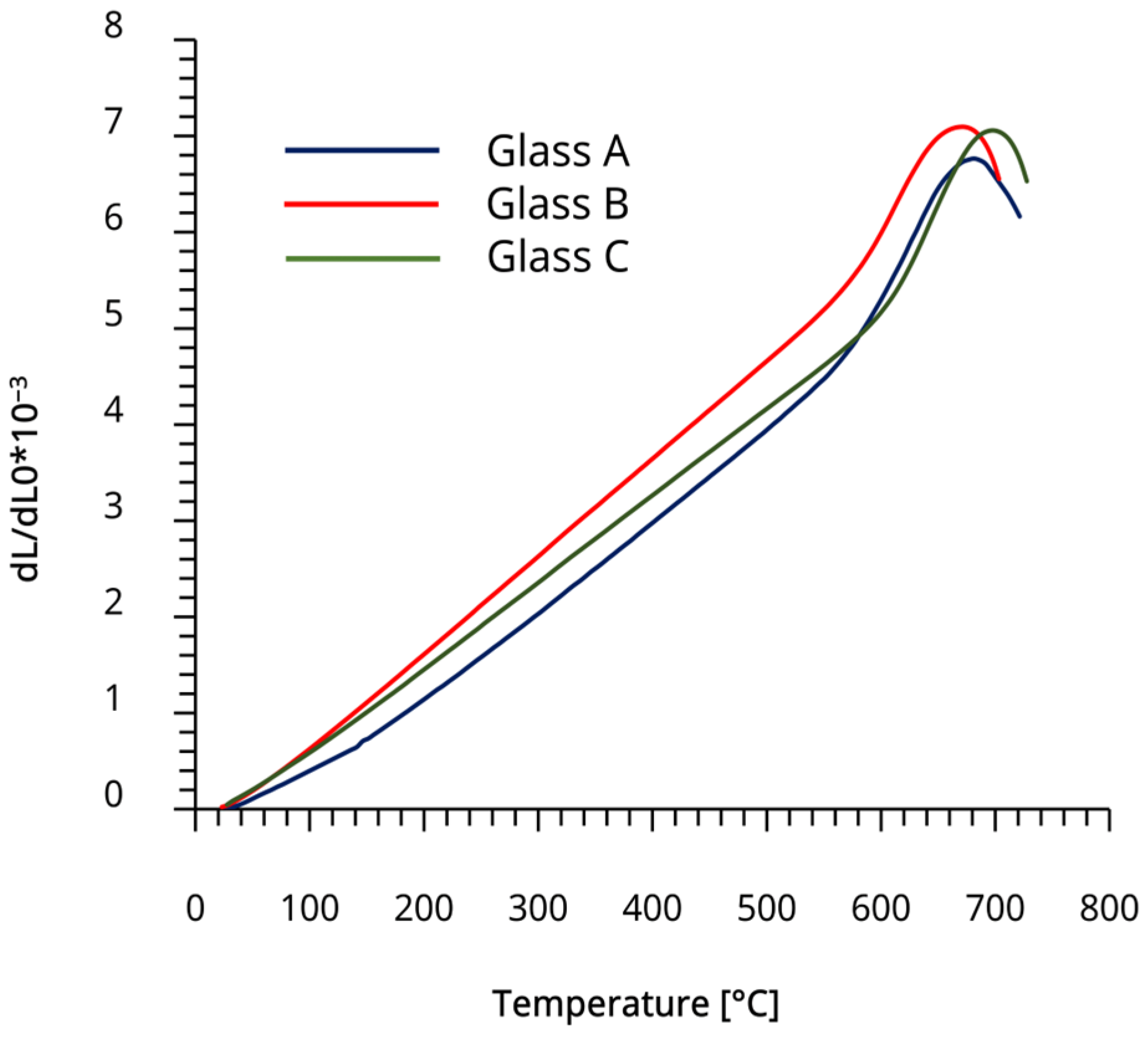
2.6. Differential Thermal Expansion Analysis
2.7. Glass Density and Refractive Index Measurement
2.8. Glass-Ceramic Specimen Preparation
2.9. Biaxial Flexural Strength Testing
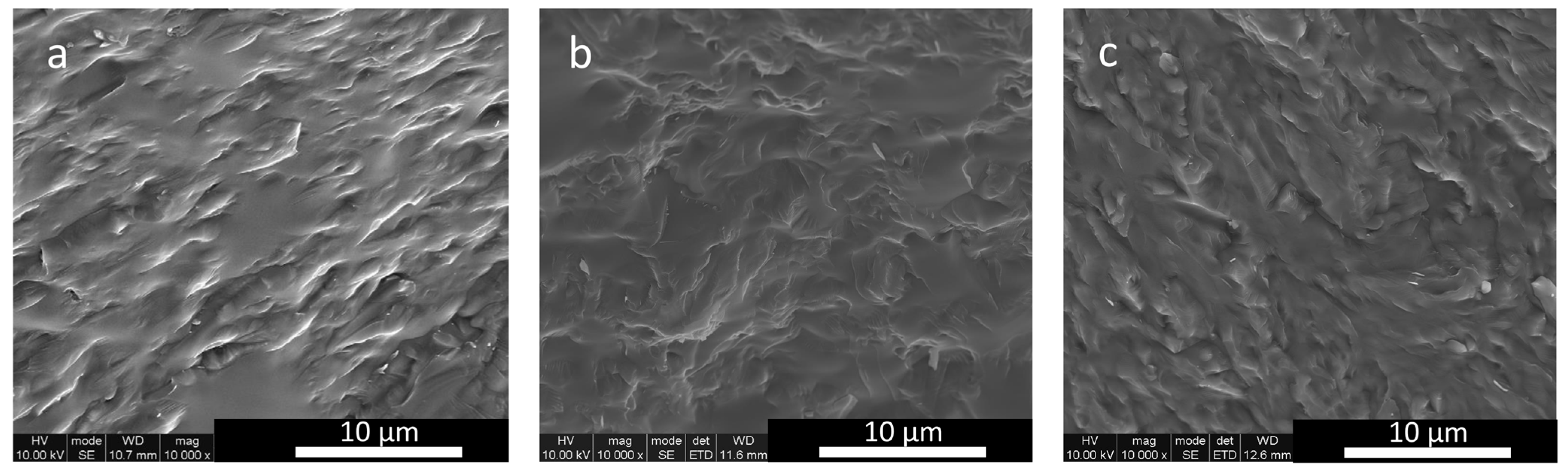
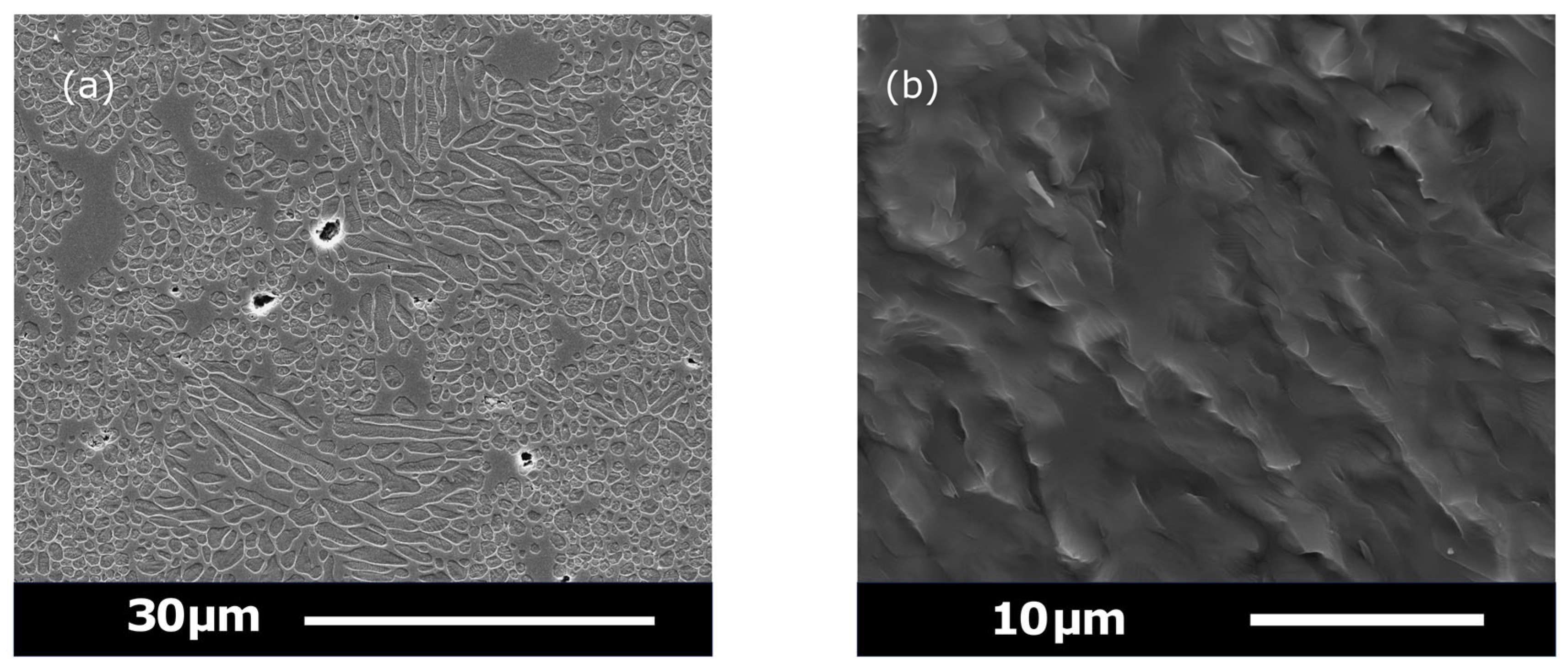
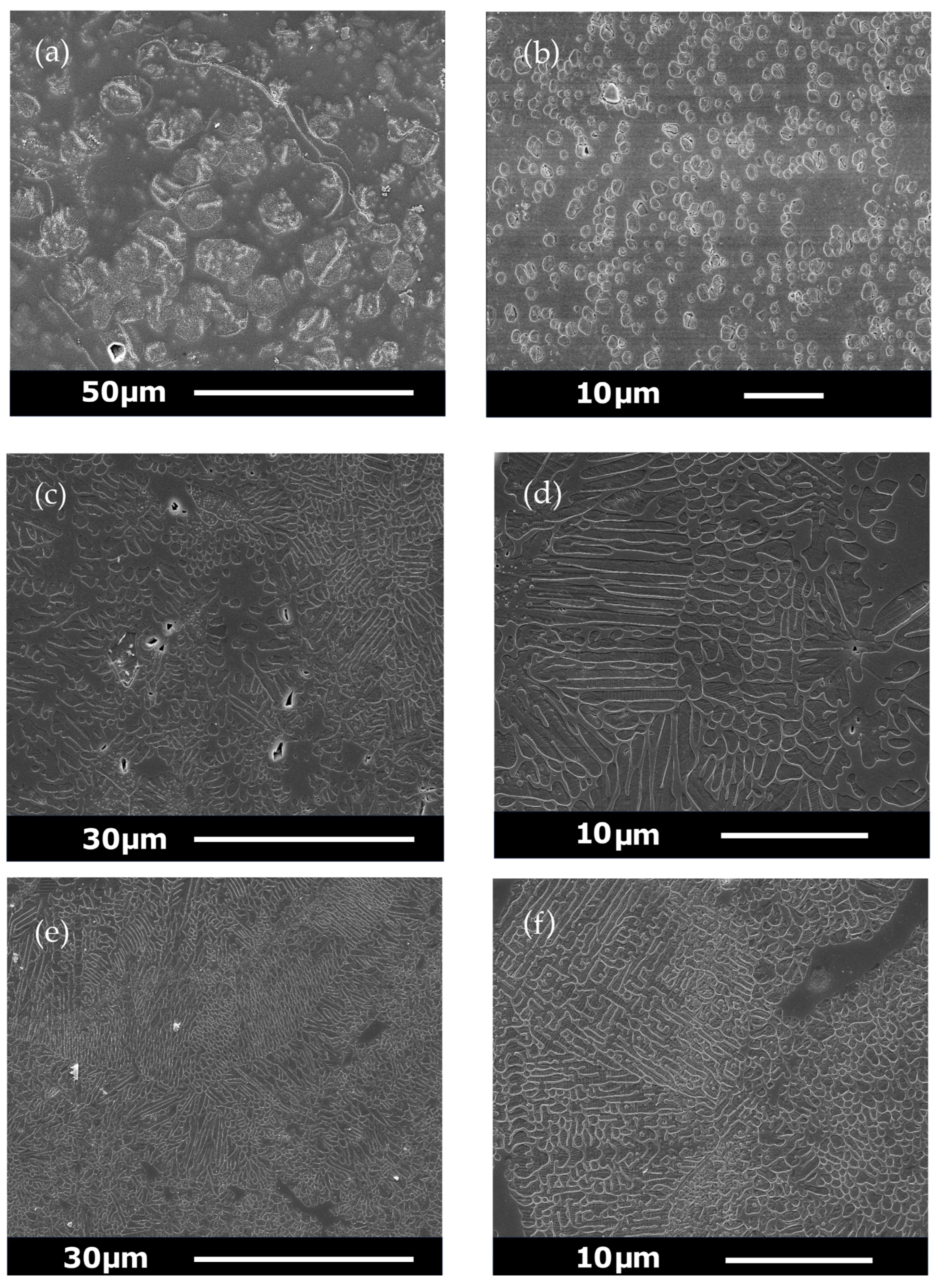
2.10. Secondary Electron Imaging
3. Results
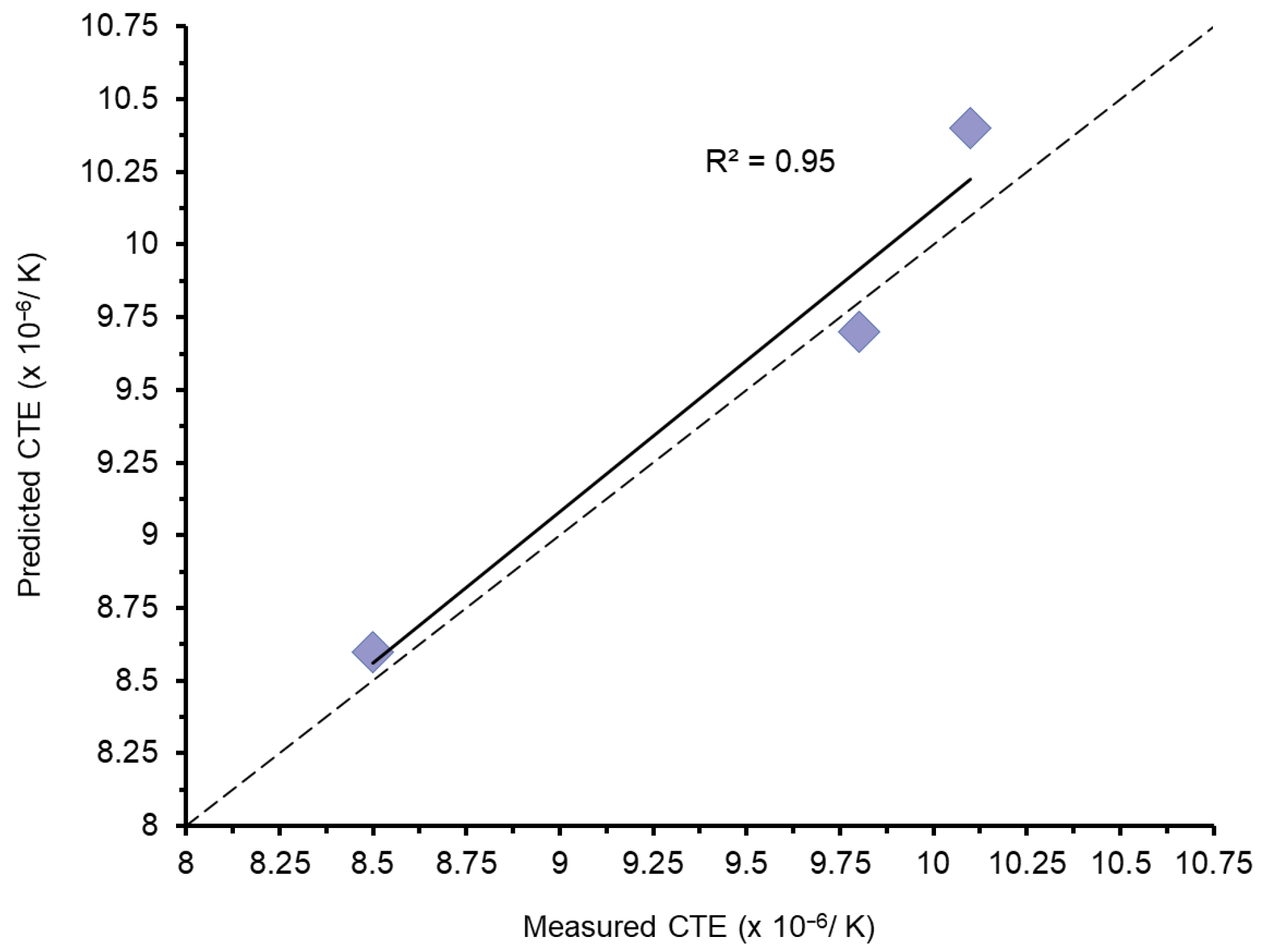
3.1. Appen-Predicted and Measured Values
3.2. Dilatometry Results
3.3. BFS Results
3.4. HTXRD Results
3.5. SEM Results
3.6. Solid State MAS-NMR Results
4. Discussion
5. Conclusions
6. Patents
- Theocharopoulos A., Chen XH., Karpukhina N., Hill, R.G., Cattell, M.J. Leucite glass ceramics. US9856165B2, 2018.
- Theocharopoulos A., Chen XH., Karpukhina N., Hill, R.G., Cattell, M.J. Leucite glass ceramics. EP3013762B1, 2022.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTE | Coefficient of Thermal Expansion |
| GC | Glass-Ceramic |
| FGMIC | Functional Glass Manufacturing Innovation Consortium |
| AI | Artificial Intelligence |
| ML | Machine Learning |
| RMSE | Root Mean Squared Error |
| R.I. | Refractive Index |
| BFS | Biaxial Flexural Strength |
| NIST SRM | National Institute of Standards Standard Reference Material |
| CCC | Critical Correlation Coefficient |
| SEM | Scanning Electron Microscopy |
| EDX | Electron Dispersive X-ray Spectroscopy |
| HTXRD/XRD | High-Temperature X-ray Diffraction/X-ray diffraction |
| DSC | Differential Scanning Calorimetry |
| MAS NMR | Magic Angle Spinning Nuclear Magnetic Resonance |
| SD | Standard Deviation |
| YTZP | Yttria Tetragonal Zirconia Polycrystals |
Appendix A
| Oxides | Thermal Expansion Coefficient (α) Appen Factors (×10−6/K, 20–400 °C) |
|---|---|
| SiO2 | 0.5 to 3.8 |
| Al2O3 | −3.0 |
| K2O | 46.5 (42.0) |
| CaO | 13.0 |
| TiO2 | −1.5 to −3.0 |
| Na2O | 39.5 |
| Li2O | 27.0 |
| MgO | 6.0 |
The following remarks apply for specific Appen Factors:
| |
References
- Zarzycki, J. Glasses and the Vitreous State; Cambridge University Press: Cambridge, UK, 1991; pp. 1, 37–64, 228–231. [Google Scholar]
- Priven, A.I. General method for calculating the properties of oxide glasses and glass forming melts from their composition and temperature. Glass Technol. 2004, 45, 244–254. Available online: https://www.semanticscholar.org/paper/General-method-for-calculating-the-properties-of-Priven/92307dc8af50a43eaa9697dd859002126862ad0d (accessed on 22 October 2025).
- Functional Glass Manufacturing Innovation Consortium. Driving Functional Glass Manufacturing Innovation: A Technology Roadmap to 2025. Available online: https://ceramics.org/wp-content/uploads/2019/05/FGMIC-roadmap.pdf (accessed on 22 October 2025).
- Winkelmann, A.A.; Schott, O. Dependence of thermal resistance coefficients of glasses on their chemical composition. Ann. Phys. 1894, 51, 730–746. [Google Scholar] [CrossRef]
- Gilard, P.; Dubrul, L. Calculation of physical properties of glass: III. Index of refraction. J. Soc. Glass Technol. 1937, 21, 476–488. [Google Scholar]
- Huggins, M.L.; Sun, K.-H. Calculation of density and optical constants of a glass from its composition in weight percentage. J. Am. Ceram. Soc. 1943, 26, 4–11. [Google Scholar] [CrossRef]
- Appen, A.A. Toward the method for calculating the properties of glass. Glass Ceram. 1961, 18, 235–237. [Google Scholar] [CrossRef]
- Hormadaly, J. Empirical methods for estimating the linear coefficient of expansion of oxide glasses from their composition. J. Non-Cryst. Solids 1986, 79, 311–324. [Google Scholar] [CrossRef]
- Volf, M.B. Mathematical Approach to Glass; Elsevier Science Pub. Comp. Inc.: Amsterdam, The Netherlands, 1988; pp. 31, 142–146. [Google Scholar]
- De Guire, E.; Bartolo, L.; Brindle, R.; Devanathan, R.; Dickey, E.C.; Fessler, J.; French, R.H.; Fotheringham, U.; Harmer, M.; Lara-Curzio, E.; et al. Data-driven glass/ceramic science research: Insights from the glass and ceramic and data science/informatics communities. J. Am. Ceram. Soc. 2019, 102, 6385–6406. [Google Scholar] [CrossRef]
- Duminis, T.; Shahid, S.; Karpukhina, N.G.; Hill, R.G. Predicting refractive index of fluoride containing glasses for aesthetic dental restorations. Dent. Mater. 2018, 34, e83–e88. [Google Scholar] [CrossRef]
- McCloy, J. Methods for prediction of refractive index in glasses for the infrared. In Proceedings of the SPIE 8016, Window and Dome Technologies and Materials XII, Orlando, FL, USA, 25–29 April 2011. [Google Scholar] [CrossRef]
- Lu, X.; Vienna, J.D.; Du, J. Glass formulation and composition optimization with property models: A review. J. Am. Ceram. Soc. 2024, 107, 1603–1624. [Google Scholar] [CrossRef]
- Youngman, R. NMR Spectroscopy in glass science: A review of the elements. Materials 2018, 11, 476. [Google Scholar] [CrossRef]
- Allu, A.R.; Gaddam, A.; Ganisetti, S.; Balaji, S.; Siegel, R.; Mather, G.C.; Fabian, M.; Pascual, M.J.; Ditaranto, N.; Milius, W.; et al. Structure and crystallization of alkaline-earth aluminosilicate glasses: Prevention of the alumina-avoidance principle. The J. Phys. Chem. B 2018, 122, 4737–4747. [Google Scholar] [CrossRef]
- Höland, W.; Rheinberger, V. Dental glass-ceramics. In Bioceramics and Their Clinical Applications; Kokubo, T., Ed.; Woodhead Publishing: Cambridge, UK, 2008; pp. 548–568. [Google Scholar]
- Chen, X.; Chadwick, T.C.; Wilson, R.M.; Hill, R.; Cattell, M.J. Crystallization of high-strength fine-sized leucite glass-ceramics. J. Dent. Res. 2010, 89, 1510–1516. [Google Scholar] [CrossRef]
- Fu, L.; Engqvist, H.; Xia, W. Glass–ceramics in Dentistry: A review. Materials 2020, 13, 1049. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals; John Wiley and Sons Inc.: London, UK, 1966. [Google Scholar]
- Timoshenko, S.P.; Woinowsky-Krieger, S. Theory of Plates and Shells, 2nd ed.; McGraw-Hill: New York, NY, USA, 1959; pp. 70–71. [Google Scholar]
- Abernethy, R.B. The New Weibull Handbook; Abernethy, R.B.: North Palm Beach, FL, USA, 1996. [Google Scholar]
- Theocharopoulos, A.; Chen, X.; Wilson, R.M.; Hill, R.; Cattell, M.J. Crystallization of high-strength nano-scale leucite glass-ceramics. Dent. Mater. 2013, 29, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Kohn, S.; Henderson, C.; Dupree, R. Si–Al ordering in leucite group minerals and ion-exchanged analogues: An MAS NMR study. Am. Mineral. 1997, 82, 1133–1140. [Google Scholar] [CrossRef]
- Cattell, M.J.; Patzig, C.; Bissasu, S.; Tsoutsos, A.; Karpukhina, N. Nucleation efficacy and flexural strength of novel leucite glass-ceramics. Dent. Mater. 2020, 36, 592–602. [Google Scholar] [CrossRef]
- Fabris, D.C.N.; Miguel, E.H.; Vargas, R.; Canto, R.B.; Villas-Boas, M.d.O.C.; Peitl, O.; Sglavo, V.M.; Zanotto, E.D. Microstructure, residual stresses, and mechanical performance of surface crystallized translucent glass-ceramics. J. Eur. Ceram. Soc. 2022, 42, 4631–4642. [Google Scholar] [CrossRef]
- Mackert, J.J.R.; Twiggs, S.W.; Williams, A.L. High-temperature X-ray diffraction measurement of sanidine thermal expansion. J. Dent. Res. 2000, 79, 1590–1595. [Google Scholar] [CrossRef]
- Mackert, J.R.; Rueggeberg, E.; Lockwood, P.E.; Evans, A.L.; Thompson, W.O. Isothermal anneal effect on microcrack density around leucite particles in dental porcelain. J. Dent. Res. 1994, 73, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J. Strength and engineering design. In An Introduction to the Mechanical Properties of Ceramics, 1st ed.; Solid State Science Series; Cambridge University Press: Cambridge, UK, 1998; pp. 248–264. [Google Scholar]
- Quinn, J.B.; Quinn, G.D.; Kelly, J.R.; Scherrer, S.S. Fractographic analyses of three ceramic whole crown restoration failures. Dent. Mater. 2005, 21, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Della Bona, A.; Anusavice, K.J.; DeHoff, P.H. Weibull analysis and flexural strength of hot-pressed core and veneered ceramic structures. Dent. Mater. 2003, 19, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Beuer, F.; Hey, J.; Schmidt, F.; Sorensen, J.A.; Prause, E. Antagonist enamel tooth wear produced by different dental ceramic systems: A systematic review and network meta-analysis of controlled clinical trials. J. Dent. 2024, 142, 104832. [Google Scholar] [CrossRef]
- Nonami, T.; Tsutsumi, S. Press-formable CaO-MgO-SiO2-TiO2-Ag2O glass as a biomaterial. J. Biomed. Mater. Res. 2000, 50, 8–15. [Google Scholar] [CrossRef]
- Cattell, M.J.; Chadwick, T.C.; Knowles, J.C.; Clarke, R.L. The crystallization of an aluminosilicate glass in the K2O-Al2O3-SiO2 System. Dent. Mater. 2005, 21, 811–822. [Google Scholar] [CrossRef]
- James, P.F. Nucleation in glass forming systems a review. In Advances in Ceramics; Simmons, J.H., Uhlmann, D.R., Beall, B.H., Eds.; The American Ceramic Society: Columbus, OH, USA, 1982; Volume 4, pp. 1–48. [Google Scholar]
- Almuhamadi, J.; Almusali, M.H.; Chen, X.; Theocharopoulos, A.L.; Alostath, H.F.; Karpukhina, N.; Cattell, M.J. Effect of TiO2 and CaO addition on the crystallization and flexural strength of novel leucite glass-ceramics. Materials 2024, 17, 3422. [Google Scholar] [CrossRef]
- Schairer, J.F.; Bowen, N.L. The system K2O-Al2O3-SiO2. Am. J. Sci. 1955, 253, 681–746. [Google Scholar] [CrossRef]
- Barreiro, M.M.; Vicente, E.E. Kinetics of isothermal phase transformations in a dental porcelain. J. Mater. Sci. Mater. Med. 1993, 4, 431–436. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-Forming Minerals: Framework Silicates; Wiley: Hoboken, NJ, USA, 1963; Volume 4. [Google Scholar]
- Garibay-Alvarado, J.A.; Pizá-Ruiz, P.; Zaragoza-Contreras, A.E.; Espinosa-Magaña, F.; Reyes-López, S.Y. SiO2-Al2O3-ZrO2-Ag composite and its signal enhancement capacity on Raman spectroscopy. Chemosensors 2025, 13, 266. [Google Scholar] [CrossRef]
- Scholze, H.; Lakin, M.J. Calculation Based on Composition. Glass: Nature, Structure and Properties, 1st ed.; Springer: London, UK, 1991; pp. 189–190. [Google Scholar]
- Priven, A.I.; Mazurin, O.V. Comparison of methods used for the calculation of density, refractive index and thermal expansion of oxide glasses. Glass Technol. 2003, 44, 156–166. Available online: https://www.semanticscholar.org/paper/Comparison-of-methods-used-for-the-calculation-of-Priven-Mazurin/579afc011415ff6711708a9b2b3442e991899677 (accessed on 22 October 2025).
- Li, H.; Liu, P.; Liu, S.; Yang, B. Deep learning-assisted attribute prediction of chalcogenide glasses based on graph classification. Sci. Rep. 2025, 15, 19360. [Google Scholar] [CrossRef]
- Cassar, D.R. GlassNet: A multitask deep neural network for predicting many glass properties. Ceram. Int. 2023, 49, 36013–36024. [Google Scholar] [CrossRef]
- Davel, C.; Bassiri-Gharb, N.; Correa-Baena, J.-P. Machine learning in X-ray diffraction for materials discovery and characterization. Matter 2025, 8, 102272. [Google Scholar] [CrossRef]
| SiO2 | Al2O3 | K2O | CaO | TiO2 | Na2O | Li2O | MgO | |
|---|---|---|---|---|---|---|---|---|
| Glass A | 72.6 | 10.7 | 7.9 | 2.1 | 0.3 | 4.7 | 1.1 | 0.5 |
| Glass B | 70.9 | 10.0 | 10.9 | 2.1 | 0.5 | 4.0 | 1.1 | 0.5 |
| Glass C | 69.3 | 10.1 | 12.5 | 2.1 | 0.5 | 4.0 | 1.1 | 0.5 |
| Experimental Glasses | Nucleation Temp. [°C] | Nucleation Hold [h] | Crystallization Temp. [°C] | Crystallization Hold [h] | Heating Rate [°C/min] |
|---|---|---|---|---|---|
| Glass A | 650 | 1 | 1120 | 1 | 10 |
| Glass B | 620 | 1 | 920 | 1 | 10 |
| Glass C | 620 | 1 | 795 | 1 | 20 |
| Glasses | CTE [10−6/K, 100–400 °C] | Density [g/cm3] | Refractive Index (R.I.) | Predicted Glass Network Connectivity | |||
|---|---|---|---|---|---|---|---|
| Predicted | Measured | Predicted | Measured | Predicted | Measured | ||
| Glass A | 8.6 | 8.5 * | 2.381 | 2.408 (0.014) | 1.50 | 1.50 | 3.90 |
| Glass B | 9.7 | 9.8 | 2.397 | 2.418 (0.006) | 1.51 | 1.51 | 3.87 |
| Glass C | 10.4 | 10.1 | 2.410 | 2.434 (0.006) | 1.51 | 1.50 | 3.88 |
| Glass—Ceramics | BFS | ||||
|---|---|---|---|---|---|
| Mean (SD) BFS [MPa] | Weibull m | C.I. for m (95%) | Characteristic Strength σ0 [MPa] | C.I. for σ0 (95%) | |
| A | 153.2 (21.7) a* | 8.5 a | 6.9–10.4 | 162.0 a | 156.0–168.2 |
| B | 131.7 (9.0) b | 17.6 b | 14.1–21.9 | 135.6 b | 133.2–138.1 |
| C | 212.3 (28.2) c | 8.5 a | 6.7–10.9 | 224.4 c | 216.2–232.9 |
| Glass—Ceramics | Mean (SD) Crystal Size [μm2] | Leucite Area Fraction (%) |
|---|---|---|
| A * | 0.99 (0.59) | 25.0 |
| B | 1.45 (2.22) | 54.2 |
| C | 0.57 (0.55) | 50.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theocharopoulos, A.L.; Chen, X.; Karpukhina, N.; Cattell, M.J. Glass Property Predictions and the Design and Characterization of Leucite Glass-Ceramics. Appl. Sci. 2025, 15, 12129. https://doi.org/10.3390/app152212129
Theocharopoulos AL, Chen X, Karpukhina N, Cattell MJ. Glass Property Predictions and the Design and Characterization of Leucite Glass-Ceramics. Applied Sciences. 2025; 15(22):12129. https://doi.org/10.3390/app152212129
Chicago/Turabian StyleTheocharopoulos, Antonios L., Xiaohui Chen, Natalia Karpukhina, and Michael J. Cattell. 2025. "Glass Property Predictions and the Design and Characterization of Leucite Glass-Ceramics" Applied Sciences 15, no. 22: 12129. https://doi.org/10.3390/app152212129
APA StyleTheocharopoulos, A. L., Chen, X., Karpukhina, N., & Cattell, M. J. (2025). Glass Property Predictions and the Design and Characterization of Leucite Glass-Ceramics. Applied Sciences, 15(22), 12129. https://doi.org/10.3390/app152212129







