Tumor-Infiltrating Immune Cells in Non-Muscle-Invasive Bladder Cancer: Prognostic Implications, Predictive Value, and Future Perspectives
Abstract
1. Introduction
2. TIL as a Potential Prognostic Tool in BUC—With a Focus on NMIBC
3. TIL as a Predictive Tool in BCG Treatment
4. Tumor-Associated Macrophages (TAM)
5. T Cells
6. NK Cells
7. B Cells
8. Tertiary Lymphoid Structures (TLS)
9. Methods of Assessment and Reproducibility
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| BCG | Bacillus Calmette–Guérin |
| CSS | Cancer-specific survival |
| HG | High grade |
| H&E | Hematoxylin and eosin |
| NMIBC | Non-muscle-invasive bladder cancer |
| OS | Overall survival |
| RFS | Recurrence-free survival |
| TAM | Tumor-associated macrophages |
| TIL | Tumor-infiltrating lymphocytes |
References
- IARC. Global Cancer Observatory: Cancer Today [Internet]. 2025. Available online: https://gco.iarc.fr/today/en/dataviz/bars?mode=cancer&group_populations=1&key=total (accessed on 31 August 2025).
- Smani, S.; DuBois, J.; Zhao, K.; Sutherland, R.; Rahman, S.N.; Humphrey, P.; Hesse, D.; Tan, W.S.; Martin, D.; Lokeshwar, S.D.; et al. Advancements in the Diagnosis, Treatment, and Risk Stratification of Non-Muscle Invasive Bladder Cancer. Curr. Oncol. Rep. 2025, 27, 236–246. [Google Scholar] [CrossRef]
- Holzbeierlein, J.M.; Bixler, B.R.; Buckley, D.I.; Chang, S.S.; Holmes, R.; James, A.C.; Kirkby, E.; McKiernan, J.M.; Schuckman, A.K. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline: 2024 Amendment. J. Urol. 2024, 211, 533–538. [Google Scholar] [CrossRef]
- Cambier, S.; Sylvester, R.J.; Collette, L.; Gontero, P.; Brausi, M.A.; van Andel, G.; Kirkels, W.J.; Da Silva, F.C.; Oosterlinck, W.; Prescott, S.; et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1–3 Years of Maintenance Bacillus Calmette-Guérin. Eur. Urol. 2016, 69, 60–69. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Pettenati, C.; Ingersoll, M.A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat. Rev. Urol. 2018, 15, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Engelmann, S.U.; Mayr, R.; Gossler, C.; Pickl, C.; Kälble, S.; Yang, Y.; Otto, W.; Hartmann, V.; Burger, M.; et al. A novel grading approach predicts worse outcomes in stage pT1 non-muscle-invasive bladder cancer. BJU Int. 2024, 134, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Rouanne, M.; Betari, R.; Radulescu, C.; Goubar, A.; Signolle, N.; Neuzillet, Y.; Allory, Y.; Marabelle, A.; Adam, J.; Lebret, T. Stromal lymphocyte infiltration is associated with tumour invasion depth but is not prognostic in high-grade T1 bladder cancer. Eur. J. Cancer 2019, 108, 111–119. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Falagario, U.G.; Zanelli, M.; Palicelli, A.; Zizzo, M.; Ascani, S.; Tortorella, S.; Mancini, V.; Cormio, A.; Carrieri, G.; et al. Clinicopathological Features and Survival Analysis in Molecular Subtypes of Muscle-Invasive Bladder Cancer. Int. J. Mol. Sci. 2023, 24, 6610. [Google Scholar] [CrossRef]
- Hülsen, S.; Lippolis, E.; Ferrazzi, F.; Otto, W.; Distel, L.; Fietkau, R.; Denzinger, S.; Breyer, J.; Burger, M.; Bertz, S.; et al. High Stroma T-Cell Infiltration is Associated with Better Survival in Stage pT1 Bladder Cancer. Int. J. Mol. Sci. 2020, 21, 8407. [Google Scholar] [CrossRef]
- Drachneris, J.; Rasmusson, A.; Morkunas, M.; Fabijonavicius, M.; Cekauskas, A.; Jankevicius, F.; Laurinavicius, A. CD8+ Cell Density Gradient across the Tumor Epithelium-Stromal Interface of Non-Muscle Invasive Papillary Urothelial Carcinoma Predicts Recurrence-Free Survival after BCG Immunotherapy. Cancers 2023, 15, 1205. [Google Scholar] [CrossRef]
- Lee, S.M. Prognosis in high-grade T1 bladder cancer: Host immune response and tumour infiltrating lymphocytes. Transl. Androl. Urol. 2019, 8, S335–S336. [Google Scholar] [CrossRef]
- De Carlo, C.; Valeri, M.; Corbitt, D.N.; Cieri, M.; Colombo, P. Non-muscle invasive bladder cancer biomarkers beyond morphology. Front. Oncol. 2022, 12, 947446. [Google Scholar] [CrossRef] [PubMed]
- Semeniuk-Wojtaś, A.; Modzelewska, M.; Poddębniak-Strama, K.; Kołaczyńska, S.; Lubas, A.; Górnicka, B.; Jakieła, A.; Stec, R. CD4, CD20 and PD-L1 as Markers of Recurrence in Non-Muscle-Invasive Bladder Cancer. Cancers 2023, 15, 5529. [Google Scholar] [CrossRef] [PubMed]
- Ledderose, S.; Rodler, S.; Eismann, L.; Ledderose, G.; Ledderose, C. Tumor-infiltrating lymphocytes predict survival in ≥ pT2 urothelial bladder cancer. Pathol. Res. Pract. 2022, 237, 154037. [Google Scholar] [CrossRef] [PubMed]
- Yaprak Bayrak, B.; Dogan, H.N.; Teke, K.; Yuvak, H.; Dillioglugil, O. The role of tumor-infiltrating lymphocytes and depth of invasion in T1 bladder cancer: A retrospective analysis. BMC Urol. 2025, 25, 135. [Google Scholar] [CrossRef]
- Eckstein, M.; Matek, C.; Wagner, P.; Erber, R.; Büttner-Herold, M.; Wild, P.J.; Taubert, H.; Wach, S.; Sikic, D.; Wullich, B.; et al. Proposal for a Novel Histological Scoring System as a Potential Grading Approach for Muscle-invasive Urothelial Bladder Cancer Correlating with Disease Aggressiveness and Patient Outcomes. Eur. Urol. Oncol. 2024, 7, 128–138. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.H.; Chuah, E. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef]
- Krpina, K.; Markić, D.; Rahelić, D.; Ahel, J.; Rubinić, N.; Španjol, J. 10-year survival of a patient with metastatic prostate cancer: Case report and literature review. Arch. Ital. Urol. Androl. 2015, 87, 252–253. [Google Scholar] [CrossRef][Green Version]
- Liu, K.; Zhao, K.; Wang, L.; Sun, E. The prognostic values of tumor-infiltrating neutrophils, lymphocytes and neutrophil/lymphocyte rates in bladder urothelial cancer. Pathol. Res. Pract. 2018, 214, 1074–1080. [Google Scholar] [CrossRef]
- Roumiguié, M.; Kamat, A.M.; Bivalacqua, T.J.; Lerner, S.P.; Kassouf, W.; Böhle, A.; Brausi, M.; Buckley, R.; Persad, R.; Colombel, M.; et al. International Bladder Cancer Group Consensus Statement on Clinical Trial Design for Patients with Bacillus Calmette-Guérin-exposed High-risk Non-muscle-invasive Bladder Cancer. Eur Urol. 2022, 82, 34–46. [Google Scholar] [CrossRef]
- Viveiros, M.M.; Barreto, M.C.; Seca, A.M.L. Laurus azorica: Valorization through Its Phytochemical Study and Biological Activities. Separations 2022, 9, 211. [Google Scholar] [CrossRef]
- Breyer, J.; Wirtz, R.M.; Otto, W.; Erben, P.; Worst, T.S.; Stoehr, R.; Eckstein, M.; Denzinger, S.; Burger, M.; Hartmann, A. High PDL1 mRNA expression predicts better survival of stage pT1 non-muscle-invasive bladder cancer (NMIBC) patients. Cancer Immunol. Immunother. 2018, 67, 403–412. [Google Scholar] [CrossRef]
- Wang, B.; Wu, S.; Zeng, H.; Liu, Z.; Dong, W.; He, W.; Chen, X.; Dong, X.; Zheng, L.; Lin, T.; et al. CD103+ Tumor Infiltrating Lymphocytes Predict a Favorable Prognosis in Urothelial Cell Carcinoma of the Bladder. J. Urol. 2015, 194, 556–562. [Google Scholar] [CrossRef]
- Drachneris, J.; Morkunas, M.; Fabijonavicius, M.; Cekauskas, A.; Jankevicius, F.; Laurinavicius, A. Spatial Distribution of Macrophage and Lymphocyte Subtypes within Tumor Microenvironment to Predict Recurrence of Non-Muscle-Invasive Papillary Urothelial Carcinoma after BCG Immunotherapy. Int. J. Mol. Sci. 2024, 25, 4776. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Roumiguié, M.; Kamat, A.M.; Shore, N.D.; Boormans, J.L.; Esen, A.A.; Lebret, T.; Kandori, S.; Bajorin, D.F.; Krieger, L.E.M.; et al. Pembrolizumab monotherapy for high-risk non-muscle-invasive bladder cancer without carcinoma in situ and unresponsive to BCG (KEYNOTE-057): A single-arm, multicentre, phase 2 trial. Lancet Oncol. 2024, 25, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Brimo, F.; Wu, C.; Zeizafoun, N.; Tanguay, S.; Aprikian, A.; Mansure, J.J.; Kassouf, W. Prognostic factors in T1 bladder urothelial carcinoma: The value of recording millimetric depth of invasion, diameter of invasive carcinoma, and muscularis mucosa invasion. Hum. Pathol. 2013, 44, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.; Kimmel, C.; Bruendl, J.; Weber, F.; Denzinger, S.; Gierth, M.; Burger, M.; Hartmann, A.; Otto, W.; Breyer, J. Tumor budding correlates with tumor invasiveness and predicts worse survival in pT1 non-muscle-invasive bladder cancer. Sci. Rep. 2021, 11, 17981. [Google Scholar] [CrossRef]
- Pichler, R.; Fritz, J.; Zavadil, C.; Schäfer, G.; Culig, Z.; Brunner, A. Tumor-infiltrating immune cell subpopulations influence the oncologic outcome after intravesical Bacillus Calmette-Guérin therapy in bladder cancer. Oncotarget 2016, 7, 39916–39930. [Google Scholar] [CrossRef]
- Miyake, M.; Tatsumi, Y.; Gotoh, D.; Ohnishi, S.; Owari, T.; Iida, K.; Ohnishi, K.; Hori, S.; Morizawa, Y.; Itami, Y.; et al. Regulatory T Cells and Tumor-Associated Macrophages in the Tumor Microenvironment in Non-Muscle Invasive Bladder Cancer Treated with Intravesical Bacille Calmette-Guérin: A Long-Term Follow-Up Study of a Japanese Cohort. Int. J. Mol. Sci. 2017, 18, 2186. [Google Scholar] [CrossRef]
- Ajili, F.; Kourda, N.; Darouiche, A.; Chebil, M.; Boubaker, S. Prognostic Value of Tumor-associated Macrophages Count in Human Non-muscle-invasive Bladder Cancer Treated by BCG Immunotherapy. Ultrastruct. Pathol. 2013, 37, 56–61. [Google Scholar] [CrossRef]
- Ayari, C.; LaRue, H.; Hovington, H.; Decobert, M.; Harel, F.; Bergeron, A.; Têtu, B.; Lacombe, L.; Fradet, Y. Bladder tumor infiltrating mature dendritic cells and macrophages as predictors of response to Bacillus Calmette-Guérin immunotherapy. Eur. Urol. 2009, 55, 1386–1395. [Google Scholar] [CrossRef]
- Eich, M.L.; Chaux, A.; Guner, G.; Taheri, D.; Mendoza Rodriguez, M.A.; Rodriguez Peña, M.D.C.; Baras, A.S.; Hahn, N.M.; Drake, C.; Sharma, R.; et al. Tumor immune microenvironment in non-muscle-invasive urothelial carcinoma of the bladder. Hum. Pathol. 2019, 89, 24–32. [Google Scholar] [CrossRef]
- Takayama, H.; Nishimura, K.; Tsujimura, A.; Nakai, Y.; Nakayama, M.; Aozasa, K.; Okuyama, A.; Nonomura, N. Increased infiltration of tumor associated macrophages is associated with poor prognosis of bladder carcinoma in situ after intravesical bacillus Calmette-Guerin instillation. J. Urol. 2009, 181, 1894–1900. [Google Scholar] [CrossRef] [PubMed]
- Vaghjiani, R.G.; Skitzki, J.J. Tertiary Lymphoid Structures as Mediators of Immunotherapy Response. Cancers 2022, 14, 3748. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-A.; Yang, B.; Tang, T.; Yang, Y.; Zhang, D.; Xiao, H.; Xu, J.; Wang, L.; Lin, L.; Jiang, J. Correlation of APE1 with VEGFA and CD163(+) macrophage infiltration in bladder cancer and their prognostic significance. Oncol. Lett. 2020, 20, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, A.; Umemoto, S.; Yokose, T.; Nakamura, Y.; Yoshihara, M.; Shoji, K.; Wada, S.; Miyagi, Y.; Kishida, T.; Sasada, T. Enhanced expression of PD-L1 in non-muscle-invasive bladder cancer after treatment with Bacillus Calmette-Guerin. Oncotarget 2018, 9, 34066–34078. [Google Scholar] [CrossRef]
- Kates, M.; Matoso, A.; Choi, W.; Baras, A.S.; Daniels, M.J.; Lombardo, K.; Brant, A.; Mikkilineni, N.; McConkey, D.J.; Kamat, A.M.; et al. Adaptive Immune Resistance to Intravesical BCG in Non-Muscle Invasive Bladder Cancer: Implications for Prospective BCG-Unresponsive Trials. Clin. Cancer Res. 2020, 26, 882–891. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Cao, N.; Li, Z.; Han, J.; Li, L. Resveratrol, an activator of SIRT1, induces protective autophagy in non-small-cell lung cancer via inhibiting Akt/mTOR and activating p38-MAPK. OncoTargets Ther. 2018, 11, 7777–7786. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Falagario, U.G.; Zanelli, M.; Palicelli, A.; Zizzo, M.; Busetto, G.M.; Cormio, A.; Carrieri, G.; Cormio, L. Integrating the PD-L1 Prognostic Biomarker in Non-Muscle Invasive Bladder Cancer in Clinical Practice—A Comprehensive Review on State-of-the-Art Advances and Critical Issues. J. Clin. Med. 2024, 13, 2182. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, Z.; Ventura, E.; Gran, B.; Shindler, K.S.; Rostami, A. The PD-1/PD-L pathway is up-regulated during IL-12-induced suppression of EAE mediated by IFN-gamma. J. Neuroimmunol. 2007, 185, 75–86. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, X.; O’Donnell, M.A. Role of Th1 and Th2 cytokines in BCG-induced IFN-γ production: Cytokine promotion and simulation of BCG effect. Cytokine 2003, 21, 17–26. [Google Scholar] [CrossRef]
- Salomé, B.; Sfakianos, J.P.; Ranti, D.; Daza, J.; Bieber, C.; Charap, A.; Hammer, C.; Banchereau, R.; Farkas, A.M.; Ruan, D.F.; et al. NKG2A and HLA-E define an alternative immune checkpoint axis in bladder cancer. Cancer Cell 2022, 40, 1027–1043.e9. [Google Scholar] [CrossRef]
- Larsson, L.; Frisén, J.; Lundeberg, J. Spatially resolved transcriptomics adds a new dimension to genomics. Nat. Methods 2021, 18, 15–18. [Google Scholar] [CrossRef]
- Esteso, G.; Felgueres, M.J.; García-Jiménez, Á.F.; Reyburn-Valés, C.; Benguría, A.; Vázquez, E.; Reyburn, H.T.; Aguiló, N.; Martín, C.; Puentes, E.; et al. BCG-activation of leukocytes is sufficient for the generation of donor-independent innate anti-tumor NK and γδ T-cells that can be further expanded in vitro. Oncoimmunology 2023, 12, 2160094. [Google Scholar] [CrossRef]
- García-Cuesta, E.M.; Esteso, G.; Ashiru, O.; López-Cobo, S.; Álvarez-Maestro, M.; Linares, A.; Ho, M.M.; Martínez-Piñeiro, L.; Reyburn, H.T.; Valés-Gómez, M. Characterization of a human anti-tumoral NK cell population expanded after BCG treatment of leukocytes. Oncoimmunology 2017, 6, e1293212. [Google Scholar] [CrossRef] [PubMed]
- de Jong, F.C.; Laajala, T.D.; Hoedemaeker, R.F.; Jordan, K.R.; van der Made, A.C.J.; Boevé, E.R.; van der Schoot, D.K.E.; Nieuwkamer, B.; Janssen, E.A.M.; Mahmoudi, T.; et al. Non-muscle-invasive bladder cancer molecular subtypes predict differential response to intravesical Bacillus Calmette-Guérin. Sci. Transl. Med. 2023, 15, eabn4118. [Google Scholar] [CrossRef] [PubMed]
- Kardoust Parizi, M.; Shariat, S.F.; Margulis, V.; Mori, K.; Lotan, Y. Value of tumour-infiltrating immune cells in predicting response to intravesical BCG in patients with non-muscle-invasive bladder cancer: A systematic review and meta-analysis. BJU Int. 2021, 127, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.; Tapia, G.; De Muga, S.; Hernández, A.; Cao, M.G.; Teixidó, C.; Urrea, V.; García, E.; Pedreño-López, S.; Ibarz, L.; et al. Combined assessment of peritumoral Th1/Th2 polarization and peripheral immunity as a new biomarker in the prediction of BCG response in patients with high-risk NMIBC. OncoImmunology 2019, 8, 1602460. [Google Scholar] [CrossRef]
- Nunez-Nateras, R.; Castle, E.P.; Protheroe, C.A.; Stanton, M.L.; Ocal, T.I.; Ferrigni, E.N.; Ochkur, S.I.; Jacobsen, E.A.; Hou, Y.-X.; Andrews, P.E.; et al. Predicting response to Bacillus Calmette-Guérin (BCG) in patients with carcinoma in situ of the bladder. Urol. Oncol. 2014, 32, 45.e23–45.e30. [Google Scholar] [CrossRef]
- Chevalier, M.F.; Trabanelli, S.; Racle, J.; Salomé, B.; Cesson, V.; Gharbi, D.; Bohner, P.; Domingos-Pereira, S.; Dartiguenave, F.; Fritschi, A.-S.; et al. ILC2-modulated T cell-to-MDSC balance is associated with bladder cancer recurrence. J. Clin. Investig. 2017, 127, 2916–2929. [Google Scholar] [CrossRef]
- Krpina, K.; Babarović, E.; Španjol, J.; Đorđević, G.; Maurer, T.; Jonjić, N. Correlation of tumor-associated macrophages and NK cells with bladder cancer size and T stage in patients with solitary low-grade urothelial carcinoma. Wien. Klin. Wochenschr. 2016, 128, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- van der Horst, G.; Bos, L.; van der Pluijm, G. Epithelial plasticity, cancer stem cells, and the tumor-supportive stroma in bladder carcinoma. Mol. Cancer Res. 2012, 10, 995–1009. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.; Oliveira, D.; Tavares, A.; Amaro, T.; Cruz, R.; Oliveira, M.J.; Ferreira, J.A.; Santos, L. The predominance of M2-polarized macrophages in the stroma of low-hypoxic bladder tumors is associated with BCG immunotherapy failure. Urol. Oncol. 2014, 32, 449–457. [Google Scholar] [CrossRef]
- Boström, M.M.; Irjala, H.; Mirtti, T.; Taimen, P.; Kauko, T.; Ålgars, A.; Jalkanen, S.; Boström, P.J. Tumor-Associated Macrophages Provide Significant Prognostic Information in Urothelial Bladder Cancer. PLoS ONE 2015, 10, e0133552. [Google Scholar] [CrossRef]
- Hori, S.; Miyake, M.; Tatsumi, Y.; Onishi, S.; Morizawa, Y.; Nakai, Y.; Tanaka, N.; Fujimoto, K. Topical and systemic immunoreaction triggered by intravesical chemotherapy in an N-butyl-N-(4-hydroxybutyl) nitorosamine induced bladder cancer mouse model. PLoS ONE 2017, 12, e0175494. [Google Scholar] [CrossRef]
- Pane, K.; Mirabelli, P.; Coppola, L.; Illiano, E.; Salvatore, M.; Franzese, M. New Roadmaps for Non-muscle-invasive Bladder Cancer with Unfavorable Prognosis. Front. Chem. 2020, 8, 600. [Google Scholar] [CrossRef]
- Hanada, T.; Nakagawa, M.; Emoto, A.; Nomura, T.; Nasu, N.; Nomura, Y. Prognostic value of tumor-associated macrophage count in human bladder cancer. Int. J. Urol. 2000, 7, 263–269. [Google Scholar] [CrossRef]
- Ibrahim, O.M.; Kalinski, P. Breaking Barriers: Modulation of Tumor Microenvironment to Enhance Bacillus Calmette-Guérin Immunotherapy of Bladder Cancer. Cells 2024, 13, 699. [Google Scholar] [CrossRef]
- Chenard, S.; Jackson, C.; Vidotto, T.; Chen, L.; Hardy, C.; Jamaspishvilli, T.; Berman, D.; Siemens, D.R.; Koti, M. Sexual Dimorphism in Outcomes of Non-muscle-invasive Bladder Cancer: A Role of CD163+ Macrophages, B cells, and PD-L1 Immune Checkpoint. Eur. Urol. Open Sci. 2021, 29, 50–58. [Google Scholar] [CrossRef]
- Rudensky, A.Y. Regulatory T cells and Foxp3. Immunol. Rev. 2011, 241, 260–268. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Murai, R.; Itoh, Y.; Kageyama, S.; Nakayama, M.; Ishigaki, H.; Teramoto, K.; Narita, M.; Yoshida, T.; Tomita, K.; Kobayashi, K.-I.; et al. Prediction of intravesical recurrence of non-muscle-invasive bladder cancer by evaluation of intratumoral Foxp3+ T cells in the primary transurethral resection of bladder tumor specimens. PLoS ONE 2018, 13, e0204745. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, Y.; Liu, Z.; Li, B.; Jiang, N.; Xu, P.; Xu, A. Identification of Signature Genes Associated with Invasiveness and the Construction of a Prognostic Model That Predicts the Overall Survival of Bladder Cancer. Front. Genet. 2021, 12, 694777. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Skok, K.; Bräutigam, K. Tumor infiltrating lymphocytes (TILs)—Pathologia, quo vadis?—A global survey. Pathol. Res. Pract. 2025, 266, 155775. [Google Scholar] [CrossRef]
- Mezheyeuski, A.; Segersten, U.; Leiss, L.W.; Malmström, P.-U.; Hatina, J.; Östman, A.; Strell, C. Fibroblasts in urothelial bladder cancer define stroma phenotypes that are associated with clinical outcome. Sci. Rep. 2020, 10, 281. [Google Scholar] [CrossRef]
- Roumiguié, M.; Compérat, E.; Chaltiel, L.; Nouhaud, F.X.; Verhoest, G.; Masson-Lecomte, A.; Colin, P.; Audenet, F.; Houédé, N.; Larré, S.; et al. PD-L1 expression and pattern of immune cells in pre-treatment specimens are associated with disease-free survival for HR-NMIBC undergoing BCG treatment. World J. Urol. 2021, 39, 4055–4065. [Google Scholar] [CrossRef]
- Audenet, F.; Farkas, A.M.; Anastos, H.; Galsky, M.D.; Bhardwaj, N.; Sfakianos, J.P. Immune phenotype of peripheral blood mononuclear cells in patients with high-risk non-muscle invasive bladder cancer. World J. Urol. 2018, 36, 1741–1748. [Google Scholar] [CrossRef]
- Brandau, S.; Riemensberger, J.; Jacobsen, M.; Kemp, D.; Zhao, W.; Zhao, X.; Jocham, D.; Ratliff, T.L.; Böhle, A. NK cells are essential for effective BCG immunotherapy. Int. J. Cancer 2001, 92, 697–702. [Google Scholar] [CrossRef]
- Li, L.; Li, A.; Jin, H.; Li, M.; Jia, Q. Inhibitory receptors and checkpoints on NK cells: Implications for cancer immunotherapy. Pathol. Res. Pract. 2024, 253, 155003. [Google Scholar] [CrossRef] [PubMed]
- Ran, G.H.; Lin, Y.Q.; Tian, L.; Zhang, T.; Yan, D.M.; Yu, J.H.; Deng, Y.C. Natural killer cell homing and trafficking in tissues and tumors: From biology to application. Signal Transduct. Target Ther. 2022, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Park, J.H.; Park, D.I.; Sohn, C.I.; Lee, J.M.; Kim, T.I. Impact of Smoking on Human Natural Killer Cell Activity: A Large Cohort Study. J. Cancer Prev. 2020, 25, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.Z.; Bastos, D.A.; Mattedi, R.L.; Dzik, C.; Jardim, D.L.; Coelho, R.; Ribeiro-Filho, L.A.; Cordeiro, M.D.; Nahas, W.C.; Mello, E.S.; et al. Infiltrating Natural Killer cells influence the efficacy of BCG immunotherapy in non-muscle-invasive bladder cancer. Pathol. Res. Pract. 2025, 270, 155997. [Google Scholar] [CrossRef]
- Lu, L.M.; Zavitz, C.C.; Chen, B.; Kianpour, S.; Wan, Y.; Stämpfli, M.R. Cigarette smoke impairs NK cell-dependent tumor immune surveillance. J. Immunol. 2007, 178, 936–943. [Google Scholar] [CrossRef]
- Yutkin, V.; Pode, D.; Pikarsky, E.; Mandelboim, O. The expression level of ligands for natural killer cell receptors predicts response to bacillus Calmette-Guerin therapy: A pilot study. J. Urol. 2007, 178, 2660–2664. [Google Scholar] [CrossRef]
- Krpina, K.; Babarović, E.; Jonjić, N. Correlation of tumor-infiltrating lymphocytes with bladder cancer recurrence in patients with solitary low-grade urothelial carcinoma. Virchows Arch. 2015, 467, 443–448. [Google Scholar] [CrossRef]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Farkas, A.M.; Gul, Z.; Sfakianos, J.P. Harnessing Natural Killer Cell Function for Genitourinary Cancers. Urol. Clin. N. Am. 2020, 47, 433–442. [Google Scholar] [CrossRef]
- Habif, G.; Crinier, A.; André, P.; Vivier, E.; Narni-Mancinelli, E. Targeting natural killer cells in solid tumors. Cell. Mol. Immunol. 2019, 16, 415–422. [Google Scholar] [CrossRef]
- Huntington, N.D.; Cursons, J.; Rautela, J. The cancer-natural killer cell immunity cycle. Nat. Rev. Cancer 2020, 20, 437–454. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Ranti, D.; Bieber, C.; Wang, Y.S.; Sfakianos, J.P.; Horowitz, A. Natural killer cells: Unlocking new treatments for bladder cancer. Trends Cancer 2022, 8, 698–710. [Google Scholar] [CrossRef]
- Chu, P.G.; Arber, D.A. CD79: A review. Appl. Immunohistochem. Mol. Morphol. 2001, 9, 97–106. [Google Scholar] [CrossRef]
- Sanz, I.; Wei, C.; Jenks, S.A.; Cashman, K.S.; Tipton, C.; Woodruff, M.C.; Hom, J.; Lee, F.E.-H. Challenges and Opportunities for Consistent Classification of Human B Cell and Plasma Cell Populations. Front. Immunol. 2019, 10, 2458. [Google Scholar] [CrossRef] [PubMed]
- Semeniuk-Wojtaś, A.; Poddębniak-Strama, K.; Modzelewska, M.; Baryła, M.; Dziąg-Dudek, E.; Syryło, T.; Górnicka, B.; Jakieła, A.; Stec, R. Tumour microenvironment as a predictive factor for immunotherapy in non-muscle-invasive bladder cancer. Cancer Immunol. Immunother. 2023, 72, 1971–1989. [Google Scholar] [CrossRef] [PubMed]
- Viveiros, N.; Flores, B.C.; Lobo, J.; Martins-Lima, C.; Cantante, M.; Lopes, P.; Deantonio, C.; Palu, C.; Sainson, R.C.; Henrique, R.; et al. Detailed bladder cancer immunoprofiling reveals new clues for immunotherapeutic strategies. Clin. Transl. Immunol. 2022, 11, e1402. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Wang, Y.; Liu, L.; Li, L.; Yeh, S.; Qi, L.; Chang, C. Tumor microenvironment B cells increase bladder cancer metastasis via modulation of the IL-8/androgen receptor (AR)/MMPs signals. Oncotarget 2015, 6, 26065–26078. [Google Scholar] [CrossRef]
- Dieu-Nosjean, M.-C.; Antoine, M.; Danel, C.; Heudes, D.; Wislez, M.; Poulot, V.; Rabbe, N.; Laurans, L.; Tartour, E.; de Chaisemartin, L.; et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol. 2008, 26, 4410–4417. [Google Scholar] [CrossRef]
- Finkin, S.; Yuan, D.; Stein, I.; Taniguchi, K.; Weber, A.; Unger, K.; Browning, J.L.; Goossens, N.; Nakagawa, S.; Gunasekaran, G.; et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat. Immunol. 2015, 16, 1235–1244. [Google Scholar] [CrossRef]
- Quinn, D.I.; Shore, N.D.; Egawa, S.; Gerritsen, W.R.; Fizazi, K. Immunotherapy for castration-resistant prostate cancer: Progress and new paradigms. Urol. Oncol. 2015, 33, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Koti, M.; Xu, A.S.; Ren, K.Y.M.; Visram, K.; Ren, R.; Berman, D.M.; Siemens, D.R. Tertiary Lymphoid Structures Associate with Tumour Stage in Urothelial Bladder Cancer. Bladder Cancer 2017, 3, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, N.; Gil-Jimenez, A.; Silina, K.; van Montfoort, M.L.; Einerhand, S.; Jonkman, L.; Voskuilen, C.S.; Peters, D.; Sanders, J.; Lubeck, Y.; et al. The Tumor Immune Landscape and Architecture of Tertiary Lymphoid Structures in Urothelial Cancer. Front. Immunol. 2021, 12, 793964. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.M.; Salgado, R.; Gevaert, T.; Russell, P.A.M.; John, T.; Thapa, B.M.; Christie, M.M.; van de Vijver, K.; Estrada, M.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non-Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv. Anat. Pathol. 2017, 24, 311–335. [Google Scholar]
- Bayrak, C.S.; Stein, D.; Jain, A.; Chaudhary, K.; Nadkarni, G.N.; Van Vleck, T.T.; Puel, A.; Boisson-Dupuis, S.; Okada, S.; Stenson, P.D.; et al. Identification of discriminative gene-level and protein-level features associated with pathogenic gain-of-function and loss-of-function variants. Am. J. Hum. Genet. 2021, 108, 2301–2318. [Google Scholar] [CrossRef]
- Bieri, U.; Scharl, M.; Sigg, S.; Szczerba, B.M.; Morsy, Y.; Rüschoff, J.H.; Schraml, P.H.; Krauthammer, M.; Hefermehl, L.J.; Eberli, D.; et al. Prospective observational study of the role of the microbiome in BCG responsiveness prediction (SILENT-EMPIRE): A study protocol. BMJ Open 2022, 12, e061421. [Google Scholar] [CrossRef]
- Hassan, W.A.; ElBanna, A.K.; Noufal, N.; El-Assmy, M.; Lotfy, H.; Ali, R.I. Significance of tumor-associated neutrophils, lymphocytes, and neutrophil-to-lymphocyte ratio in non-invasive and invasive bladder urothelial carcinoma. J. Pathol. Transl. Med. 2023, 57, 88–94. [Google Scholar] [CrossRef]
- Chen, L.; Xu, L.; Zhang, X.; Zhang, J.; Bai, X.; Peng, Q.; Guo, E.; Lu, X.; Yu, S.; Jin, Z.; et al. Diagnostic value of dual-layer spectral detector CT parameters for differentiating high- from low-grade bladder cancer. Insights Imaging 2025, 16, 6. [Google Scholar] [CrossRef]
- Damrauer, J.S.; Roell, K.R.; Smith, M.A.; Sun, X.; Kirk, E.L.; Hoadley, K.A.; Benefield, H.C.; Iyer, G.; Solit, D.B.; Milowsky, M.I.; et al. Identification of a Novel Inflamed Tumor Microenvironment Signature as a Predictive Biomarker of Bacillus Calmette-Guérin Immunotherapy in Non-Muscle-Invasive Bladder Cancer. Clin. Cancer Res. 2021, 27, 4599–4609. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Cormio, A.; Zanelli, M.; Zizzo, M.; Palicelli, A.; Falagario, U.G.; Milanese, G.; Galosi, A.B.; Mazzucchelli, R.; Cormio, L.; et al. Diagnostic, Prognostic, and Predictive Tissue Biomarkers in Urothelial Carcinoma In Situ: A Narrative Review. Diagnostics 2025, 15, 2163. [Google Scholar] [CrossRef]
- Rohaan, M.W.; Wilgenhof, S.; Haanen, J. Adoptive cellular therapies: The current landscape. Virchows Arch. 2019, 474, 449–461. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Restifo, N.P.; Yang, J.C.; Morgan, R.A.; Dudley, M.E. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 2008, 8, 299–308. [Google Scholar] [CrossRef]
- Piombino, C.; Tonni, E.; Oltrecolli, M.; Pirola, M.; Pipitone, S.; Baldessari, C.; Dominici, M.; Sabbatini, R.; Vitale, M.G. Immunotherapy in urothelial cancer: Current status and future directions. Expert Rev. Anticancer Ther. 2023, 23, 1141–1155. [Google Scholar] [CrossRef]
- Kim, J.W.; Tomita, Y.; Trepel, J.; Apolo, A.B. Emerging immunotherapies for bladder cancer. Curr. Opin. Oncol. 2015, 27, 191–200. [Google Scholar] [CrossRef]
- Tran, E.; Robbins, P.F.; Rosenberg, S.A. ‘Final common pathway’ of human cancer immunotherapy: Targeting random somatic mutations. Nat. Immunol. 2017, 18, 255–262. [Google Scholar] [CrossRef]

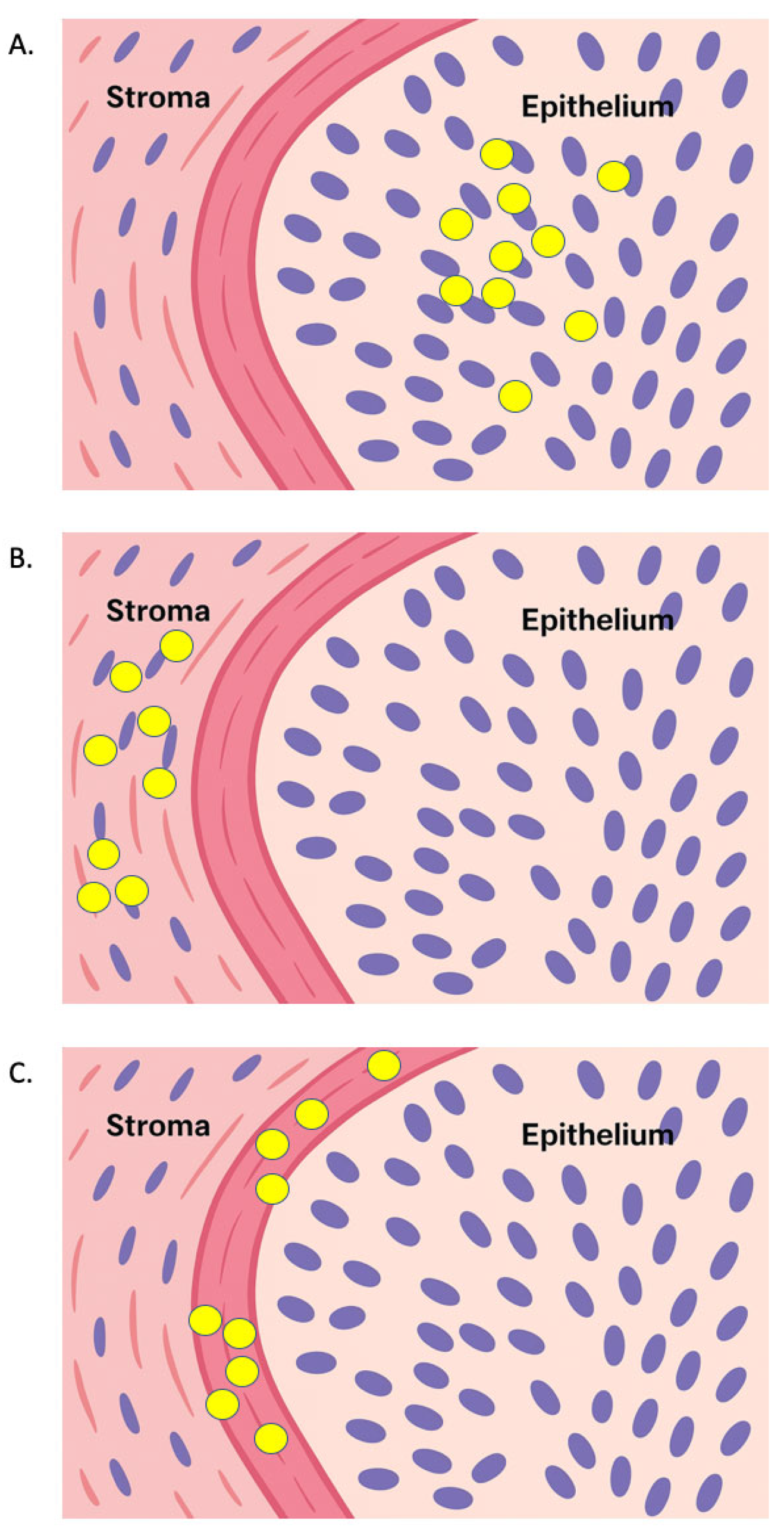
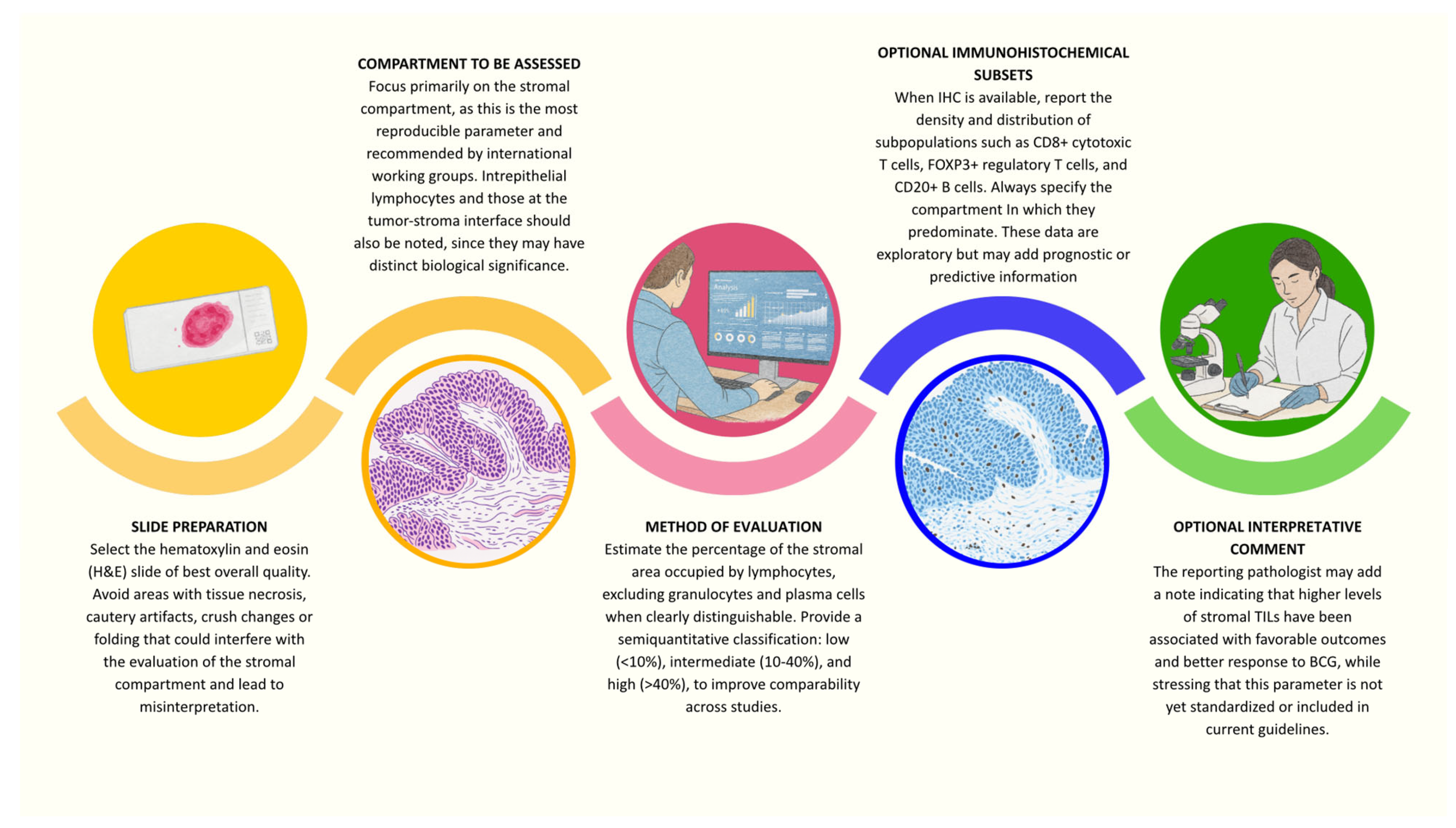
| Study | Cohort | TIL Subsets/Methodology | Significant Association | Prognostic Endpoint(s) |
|---|---|---|---|---|
| Hülsen S et al., 2020 [10] | 167 pT1 NMIBC | Stromal vs. intraepithelial TIL; CD3+, CD8+ | High stromal CD3+ infiltration—favorable outcome (OS, RFS) | OS, RFS |
| Rouanne M et al., 2019 [8] | 147 pT1 HG NMIBC | Stromal TIL density (%) | Higher stromal TIL density—tumor invasion depth | CSS |
| Drachneris J et al., 2023 [11] | 157 papillary NMIBC treated with BCG | Spatial CD8+ gradient at epithelium–stroma interface (digital analysis) | CD8+ gradient—RFS | RFS |
| Yaprak Bayrak B et al., 2025 [16] | 154 pT1 NMIBC | Semi-quantitative H&E TIL scoring | Higher TILs ratios—increased tumor thickness and more extensive invasion | RFS, PFS |
| Pichler R et al., 2016 [29] | 40 NMIBC treated with BCG | TAM, B-cell, T-cell densities at different compartments | Higher CD4+ and GATA3+ count—prolonged RFS. Higher Tregs and TAMs—BCG failure | RFS |
| Miyake M et al., 2017 [30] | 71 NMIBC treated with BCG | Peritumoral FOXP3+ Tregs and CD68+ TAM | High counts of Treg and TAMs—shorter RFS | RFS |
| Drachneris J et al., 2024 [25] | 165 papillary NMIBC treated with BCG | Epithelial-stromal Interface Density Ratios for CD8+, ICOS+, CD11c+, CD20+, CD163+ | CD11c IDR > CD8 and ICOS IDR—longer RFS | RFS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzucchelli, R.; Cormio, A.; Zanelli, M.; Zizzo, M.; Palicelli, A.; Galosi, A.B.; Sanguedolce, F. Tumor-Infiltrating Immune Cells in Non-Muscle-Invasive Bladder Cancer: Prognostic Implications, Predictive Value, and Future Perspectives. Appl. Sci. 2025, 15, 12032. https://doi.org/10.3390/app152212032
Mazzucchelli R, Cormio A, Zanelli M, Zizzo M, Palicelli A, Galosi AB, Sanguedolce F. Tumor-Infiltrating Immune Cells in Non-Muscle-Invasive Bladder Cancer: Prognostic Implications, Predictive Value, and Future Perspectives. Applied Sciences. 2025; 15(22):12032. https://doi.org/10.3390/app152212032
Chicago/Turabian StyleMazzucchelli, Roberta, Angelo Cormio, Magda Zanelli, Maurizio Zizzo, Andrea Palicelli, Andrea Benedetto Galosi, and Francesca Sanguedolce. 2025. "Tumor-Infiltrating Immune Cells in Non-Muscle-Invasive Bladder Cancer: Prognostic Implications, Predictive Value, and Future Perspectives" Applied Sciences 15, no. 22: 12032. https://doi.org/10.3390/app152212032
APA StyleMazzucchelli, R., Cormio, A., Zanelli, M., Zizzo, M., Palicelli, A., Galosi, A. B., & Sanguedolce, F. (2025). Tumor-Infiltrating Immune Cells in Non-Muscle-Invasive Bladder Cancer: Prognostic Implications, Predictive Value, and Future Perspectives. Applied Sciences, 15(22), 12032. https://doi.org/10.3390/app152212032






