Use Organic Polymers Polyvinyl Alcohol (PVA) and Polyethylene Oxide (PEO) in Diesel Heating Fuel to Reduce Humidity and Sulfur and Enhance Combustion Efficiency
Abstract
1. Introduction
2. Experimental Study
2.1. Experimental Procedure
2.2. Thermal Gravimetric Analysis (TGA)
2.3. Differential Scanning Calorimetry (DSC)
3. Results and Discussion
3.1. Thermal Stability (TGA)
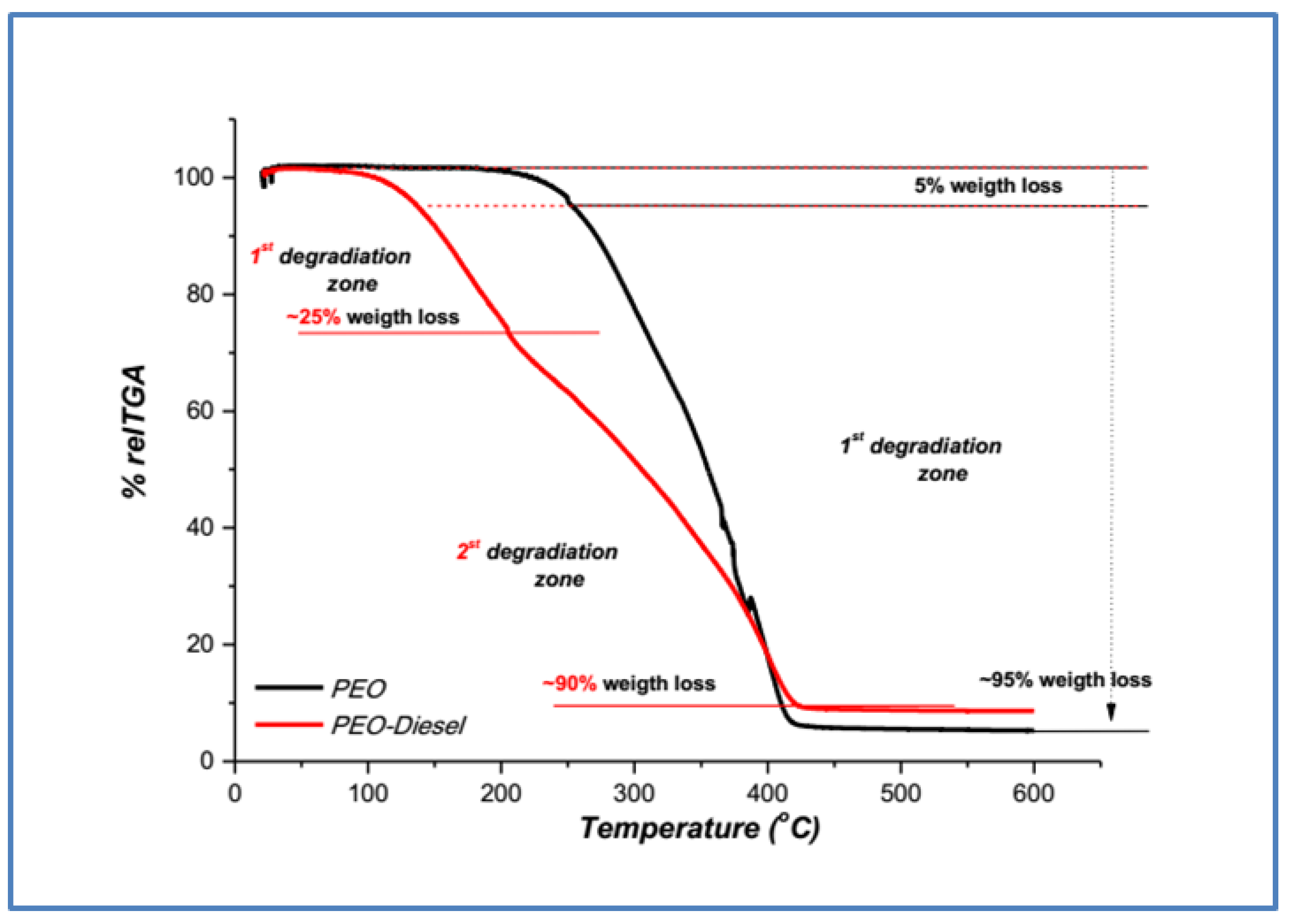
3.1.1. Poly(ethylene Oxide) TGA Samples Characterization
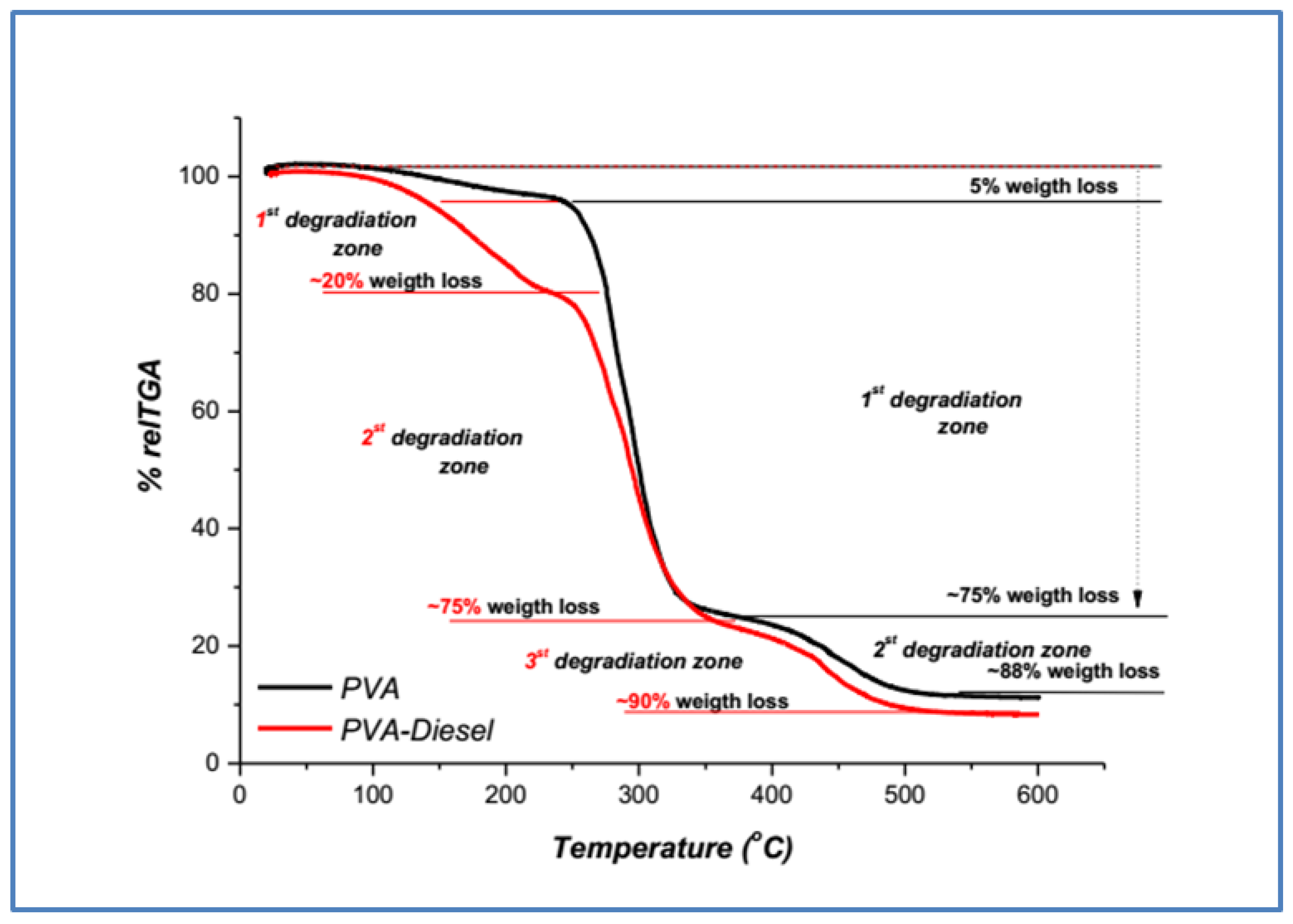
3.1.2. Poly(vinyl Alcohol) TGA Samples Characterization
3.2. DSC Analysis
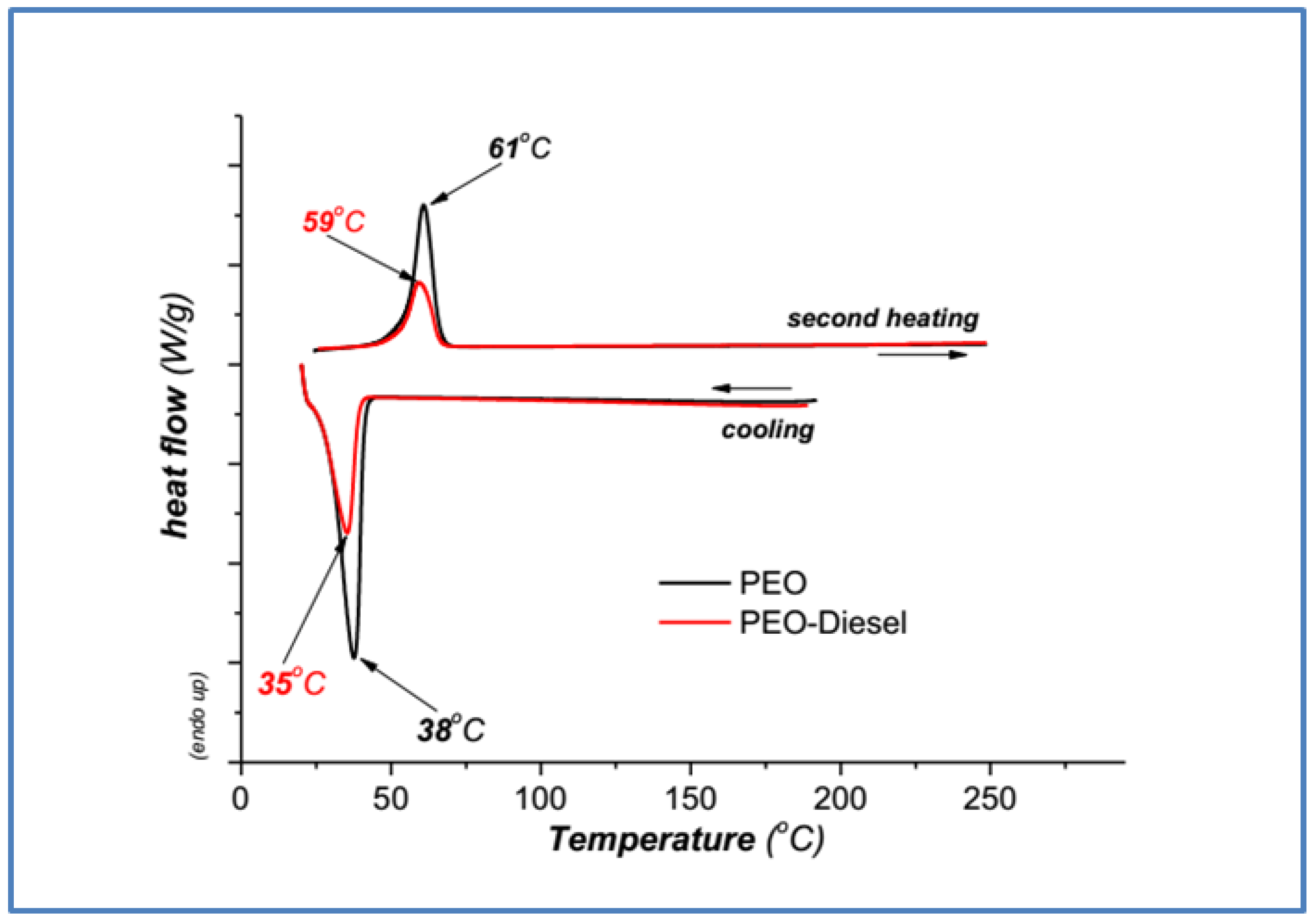
3.2.1. Poly(ethylene Oxide) DSC Samples Characterization
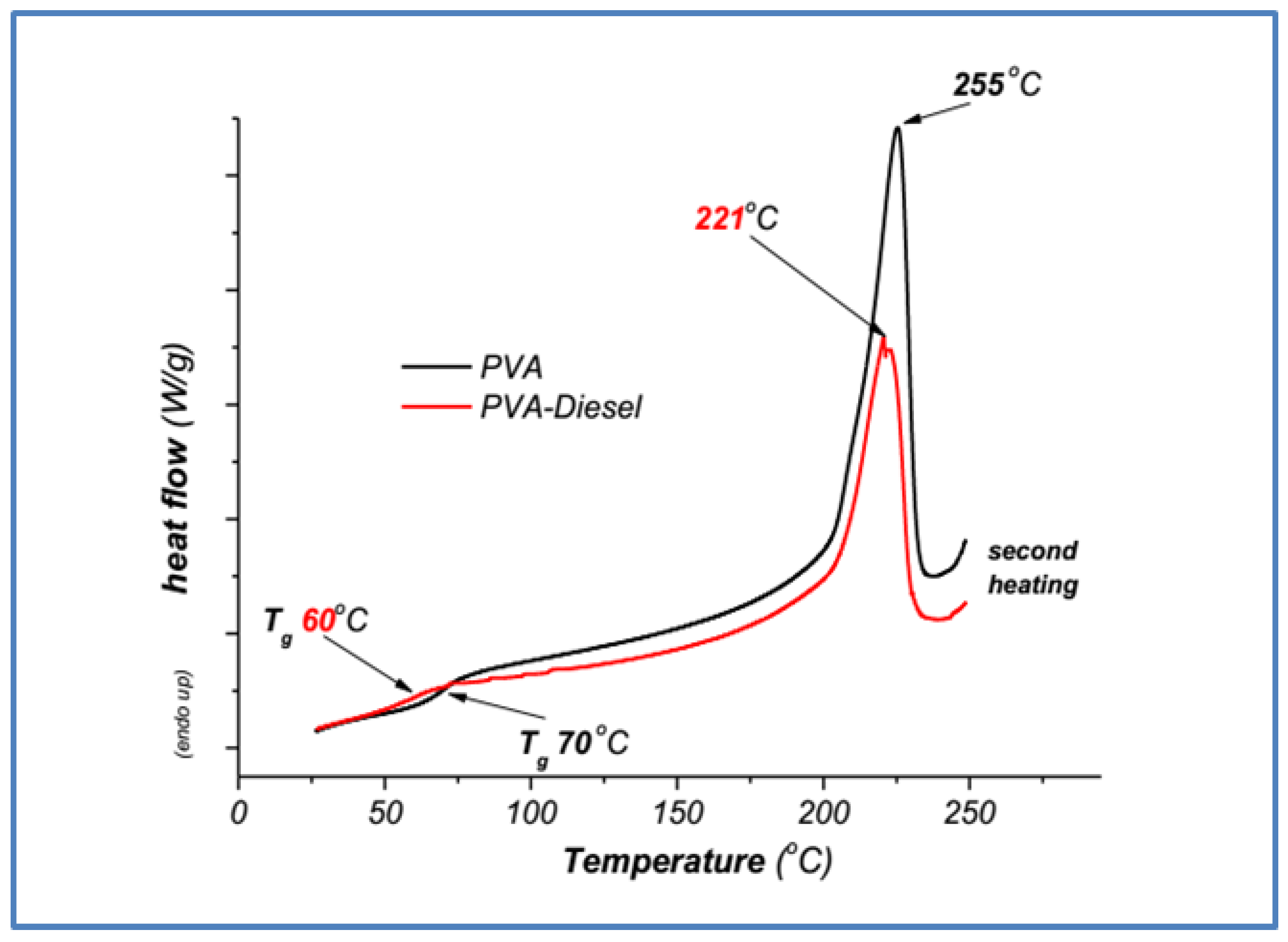
3.2.2. Poly(vinyl Alcohol) DSC Samples Characterization
3.3. Effect of Polymer Addition and Humidity of Diesel
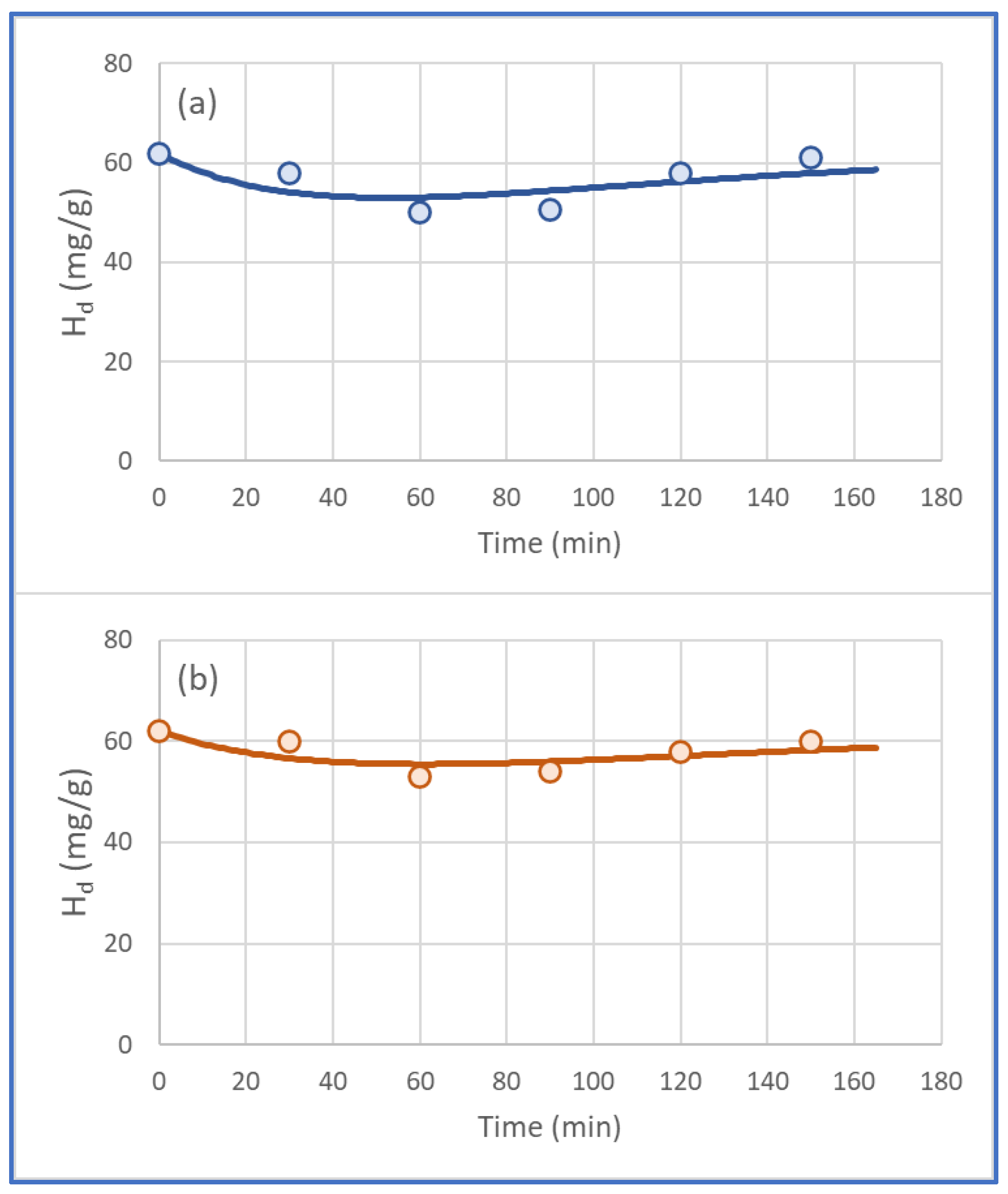
4. Theoretical Prediction of Humidity Content vs. Time
5. Conclusions
- The polymer does not remain in the fuel after its use.
- The amount of polymer used is of the order of additives in fuels.
- The method can be applied at any point in the production process and transportation of petroleum products but also in their consumption areas.
- The raw material for the synthesis of the polymer, polyvinyl alcohol (PVA) MW 85,000–125,000 fully hydrolyzed (99+%), polyethylene oxide (PEO) MW > 100,000 fully hydrolyzed (99+%), is harmless and environmentally friendly.
- The cost of acquiring the polymer is low, while it can be synthesized in the laboratory and used more than once.
- Applications on a large scale of thermal treatments of crude oil that may alter the characteristics of the final products are avoided. Of course, the application of the proposed methodology to the production process of petroleum products must undergo further research.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ershov, M.A.; Savelenko, V.D.; Shvedova, N.S.; Kapustin, V.M.; Abdellatief, T.M.M.; Karpov, N.V.; Dutlov, E.V.; Borisan, D.V. An evolving research agenda of merit function calculations for new gasoline compositions. Fuel 2022, 322, 124209. [Google Scholar] [CrossRef]
- Abdellatief, T.M.M.; Ershov, M.A.; Kapustin, V.M.; Abdelkareem, M.A.; Kamil, M.; Olabi, A.G. Recent trends for introducing promising fuel components to enhance the anti-knock quality of gasoline: A systematic review. Fuel 2021, 291, 120112. [Google Scholar] [CrossRef]
- Gary, J.H.; Handwerk, J.H.; Glenn, E. Petroleum Refining: Technology and Economics, 4th ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Knothe, G.; Sharp, C.A.; Ryan, T.W. Exhaust Emissions of Biodiesel, Petrodiesel, Neat Methyl Esters, and Alkanes in a New Technology Engine. Energy Fuels 2006, 20, 403–408. [Google Scholar] [CrossRef]
- Sadeq, A.M. Combustion Advancements: From Molecules to Future Challenges, 1st ed.; Lulu Press, Inc.: Morrisville, CA, USA, 2023; ISBN 979-8-9907836-1-4. [Google Scholar]
- AFS. Water Contamination in Fuel: Cause and Effect—American Filtration and Separations Society; Archived from The Original on 23 March 2015; AFS: New York, NY, USA, 2015. [Google Scholar]
- Bhan Opinder, K.; Tang Sheng, Y.; Brinkman Dennis, W.; Carley, B. Causes of poor filterability in jet fuels. Fuel 1998, 67, 227–237. [Google Scholar] [CrossRef]
- Saleh, J.; Tremblay Andrι, Y.; Dubι Marc, A. Glycerol removal from biodieselusing membrane separation technology. Fuel 2010, 89, 2260–2266. [Google Scholar] [CrossRef]
- Chastek, T.Q. Improving cold flow properties of canola-based biodiesel. Biomass Bioenergy 2011, 35, 600–607. [Google Scholar] [CrossRef]
- Nair, S.; Tatarchuk, B.J. Supported silver adsorbents for Sulphur removal from hydrocarbon fuels. Fuel 2010, 89, 3218–3225. [Google Scholar] [CrossRef]
- Tsanaktsidis, C.G.; Favvas, E.P.; Tzilantonis, G.T.; Scaltsoyiannes, A.V. A new fuel (D-BD-J) from the blending of conventional diesel, biodiesel and JP8. Fuel Proc. Techn. 2014, 127, 66–71. [Google Scholar] [CrossRef]
- Tsanaktsidis, C.G.; Christidis, S.G.; Favvas, E.P. A novel method for improving the physicochemical properties of diesel and jet fuel using polyaspartate polymer additives. Fuel 2013, 104, 155–162. [Google Scholar] [CrossRef]
- Hallensleben, M.L. Polyvinyl Compounds, Others. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Tang, X.; Alavi, S. Recent Advances in Starch, Polyvinyl Alcohol Based Polymer Blends, Nanocomposites and Their Biodegradability. Carbohydr. Polym. 2011, 85, 7–16. [Google Scholar] [CrossRef]
- Adelnia, H.; Ensandoost, R.; Moonshi, S.S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Polyethylene Glycol as Pharmaceutical Excipient. Available online: https://pharmaceutical.basf.com (accessed on 27 April 2021).
- Kahovec, J.; Fox, R.B.; Hatada, K. Nomenclature of regular single-strand organic polymers. Pure Appl. Chem. 2002, 74, 1921–1956. [Google Scholar] [CrossRef]
- Ma, Y.; Ji, Y.; Zhong, T.; Wan, W.; Yang, Q.; Li, A.; Zhang, X.; Lin, M. Bioprinting-Based PDLSC-ECM Screening for in Vivo Repair of Alveolar Bone Defect Using Cell-Laden, Injectable and Photocrosslinkable Hydrogels. ACS Biomater. Sci. Eng. 2017, 3, 3534–3545. [Google Scholar] [CrossRef] [PubMed]
- ISO 12185:2024; Crude Petroleum, Petroleum Products and Related Products—Determination of Density—Laboratory Density Meter with an Oscillating U-Tube Sensor. 2nd ed. ISO: Geneva, Switzerland, 2024.
- ISO 3405:2019; Petroleum and Related Products from Natural or Synthetic Sources—Determination of Distillation Characteristics at Atmospheric Pressure. 5th ed. ISO: Geneva, Switzerland, 2019.
- ISO 2719:2016; Determination of Flash Point—Pensky-Martens Closed Cup Method. 4th ed. ISO: Geneva, Switzerland, 2016.
- ISO 12937:2000; Petroleum Products—Determination of Water—Coulometric Karl Fischer Titration Method. 1st ed. ISO: Geneva, Switzerland, 2000.
- ISO 20846:2019; Petroleum Products—Determination of Sulfur Content of Automotive Fuels—Ultraviolet Fluorescence Method. 3rd ed. ISO: Geneva, Switzerland, 2019.
- Borges, M.E.; Díaz, L.; Gavín, J.; Brito, A. Estimation of the content of fatty acid methyl esters (FAME) in biodiesel samples from dynamic viscosity measurements. Fuel Process. Technol. 2011, 92, 597–599. [Google Scholar] [CrossRef]
- ISO 4264:2018; Petroleum Products—Calculation of Cetane Index of Middle-Distillate Fuels by the Four Variable Equation. 3rd ed. ISO: Geneva, Switzerland, 2018.
- Kjellander, R.; Florin, E. Water structure and changes in thermal stability of the system poly(ethylene oxide)–water. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1989, 12, 3901–4366. [Google Scholar] [CrossRef]
- Awada, H.; Daneault, C. Chemical Modification of Poly(Vinyl Alcohol) in Water. Appl. Sci. 2015, 5, 840–850. [Google Scholar]
- Takei, T.; Kurosaki, K.; Nishimoto, Y.; Sugitani, Y. Behavior of Bound Water in Polyethylene Oxide Studied by DSC and High-Frequency Spectroscopy. Anal. Sci. 2002, 18, 681–684. [Google Scholar] [CrossRef][Green Version]
- Jin, J.; Song, M.; Pan, F. A DSC study of effect of carbon nanotubes on crystallization behavior of poly(ethylene oxide). Thermochim. Acta 2007, 456, 25–31. [Google Scholar] [CrossRef]
- Anyfantis, G.C.; Cingolani, R.; Athanassiou, A.; Bayer, I.S. Effect of trifluoroacetic acid on the properties of polyvinyl alcohol and polyvinyl alcohol–cellulose composites. Chem. Eng. J. 2015, 277, 242–251. [Google Scholar] [CrossRef]
- Perez, I.D.; dos Santos, F.B.; Miranda, N.T.; Vieira, M.G.A.; Fregolente, L.V. Polymer hydrogel for water removal from naphthenic insulating oil and marine diesel. Fuel 2022, 324, 124702. [Google Scholar] [CrossRef]
- Favvas, E.P.; Tsanaktsidis, C.G.; Tzilantonis, G.T.; Christidis, S.G. H2O removal from diesel and JP8 fuels: A comparison study between synthetic and natural dehydration agents. J. Engin. Sci. Techn. Rev. 2014, 4, 104–108. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Emission Facts: Average Carbon Dioxide Emissions Resulting from Gasoline and Diesel Fuel; US Environmental Protection Agency: Washington, DC, USA, 2005. [Google Scholar]
- Date Anil, W. Analytic Combustion: With Thermodynamics, Chemical Kinetics and Mass Transfer; Google eBook; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar]
- Hellenic Statistical Authority. Consumption of Petroleum Products, Under the Link “Environment and Energy > Energy > Petroleum Products (Consumption)”. 2022. Available online: https://www.statistics.gr/el/statistics/-/publication/SDE15/- (accessed on 4 July 2025).
- Reichert, P. Aquasim 2.0-User Manual, Computer Program for the Identification and Simulation of Aquatic Systems; EAWAG: Dübendorf, Switzerland, 1998; ISBN 3-906484-16-5. [Google Scholar]
- Petzold, L. A description of DASSL: A differential/algebraic system solver. In Scientific Computing; Stepleman, R.E., Ed.; IMACS/North-Holland: Amsterdam, The Netherlands, 1983; pp. 65–68. [Google Scholar]
- Vasiliadou, I.A.; Bari Chowdhury, A.K.M.M.; Akratos, C.S.; Tekerlekopoulou, A.G.; Pavlou, S.; Vayenas, D.V. Mathematical modeling of olive mill waste composting process. Waste Manag. 2015, 43, 61–71. [Google Scholar] [CrossRef]
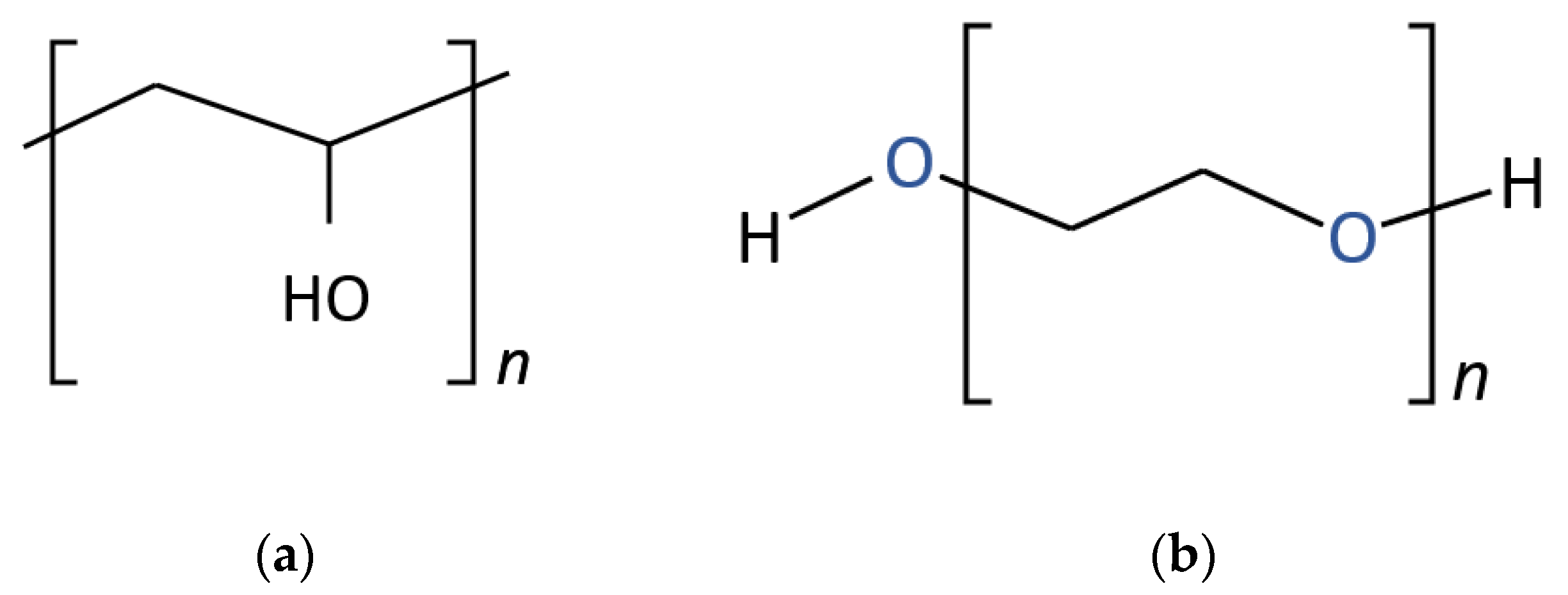
| Parameters | Units | Limits | Methods | Diesel |
|---|---|---|---|---|
| Density 15 °C | gr/mL | - | ISO 12185:2024 [19] | 0.8509 |
| Distillation 350 °C | % v/v | min 85.0 | EN ISO 3405:2019 [20] | 87.2 |
| 10% | % v/v | - | 202.2 | |
| 50% | % v/v | - | 280.4 | |
| 90% | % v/v | - | 360.0 | |
| Flash point | °C | min 55.0 | ISO 2719:2016 [21] | 60.2 |
| Humidify | mg/kg | max 1.000 | PrEN ISO 12937 [22] | 62 |
| Color | - | red/clear | VISUAL | red/clear |
| Sulfur | mg/kg | max 1.000 | ISO 20846:2019 [23] | 941 |
| F.A.M.E (Fatty Acid Methyl Esters) | % v/v | 0 | --- [24] | 0.6 |
| Cetane index | - | min 40.0 | EN ISO 4264 [25] | 47.8 |
| Polymer | Τ5% (°C) | Τmax (°C) | Weight Loss (%) | |||
|---|---|---|---|---|---|---|
| Poly(ethylene oxide) (PEO) | 251 | 348 | 95% | |||
| PEO—Diesel | 135 | 1st 161 | 2nd 337 | 90% | ||
| Poly(vinyl alcohol) (PVA) | 245 | 1st 291 | 2nd 460 | 88% | ||
| PVA—Diesel | 137 | 1st 163 | 2nd 291 | 3rd 439 | 90% | |
| Polymer | Tg (°C) | Tm (°C) |
|---|---|---|
| Poly(ethylene oxide) (PEO) | 38 | 61 |
| PEO—diesel | 35 | 59 |
| Poly(vinyl alcohol) (PVA) | 70 | 255 |
| PVA—diesel | 60 | 221 |
| Diesel (mL) + PAV (g) | t (min) | HUMIDITY (mg/g) | Diesel (mL) + PAV (g) | t (min) | HUMIDITY (mg/g) | Diesel (mL) + PAV (g) | t (min) | HUMIDITY (mg/g) |
| 20 + 0 | 0 | 62 | 20 + 0.1 | 60 | 50 | 20 + 0.1 | 60 | 50 |
| 20 + 0.1 | 30 | 58 | 20 + 0.2 | 60 | 56 | 40 + 0.1 | 60 | 58 |
| 20 + 0.1 | 60 | 50 | 20 + 0.3 | 60 | 58 | 60 + 0.1 | 60 | 60 |
| 20 + 0.1 | 90 | 50.7 | ||||||
| 20 + 0.1 | 120 | 58 | ||||||
| 20 + 0.1 | 150 | 61 | ||||||
| Diesel (mL) + POE (g) | t (min) | HUMIDITY (mg/g) | Diesel (mL) + PAV (g) | t (min) | HUMIDITY (mg/g) | Diesel (mL) + POE (g) | t (min) | HUMIDITY (mg/g) |
| 20 + 0 | 0 | 62 | 20 + 0.1 | 60 | 53 | 20 + 0.1 | 60 | 53 |
| 20 + 0.1 | 30 | 60 | 20 + 0.2 | 60 | 57 | 40 + 0.1 | 60 | 59 |
| 20 + 0.1 | 60 | 53 | 20 + 0.3 | 60 | 60 | 60 + 0.1 | 60 | 61 |
| 20 + 0.1 | 90 | 54 | ||||||
| 20 + 0.1 | 120 | 58 | ||||||
| 20 + 0.1 | 150 | 60 |
| Parameters | Units | Limits | Methods | Diesel | Diesel +PVA | Diesel +POE |
|---|---|---|---|---|---|---|
| Density at 15 °C | gr/ml | - | ISO 12185:2024 [19] | 0.8509 | 0.8501 | 0.8504 |
| Distillation at 350 °C | % v/v | min 85.0 | EN ISO 3405:2019 [20] | 87.2 | 87.3 | 87.1 |
| 10% | % v/v | - | 202.2 | 203.0 | 202.1 | |
| 50% | % v/v | - | 280.4 | 282.2 | 280.3 | |
| 90% | % v/v | - | 360.0 | 360.4 | 360.2 | |
| Flash point | °C | min 55.0 | ISO 2719:2016 [21] | 60.2 | 61.2 | 59.4 |
| Humidify | mg/kg | max 1.000 | PrEN ISO 12937 [22] | 62 | 50 | 53 |
| Color | - | red/clear | VISUAL | red/clear | red/clear | red/clear |
| Sulfur | mg/kg | max 1.000 | ISO 20846: 2019 [23] | 941 | 937 | 940 |
| F.A.M.E (fatty acid methyl esters) | % v/v | 0 | --- [24] | 0.6 | 0.5 | 0.5 |
| Cetane index | - | min 40.0 | EN ISO 4264 [25] | 47.8 | 47.9 | 47.7 |
| Polymer | (1/min) | (1/min) | (mg/g) | (mg/g) | R2 |
|---|---|---|---|---|---|
| PVA | 0.0206 | 0.0174 | 22.52 | 62 | 0.642 |
| POE | 0.0157 | 0.0170 | 18.43 | 62 | 0.639 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzilantonis, G.; Stimoniaris, A.; Vasiliadou, I.A.; Kanapitsas, A.; Tsanaktsidis, C.G. Use Organic Polymers Polyvinyl Alcohol (PVA) and Polyethylene Oxide (PEO) in Diesel Heating Fuel to Reduce Humidity and Sulfur and Enhance Combustion Efficiency. Appl. Sci. 2025, 15, 11945. https://doi.org/10.3390/app152211945
Tzilantonis G, Stimoniaris A, Vasiliadou IA, Kanapitsas A, Tsanaktsidis CG. Use Organic Polymers Polyvinyl Alcohol (PVA) and Polyethylene Oxide (PEO) in Diesel Heating Fuel to Reduce Humidity and Sulfur and Enhance Combustion Efficiency. Applied Sciences. 2025; 15(22):11945. https://doi.org/10.3390/app152211945
Chicago/Turabian StyleTzilantonis, George, Adamos Stimoniaris, Ioanna A. Vasiliadou, Athanasios Kanapitsas, and Constantinos G. Tsanaktsidis. 2025. "Use Organic Polymers Polyvinyl Alcohol (PVA) and Polyethylene Oxide (PEO) in Diesel Heating Fuel to Reduce Humidity and Sulfur and Enhance Combustion Efficiency" Applied Sciences 15, no. 22: 11945. https://doi.org/10.3390/app152211945
APA StyleTzilantonis, G., Stimoniaris, A., Vasiliadou, I. A., Kanapitsas, A., & Tsanaktsidis, C. G. (2025). Use Organic Polymers Polyvinyl Alcohol (PVA) and Polyethylene Oxide (PEO) in Diesel Heating Fuel to Reduce Humidity and Sulfur and Enhance Combustion Efficiency. Applied Sciences, 15(22), 11945. https://doi.org/10.3390/app152211945









