Insight into Current Research on Luminescent Metal–Organic Frameworks (MOFs) Based on the 1,2,4-Triazole Scaffold
Abstract
1. Introduction
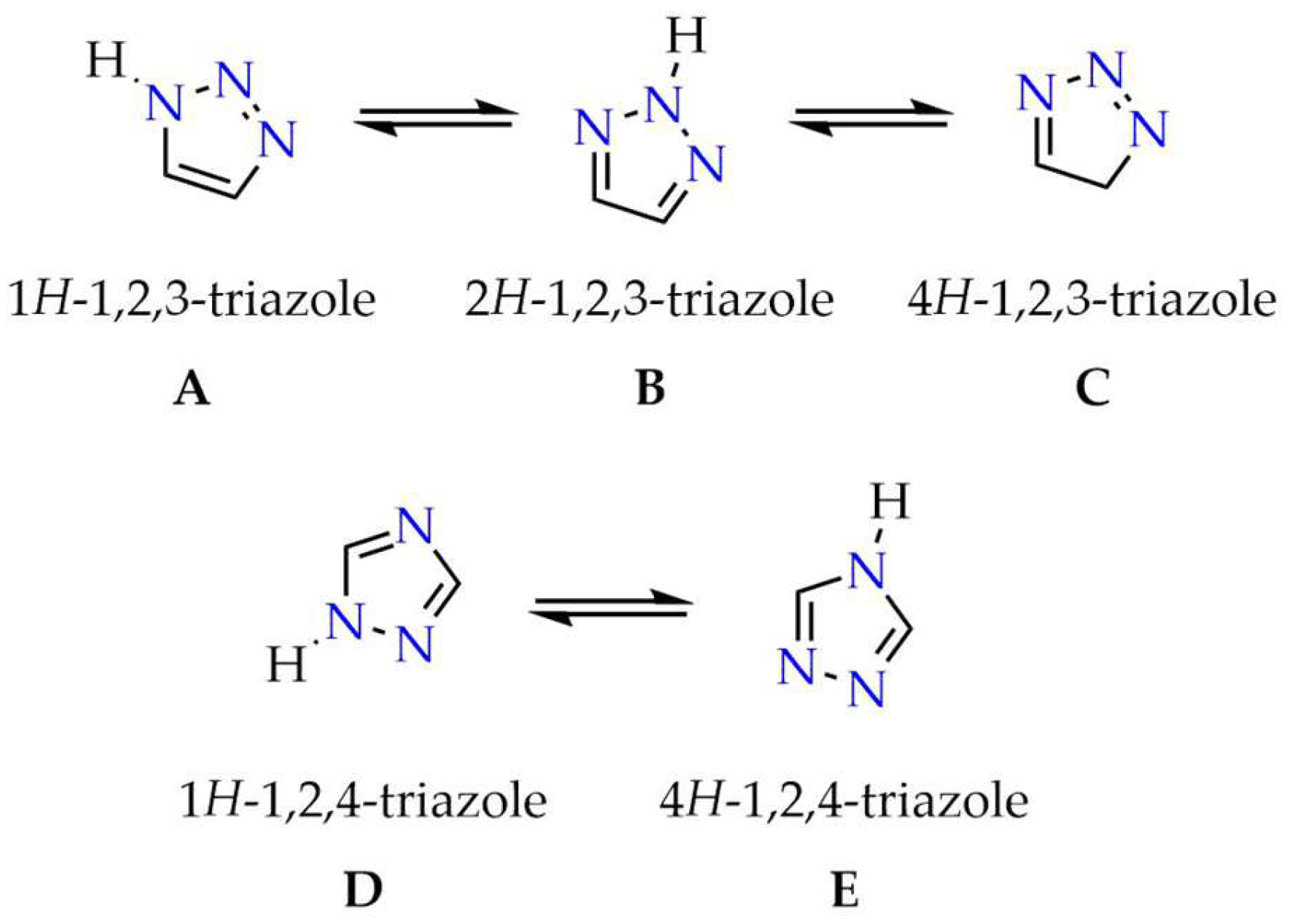
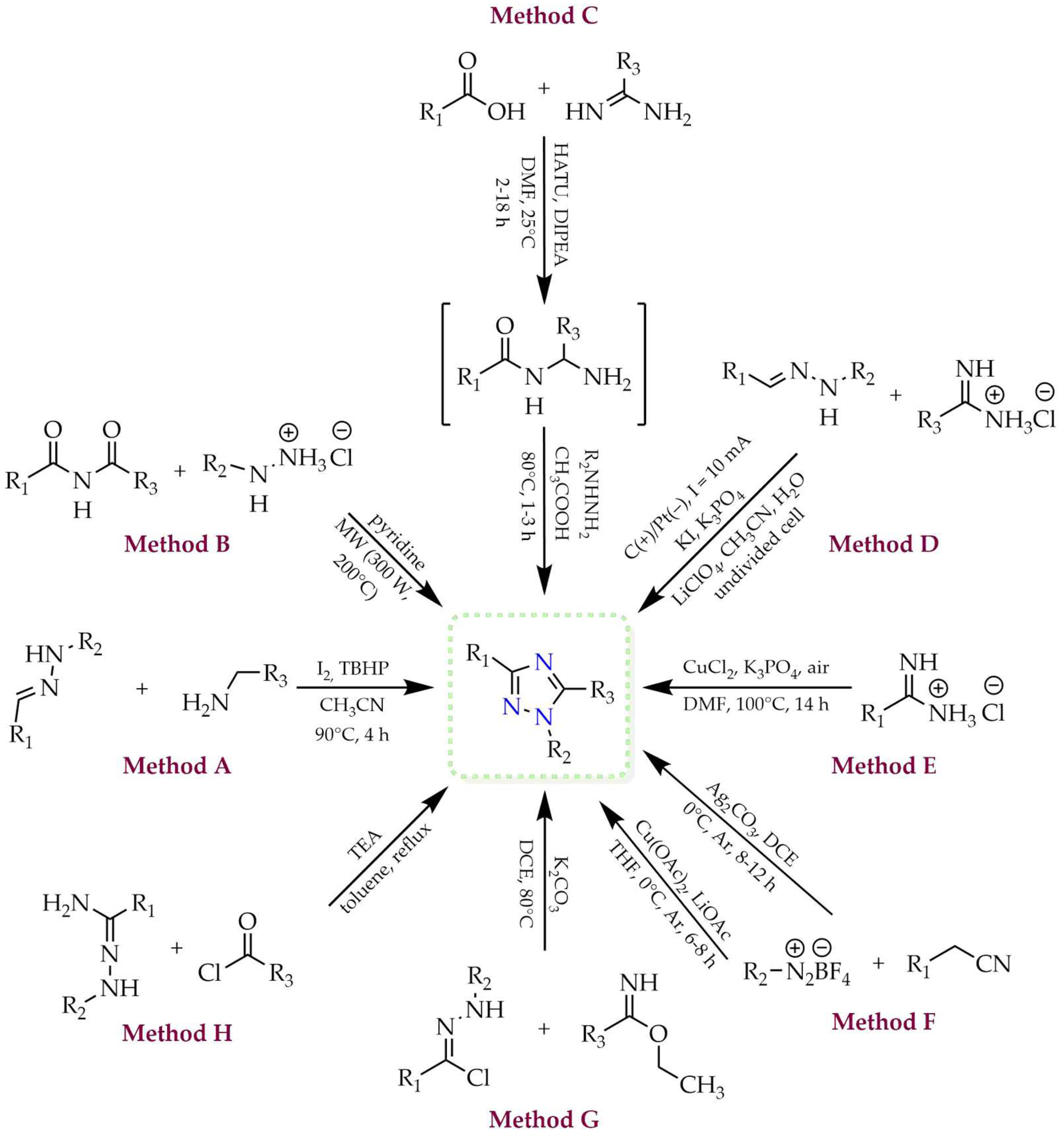
2. Selected Methods for Obtaining 1,2,4-Triazoles
2.1. Synthesis of 1H-1,2,4-Triazoles
2.2. Synthesis of 4H-1,2,4-Triazoles
3. Triazole-Based Luminescent Metal—Organic Frameworks (LMOFs)
3.1. Metal—Organic Frameworks (MOFs)
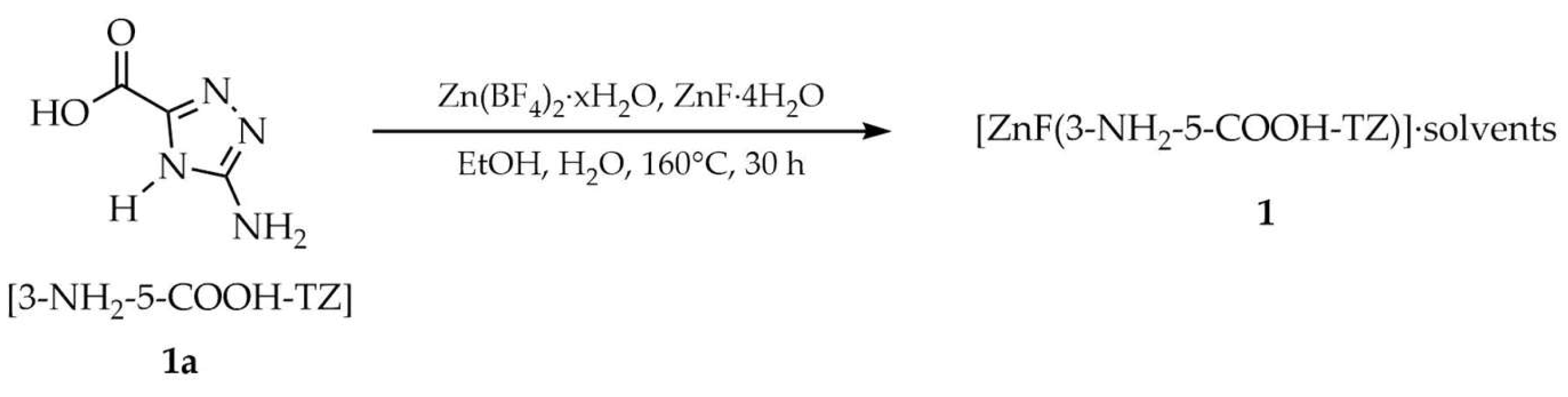
3.1.1. Zinc-Based Metal–Organic Frameworks Containing 1,2,4-Triazole Organic Linker
| Zn-MOFs | ||||
|---|---|---|---|---|
| MOF | λabs [nm] | λex [nm] | λem [nm] | Ref. |
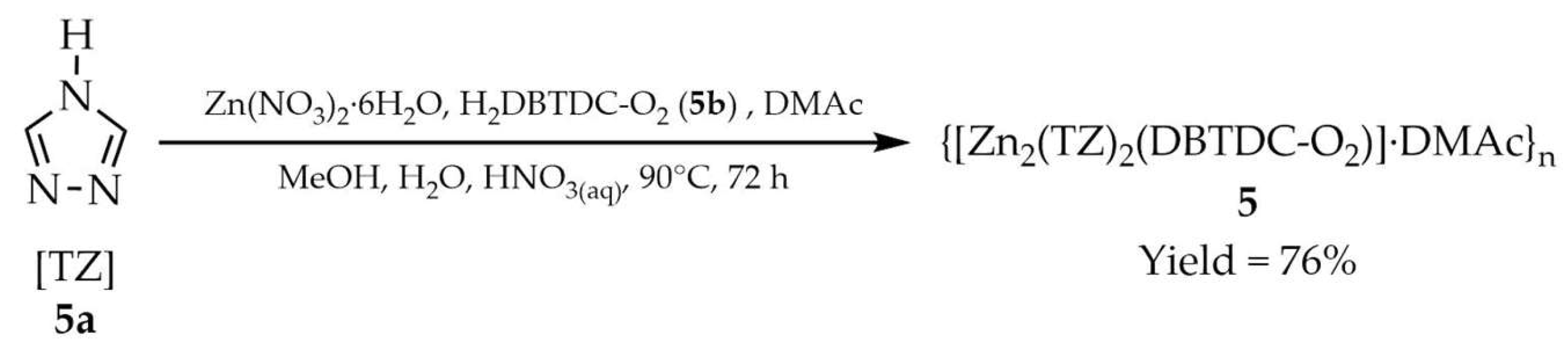
| 5 a | - | 327 | 392 | [90] |
| 5a a | - | 350 | 421 | |
| 5b a | - | 345 | 394 | |
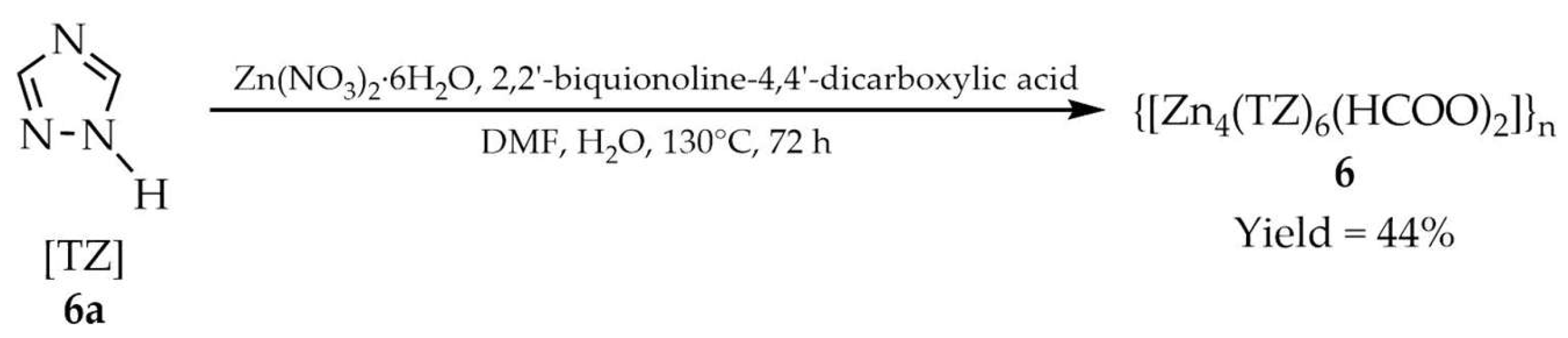
| 6 a | ~290 | 270 | 295 | [91] |
| 6a a | - | 270 | 274 | |
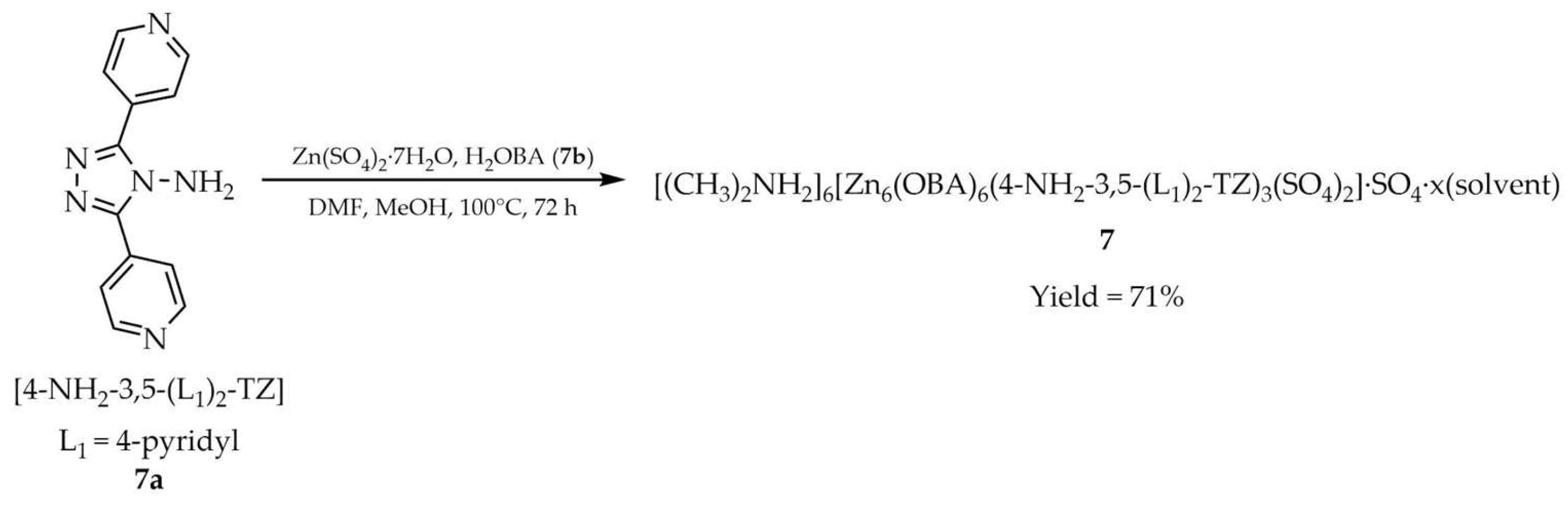
| 7 a | - | 310 | 375; 474 | [92] |
| 7a a | - | 290 | 354 | |
| 7b a | - | 346 | 563 | |
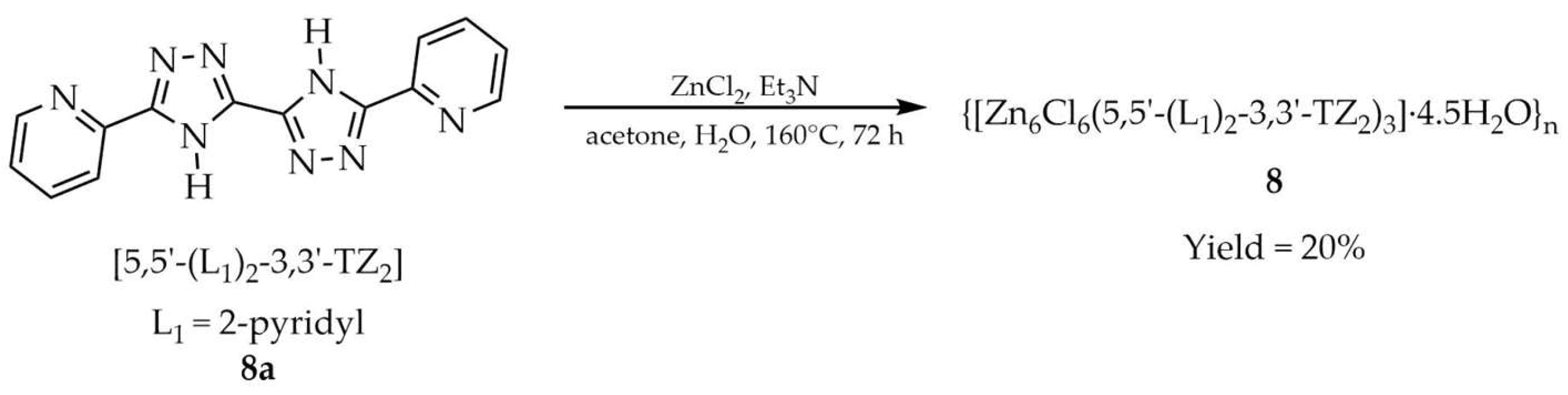
| 8 b | 420 | 326 | 420 | [93] |
| 8a a | - | 380 | ~482 | |
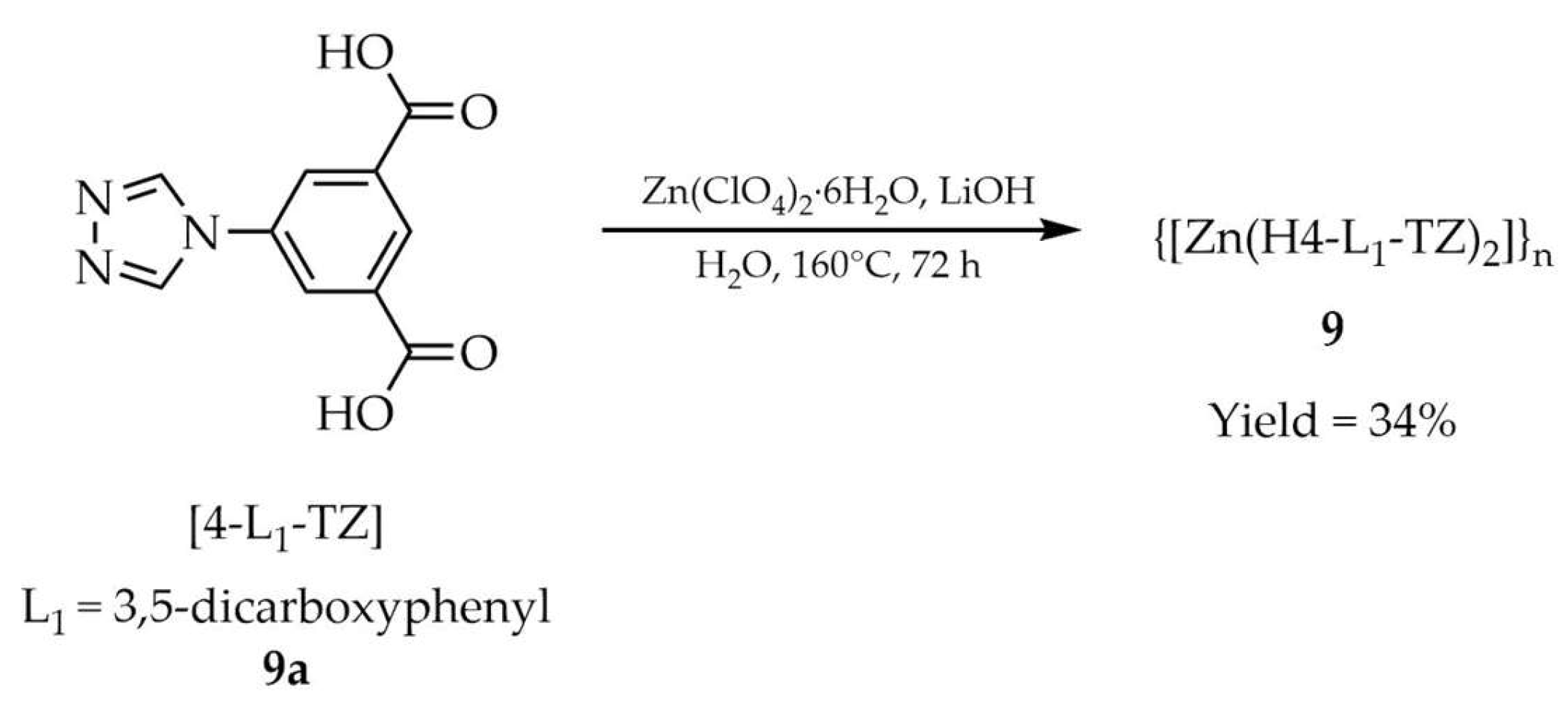
| 9 c | 310 | 310 | 410 | [94] |
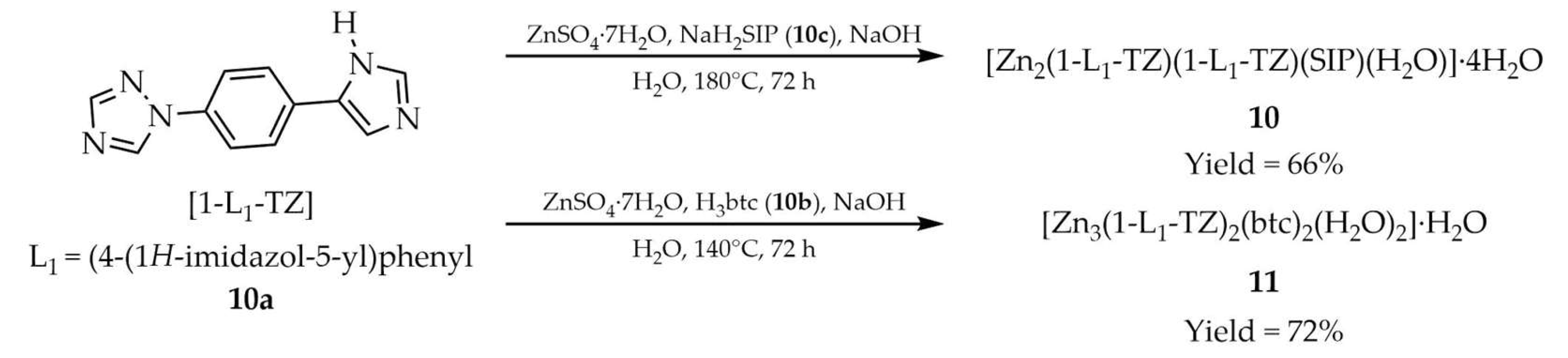
| 10 a | 280–340 | 335 | 396 | [95] |
| 10a a | ~315 | - | - | |
| 11 a | 280–340 | 330 | 384 | |
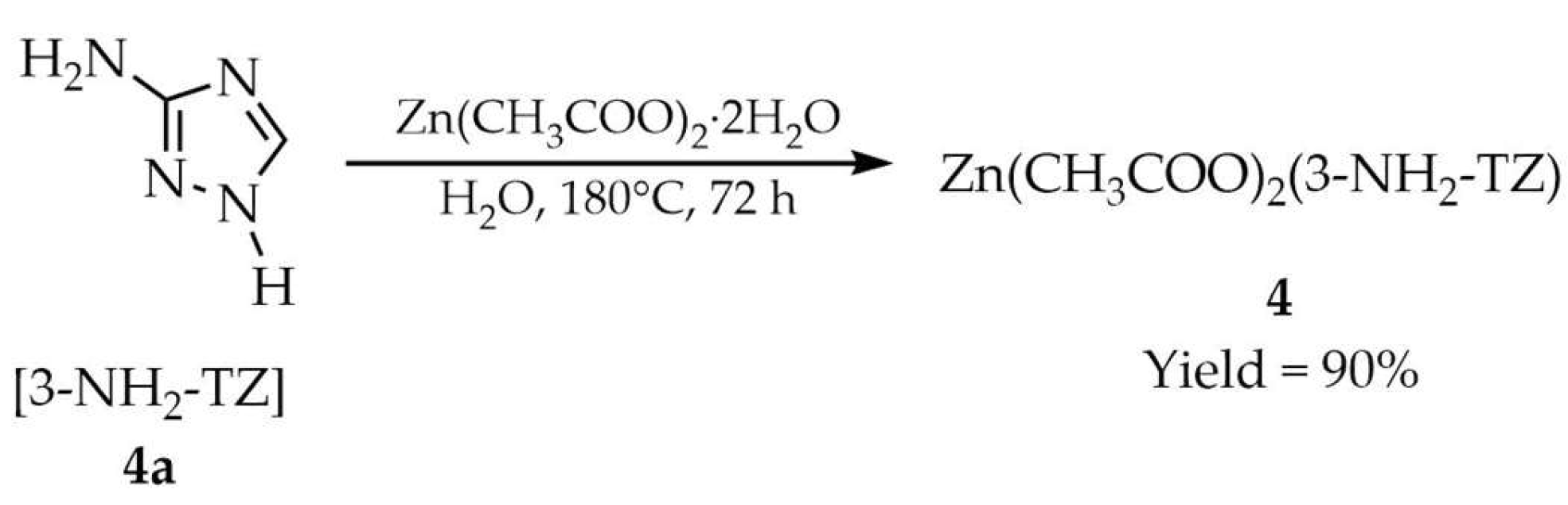
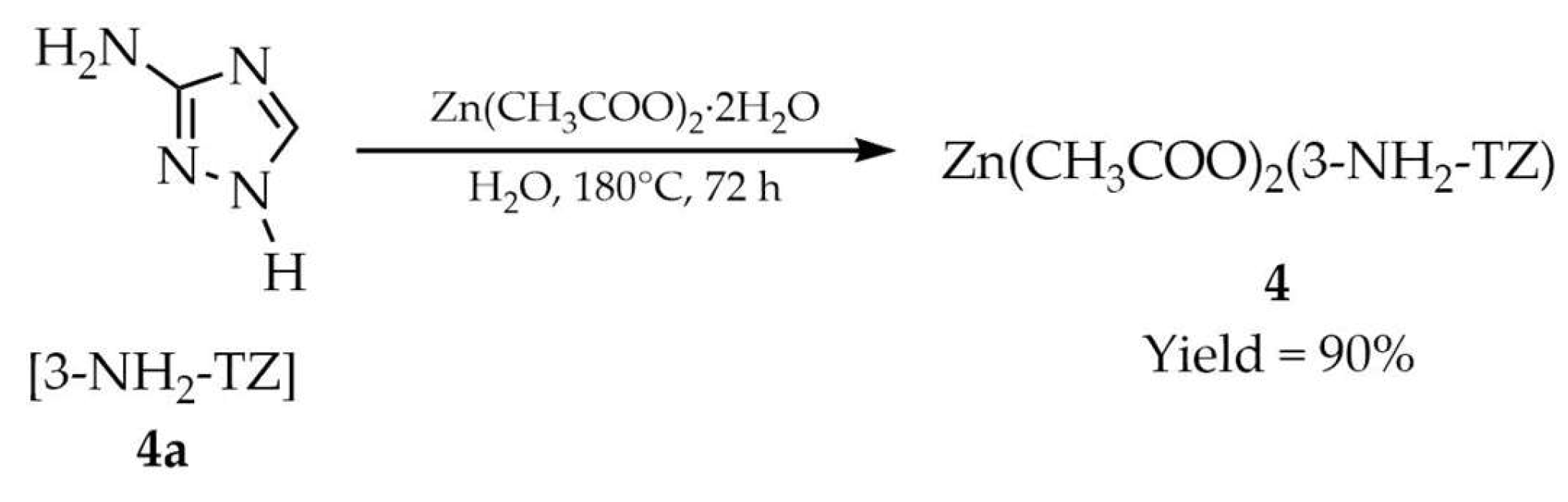
| 4 a | - | 382 | 433, 487–600 | [52] |
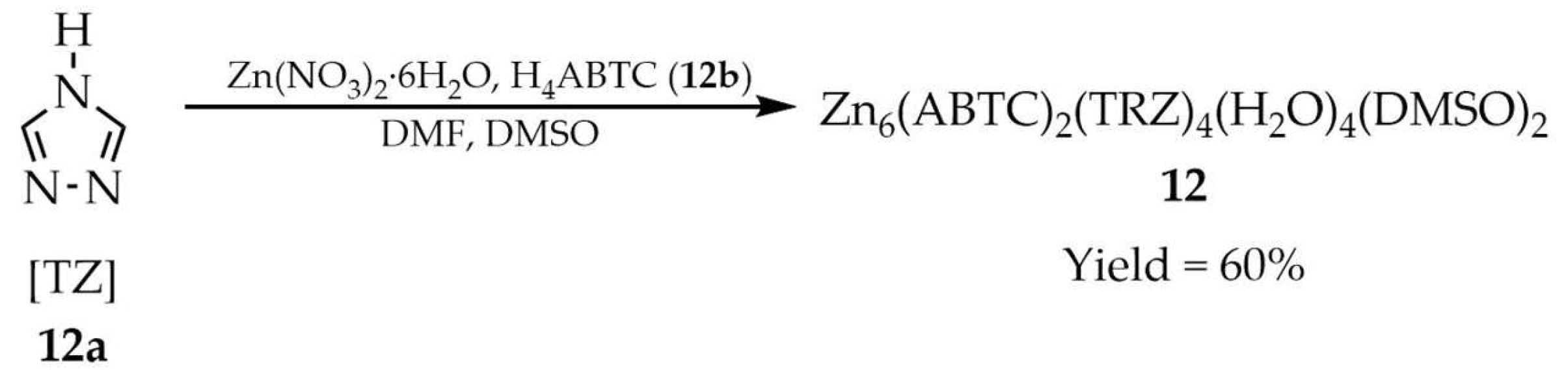
| 12 a | 337, 450 | 280 | 392 | [96] |
| 12b a | 331, 450 | 280 | 389 | |
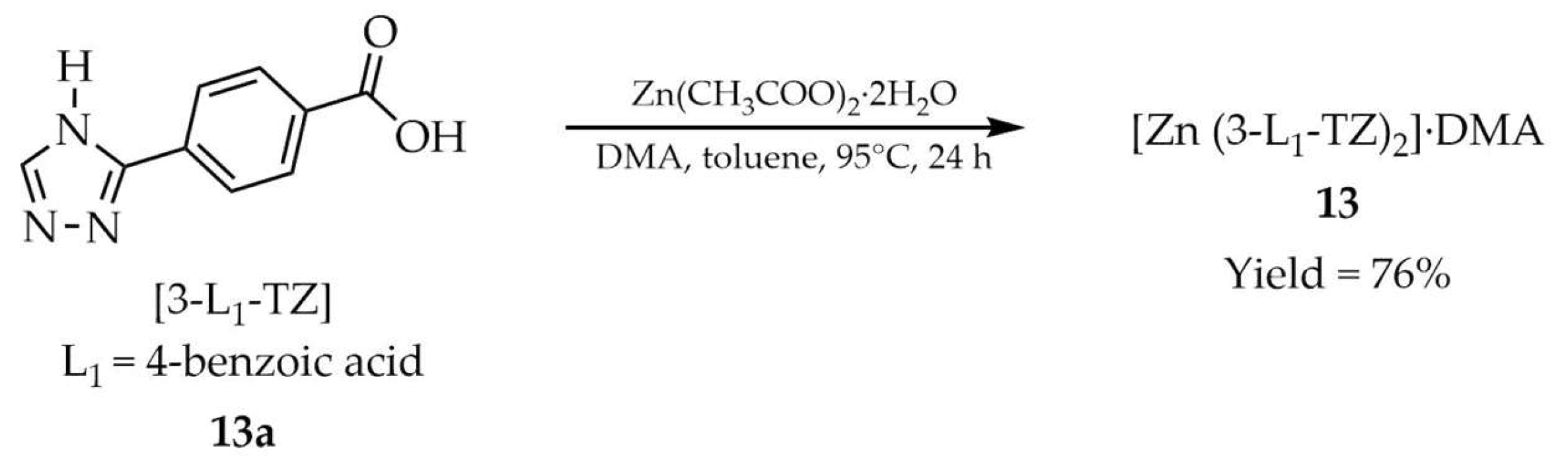
| 13 a | - | 322 a, 302 d | 408 a, 333 d | [97] |
| 13a a | - | 299 a, 291 d | 357 a, 333 d | |
| 14 e | - | 340 | 459 | [98] |
| 14a e | - | 340 | 366, 423 | |
| 14b e | - | 340 | 524 | |
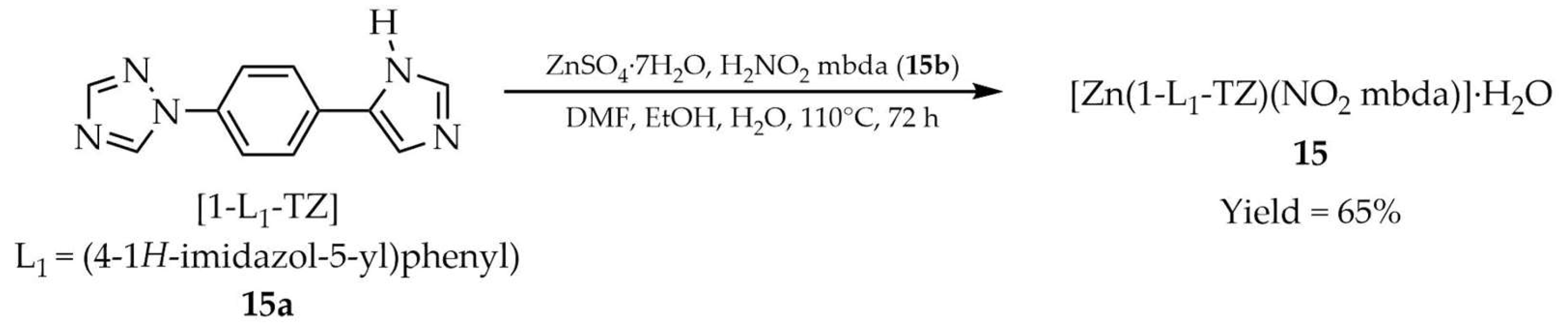
| 15 a | - | 328 | 418 | [99] |
| 15a a | - | 330 | 410 | |
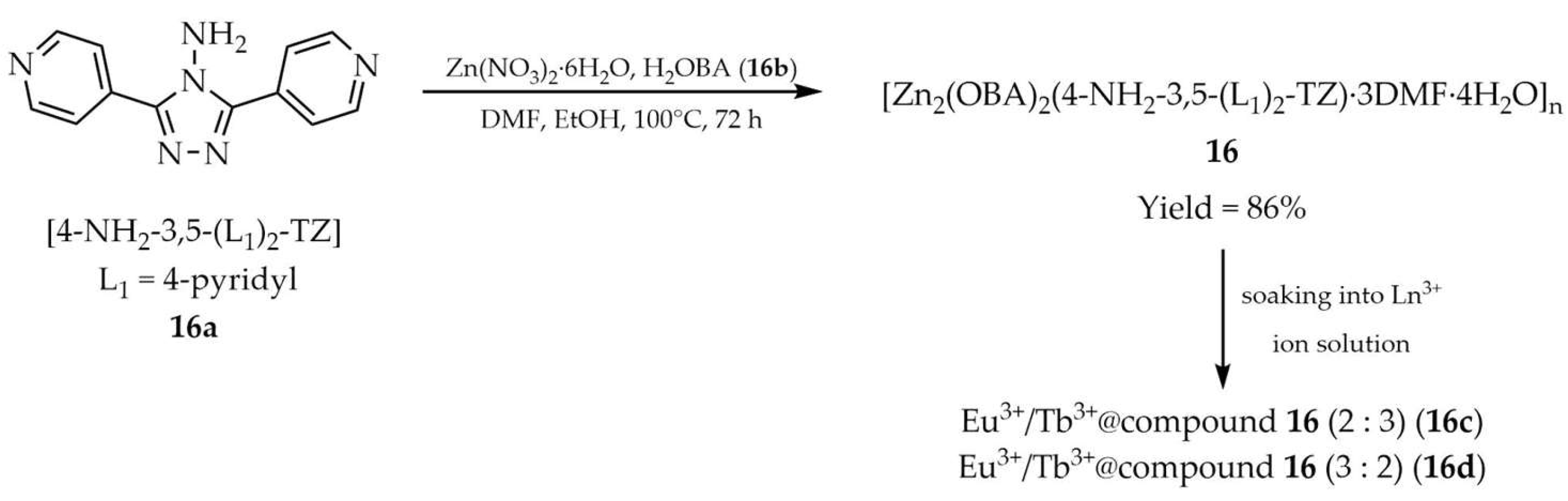
| 16 a | - | 359 a, 290 f | 415 a, 370 f | [100] |
| 16a a | - | 290 | 354 | |
| 16b a | - | 346 | 563 | |
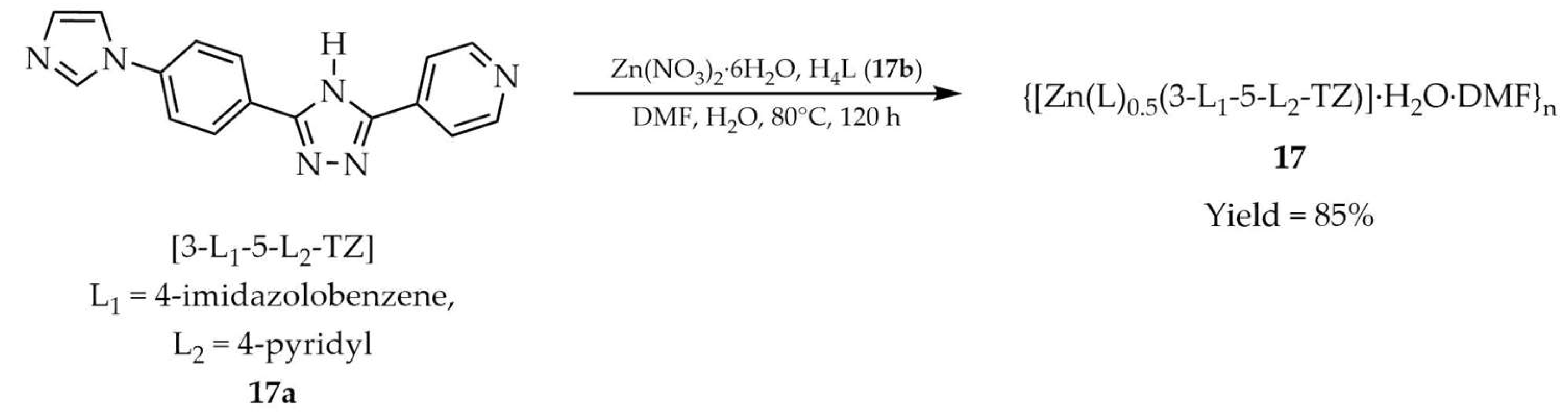
| 17 a | - | 335 | 395 | [101] |
| 17a a | - | 278 | 429 | |
| 17b a | - | 320 | 398 | |
| 18 g | - | 285 | 370 | [102] |
| 18a g | - | 285 | 348 | |
| 18b h | - | 285 | 359 | |
| 19a a | - | 340 | 400 | [103] |
| 19b a | - | 350 | 430 | |
| 19c a | - | 340 | 451 | |
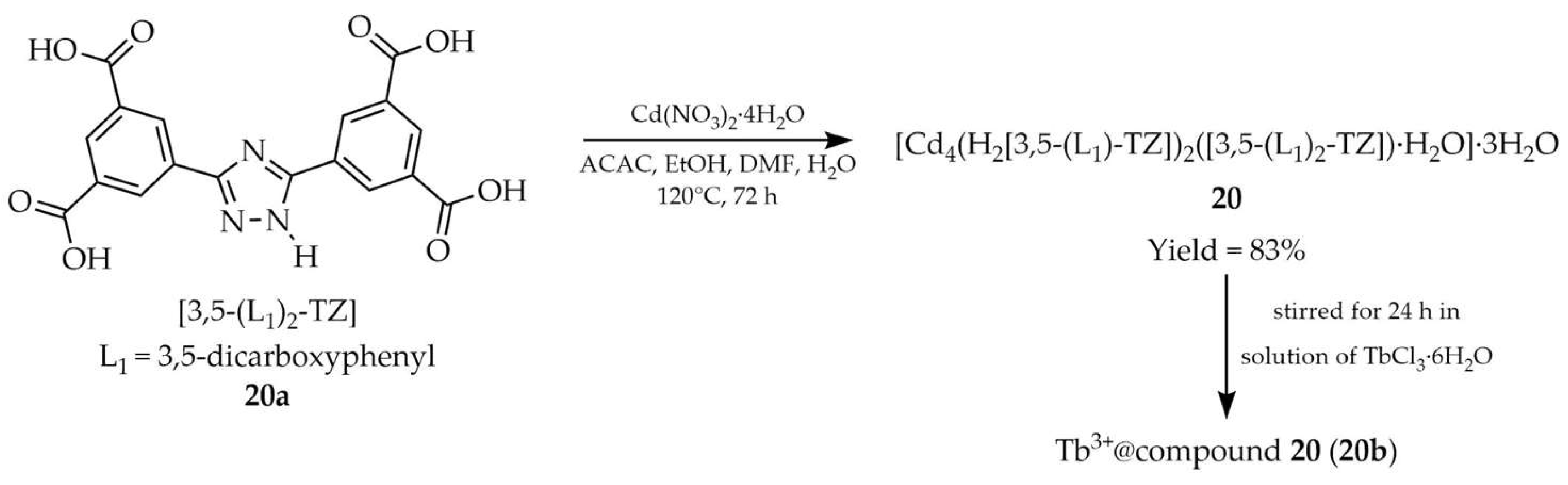
3.1.2. Cadmium-Based Metal–Organic Frameworks Containing 1,2,4-Triazole Linker
| Cd-MOFs | ||||
|---|---|---|---|---|
| MOF | λabs [nm] | λex [nm] | λem [nm] | Ref. |
| 20 a | - | 323 | 458 | [104] |
| 20a a | - | 323 | 470 | |
| 20b a | - | 285 | 490, 546, 585, 620 | |
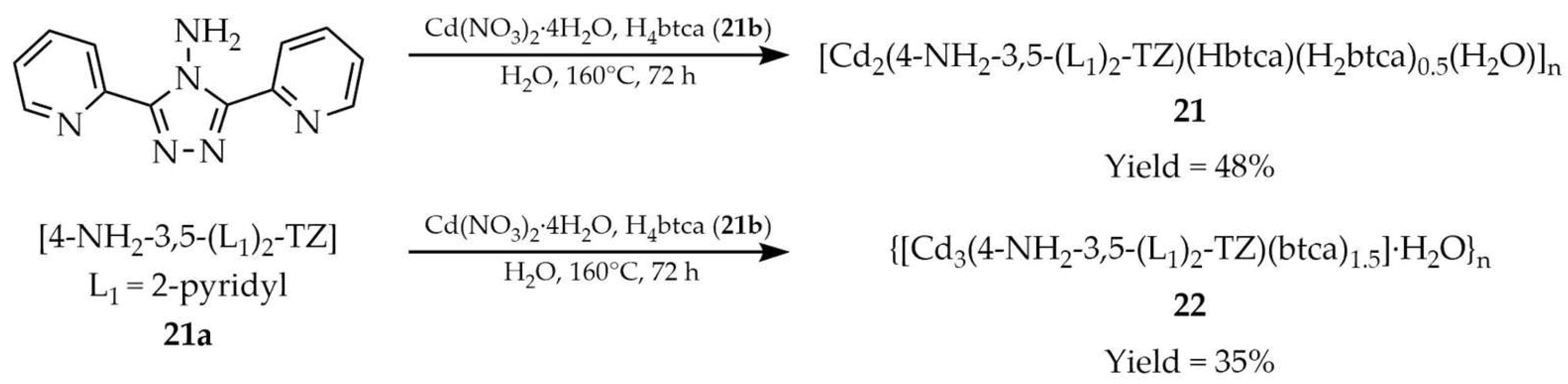
| 21 a | - | 357 | 427 | [105] |
| 21a a | - | 357 | 404 | |
| 22 a | - | 357 | 420 | |
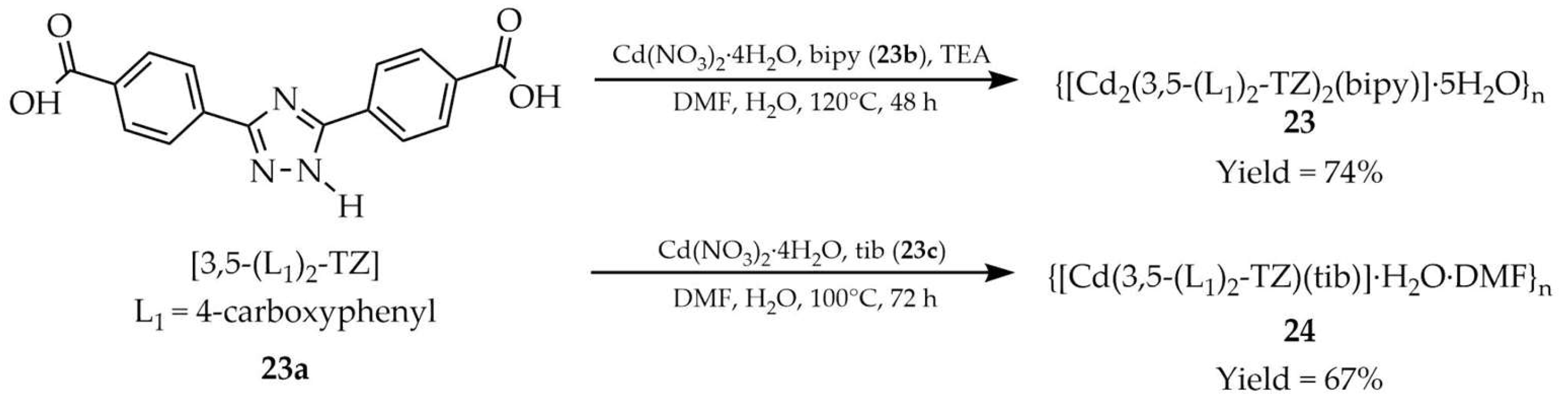
| 23 a | - | 425 | 369 | [106] |
| 23a a | - | 273 | 376 | |
| 23b a | - | 344 | 415 | |
| 23c a | - | 360 | 407 | |
| 24 a | - | 450 | 368 | |
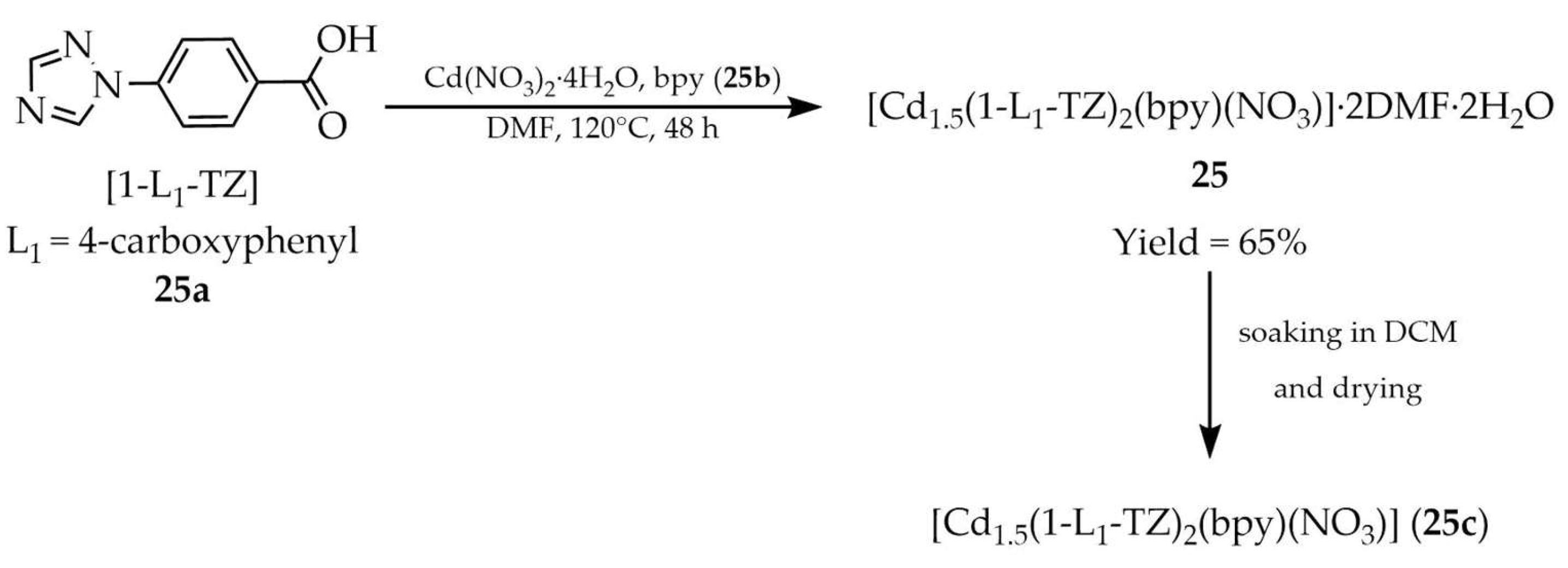
| 25c b | - | 265 | 406 | [107] |
| 26 a | 280–340 | 336 | 428 | [95] |
| 10a a | 315 | - | - | |
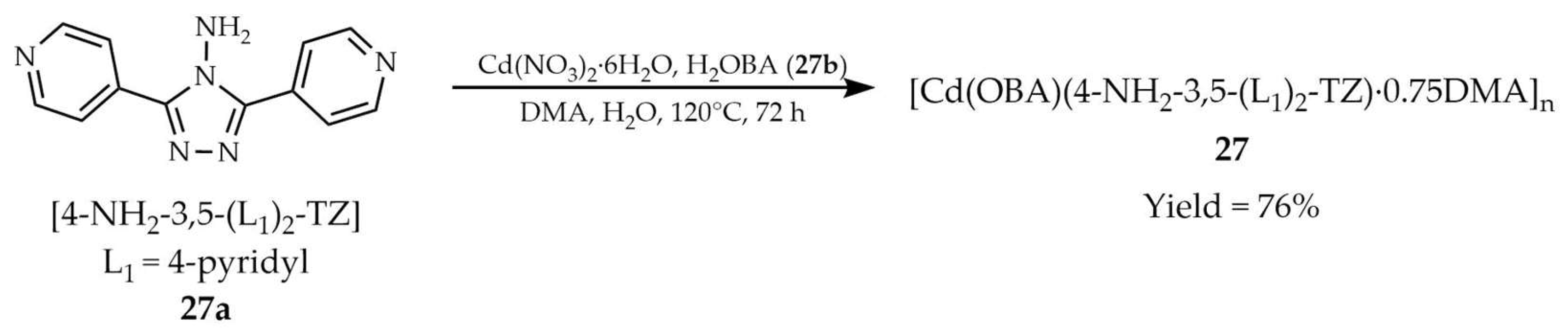
| 27 a | - | 368 | 420 | [108] |
| 27a a | - | 290 | 354 | |
| 27b a | - | 346 | 563 | |
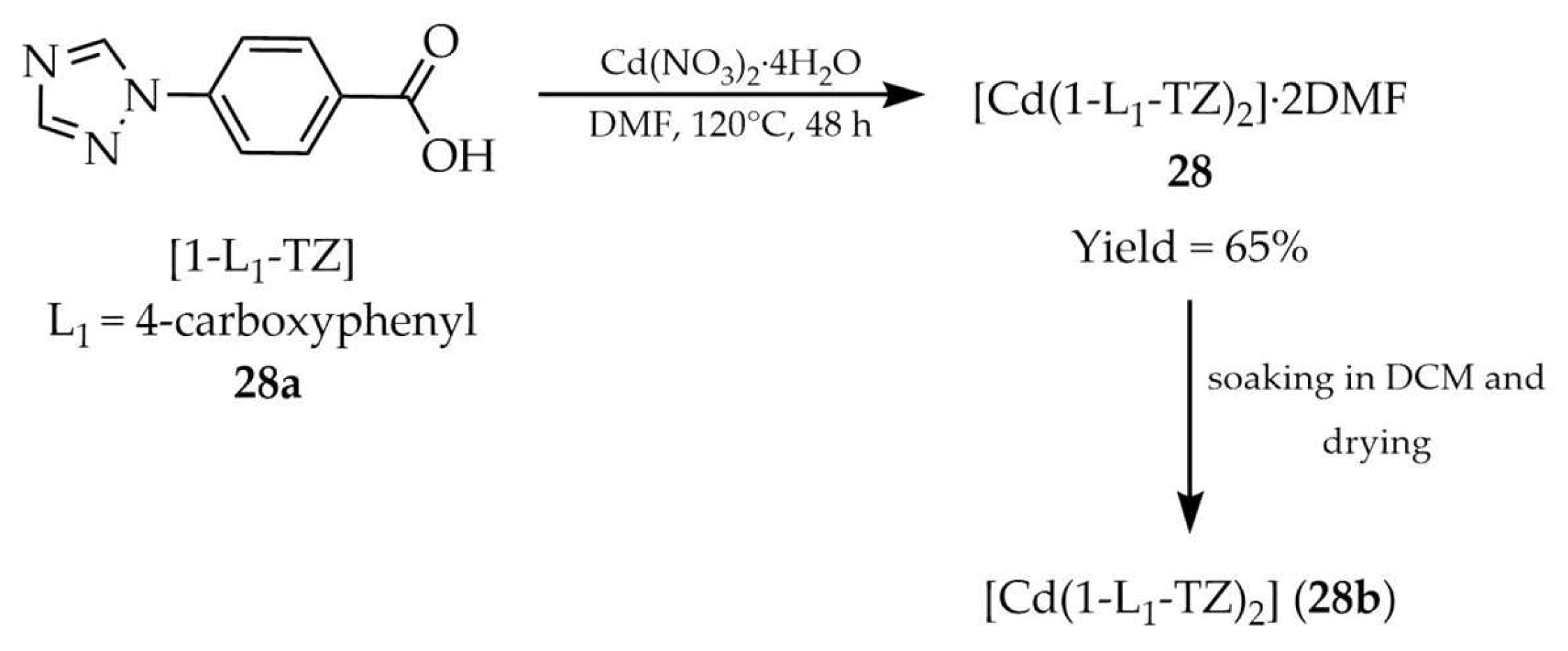
| 28b c | - | 272 | 329 | [109] |
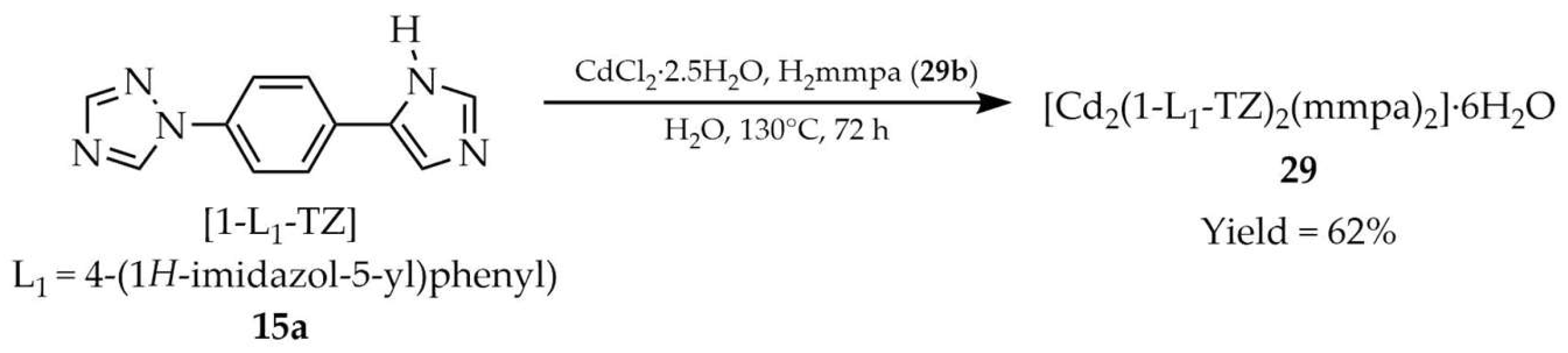
| 29 a | - | 323 | 375 | [99] |
| 15a a | - | 330 | 410 | |
| 29b a | - | 280 | ~380 | |
| 30 a | - | 330 | 428 | [110] |
| 30a a | - | 330 | 440 | |
| 31 a | - | 330 | 434 | |
3.1.3. Lanthanide-Based Metal–Organic Frameworks Containing 1,2,4-Triazole Linker
| Ln-MOFs | ||||
|---|---|---|---|---|
| MOF | λabs [nm] | λex [nm] | λem [nm] | Ref. |
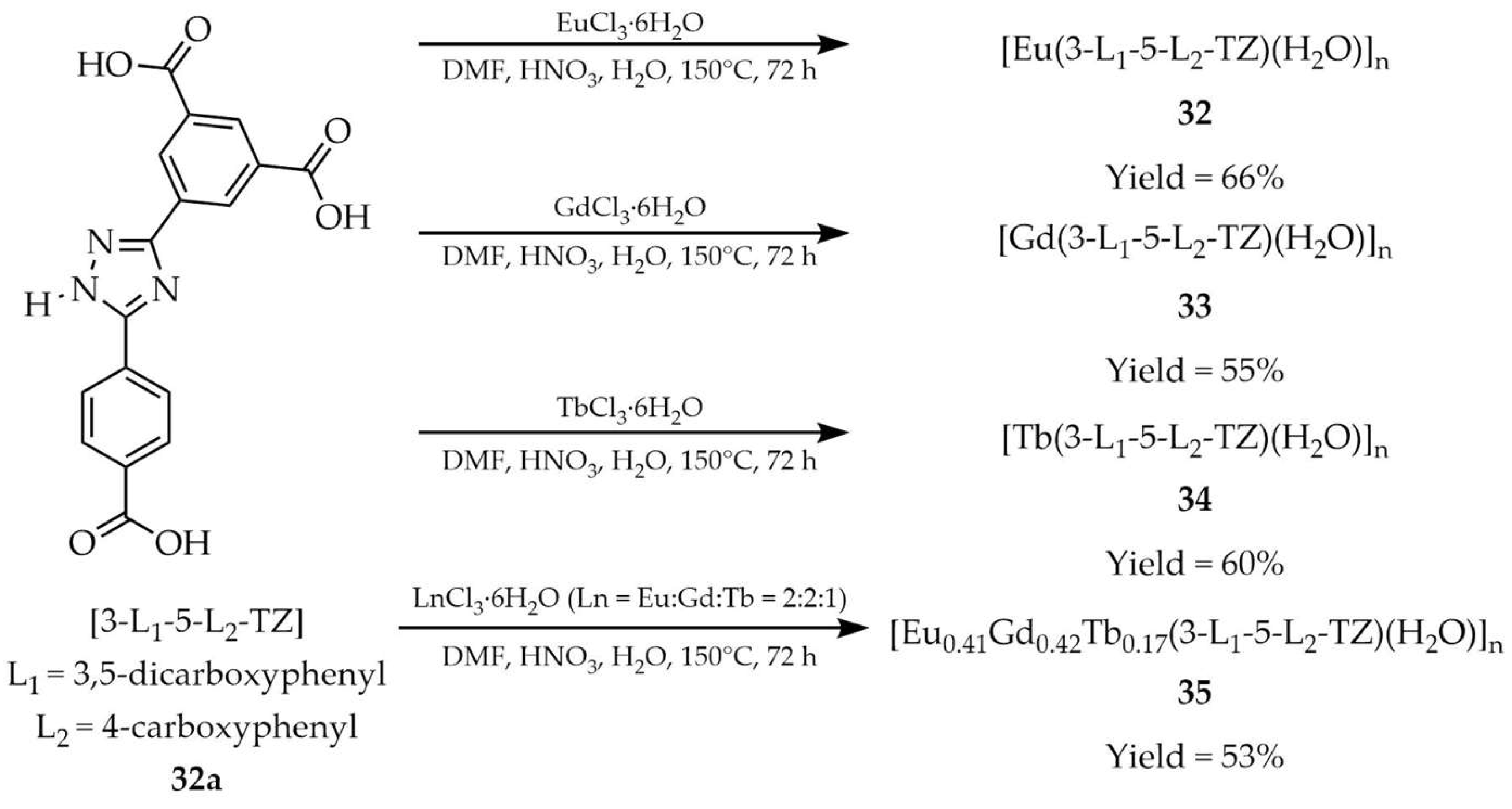
| 32 | 204 b | 350 a | 485, 591, 614, 651, 699 a | [111] |
| 33 | 204 b | 390 a | 479 a | |
| 34 | 204 b | 347 | 491, 545, 584, 619 a | |
| 35 | 204 b | 320 a | 439, 490, 545, 590, 613 a | |
| 32a | - | 372 a | 420–550 a | |
| 36 | - | - | 594, 617, 652, 699 c | [112] |
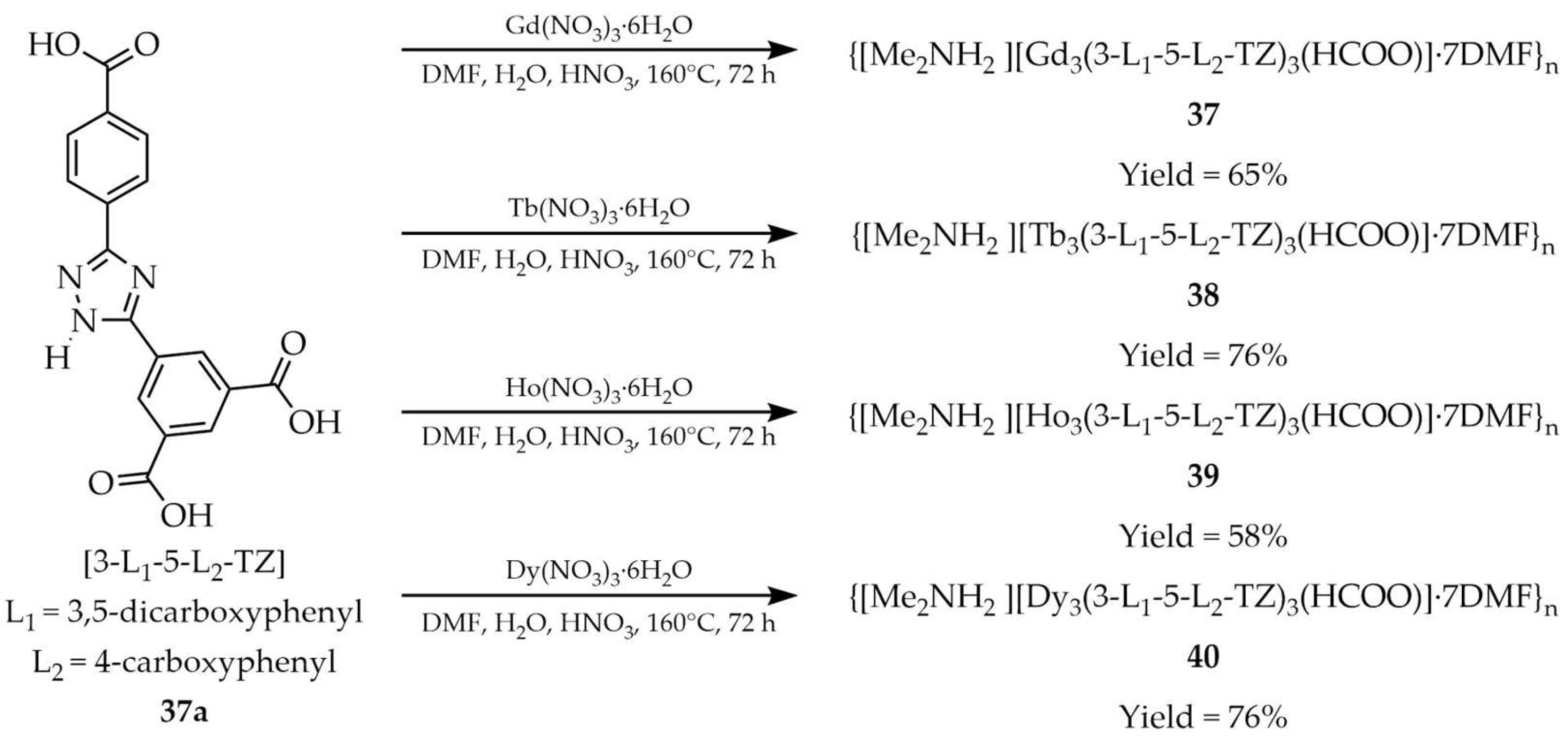
| 38 a | - | 314 | 543, 584, 621 | [113] |
| 37a a | - | 351 | 408, 468, 520 | |
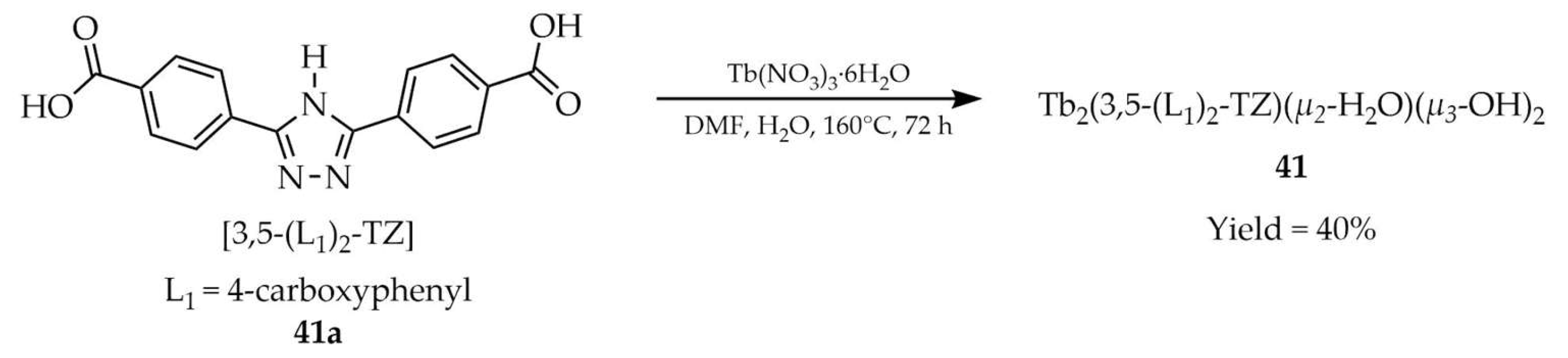
| 41 a | 280 | 493, 548, 589, 623 | [114] | |
| 42 a | - | 250 | 491, 547, 586, 623 | [115] |
| 42 c | - | 250 | 380, 491, 547, 586, 623 | |
| 42a c | - | 250 | ~380 | |
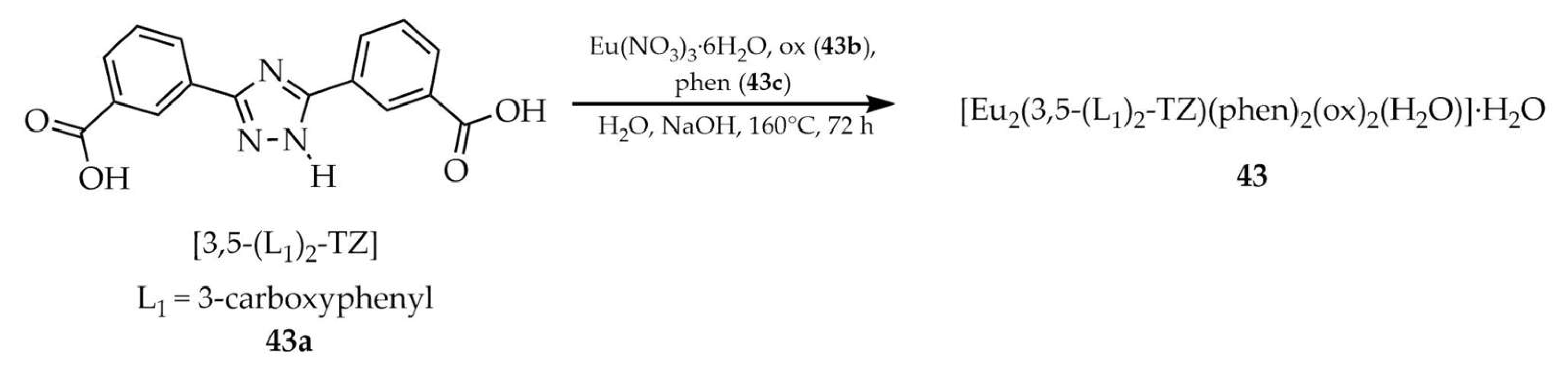
| 43 a | - | 622 | 336, 395, 466, 536 | [116] |
| 43 a | - | 336 | 583, 597, 622, 655, 698 | |
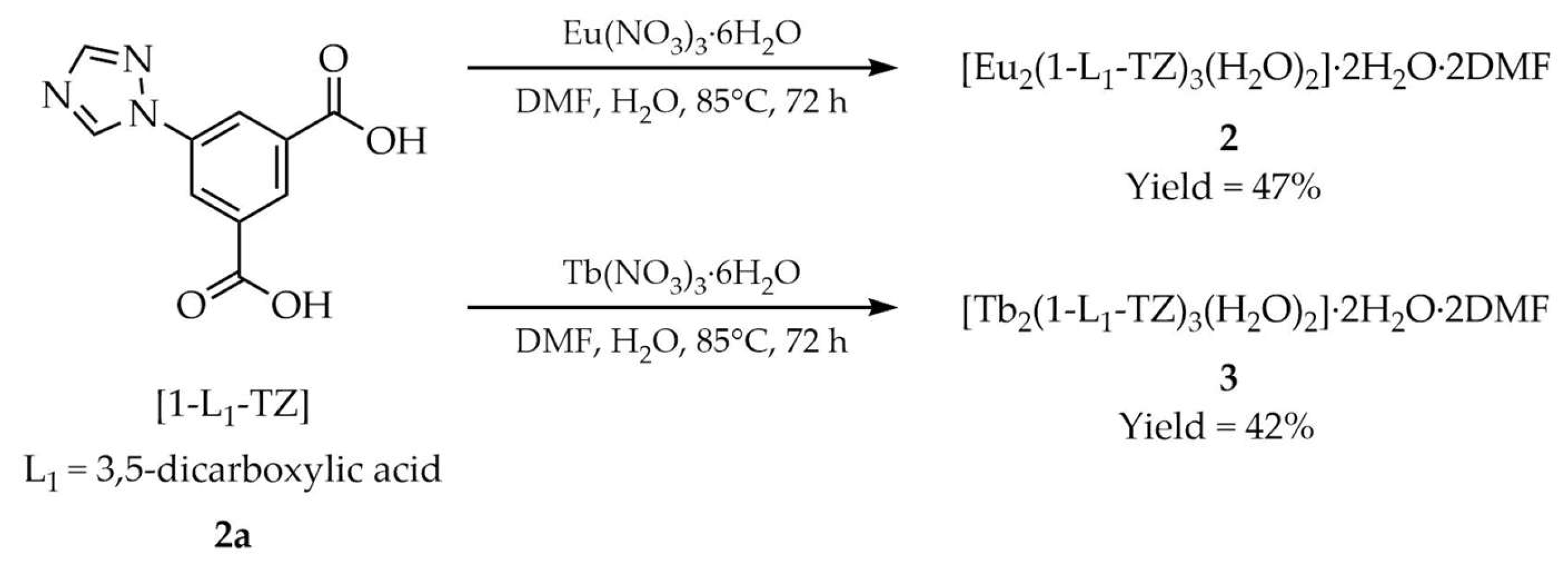
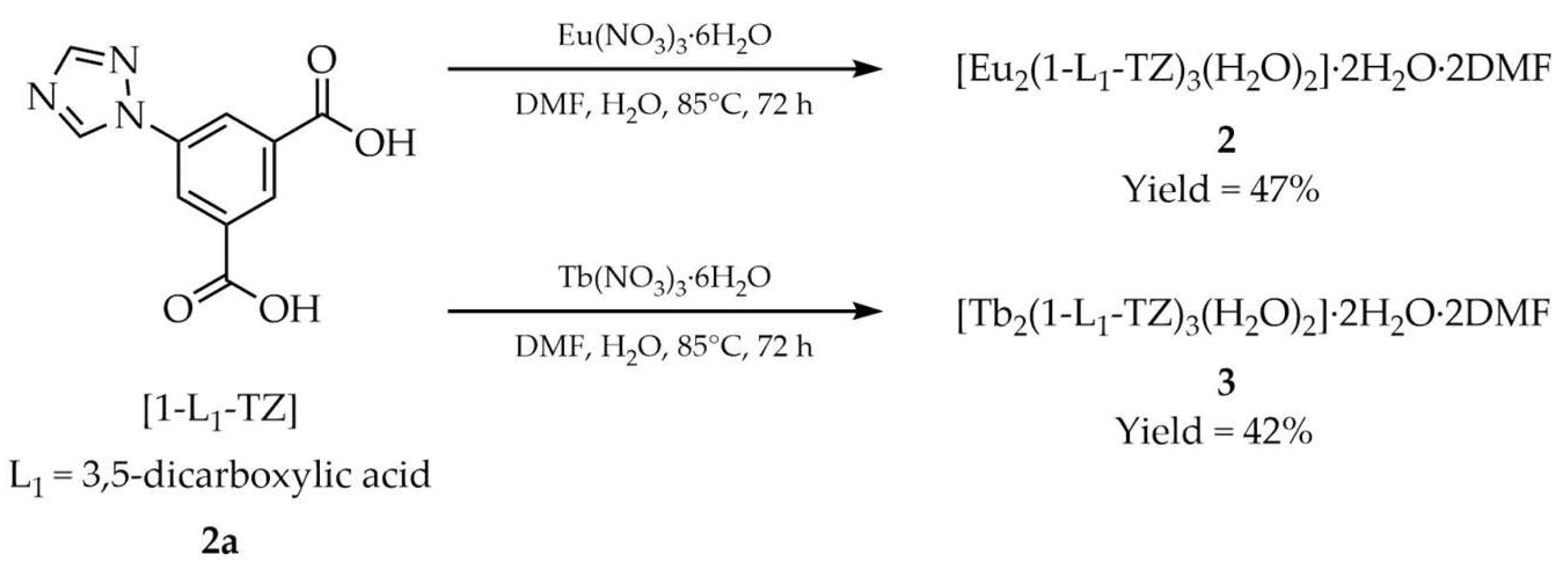
| 2 a | - | 350 | 593, 613, 651, 701 | [51] |
| 3 a | - | 350 | 489, 545, 588, 621 | |
| 44 c | - | 250 | ~350, 492, 543, 583, 617 | [117] |
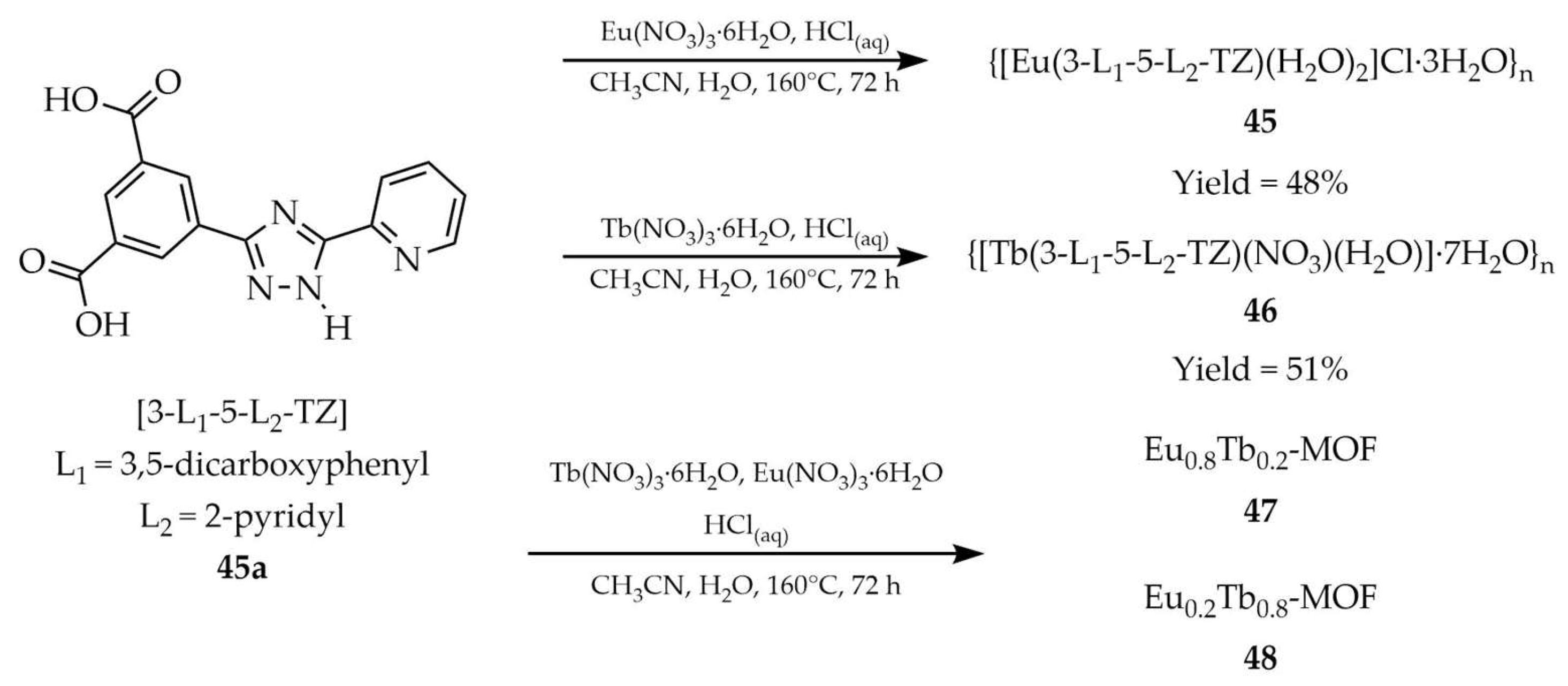
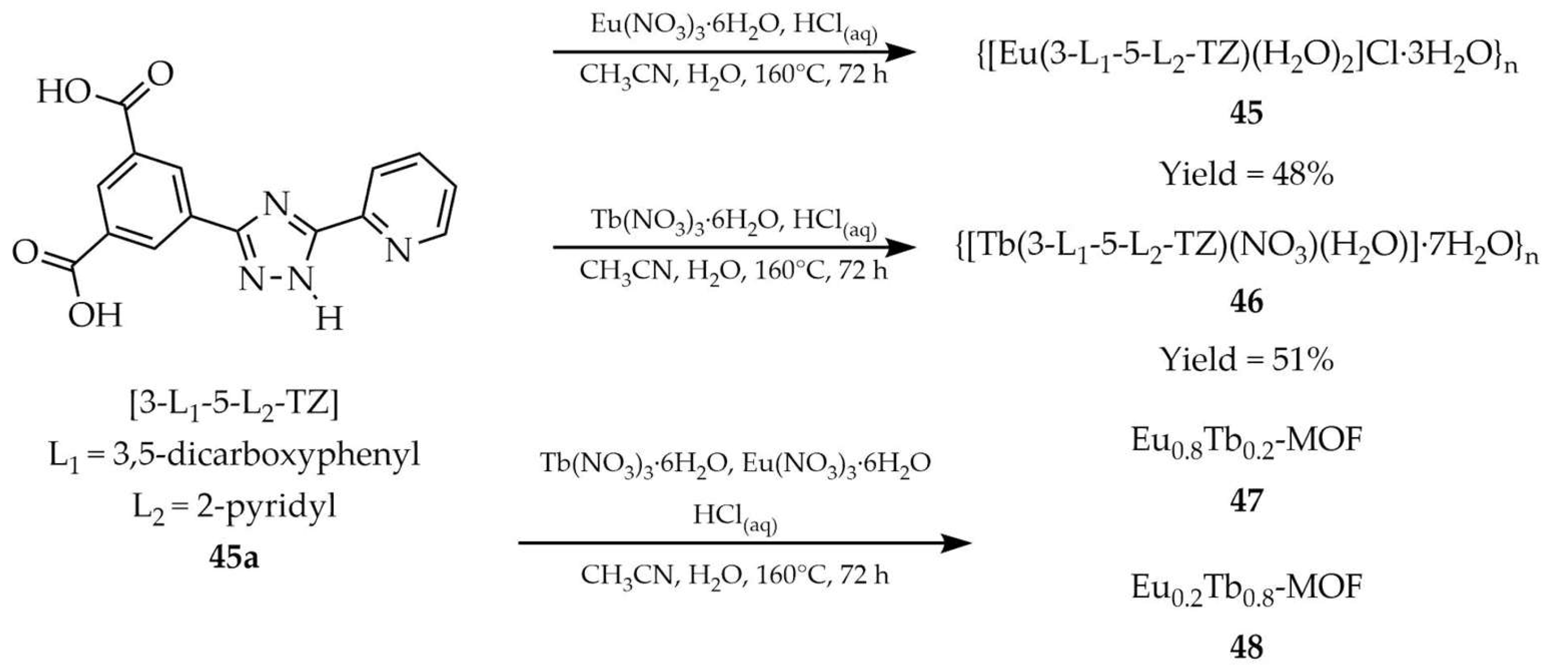
| 45 a | - | 300 | 580, 593, 617, 651, 700 | [118] |
| 46 a | - | 337 | 490, 545, 584, 622 | |
| 45a a | - | 280 | 460 | |
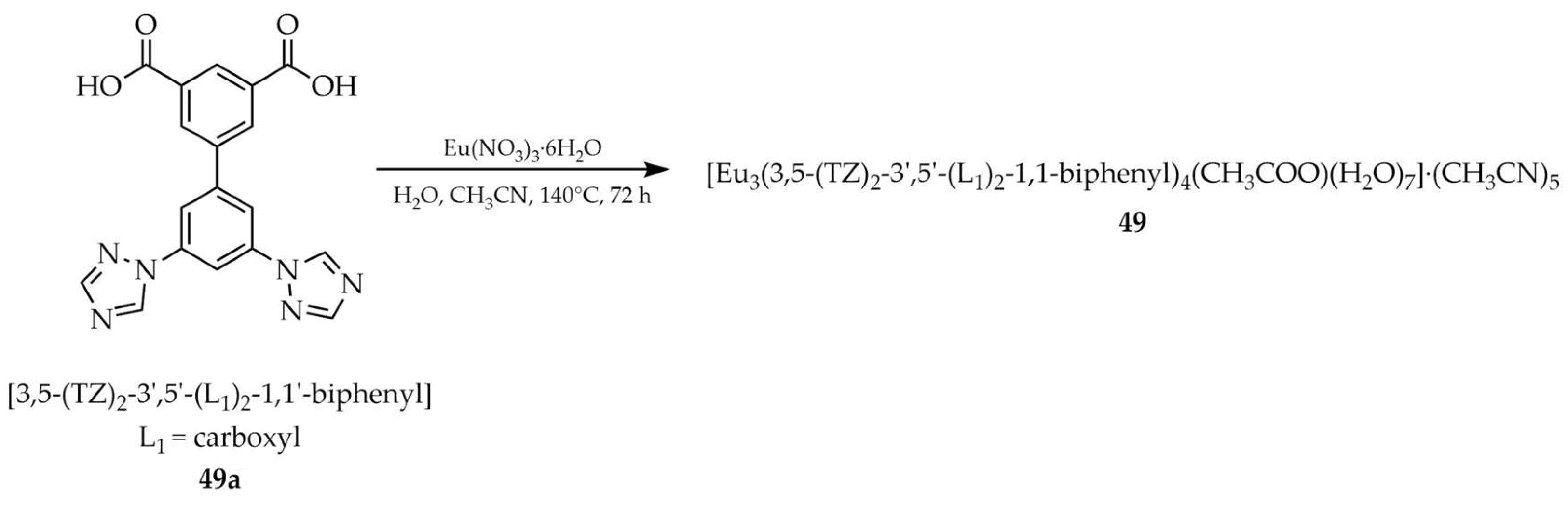
| 49 a | - | 327 | 589, 614, 648, 696 | [119] |
| 49a a | - | 321 | 350 | |
3.1.4. Cluster-Based Metal–Organic Frameworks Containing 1,2,4-Triazole Linker
| Cluster-Based Metal–Organic Frameworks | ||||
|---|---|---|---|---|
| MOF | λabs [nm] | λex [nm] | λem [nm] | Ref. |
| 50 | - | 284 a, 334 b | 339 a, 382 b | [121] |
| 50a | - | 284 a, 334 b | 355 a, 390 b | |
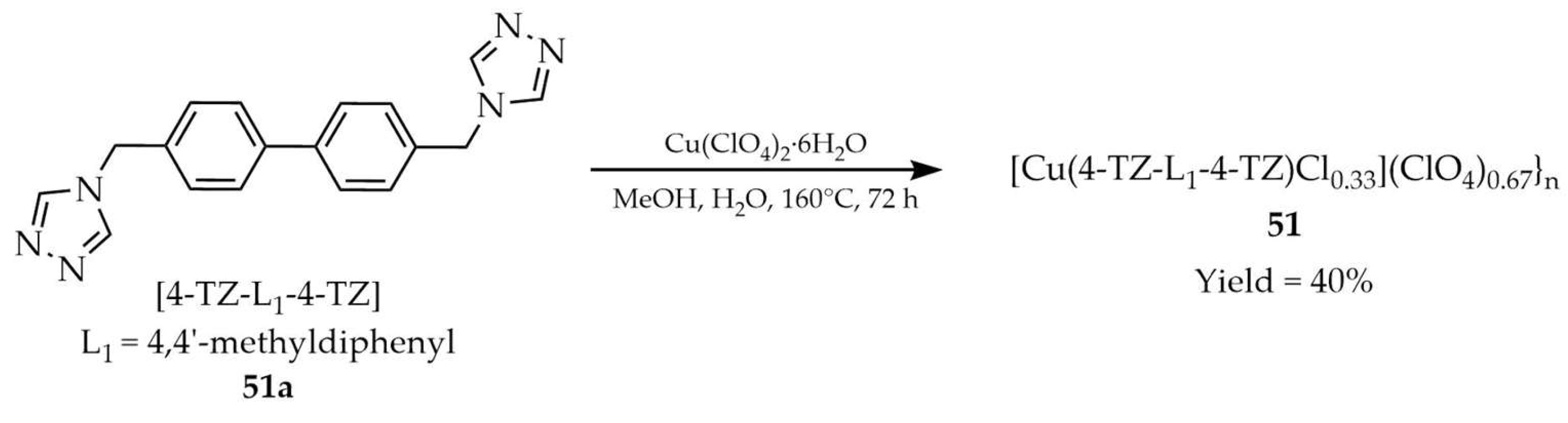
| 51 | - | 320 c, 320 ± 5 e | 422 c, 450 e | [122] |
| 51a | - | 320 d, 320 ± 5 e | 388 d, 402 e | |
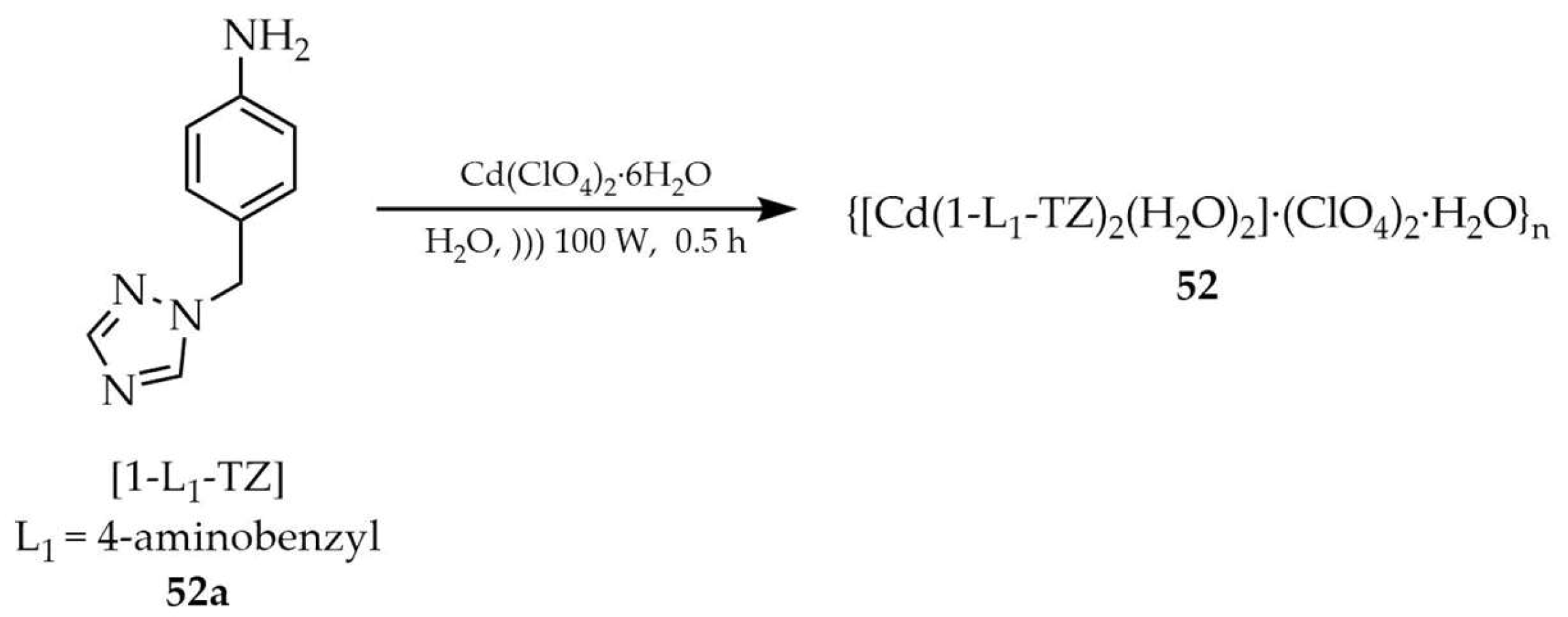
| 52 e | - | 284 | 347 | [123] |
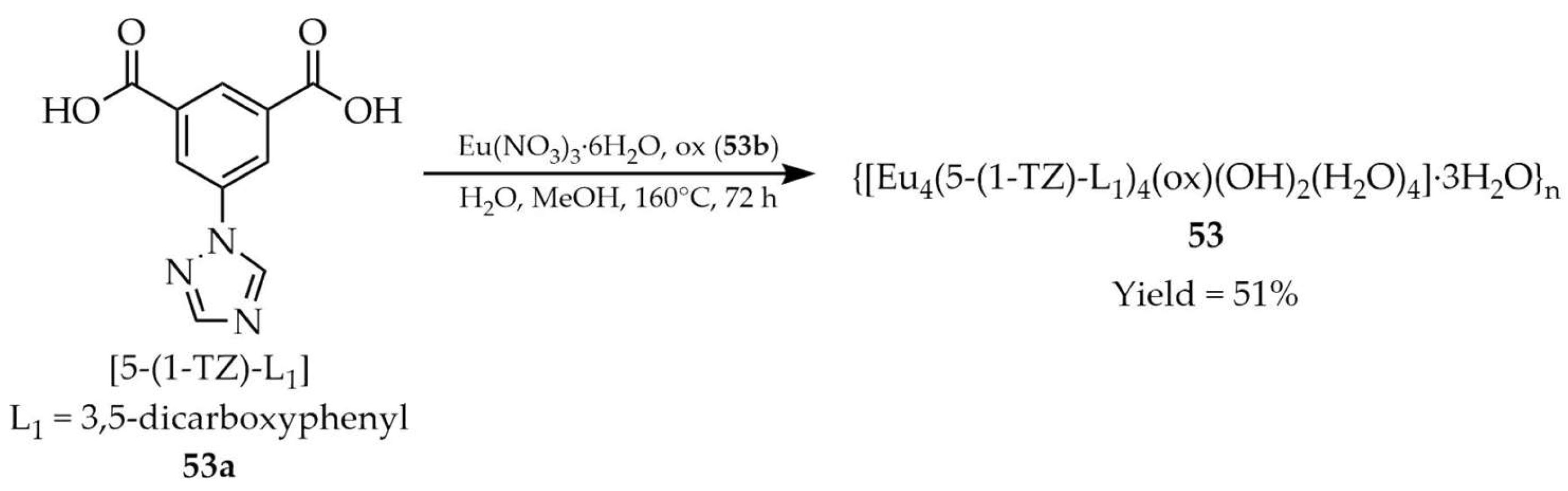
| 53 e | - | 357 | 595, 620, 650, 700 | [124] |
| 53a e | - | 370 | 504 | |
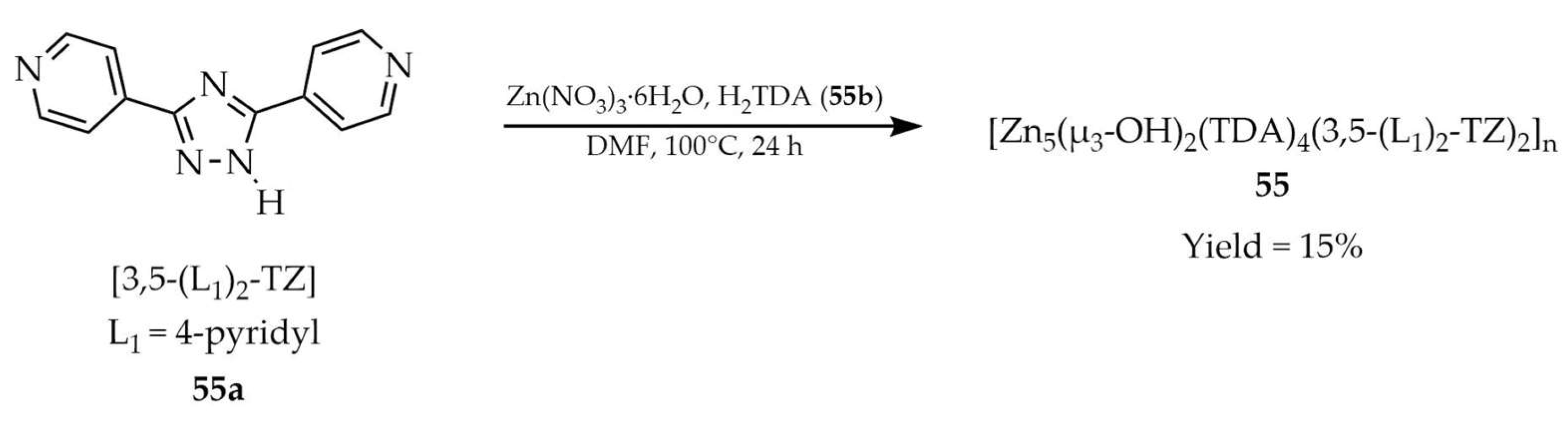
| 55 e | - | 366 | 428 | [126] |
| 55a e | - | 442 | 493 | |
| 55b e | - | 349 | 402 | |
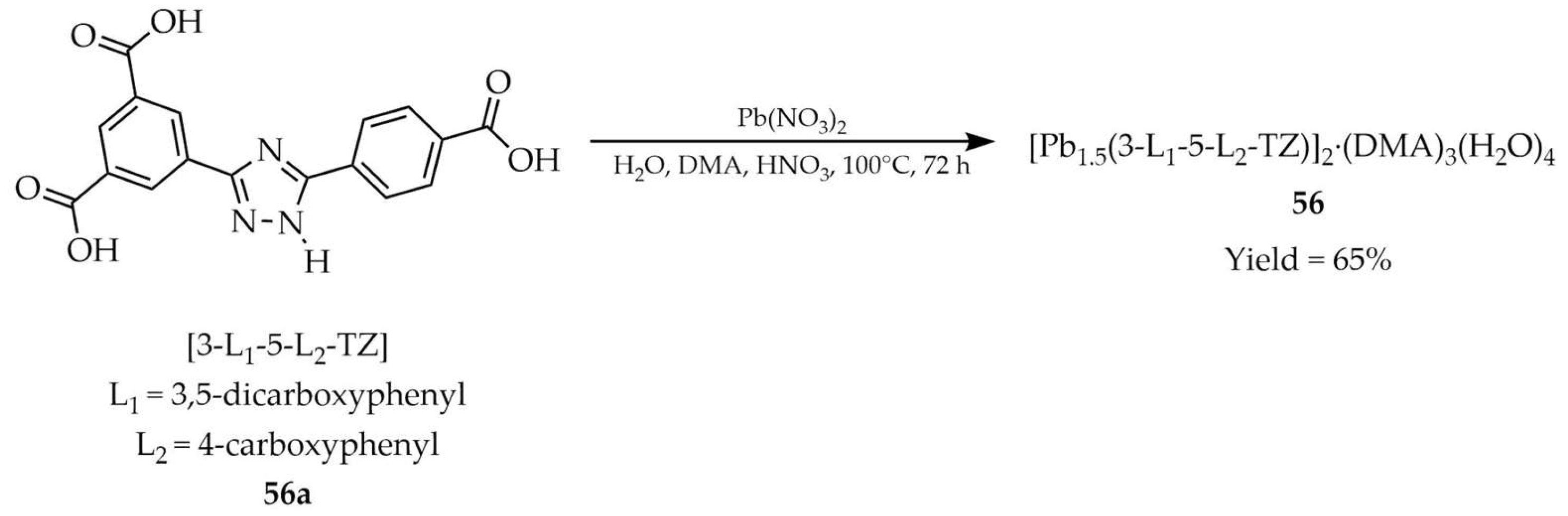
3.1.5. Lead-Based Metal–Organic Frameworks Containing 1,2,4-Triazole Linker
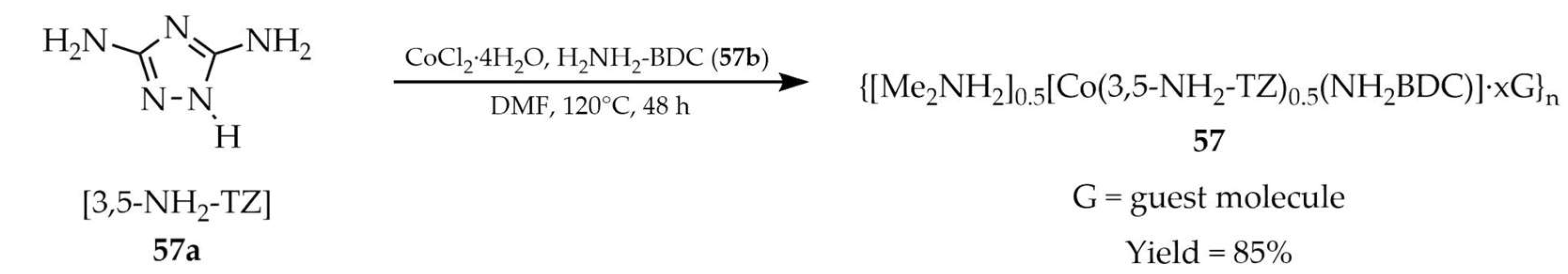
3.1.6. Cobalt-Based Metal–Organic Frameworks Containing 1,2,4-Triazole Linker
3.1.7. Copper-Based Metal–Organic Frameworks Containing 1,2,4-Triazole Linker
| Other M-Based Metal–Organic Frameworks | |||||
|---|---|---|---|---|---|
| M | MOF | λabs [nm] | λex [nm] | λem [nm] | Ref. |
| Pb | 56 a | - | 280 | 380 | [127] |
| 56 a | - | 380 | 540 | ||
| 56a a | - | 280 | ~400 | ||
| Co | 57 a | - | 340 | 430 | [128] |
| Cu | 58 a | 512 | 330 | 412 | [129] |
| 58a a | - | 310 | 411 | ||
| Entry | MOF | Sensing of Cations | Sensing of Anions | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Cation | Ksv [L/mol] | Detection Limit [mol/L] or ppm | Anion | Ksv [L/mol] | Detection Limit [mol/L] or ppm | |||
| 1 | 5 a | Fe3+ | 1.01 × 104 | 4.61 × 10−6 | Cr2O72− | 1.24 × 104 | 2.55 × 10−6 | [90] |
| Al3+ | - | 4.43 × 10−6 | SiF62− | - | 1.35 × 10−5 | |||
| 2 | 6 a | - | - | - | Cr2O72− | 9.72 × 103 | 5.82 × 10−4 | [91] |
| 3 | 7 b | Ni2+ | 9.81 × 104 | 0.46 × 10−6 | PO43− | 6.91 × 103 | 6.5 × 10−6 | [92] |
| 4 | 8 c | Cu2+ | 5.96 × 104 | 0.76 × 10−6 | MnO4− | 2.00 × 105 | 6.14 × 10−6 | [93] |
| Ag+ | 1,67 × 104 | 6.40 × 10−6 | Cr2O72− | 1.85 × 105 | 13.64 × 10−6 | |||
| - | - | - | CrO42− | 5.89 × 104 | 12.33 × 10−6 | |||
| 5 | 13 d | Fe3+ | 4.85 × 104 | - | - | - | - | [97] |
| 6 | 14 e | Fe3+ | 4.22 × 103 | 0.92 × 10−9 | - | - | - | [98] |
| 7 | 16 f | Fe3+ | 7.69 × 104 | 0.58 × 10−6 | Cr2O72− | 3.28 × 104 | 1.37 × 10−6 | [100] |
| 8 | 16d f | Fe3+ | 4.61 × 104 | 0.98 × 10−6 | Cr2O72− | 2.16 × 104 | 2.08 × 10−6 | |
| 9 | 17 a | Fe3+ | 2.21 × 104 | 1.85 × 10−6 | Cr2O72− | 1.02 × 104 | 2.21 × 10−6 | [101] |
| - | - | - | CrO42− | 1.26 × 104 | 3.78 × 10−6 | |||
| 10 | 21 a | Cu2+ | 3.83 × 104 | 1.39 × 10−6 | - | - | - | [105] |
| 11 | 25c a | Fe3+ | 1.13 × 104 | 0.098 | Cr2O72− | 5.42 × 104 | 0.32 | [107] |
| - | - | - | CrO42− | 1.73 × 104 | 0.28 | |||
| 12 | 27 e | Cr3+ | 4.39 × 103 | 10.25× 10−6 | Cr2O72− | 1.49 × 105 | 9.18 × 10−6 | [108] |
| - | - | - | CrO42− | 4.90 × 103 | 0.3 × 10−6 | |||
| 13 | 29 a | Ag+ | 8.16 × 103 | 1.4 × 10−6 | - | - | - | [99] |
| 14 | 36 a | - | - | - | PO43− | 1.27 × 104 | 2.74 × 10−4 | [112] |
| 15 | 41 e | - | - | - | MnO4− | 1.69 × 104 | 1.6 × 10−4 | [114] |
| 16 | 2 f,g | Fe3+ | 1.03 × 104 | 8.78 × 10−3 | - | - | - | [51] |
| 17 | 3 f,h | Fe3+ | 1.49 × 104 | 8.60 × 10−3 | - | - | - | |
| 18 | 45 a | Fe3+ | 7.36 × 103 | 0.57 × 10−6 | Cr2O72− | 4.85 × 103 | 2.29 × 10−6 | [118] |
| 19 | 46 a | Fe3+ | 1.18 × 104 | 0.93 × 10−6 | Cr2O72− | 1.37 × 104 | 1.54 × 10−6 | |
| 20 | 54 a | - | - | - | Cr2O72− | 1.22 × 104 | 0.023 × 10−6 | [125] |
| 21 | 55 | Ag+ a | 1.751 × 105 | 5.96 × 10−6 | Cr2O72− f | 7.397 × 104 | 4.34 × 10−6 | [126] |
| 22 | 56 a | Fe3+ | 1.2 × 105 i | 2.5 i | - | - | - | [127] |
| 4.2 × 103 j | 71 j | - | - | - | ||||
| 56 a | In3+ | 1.6 × 105 i | - | - | - | - | ||
| 56 a | Zr4+ | 1.6 × 105 i | - | - | - | - | ||
| 56 a | Al3+ | 4.3 × 104 i | - | - | - | - | ||
| 23 | 57 f | Al3+ | - | 0.0175 | - | - | - | [128] |
| Entry | MOF | Detected Compound | KSV or KEC [L/mol] | Detection Limit [mol/L] or ppm | Ref. |
|---|---|---|---|---|---|
| 1 | 5 a | NZF | 5.2 × 104 | 4.04 × 10−7 | [90] |
| NFT | 1.8 × 105 | 3.53 × 10−7 | |||
| 2 | 13 b | NB | 8.58 × 103 | - | [97] |
| 3 | 17 a | PAP | 1.95 × 104 | 3.53 × 10−6 | [101] |
| NZF | 5.50 × 104 | 0.46 × 10−6 | |||
| 4 | 18b c | DCF | 4.32 × 104 | 0.62 × 10−6 | [102] |
| TMTD | 3.10 × 104 | 0.76 × 10−6 | |||
| 5 | 21b a | arginine | 2.76 × 104 | 0.69 | [104] |
| 6 | 18 a | TNP | 1.63 × 104 | 3.11 × 10−7 | [105] |
| 7 | 18 d | TNP | 2.99 × 104 | 5.95 × 10−7 | |
| 4-NP | 3.84 × 103 | 2.53 × 10−6 | |||
| 2,4-DNT | 3.76 × 103 | 3.47 × 10−6 | |||
| NB | 5.49 × 103 | 2.61 × 10−6 | |||
| 4-NT | 4.54 × 103 | 3.31 × 10−6 | |||
| 1,3-DNB | 5.21 × 103 | 2.58 × 10−6 | |||
| 8 | 24 a | acetone | 12.89 | - | [106] |
| 9 | 28b d | DEP | 3.74 × 105 | 79.8 × 10−9 | [109] |
| IPT | 1.82 × 103 | 2.26 × 10−7 | |||
| dichloran | 3.88 × 104 | 1.24 × 10−7 | |||
| 10 | 29 d | 4-NP | 4.37 × 104 | 3.29 × 10−7 | [99] |
| 11 | 35 a | TC e | 4.06 × 104 | 0.887 | [111] |
| NZF e | 1.03 × 104 | 2.770 | |||
| NFT e | 1.90 × 105 | 0.189 | |||
| SDZ e | 1.90 × 104 | 1.890 | |||
| CBZ e | 9.64 × 104 | 0.373 | |||
| MNZ f | 1.66 × 105 | 0.217 | |||
| DTZ f | 1.64 × 105 | 0.219 | |||
| ODZ f | 2.54 × 105 | 0.142 | |||
| 12 | 36 a | acetylacetone | 3.22 × 102 | 1.21 × 10−4 | [112] |
| 13 | 41 d | m-DNB | 3.08 × 102 | 0.23 × 10−3 | [114] |
| NB | 2.26 × 103 | 0.19 × 10−3 | |||
| 4-NT | 4.01 × 103 | 0.10 × 10−3 | |||
| 2,4-DNT | 4.45 × 103 | 0.22 × 10−3 | |||
| TNP | 9.10 × 103 | 0.17× 10−3 | |||
| 4-NP | 3.38 × 104 | 0.20 × 10−3 | |||
| 14 | 42 a | creatinine | 5.1 × 10−3 | 1.7 × 10−6 | [115] |
| 15 | 43 a | colchicine | 57,963 | 2.43 × 10−5 | [116] |
| 16 | 2 d,g | 2-NT | 9.01 × 103 | 4.58 × 10−5 | [51] |
| 3-NT | 28.43 × 103 | 1.53 × 10−5 | |||
| 4-NT | 27.87 × 103 | 2.01 × 10−5 | |||
| 4-NP | 10.76 × 103 | 7.68 × 10−6 | |||
| 17 | 3 d,e | 2-NT | 8.01 × 103 | 1.44 × 10−5 | |
| 3-NT | 40.87 × 103 | 5.60 × 10−6 | |||
| 4-NT | 30.59 × 103 | 8.30 × 10−6 | |||
| 4-NP | 11.81 × 103 | 2.85 × 10−6 | |||
| 18 | 44 h | hydroxyflutamide | 4.98 × 1012 | 8.37 × 10−15 | [117] |
| 19 | 44b i | PSA | 7.9 × 1010 | 4.99 × 10−13 | |
| AR | 2.9 × 108 | 0.14 × 10−9 | |||
| 20 | 45 a | TNP | 6.93 × 103 | 1.63 × 10−6 | [118] |
| 21 | 46 a | TNP | 1.21 × 104 | 1.35 × 10−6 | |
| 22 | 49 d | CA | 2.49 × 105 | 36.4 × 10−9 | [119] |
| 23 | 50 | TNP | 7.66 × 104 j 6.36 × 104 a | 0.81 × 10−6 j 0.66 × 10−6 a | [121] |
| NB | 3.94 × 104 j | 1.00 × 10−6 j | |||
| 2,4-DNT | 3.10 × 104 j | 1.11 × 10−6 j | |||
| TNT | 1.18 × 104 j | 2.15 × 10−6 j | |||
| 24 | 52 a | 5-HT | 6.00 × 104 | 0.040 × 10−3 | [123] |
| DPA | 6.30 × 104 | 0.078 × 10−3 | |||
| 25 | 53 k | EDA | 8.08 × 103 | 4.29 × 10−7 | [124] |
| DEA | 6.15 × 103 | 4.94 × 10−7 | |||
| TMA | 4.83 × 103 | 5.89 × 10−7 | |||
| TEA | 2.92 × 103 | 9.84 × 10−7 | |||
| aniline | 2.46 ×103 | 1.012 × 10−6 | |||
| 26 | 54 a | DPA | 1.48 × 105 | 0.298 × 10−6 | [125] |
| 27 | 55 d | TNP | 1.856 × 105 | 1.59 × 10−6 | [126] |
| 28 | 56 a | TNP | 3.3 × 104 k | 9 | [127] |
| 3.6 × 104 l | 8 | ||||
| 4-NA | 2.3 × 104 k | 13 | |||
| 2.4 × 104 l | 12.5 | ||||
| NB | 1.5 × 104 k | 20 | |||
| 0.7 × 104 l | 43 | ||||
| MNZ | 1.3 × 104 k | 23 | |||
| 1.5 × 104 l | 20 | ||||
| DTZ | 0.9 × 104 k | 33 | |||
| 1.5 × 104 l | 20 |
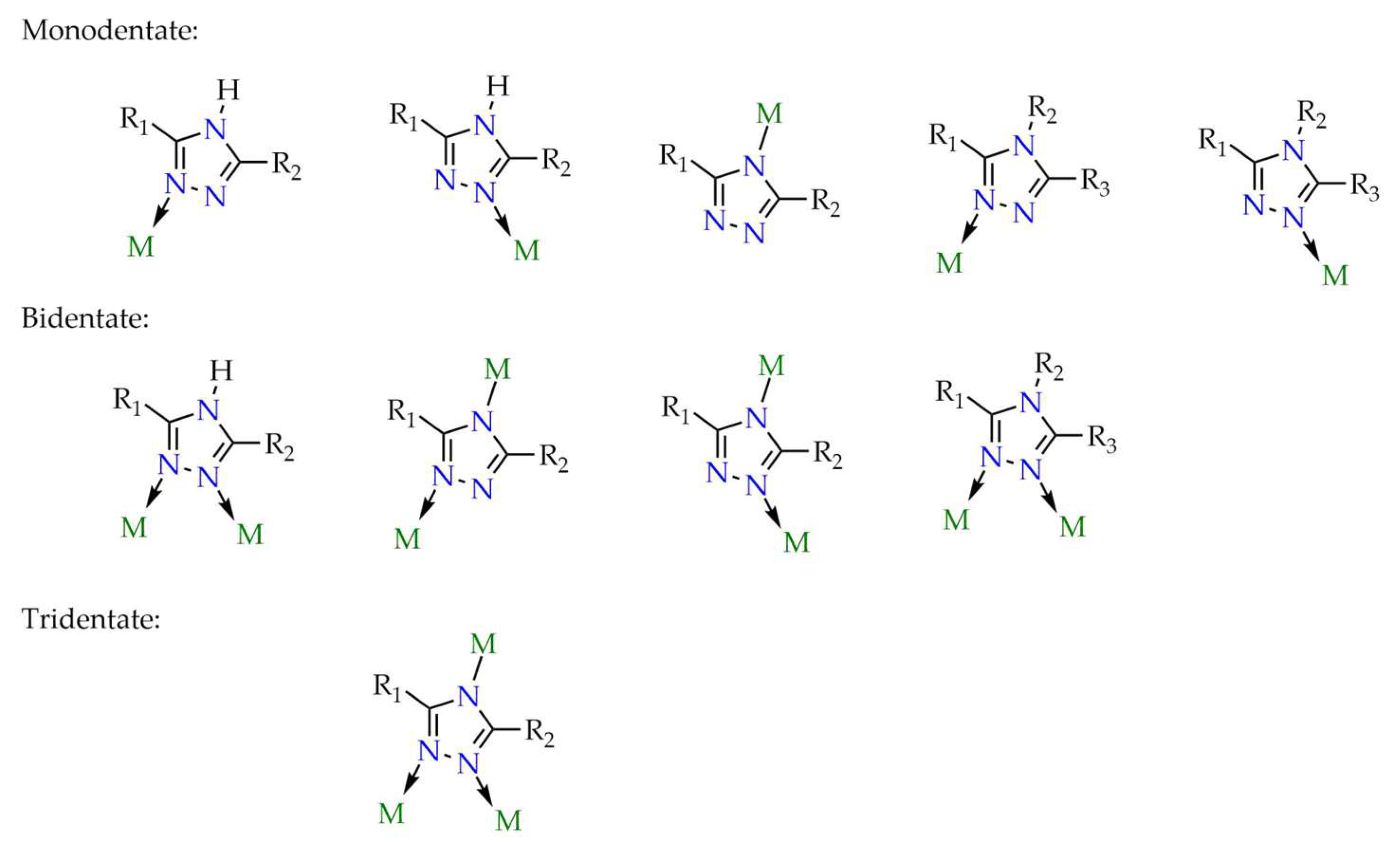
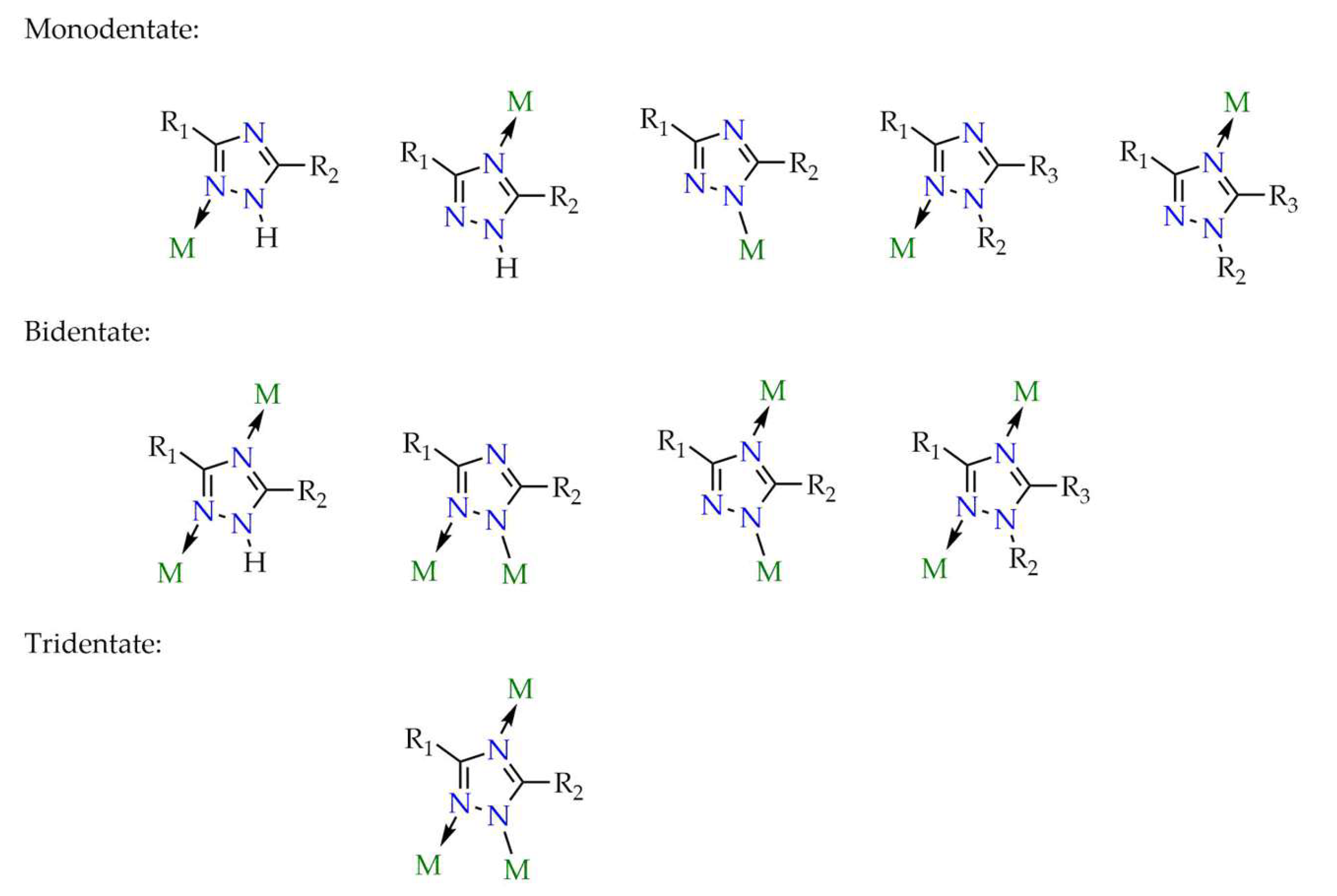
4. General Remarks on Structure-Property Relationships and Current Challenges for 1,2,4-Triazole-Based LMOFs
| Metal | Ligand | Medium | MOF | Quantum Yield [%] | Luminescence Lifetime [ns] | Ref. |
|---|---|---|---|---|---|---|
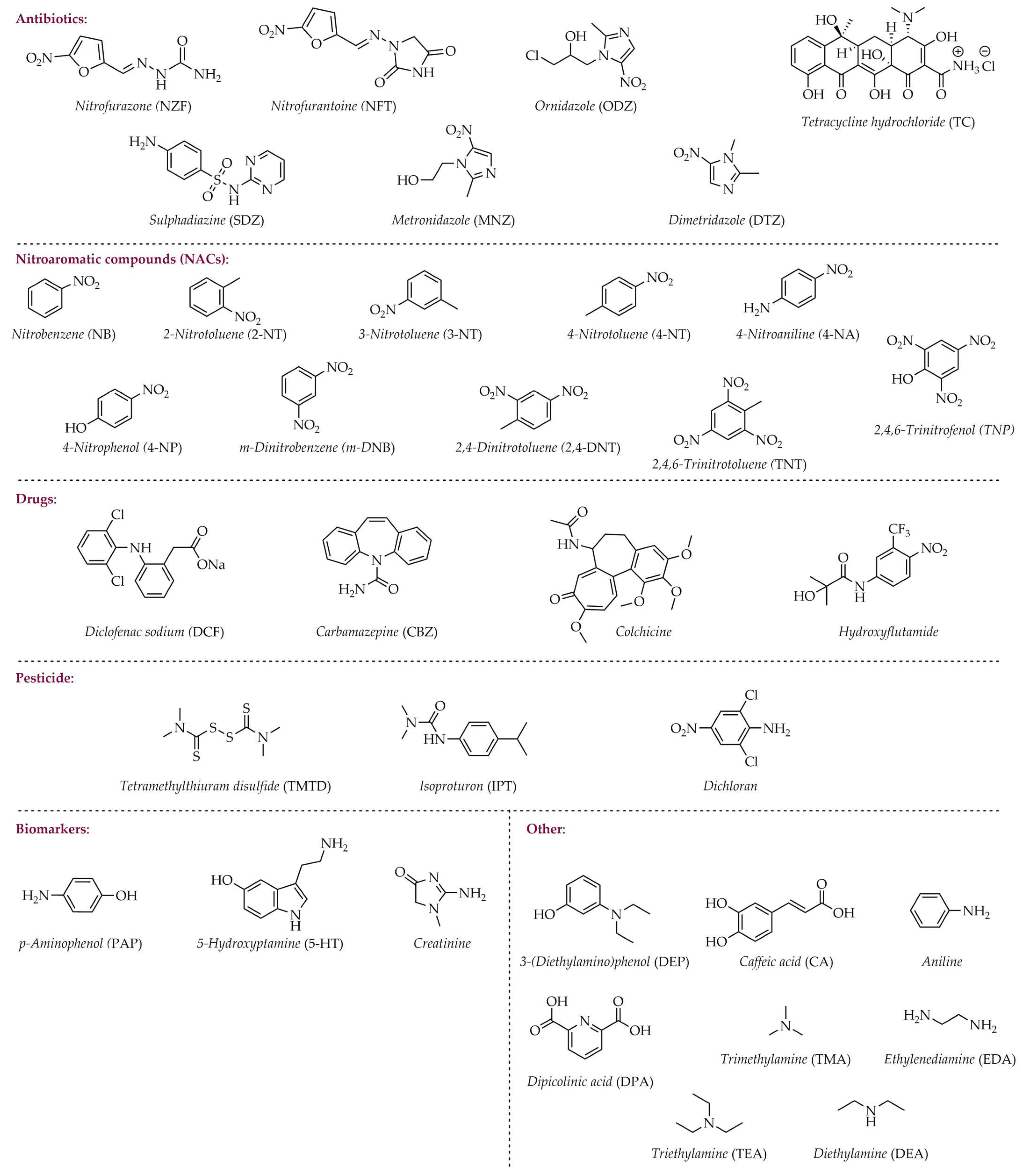
| Zn | 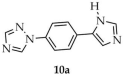 | H2O | 10 | 5.24 | 29.56 | [95] |
| 11 | 3.18 | 18.53 | ||||
 | DMA/ toluene | 13 | 7.4 | - | [97] | |
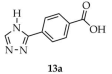
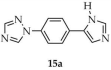 | DMF/ EtOH/ H2O | 15 | 2.15 | 8 | [99] | |
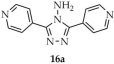 | DMF/ EtOH | 16 | 18 | 6.27 | [100] | |
| 16c | 25.3 | 484,500, 1,260,700 | ||||
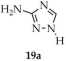 | DMF/ H2O | 19c | 3.02 | 2.7241 | [103] | |
| Cd | 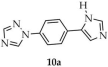 | H2O | 26 | 2.62 | 14.62 | [95] |
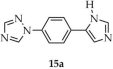 | H2O | 29 | 5.32 | 16 | [99] | |
| Tb | 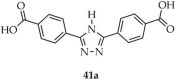 | DMF/ H2O | 41 | 9.5 | 143,000 | [114] |
| Eu | 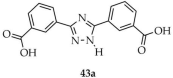 | H2O | 43 | 52.51 | 726,000 | [116] |
| Eu, Tb | 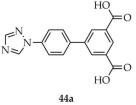 | DMF/ H2O | 44 | 42 | 544,000 | [117] |
| Eu | 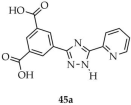 | CH3CN/H2O | 45 | 9.8 | 1,150,000 | [118] |
| Tb | 46 | 10.1 | 940,000 | |||
| Cu | 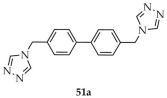 | MeOH/ H2O | 51 | 4.81 | 1.46 | [122] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balabin, R.M. Tautomeric Equilibrium and Hydrogen Shifts in Tetrazole and Triazoles: Focal-Point Analysis and Ab Initio Limit. J. Chem. Phys. 2009, 131, 154307. [Google Scholar] [CrossRef]
- Lei, Y.; Peng, Z.; He, F.; Wang, G. Structure-Activity Relationships and Mechanism Studies of Novel 1,2,4-Triazole-Schiff Base Derivatives as Urease Inhibitors: Synthesis, Enzyme Kinetics, Spectroscopy, Thermodynamics, and Molecular Docking. Bioorg. Med. Chem. 2025, 128, 118289. [Google Scholar] [CrossRef] [PubMed]
- Rajesab, P.; Mathada, B.S.; Niranjan, V.; Sinha, L.; Setlur, A.S.; Maurya, A.; Chandrashekar, K.; Prasad, O. Unveiling a Novel Thiazolo[2,3-c][1,2,4]Triazole Scaffolds as a Dual Cyclooxygenase and Cholinesterase Inhibitors: Spectral Analysis, DFT Calculations, in Vitro and in Silico Studies. J. Mol. Struct. 2025, 1345, 143166. [Google Scholar] [CrossRef]
- Gao, F.; Wang, T.; Xiao, J.; Huang, G. Antibacterial Activity Study of 1,2,4-Triazole Derivatives. Eur. J. Med. Chem. 2019, 173, 274–281. [Google Scholar] [CrossRef] [PubMed]
- El-Sebaey, S.A. Recent Advances in 1,2,4-Triazole Scaffolds as Antiviral Agents. ChemistrySelect 2020, 5, 11654–11680. [Google Scholar] [CrossRef]
- Koparir, P. Synthesis, Antioxidant and Antitumor Activities of Some of New Cyclobutane Containing Triazoles Derivatives. Phosphorus Sulfur. Silicon Relat. Elem. 2019, 194, 1028–1034. [Google Scholar] [CrossRef]
- Khaliullin, F.A.; Klen, E.E.; Nikitina, I.L.; Pavlov, V.N.; Rozit, G.A.; Gaisina, G.G.; Samorodov, A.V. Synthesis and Antidepressant Activity of Thietane-Containing 4-(2-Oxo-2-Phenylethyl)-1H-1,2,4-Triazol-4-Ium Bromides. Pharm. Chem. J. 2023, 56, 1596–1603. [Google Scholar] [CrossRef]
- Manjunath, R.; Hakkimane, S.S.; Shashikala, B.S.; Gaonkar, S.L. Design, Synthesis and Evaluation of Benzodioxole and Bromofuran Tethered 1,2,4-Triazole Hybrids as Potential Anti Breast Cancer Agents with Computational Insights. Sci. Rep. 2025, 15, 25680. [Google Scholar] [CrossRef]
- Marzouk, M.A.; Elsayed, M.M.; Shehab, W.S.; Fawzy, S.M.; Mohammed, S.M.; Abdel-Razek, M.A.; Khedr, G.E.; Elsayed, D.A. Dual α-Amylase and α-Glucosidase Inhibition by 1,2,4-Triazole Derivatives for Diabetes Treatment. Sci. Rep. 2025, 15, 27172. [Google Scholar] [CrossRef]
- Lamb, H.M.; Adkins, J.C. Letrozole: A Review of Use in Postmenopausal Women with Advanced Breast Cancer. Drugs 1998, 56, 1125–1140. [Google Scholar] [CrossRef]
- Parker, W.B. Metabolism and Antiviral Activity of Ribavirin. Virus Res. 2005, 107, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Wellington, K.; Plosker, G.L. Rizatriptan: An Update of Its Use in the Management of Migraine. Drugs 2002, 62, 1539–1574. [Google Scholar] [CrossRef] [PubMed]
- Desta, B.; Amare, G. Paclobutrazol as a Plant Growth Regulator. Chem. Biol. Technol. Agric. 2021, 8, 1. [Google Scholar] [CrossRef]
- Saito, S.; Okamoto, M.; Shinoda, S.; Kushiro, T.; Koshiba, T.; Kamiya, Y.; Hirai, N.; Todoroki, Y.; Sakata, K.; Nambara, E.; et al. A Plant Growth Retardant, Uniconazole, is a Potent Inhibitor of ABA Catabolism in Arabidopsis. Biosci. Biotechnol. Biochem. 2006, 70, 1731–1739. [Google Scholar] [CrossRef]
- Quinn, J.A.; Fujimoto, T.T.; Egan, A.R.; Shaber, S.H. The Properties of RH 3866, a New Triazole Fungicide. Pestic. Sci. 1986, 17, 357–362. [Google Scholar] [CrossRef]
- Minaev, B.; Stakhira, P.; Panchenko, O.; Minaeva, V.; Kutsiy, S.; Melnykov, S.; Danyliv, I.; Volyniuk, D.; Grazulevicius, J.V.; Kudelko, A.; et al. Theoretical and Experimental Investigation of Exciplex-Forming and Electroluminescent Properties of 4-Ethyl-3,5-Bis[4-(N,N-Diphenylamine)Biphenyl-4-yl]-4H-1,2,4-Triazole. Opt. Mater. 2024, 147, 114606. [Google Scholar] [CrossRef]
- Xu, L.; Sun, M.; Zhou, Y.; Lou, J.; Xie, M.; Li, Z.; Sun, Q.; Pan, Y.; Xue, S.; Yang, W. A New Multifunctional Fluorescent Molecule for Highly Efficient Non-Doped Deep-Blue Electro-Fluorescence with High Color-Purity and Efficient Phosphorescent OLEDs. Org. Chem. Front. 2023, 10, 490–498. [Google Scholar] [CrossRef]
- Koodalingam, M.; Gao, M.; Jang, J.; Burn, P.L.; Kistemaker, J.C.M.; Puttock, E.V.; Shaw, P.E. Effect of Emissive Ligand Number on the Optoelectronic Properties of Dendronised Heteroleptic Green Emitting Iridium(III) Complexes. Org. Electron. 2025, 138, 107192. [Google Scholar] [CrossRef]
- Ryu, C.H.; Lim, J.; Kim, M.B.; Lee, J.H.; Hwang, H.; Lee, J.Y.; Lee, K.M. Tris(5-Phenyl-1H-1,2,4-Triazolyl)Iridium(III) Complex and Its Use in Blue Phosphorescent Organic Light-Emitting Diodes to Provide an External Quantum Efficiency of up to 27.8%. Adv. Opt. Mater. 2021, 9, 2001957. [Google Scholar] [CrossRef]
- Erer, H.; Demir, S.; Karaman, B.; Arıcı, M.; Yeşilel, O.Z. Synthesis and Characterization of Cobalt(II) and Zinc(II) Coordination Polymers Constructed from Bis(Isophthalic Acid) and Bis(1,2,4-Triazole) Linkers. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2386–2398. [Google Scholar] [CrossRef]
- Hilmi, A.; Shoker, T.A.; Ghaddar, T.H. Universal Low-Temperature MWCNT-COOH-Based Counter Electrode and a New Thiolate/Disulfide Electrolyte System for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 8744–8753. [Google Scholar] [CrossRef]
- Tang, H.; Shen, J.; Dai, J.; Rajalakshmi, K.; Muthusamy, S.; Kannan, P.; Zhu, D.; You, B.; Liu, X. Sensitive Determination of Caffeoylquinic Acid in Food and Blood Samples Using Nanostructured Conducting Polymer Composite Modified Electrode. Food Chem. 2025, 492, 145337. [Google Scholar] [CrossRef]
- Bakov, V.V.; Georgiev, N.I.; Donkova, N.G.; Bojinov, V.B. Ratiometric 1,8-Naphthalimide/1,2,4-Triazole Conjugates as Highly Sensitive and Self-Regenerating Solid-State “Naked Eye” Probes for Ammonia and Low Molecular Weight Volatile Amines. J. Photochem. Photobiol. A Chem. 2025, 469, 116590. [Google Scholar] [CrossRef]
- Venugopal, S.; Saha, S.; Kumbhakarna, N.; Vargeese, A.A. Insights into Triazole-Based Energetic Material Design from Decomposition Pathways of Triazole Derivatives. Phys. Chem. Chem. Phys. 2025, 27, 4269–4277. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, G.; Supriya, N.; Vijayalakshmi, K.P.; Deepthi, T. Synthesis, Characterization, Pyrolysis and Thermokinetic Studies of an Energetic Ionic Liquid: 4-Amino-1-Methyl-1,2,4-Triazolium Nitrate. J. Ion. Liq. 2025, 5, 100161. [Google Scholar] [CrossRef]
- Özkan, C.; Armaki, A.M.; Rahimi, E.; Anusuyadevi, P.R.; Nie, H.Y.; Hedberg, Y.; Taheri, P.; Mol, A. Quasi-Stable Adsorption as a Stepping Stone to Stable Corrosion Inhibition. Appl. Surf. Sci. 2025, 712, 164060. [Google Scholar] [CrossRef]
- Al-Ahmed, Z.A.M.; Kamel, M.M.; Mahmoud, M.A.A.; Ali, S.A.M.; Ibrahim, A.Z.; Alshehri, B.M. 4-Amino-5-(Trifluoromethyl)-4H-1,2,4-Triazole-3-Thiol as an Effective Corrosion Inhibitor for Low Carbon Steel in HCl Environment: Experimental and Theoretical Studies. BMC Chem. 2025, 19, 205. [Google Scholar] [CrossRef]
- Chen, Z.; Li, H.; Dong, W.; Miao, M.; Ren, H. I2-Catalyzed Oxidative Coupling Reactions of Hydrazones and Amines and the Application in the Synthesis of 1,3,5-Trisubstituted 1,2,4-Triazoles. Org. Lett. 2016, 18, 1334–1337. [Google Scholar] [CrossRef]
- Lee, J.; Hong, M.; Jung, Y.; Cho, E.J.; Rhee, H. Synthesis of 1,3,5-Trisubstituted-1,2,4-Triazoles by Microwave-Assisted N-Acylation of Amide Derivatives and the Consecutive Reaction with Hydrazine Hydrochlorides. Tetrahedron 2012, 68, 2045–2051. [Google Scholar] [CrossRef]
- Castanedo, G.M.; Seng, P.S.; Blaquiere, N.; Trapp, S.; Staben, S.T. Rapid Synthesis of 1,3,5-Substituted 1,2,4-Triazoles from Carboxylic Acids, Amidines, and Hydrazines. J. Org. Chem. 2011, 76, 1177–1179. [Google Scholar] [CrossRef]
- Jiang, R.; Mu, Y.; Hou, J.; Wan, Y.; Hong, Y.; Yang, Z.; Tang, D. Electrochemical Cyclization of Hydrazones and Amidines to Access Trisubstituted 1,2,4-Triazoles. Synlett 2023, 34, 1804–1808. [Google Scholar] [CrossRef]
- Huang, H.; Guo, W.; Wu, W.; Li, C.J.; Jiang, H. Copper-Catalyzed Oxidative C(sp3)–H Functionalization for Facile Synthesis of 1,2,4-Triazoles and 1,3,5-Triazines from Amidines. Org. Lett. 2015, 17, 2894–2897. [Google Scholar] [CrossRef]
- Liu, J.Q.; Shen, X.; Wang, Y.; Wang, X.S.; Bi, X. Cycloaddition of Isocyanides with Aryl Diazonium Salts: Catalyst-Dependent Regioselective Synthesis of 1,3- and 1,5-Disubstituted 1,2,4-Triazoles. Org. Lett. 2018, 20, 6930–6933. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, J.L.; Chen, Z.; Wang, R. Base-Promoted (3+2) Cycloaddition of Trifluoroacetohydrazonoyl Chlorides with Imidates En Route to Trifluoromethyl-1,2,4-Triazoles. J. Org. Chem. 2022, 87, 14514–14522. [Google Scholar] [CrossRef]
- Li, S.M.; Tsai, S.E.; Chiang, C.Y.; Chung, C.Y.; Chuang, T.J.; Tseng, C.C.; Jiang, W.P.; Huang, G.J.; Lin, C.Y.; Yang, Y.C.; et al. New Methyl 5-(Halomethyl)-1-Aryl-1H-1,2,4-Triazole-3-Carboxylates as Selective COX-2 Inhibitors and Anti-Inflammatory Agents: Design, Synthesis, Biological Evaluation, and Docking Study. Bioorg. Chem. 2020, 104, 104333. [Google Scholar] [CrossRef]
- Olesiejuk, M.; Kudelko, A.; Swiatkowski, M.; Kruszynski, R. Synthesis of 4-Alkyl-4H-1,2,4-Triazole Derivatives by Suzuki Cross-Coupling Reactions and Their Luminescence Properties. Molecules 2019, 24, 652. [Google Scholar] [CrossRef]
- Bechara, W.S.; Khazhieva, I.S.; Rodriguez, E.; Charette, A.B. One-Pot Synthesis of 3,4,5-Trisubstituted 1,2,4-Triazoles via the Addition of Hydrazides to Activated Secondary Amides. Org. Lett. 2015, 17, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.N.; Zhang, J.; Li, J.; Chen, Z.; Wu, X.F. Metal-Free Synthesis of 3-Trifluoromethyl-1,2,4-Triazoles via Oxidative Cyclization of Trifluoroacetimidohydrazides with N,N-Dimethylformamide as Carbon Synthons. Green Synth. Catal. 2022, 3, 385–388. [Google Scholar] [CrossRef]
- Yocca, S.R.; Yount, J.; Zeller, M.; Byrd, E.F.C.; Piercey, D.G. 1,3,4,5-Tetraamino-1,2,4-Triazolium Cation: An Energetic Moiety. Inorg. Chem. 2021, 60, 9645–9652. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Ullah, H.; Taha, M.; Hussain, R.; Sarfraz, M.; Iqbal, R.; Iqbal, N.; Khan, S.; Ali Shah, S.A.; Albalawi, M.A.; et al. Synthesis of New Triazole-Based Thiosemicarbazone Derivatives as Anti-Alzheimer’s Disease Candidates: Evidence-Based In Vitro Study. Molecules 2023, 28, 21. [Google Scholar] [CrossRef]
- Hutchinson, S.M.; Ardón-Muñoz, L.G.; Ratliff, M.L.; Bolliger, J.L. Catalytic Preparation of 1-Aryl-Substituted 1,2,4-Triazolium Salts. ACS Omega 2019, 4, 17923–17933. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, A.; Guin, S.; Rajamanickam, S.; Rout, S.K.; Patel, B.K. Synthesis of 1,2,4-Triazoles via Oxidative Heterocyclization: Selective C–N Bond Over C–S Bond Formation. J. Org. Chem. 2015, 80, 9016–9027. [Google Scholar] [CrossRef] [PubMed]
- Nakka, M.; Tadikonda, R.; Rayavarapu, S.; Sarakula, P.; Vidavalur, S. A Simple and Efficient Synthesis of 3,4,5-Trisubstituted/N-Fused 1,2,4-Triazoles via Ceric Ammonium Nitrate Catalyzed Oxidative Cyclization of Amidrazones with Aldehydes Using Polyethylene Glycol as a Recyclable Reaction Medium. Synthesis 2015, 47, 517–525. [Google Scholar] [CrossRef]
- James, S.L. Metal-Organic Frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef]
- Su, C.Y.; Goforth, A.M.; Smith, M.D.; Pellechia, P.J.; Zur Loye, H.C. Exceptionally Stable, Hollow Tubular Metal-Orgarnic Architectures: Synthesis, Characterization, and Solid-State Transformation Study. J. Am. Chem. Soc. 2004, 126, 3576–3586. [Google Scholar] [CrossRef]
- Dutt, S.; Kumar, A.; Singh, S. Synthesis of Metal Organic Frameworks (MOFs) and Their Derived Materials for Energy Storage Applications. Clean Technol. 2023, 5, 140–166. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Hunt, J.R.; Yaghi, O.M. Room Temperature Synthesis of Metal-Organic Frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 2008, 64, 8553–8557. [Google Scholar] [CrossRef]
- Rabenau, A. The Role of Hydrothermal Synthesis in Preparative Chemistry. Angew. Chem. Int. Ed. Engl. 1985, 24, 1026–1040. [Google Scholar] [CrossRef]
- Yoshimura, M.; Byrappa, K. Hydrothermal Processing of Materials: Past, Present and Future. J. Mater. Sci. 2008, 43, 2085–2103. [Google Scholar] [CrossRef]
- Férey, G. Metal-Organic Frameworks: The Young Child of the Porous Solids Family. Stud. Surf. Sci. Catal. 2007, 170, 66–84. [Google Scholar] [CrossRef]
- Sun, J.; Xi, Y.; Gao, L.; Hu, M.; Liu, W.; Ma, E.; Huang, R.; Qin, W.; Wu, G. Two Isostructural Ln-MOFs Containing Triazole Groups as Luminescent Probes for Efficient Sensing of NACs and Fe3+. Inorganica Chim. Acta 2023, 547, 121376. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.Y.; Luo, F. A New Zn-Triazole MOF Showing Very Long-Lived Luminescence up to 3 s. J. Solid State Chem. 2021, 301, 122369. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Kalmutzki, M.J.; Diercks, C.S. Introduction to Reticular Chemistry: Metal-Organic Frameworks and Covalent Organic Frameworks; Wiley: Hoboken, NJ, USA, 2019; pp. 1–509. [Google Scholar] [CrossRef]
- Abid, H.R.; Azhar, M.R.; Iglauer, S.; Rada, Z.H.; Al-Yaseri, A.; Keshavarz, A. Physicochemical Characterization of Metal Organic Framework Materials: A Mini Review. Heliyon 2024, 10, e23840. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.G.; Salinger, J.L.; Bingel, L.W.; Walton, K.S. Determining Surface Areas and Pore Volumes of Metal-Organic Frameworks. J. Vis. Exp. 2024, 7, e65716. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Razavi, S.A.A.; Piroozzadeh, M.; Morsali, A. Sensing Organic Analytes by Metal–Organic Frameworks: A New Way of Considering the Topic. Inorg. Chem. Front. 2020, 7, 1598–1632. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, H.; Zhu, X.W.; Lu, W.; Li, D. Metal–Organic Frameworks as Photoluminescent Biosensing Platforms: Mechanisms and Applications. Chem. Soc. Rev. 2021, 50, 4484–4513. [Google Scholar] [CrossRef]
- Raptopoulou, C.P. Metal-Organic Frameworks: Synthetic Methods and Potential Applications. Materials 2021, 14, 310. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-Organic Framework Functionalization and Design Strategies for Advanced Electrochemical Energy Storage Devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef]
- Pham, T.D.; Sengupta, D.; Farha, O.K.; Snurr, R.Q. Investigation of Anionic Metal-Organic Frameworks with Extra-Framework Cations for Room Temperature Hydrogen Storage. Chem. Mater. 2024, 36, 3794–3802. [Google Scholar] [CrossRef]
- Xie, W.; Fu, Q.; Yang, L.-Z.; Yan, L.; Zhang, J.; Zhao, X. Methane Storage and Purification of Natural Gas in Metal-Organic Frameworks. ChemSusChem 2025, 18, e202401382. [Google Scholar] [CrossRef]
- Wang, J.W.; Fan, S.C.; Li, H.P.; Bu, X.; Xue, Y.Y.; Zhai, Q.G. De-Linker-Enabled Exceptional Volumetric Acetylene Storage Capacity and Benchmark C2H2/C2H4 and C2H2/CO2 Separations in Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2023, 62, e202217839. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, X.; Liu, S.; Liu, X.; Liu, G.; Liu, R.; Niu, J.; Yuan, Z.; Zhang, J.; Yi, X.; et al. Melamine-Derived Nitrogen-Doped Carbon Foam Supported Bimetallic NiZn-MOFs as an Efficient Adsorbent for CH4 Storage. J. Porous Mater. 2025, 32, 1309–1319. [Google Scholar] [CrossRef]
- Li, Y.Z.; Wang, G.D.; Xu, F.; Yin, Q.; Zhao, D.; Qi, J.; Sui, Y.; Hou, L.; Wang, Y.Y. A Robust Indium-Organic Framework with Open Tubular Channels for Efficient Separation of Acetylene. Nano Res. 2024, 17, 3139–3146. [Google Scholar] [CrossRef]
- Hou, W.; Cheng, J.; Hu, L.; Wu, Y.; Zhou, J. Mixed Matrix Membranes Based on NbOF52− Anion-Pillared Porous MOFs for Efficient CO2 Separation. J. Memb. Sci. 2024, 693, 122323. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, S.; Ye, P.; Zhang, L.; Shen, X.; Huang, Q.; Xu, H.; Liu, H.; Li, S. In Situ Synthesis of Hierarchical Porous Zr-MOFs on Columnar Activated Carbon and Application in Toxic Gas Adsorption. Inorg. Chem. 2022, 61, 18355–18364. [Google Scholar] [CrossRef]
- Obeso, J.L.; Amaro, D.R.; Flores, C.V.; Gutiérrez-Alejandre, A.; Peralta, R.A.; Leyva, C.; Ibarra, I.A. Chemical Transformations of Highly Toxic H2S to Promising Clean Energy in MOFs. Coord. Chem. Rev. 2023, 485, 215135. [Google Scholar] [CrossRef]
- Kim, D.W.; Kang, D.W.; Kang, M.; Lee, J.H.; Choe, J.H.; Chae, Y.S.; Choi, D.S.; Yun, H.; Hong, C.S. High Ammonia Uptake of a Metal–Organic Framework Adsorbent in a Wide Pressure Range. Angew. Chem. Int. Ed. 2020, 59, 22531–22536. [Google Scholar] [CrossRef]
- Cheng, L.; Dang, Y.; Wang, Y.; Chen, K.J. Recent Advances in Metal–Organic Frameworks for Water Absorption and Their Applications. Mater. Chem. Front. 2024, 8, 1171–1194. [Google Scholar] [CrossRef]
- Chen, W.; Cai, P.; Zhou, H.C.; Madrahimov, S.T. Bridging Homogeneous and Heterogeneous Catalysis: Phosphine-Functionalized Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2024, 63, e202315075. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, R.; Pramanik, B.; Mondal, S.; Das, M.C. A Highly Chemically Robust 3D Interpenetrated MOF Heterogeneous Catalyst for the Synthesis of Hantzsch 1,4-Dihydropyridines and Drug Molecules. Small 2024, 20, 2309281. [Google Scholar] [CrossRef] [PubMed]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal-Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef]
- Sun, N.; Shah, S.S.A.; Lin, Z.; Zheng, Y.Z.; Jiao, L.; Jiang, H.L. MOF-Based Electrocatalysts: An Overview from the Perspective of Structural Design. Chem. Rev. 2025, 125, 2703–2792. [Google Scholar] [CrossRef] [PubMed]
- Juma, A.K.; Merican, Z.M.A.; Haruna, A. Recent Progress of MOF-Based Photocatalysts for Environmental Application and Sustainability Considerations. Chem. Eng. Res. Des. 2024, 208, 391–435. [Google Scholar] [CrossRef]
- Bunzen, H.; Jirák, D. Recent Advances in Metal-Organic Frameworks for Applications in Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2022, 14, 50445–50462. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Gao, J.; Sun, Y.; Duan, F.; Chen, B.; Lv, G.; Li, H.; Jiang, X.; Wu, Y.; Zhang, J.; et al. Fusing Positive and Negative CT Contrast Nanoagent for the Sensitive Detection of Hepatoma. Adv. Sci. 2023, 10, e2304668. [Google Scholar] [CrossRef]
- Duan, D.; Liu, H.; Xu, M.; Chen, M.; Han, Y.; Shi, Y.; Liu, Z. Size-Controlled Synthesis of Drug-Loaded Zeolitic Imidazolate Framework in Aqueous Solution and Size Effect on Their Cancer Theranostics in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 42165–42174. [Google Scholar] [CrossRef]
- Yu, J.; Li, Q.; Wei, Z.; Fan, G.; Wan, F.; Tian, L. Ultra-Stable MOF@MOF Nanoplatform for Photodynamic Therapy Sensitized by Relieved Hypoxia Due to Mitochondrial Respiration Inhibition. Acta Biomater. 2023, 170, 330–343. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, C.; Mei, Z.; Yang, H.; Wang, B.; Xie, C.; Xu, Y.; Tian, J. Oxygen-Enriched MOF-Hemoglobin X-Ray Nanosensitizer for Enhanced Cancer Radio-Radiodynamic Therapy. ACS Mater. Lett. 2023, 5, 3237–3247. [Google Scholar] [CrossRef]
- Yadav, P.; Kumari, S.; Yadav, A.; Bhardwaj, P.; Maruthi, M.; Chakraborty, A.; Kanoo, P. Biocompatible Drug Delivery System Based on a MOF Platform for a Sustained and Controlled Release of the Poorly Soluble Drug Norfloxacin. ACS Omega 2023, 8, 28367–28375. [Google Scholar] [CrossRef]
- Timofeeva, M.; Kenzhebayeva, Y.; Burzak, N.; Bazhenova, A.; Lunev, A.; Novikov, A.S.; Bondarenko, A.B.; Shipilovskikh, S.A.; Dyachuk, V.A.; Milichko, V.A. A Light-Driven Ultrafast Sensor Based on Biocompatible Solvatochromic Metal-Organic Frameworks. Mater. Horizons 2024, 12, 1255–1261. [Google Scholar] [CrossRef]
- Panda, S.K.; Mishra, S.; Singh, A.K. Recent Progress in the Development of MOF-Based Optical Sensors for Fe3+. Dalt. Trans. 2021, 50, 7139–7155. [Google Scholar] [CrossRef]
- Ghommem, M.; Hemid, M.; Alattar, B.; Sabouni, R.; Elhady, A.; Shama, Y.S.; Arabi, M.; Abdel-Rahman, E.M. Development of MEMS Gas Sensors Equipped with Metal Organic Frameworks. Sens. Actuators A Phys. 2024, 371, 115296. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, H.; Song, B.; Yuan, K.; Xiao, H.; Cao, Y.; Cao, Q. Metal–Organic Framework (MOF) Derivatives as Promising Chemiresistive Gas Sensing Materials: A Review. Int. J. Environ. Res. Public Health 2023, 20, 4388. [Google Scholar] [CrossRef]
- Daniel, M.; Mathew, G.; Anpo, M.; Neppolian, B. MOF Based Electrochemical Sensors for the Detection of Physiologically Relevant Biomolecules: An Overview. Coord. Chem. Rev. 2022, 468, 214627. [Google Scholar] [CrossRef]
- Yuan, H.; Cui, J.; Li, N.; Li, M.; Yu, X.; Fan, W.; Karmakar, A.; Dong, J.; Pennycook, S.J.; Cai, H.; et al. On-Chip Template-Directed Conversion of Metal Hydroxides to Metal-Organic Framework Films with Enhanced Adhesion. ACS Appl. Mater. Interfaces 2020, 12, 36715–36722. [Google Scholar] [CrossRef]
- Banning, D.H.; Davenport, A.M.; Lakanen, N.M.; Huang, J.; Brozek, C.K.; Johnson, D.W. ChemFET Anion Sensor Based on MOF Nanoparticles. Chempluschem 2024, 90, e202400622. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, H.; Yan, S.; Zhou, Y.; Guo, X.; An, D.; Zhong, W.; Zhang, Y. Fabrication of MOF-Based Nanozyme Sensor Arrays and Their Application in Disease Diagnosis. Coord. Chem. Rev. 2025, 532, 216506. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, J.; Xu, J.; Ma, J.; Bai, Y.; Cao, S.; Zhang, S.; Pang, H. Metal-Organic Framework (MOF) Composites as Promising Materials for Energy Storage Applications. Adv. Colloid Interface Sci. 2022, 307, 102732. [Google Scholar] [CrossRef]
- He, H.; Zhu, Q.Q.; Li, C.P.; Du, M. Design of a Highly-Stable Pillar-Layer Zinc(II) Porous Framework for Rapid, Reversible, and Multi-Responsive Luminescent Sensor in Water. Cryst. Growth Des. 2019, 19, 694–703. [Google Scholar] [CrossRef]
- Zou, J.Y.; Li, L.; You, S.Y.; Zhang, S.W. A Zinc(II) Triazolate Framework with Luminescence Response toward Dichromate Anion in Aqueous Solution. Inorganica Chim. Acta 2019, 498, 119126. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, X.; Zhang, J. A New Multifunctional Zinc-Organic Framework with Rare Interpenetrated Tripillared Bilayers as a Luminescent Probe for Detecting Ni2+ and PO43−in Water. Cryst. Growth Des. 2020, 20, 5120–5128. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Y.; Gu, L.W.; Qin, X.M.; Li, H.Y.; Bian, H.D.; Huang, F.P. A Zinc2+-Dpbt Framework: Luminescence Sensing of Cu2+, Ag+, MnO4− and Cr(VI) (Cr2O72−and CrO42−) Ions. New J. Chem. 2020, 44, 10681–10688. [Google Scholar] [CrossRef]
- Liu, F.; Kumar, S.; Li, S.; You, H.; Ren, P.; Zhao, L. Bifunctional Design of Stable Metal-Organic Framework Bearing Triazole–Carboxylate Mixed Ligand: Highly Efficient Heterogeneous Catalyst for Knoevenagel Condensation Reaction under Mild Conditions. Catal. Commun. 2020, 142, 106032. [Google Scholar] [CrossRef]
- Li, W.D.; Chen, S.S.; Han, S.S.; Zhao, Y. The Syntheses, Structures, and Properties of Metal-Organic Frameworks Based on Mixed Multi-N Donor and Carboxylate Ligands. J. Solid State Chem. 2020, 283, 121133. [Google Scholar] [CrossRef]
- Yu, R.Y.; Zhang, J.W.; Qu, P.; Liu, D.S.; Wang, R. Rational Design of a Rare Zn-MOF Material Based on Mixed Carboxylate-Azolate Ligands and Its Strong Blue Luminescence. Inorg. Nano-Met. Chem. 2021, 54, 30–34. [Google Scholar] [CrossRef]
- Fang, X.D.; Yao, J.; Fan, R.; Bai, X.F.; Liu, Y.E.; Hou, C.F.; Xu, Q.Q.; Zhu, A.X.; Huang, B. A Luminescent Zinc-Organic Framework as Bifunctional Chemosensors for Detection of Nitrobenzene and Fe3+. J. Solid State Chem. 2021, 294, 121854. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, P.; Dong, Y. New Luminescent Zn(II) Compound as a Highly Selective and Sensitive Luminescence Probe for the Detection of Fe3+ in Aqueous Solution. Inorg. Nano-Met. Chem. 2021, 51, 218–223. [Google Scholar] [CrossRef]
- Chen, S.S.; Han, S.S.; Ma, C.B.; Li, W.D.; Zhao, Y. A Series of Metal-Organic Frameworks: Syntheses, Structures and Luminescent Detection, Gas Adsorption, Magnetic Properties. Cryst. Growth Des. 2021, 21, 869–885. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, X.; Dang, J.; Yue, D.; Zhang, B.; Li, W.; Gahungu, G.; Wang, Z.; Zhang, J. A 2-Fold Interpenetrated Zinc-Organic Framework with Lewis Basic Triazole Sites: Luminescence Sensing of Fe3+ and Cr2O72−, and Warm White-Light Emission by Encapsulated Ln3+ Ions. CrystEngComm 2022, 24, 7058–7065. [Google Scholar] [CrossRef]
- Xian, G.; Wang, L.; Wan, X.; Yan, H.; Cheng, J.; Chen, Y.; Lu, J.; Li, Y.; Li, D.; Dou, J.; et al. Two Multiresponsive Luminescent Zn-MOFs for the Detection of Different Chemicals in Simulated Urine and Antibiotics/Cations/Anions in Aqueous Media. Inorg. Chem. 2022, 61, 7238–7250. [Google Scholar] [CrossRef]
- Yu, C.X.; Jiang, W.; Wang, K.Z.; Liang, A.P.; Song, J.G.; Zhou, Y.L.; Sun, X.Q.; Liu, L.L. Luminescent Two-Dimensional Metal-Organic Framework Nanosheets with Large π-Conjugated System: Design, Synthesis, and Detection of Anti-Inflammatory Drugs and Pesticides. Inorg. Chem. 2022, 61, 982–991. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Liu, Y.; Shi, P.; Xu, S.; Bin, Y. A Bifunctional Three-Dimensional Zn(II) Metal-Organic Framework with Strong Luminescence and Adsorption Cr(VI) Properties. ACS Omega 2024, 9, 18429–18437. [Google Scholar] [CrossRef]
- Cui, R.; Wan, Y.; Ji, G.; Liu, Z. A Highly Selective and Sensitive Fluorescent Sensor Based on Tb3+-Functionalized MOFs to Determine Arginine in Urine: A Potential Application for the Diagnosis of Cystinuria. Analyst 2019, 144, 5875–5881. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, W.; Wu, J.; Tang, G.; Zhang, C. Two Mixed-Ligand Cd(II)-Organic Frameworks with Unique Topologies: Selective Luminescence Sensing of TNP and Cu2+ Ions with Recyclable Performances. New J. Chem. 2019, 43, 16078–16088. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Lin, H.; Wen, Y.; Zhu, Q.L. Metal-Organic Frameworks Based on a Bent Triazole Dicarboxylic Acid: Magnetic Behaviors and Selective Luminescence Sensing Properties. Cryst. Growth Des. 2019, 19, 1057–1063. [Google Scholar] [CrossRef]
- Singh, M.; Senthilkumar, S.; Rajput, S.; Neogi, S. Pore-Functionalized and Hydrolytically Robust Cd(II)-Metal-Organic Framework for Highly Selective, Multicyclic CO2 Adsorption and Fast-Responsive Luminescent Monitoring of Fe(III) and Cr(VI) Ions with Notable Sensitivity and Reusability. Inorg. Chem. 2020, 59, 3012–3025. [Google Scholar] [CrossRef]
- Qin, B.W.; Zhang, X.Y.; Zhang, J.P. A Stable Multifunctional Cadmium-Organic Framework Based on 2D Stacked Layers: Effective Gas Adsorption, and Excellent Detection of Cr3+, CrO42−, and Cr2O72−. Dye. Pigment. 2020, 174, 108011. [Google Scholar] [CrossRef]
- Singh, M.; Palakkal, A.S.; Pillai, R.S.; Neogi, S. N-Functionality Actuated Improved CO2 adsorption and Turn-on Detection of Organo-Toxins with Guest-Induced Fluorescence Modulation in Isostructural Diamondoid MOFs. J. Mater. Chem. C 2021, 9, 7142–7153. [Google Scholar] [CrossRef]
- Hong, B.Q.; Qi, Y.J.; Lai, R.D.; Ge, R.; Zheng, S.T.; Li, X.X. Two Luminescent Metal-Organic Frameworks with Temperature-Dependent Emission. J. Solid State Chem. 2022, 309, 122967. [Google Scholar] [CrossRef]
- Yu, M.; Yao, X.; Wang, X.; Li, Y.; Li, G. White-Light-Emitting Decoding Sensing for Eight Frequently-Used Antibiotics Based on a Lanthanide Metal-Organic Framework. Polymers 2019, 11, 99. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.Y.; You, S.Y.; Liu, Y.W.; Cui, H.M.; Zhang, S.W. A Dual Luminescent Chemosensor Derived from a Europium(III) Metal-Organic Framework for Quantitative Detection of Phosphate Anions and Acetylacetone in Aqueous Solution. Dye. Pigment. 2020, 173, 108004. [Google Scholar] [CrossRef]
- Zhan, C.H.; Huang, D.P.; Wang, Y.; Mao, W.T.; Wang, X.J.; Jiang, Z.G.; Feng, Y.L. Four Anionic Ln-MOFs for Remarkable Separation of C2H2-CH4/CO2-CH4 and Highly Sensitive Sensing of Nitrobenzene. CrystEngComm 2021, 23, 2788–2792. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, G.; Zhang, H.; Wang, G.; Han, H. A Stable Terbium(III) Metal-Organic Framework as a Dual Luminescent Sensor for MnO4− Ions and Nitroaromatic Explosives. J. Solid State Chem. 2021, 295, 121924. [Google Scholar] [CrossRef]
- Luo, L.; Xie, Y.; Hou, S.L.; Ma, Y.; Zhao, B. Recyclable Luminescent Sensor for Detecting Creatinine Based on a Lanthanide-Organic Framework. Inorg. Chem. 2022, 61, 9990–9996. [Google Scholar] [CrossRef]
- Wang, H.; Liu, D.; Wei, M.; Qi, W.; Li, X.; Niu, Y. A Stable and Highly Luminescent 3D Eu(III)-Organic Framework for the Detection of Colchicine in Aqueous Environment. Environ. Res. 2022, 208, 112652. [Google Scholar] [CrossRef]
- Wang, X.; Gopalsamy, K.; Clavier, G.; Maurin, G.; Ding, B.; Tissot, A.; Serre, C. Lanthanide MOF-Based Luminescent Sensor Arrays for the Detection of Castration-Resistant Prostate Cancer Curing Drugs and Biomarkers. Chem. Sci. 2024, 15, 6488–6499. [Google Scholar] [CrossRef]
- Zhang, S.R.; Zhang, W.T.; Li, X.; Xu, G.J.; Xie, W.; Xu, Y.H.; Xu, N.; Su, Z.M. Multifunctional Lanthanide Metal-Organic Frameworks Act as Fluorescent Probes for the Detection of Cr2O72−, Fe3+, and TNP, White Light-Emitting Diodes, and Luminescence Thermometers. Inorg. Chem. 2025, 64, 2990–2999. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Tan, Z.; Liu, L.; Wang, Q.; Yang, T.; Zhou, X.; Niu, Z. A Luminescent Lanthanide MOF for the Selective and Ultra-High Sensitivity Detection of Caffeic Acid. Appl. Organomet. Chem. 2025, 39, e8002. [Google Scholar] [CrossRef]
- Keister, J.B. Organometallic Clusters. In Comprehensive Organometallic Chemistry III; Elsevier: Amsterdam, The Netherlands, 2007; pp. 755–780. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Feng, J.; Gong, L.; Humphrey, M.G.; Zhang, C. Decanuclear Cluster-Based Metal-Organic Framework with a (3,11)-Connected Topology and Highly Sensitive 2,4,6-Trinitrophenol Detection. Inorg. Chem. 2019, 58, 9749–9755. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.N.; Liang, Y.; Cheng, L.; Fang, Y. Chiral Fluorescent Metal-Organic Framework with a Pentanuclear Copper Cluster as an Efficient Luminescent Probe for Dy3+Ion and Cyano Compounds. Inorg. Chem. 2021, 60, 15085–15090. [Google Scholar] [CrossRef]
- An, J.D.; Wang, T.T.; Shi, Y.F.; Huo, J.Z.; Wu, X.X.; Liu, Y.Y.; Ding, B. Convenient Ultrasonic Preparation of a Water Stable Cluster-Based Cadmium(II) Coordination Material and Highly Sensitive Fluorescent Sensing for Biomarkers DPA and 5-HT. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119092. [Google Scholar] [CrossRef]
- Wang, J.R.; Fu, J.; Zhang, Y.J.; Liang, J.C.; Zhou, R.S.; Gong, S.M.; Song, J.F. A Butterfly Shaped Eu4(OH)2 Cluster-Based Luminescent Metal-Organic Framework with Lewis Basic Triazole Sites Demonstrating Turn off Sensing in the Presence of Organic Amines. Dalt. Trans. 2022, 52, 136–146. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.Y.; Wang, T.T.; Liu, Y.Y.; Zhang, L.X.; Huo, J.Z.; Ding, B. Solvo-Thermal Synthesis of a Unique Cluster-Based Nano-Porous Zinc(II) Luminescent Metal-Organic Framework for Highly Sensitive Detection of Anthrax Biomarker and Dichromate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 274, 121132. [Google Scholar] [CrossRef]
- Liu, H.F.; Tao, Y.; Wu, T.X.; Li, H.Y.; Zhang, X.Q.; Huang, F.P.; Bian, H.D. A {Zn5} Cluster-Based Metal–Organic Framework: Multifunctional Detection of Ag+, Cr2O72−, and 2,4,6-Trinitrophenol (TNP). Appl. Organomet. Chem. 2022, 36, e6456. [Google Scholar] [CrossRef]
- Sun, Y.; Dong, B.X.; Liu, W.L. An Adjustable Dual-Emission Fluorescent Metal-Organic Framework: Effective Detection of Multiple Metal Ions, Nitro-Based Molecules and DMA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117283. [Google Scholar] [CrossRef]
- Chand, S.; Verma, G.; Pal, A.; Pal, S.C.; Ma, S.; Das, M.C. Porous Anionic Co(II) Metal-Organic Framework, with a High Density of Amino Groups, as a Superior Luminescent Sensor for Turn-on Al(III) Detection. Chem. A Eur. J. 2021, 27, 11804–11810. [Google Scholar] [CrossRef]
- Qian, L.L.; Wang, Z.X.; Zhu, L.M.; Li, K.; Li, B.L.; Wu, B. Synthesis, Structure, Spectral Characteristic and Photocatalytic Degradation of Organic Dyes of a Copper Metal-Organic Framework Based on Tri(Triazole) and Pimelate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 372–377. [Google Scholar] [CrossRef]

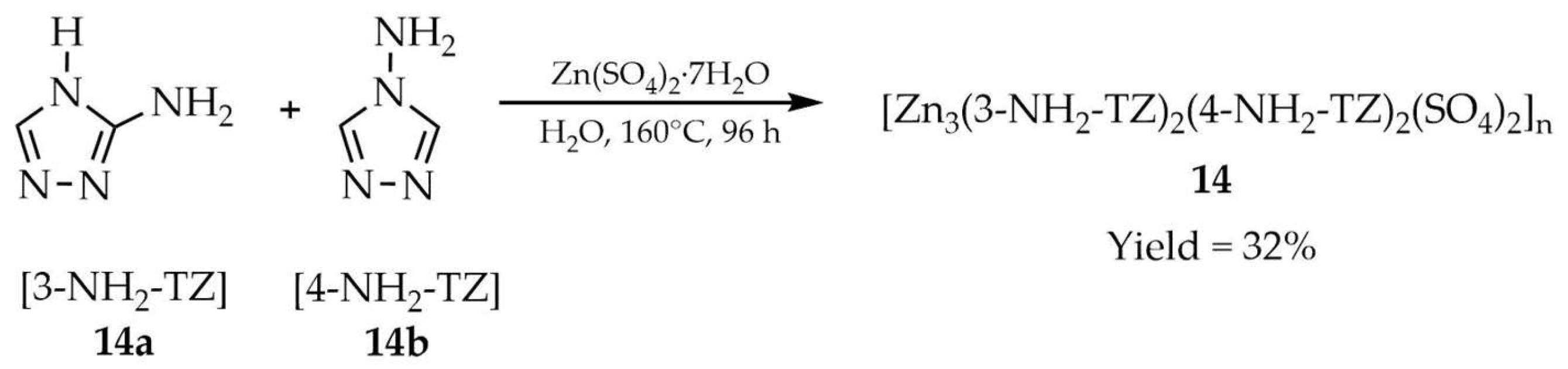

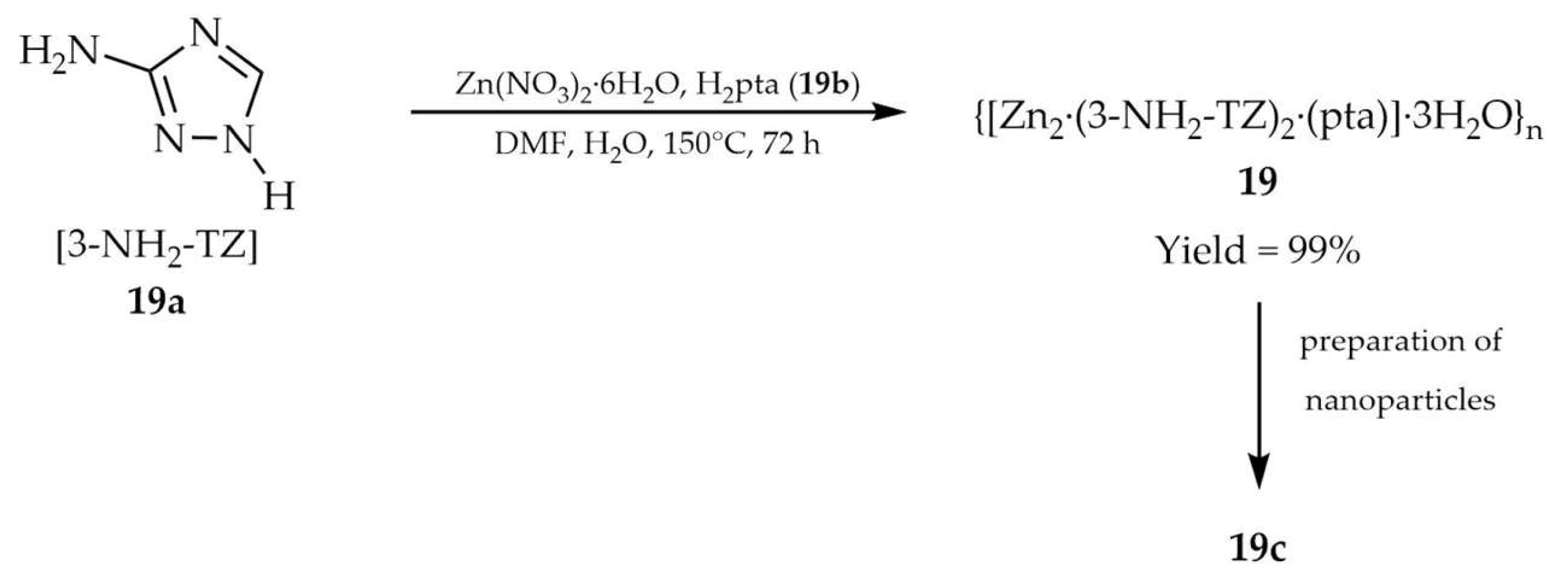




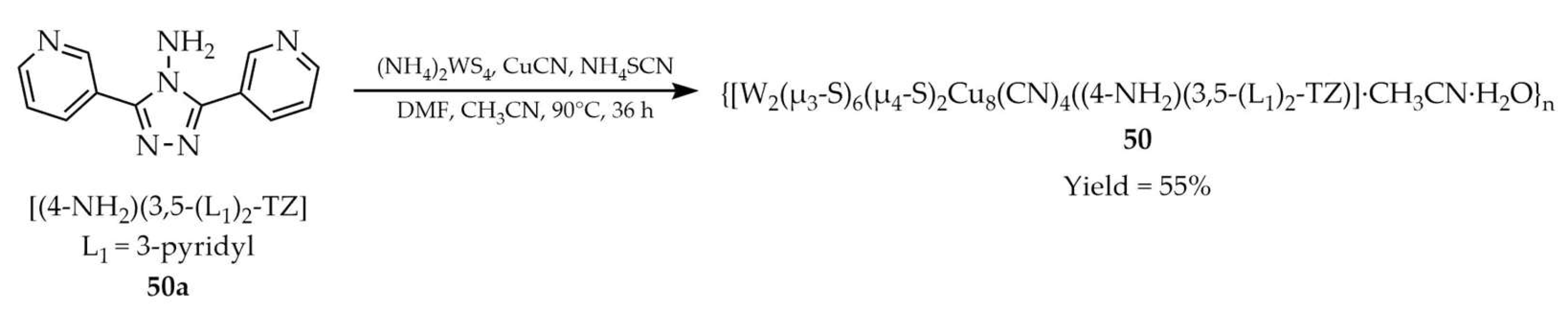

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubiesa, K.; Kudelko, A. Insight into Current Research on Luminescent Metal–Organic Frameworks (MOFs) Based on the 1,2,4-Triazole Scaffold. Appl. Sci. 2025, 15, 11943. https://doi.org/10.3390/app152211943
Kubiesa K, Kudelko A. Insight into Current Research on Luminescent Metal–Organic Frameworks (MOFs) Based on the 1,2,4-Triazole Scaffold. Applied Sciences. 2025; 15(22):11943. https://doi.org/10.3390/app152211943
Chicago/Turabian StyleKubiesa, Kornelia, and Agnieszka Kudelko. 2025. "Insight into Current Research on Luminescent Metal–Organic Frameworks (MOFs) Based on the 1,2,4-Triazole Scaffold" Applied Sciences 15, no. 22: 11943. https://doi.org/10.3390/app152211943
APA StyleKubiesa, K., & Kudelko, A. (2025). Insight into Current Research on Luminescent Metal–Organic Frameworks (MOFs) Based on the 1,2,4-Triazole Scaffold. Applied Sciences, 15(22), 11943. https://doi.org/10.3390/app152211943







