Revealing the Stabilization Mechanism of ZnO Polar Facets with 17O NMR Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Characterizations
2.3. Exposure to Closed Container
2.4. 17O Isotopic Labeling
2.5. Solid-State NMR Spectroscopy
2.6. DFT Calculations
3. Results and Discussion
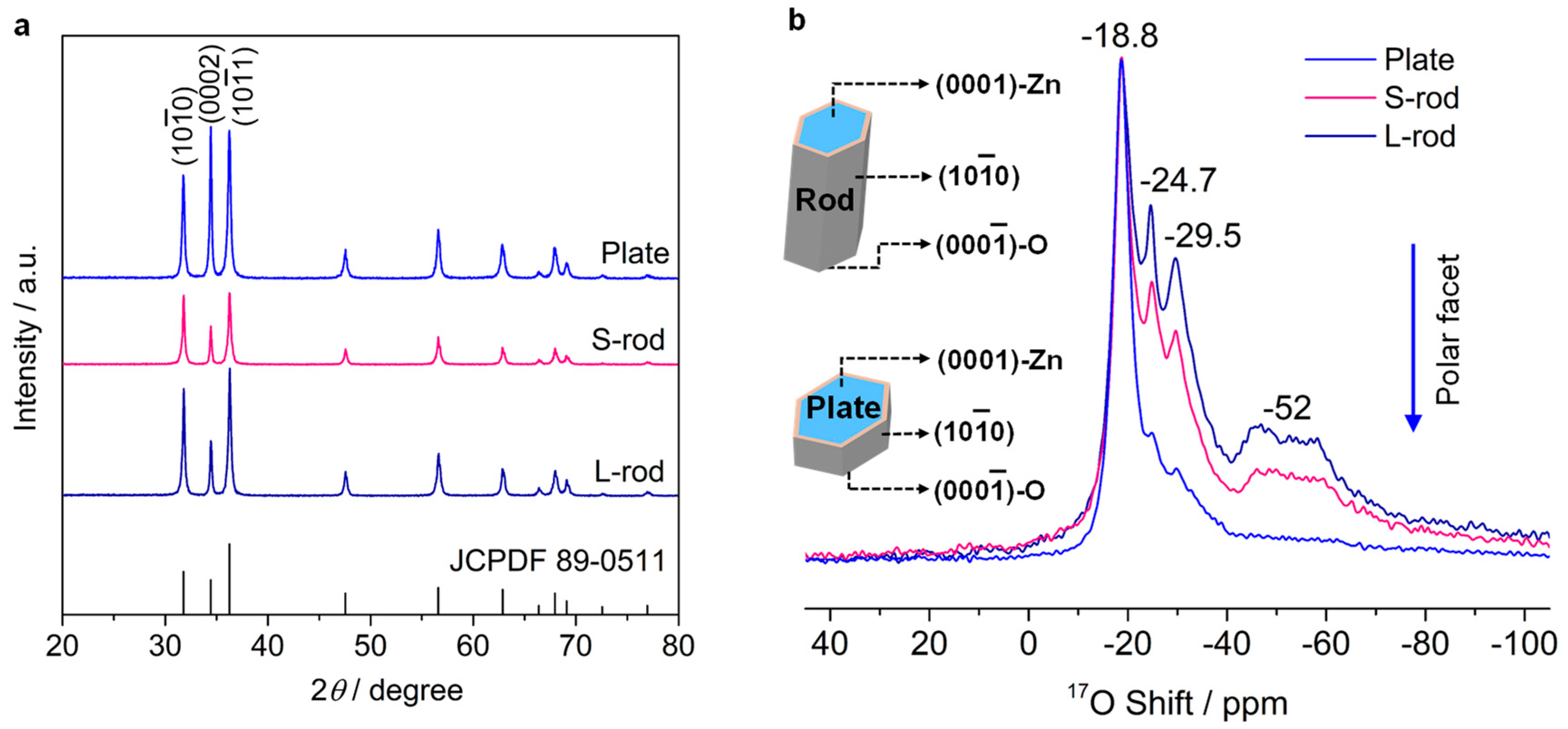
3.1. Morphology of the ZnO Sample
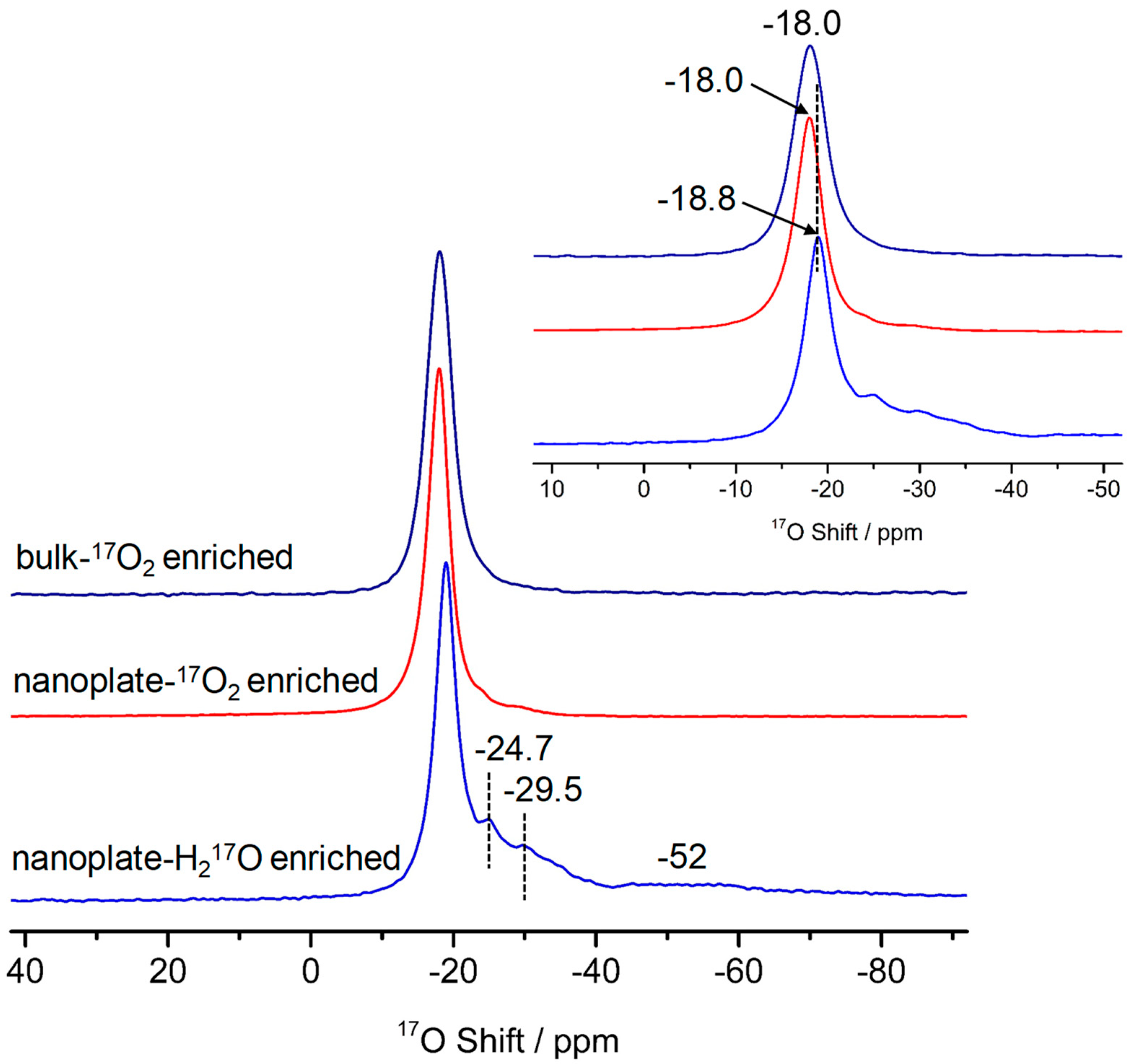
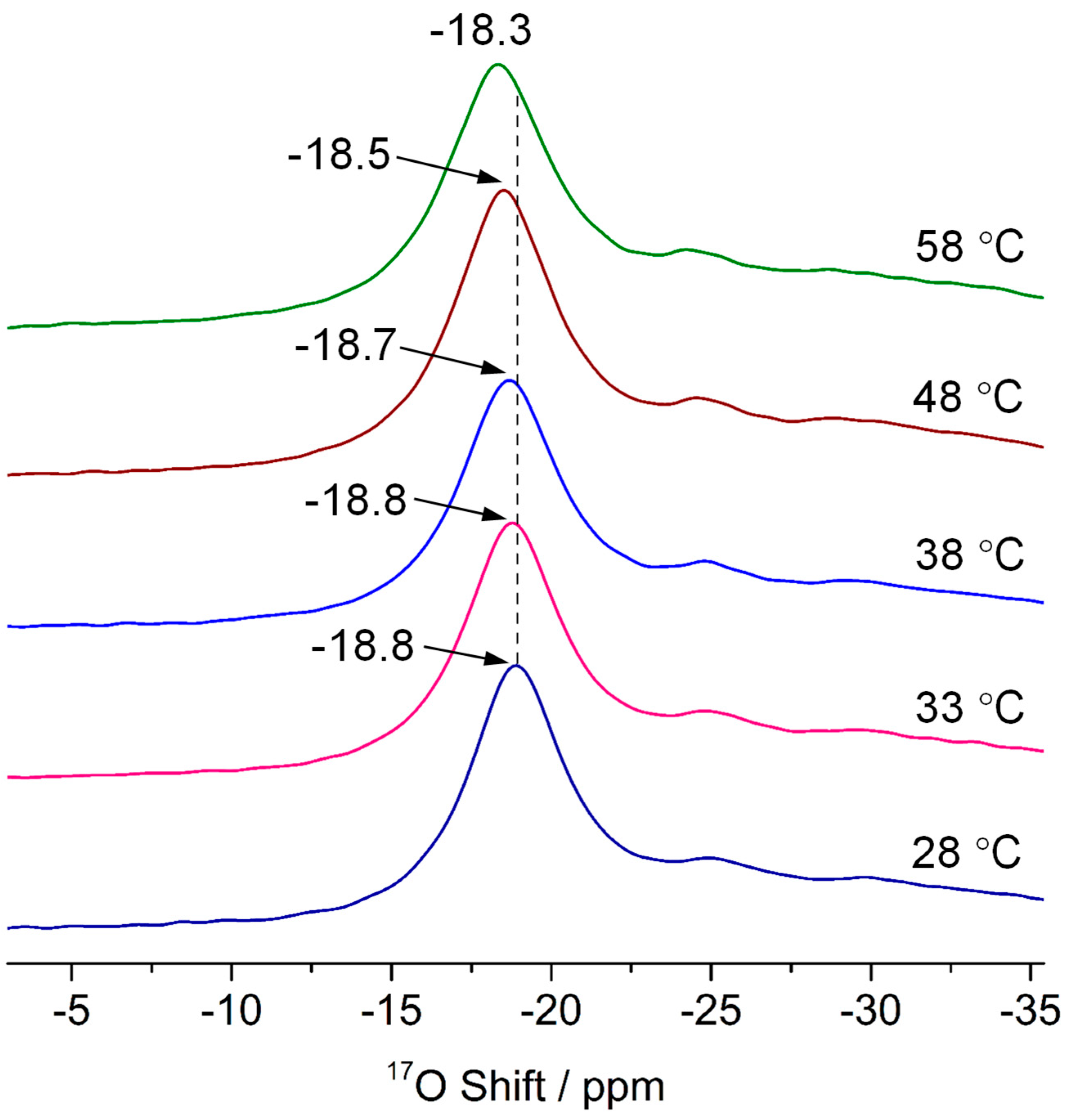
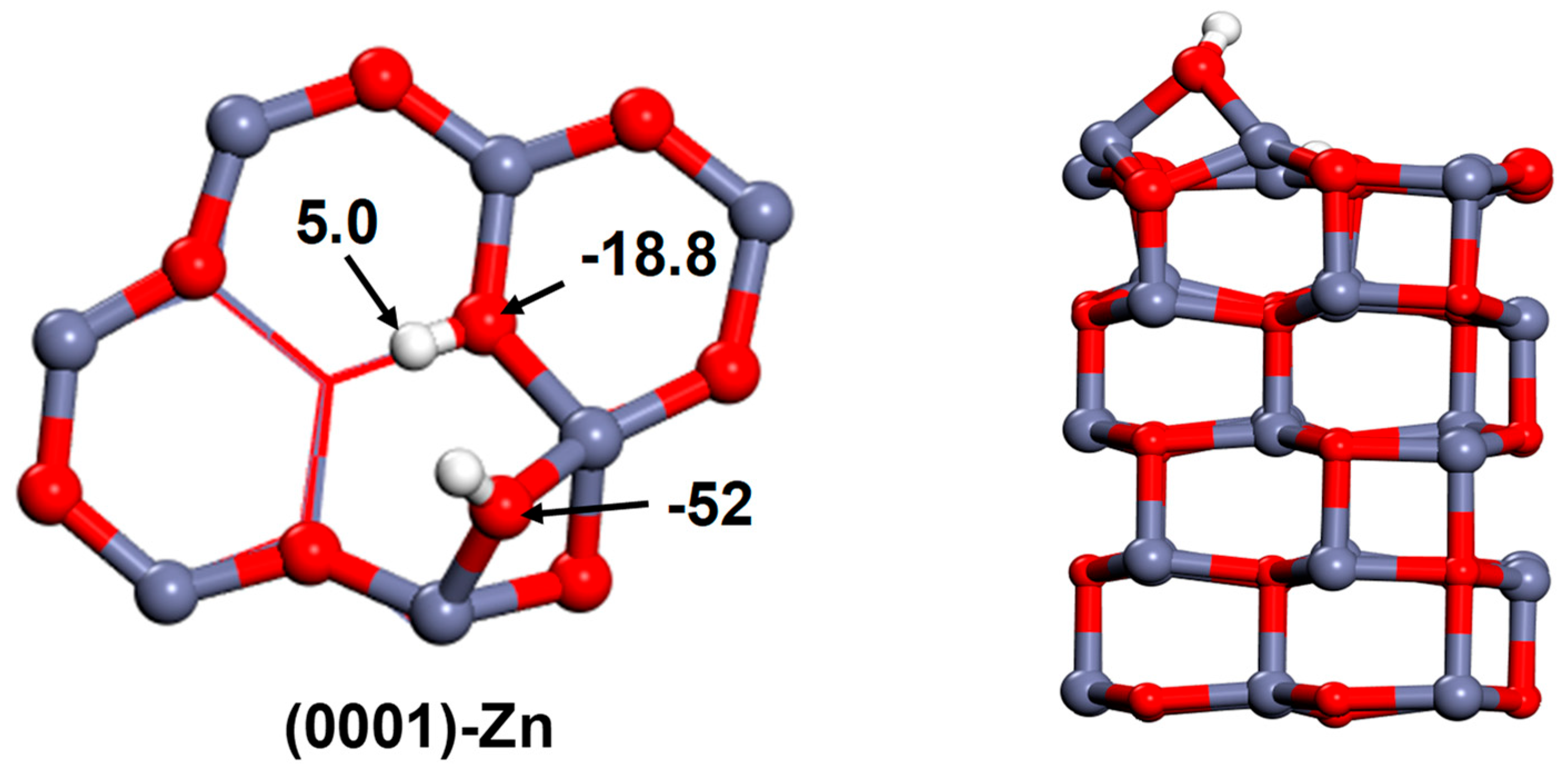
3.2. Oxygen Species on the Polar Surface of ZnO Nanoplates
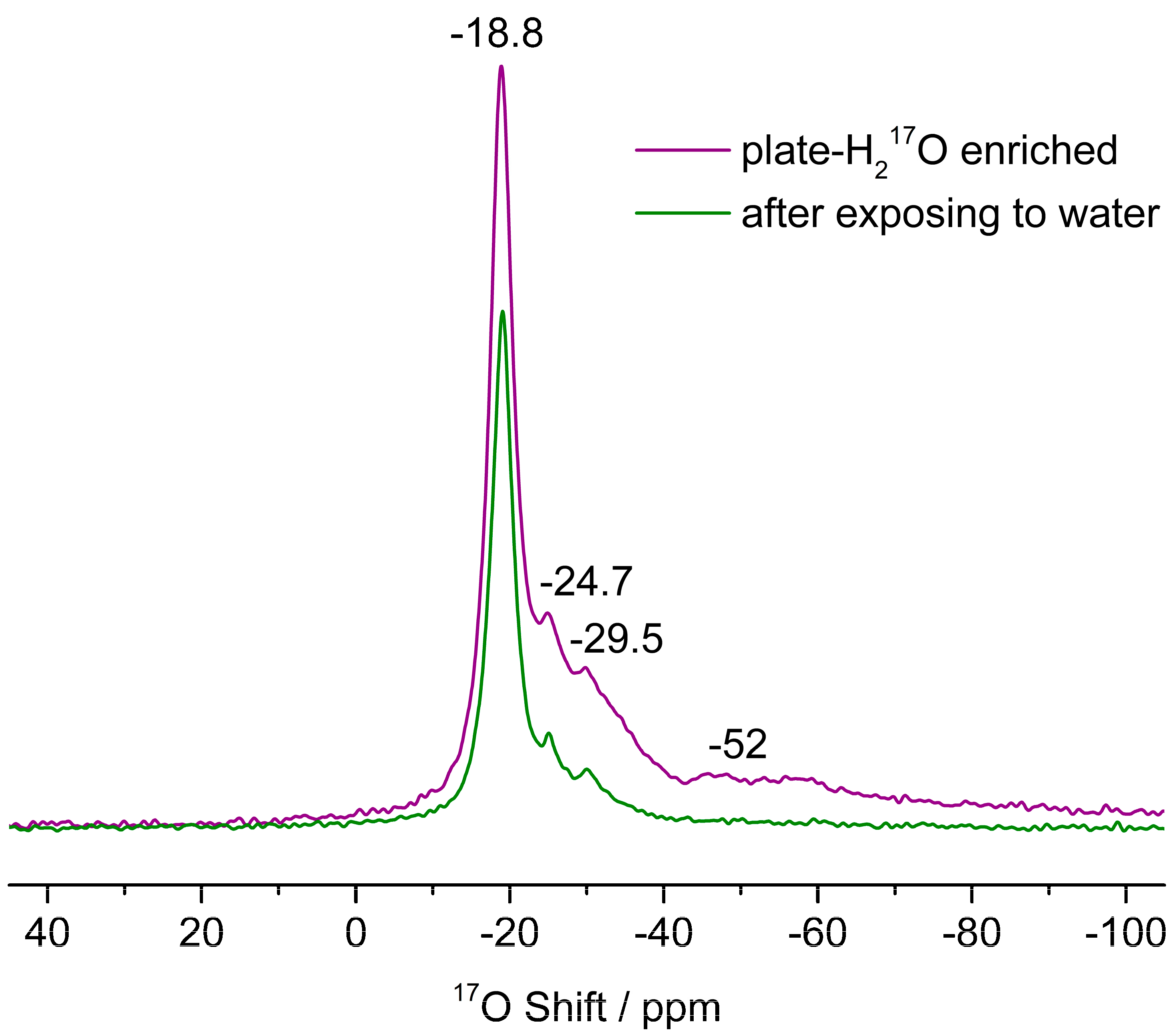
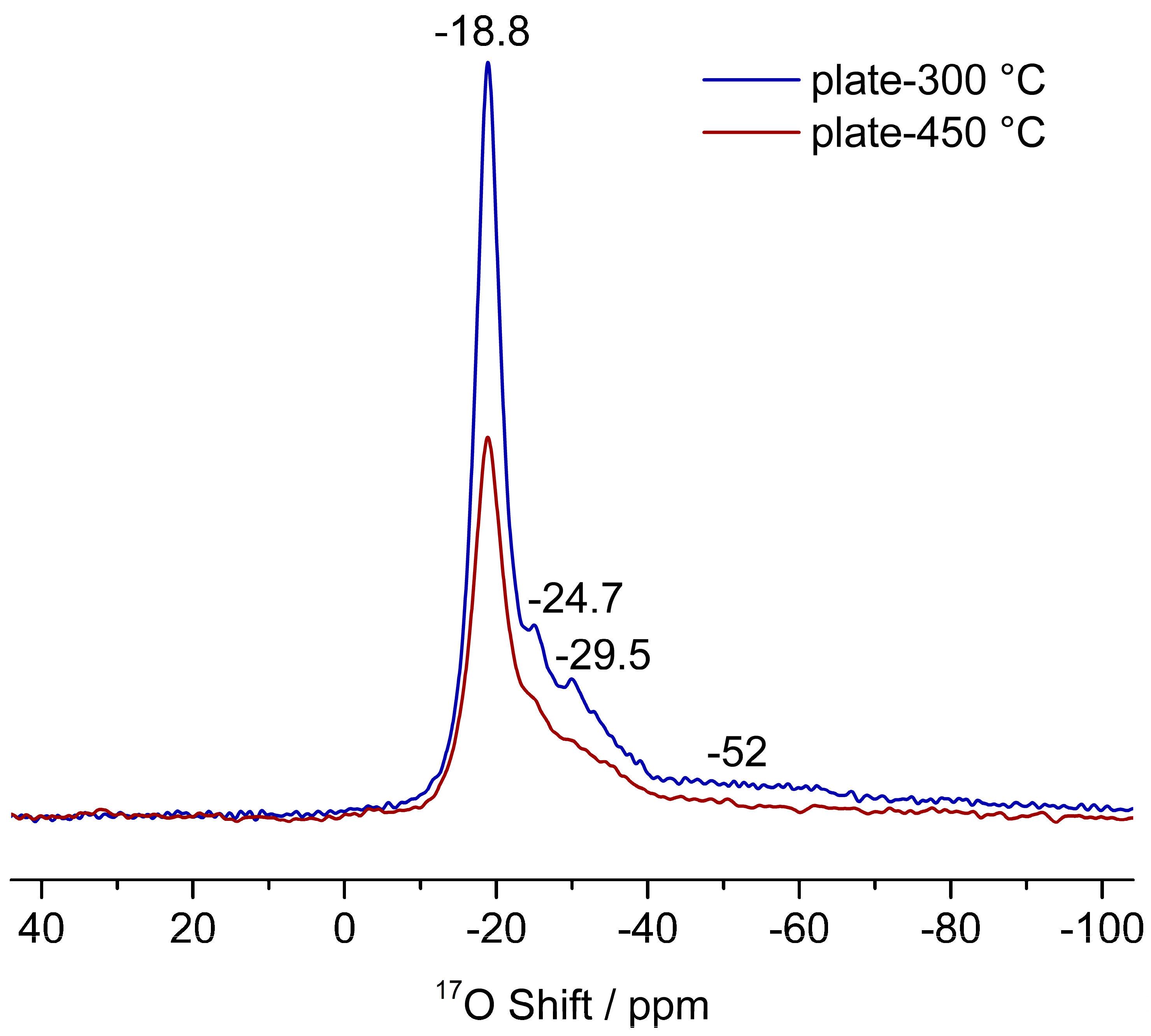
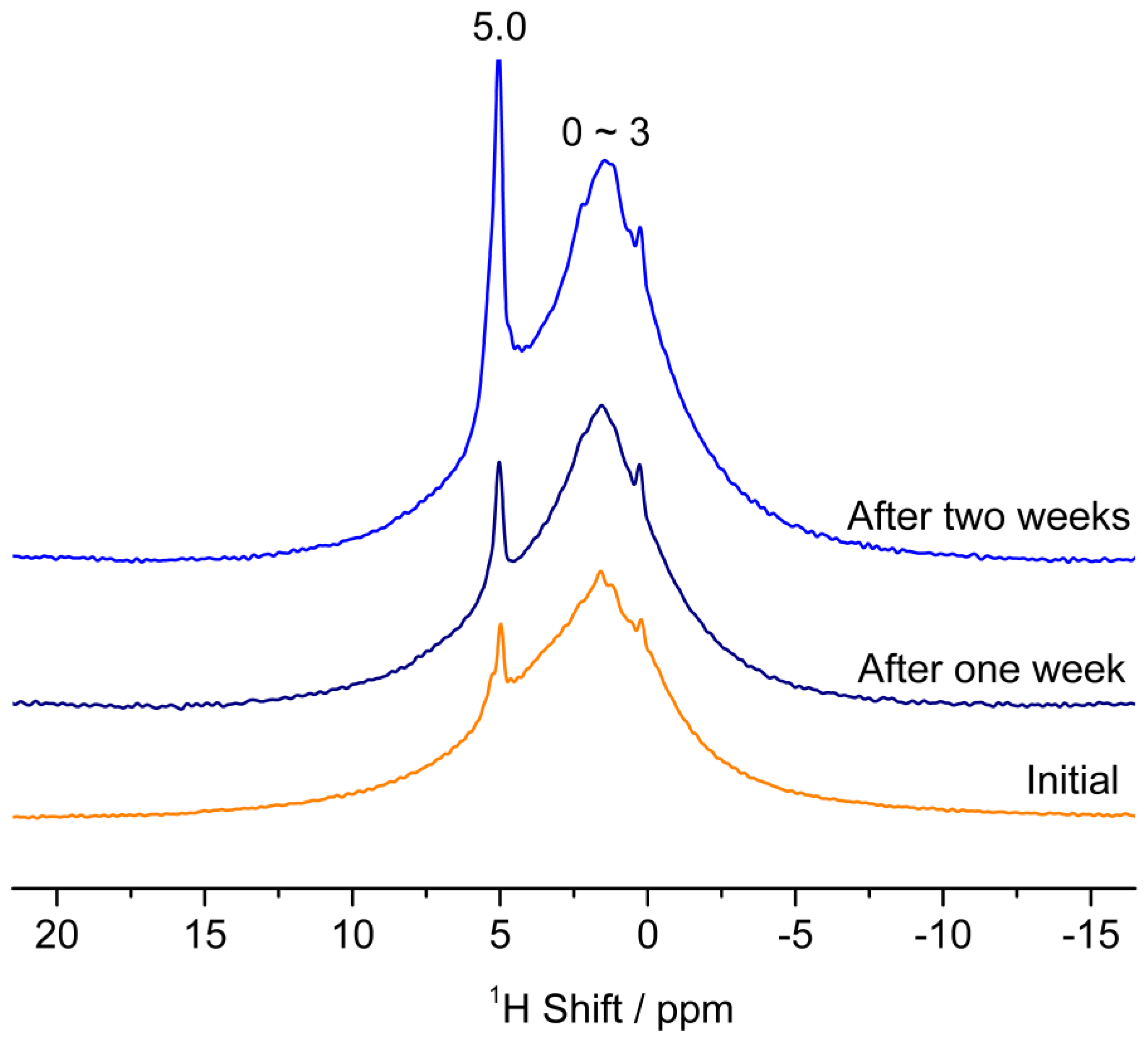
3.3. The Interactions of ZnO Polar Surfaces with Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.F.; Dandeneau, C.S.; Zhou, X.Y.; Cao, G.Z. ZnO nanostructures for dye-sensitized solar cells. Adv. Mater. 2009, 21, 4087–4108. [Google Scholar] [CrossRef]
- Wöll, C. The chemistry and physics of zinc oxide surfaces. Prog. Surf. Sci. 2007, 82, 55–120. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Bu, Y.B.; Weststrate, C.J.; Niemantsverdriet, J.W.; Fredriksson, H.O.A. Role of ZnO and CeOx in Cu-based model catalysts in activation of H2O and CO2 dynamics studied by in situ ultraviolet–visible and X-ray photoelectron spectroscopy. ACS Catal. 2016, 6, 7994–8003. [Google Scholar] [CrossRef]
- Behrens, M.; Studt, F.; Kasatkin, I.; Kühl, S.; Hävecker, M.; Abild-Pedersen, F.; Zander, S.; Girgsdies, F.; Kurr, P.; Kniep, B.-L.; et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 2012, 336, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Li, J.J.; Pan, X.L.; Xiao, J.P.; Li, H.B.; Ma, H.; Wei, M.M.; Pan, Y.; Zhou, Z.Y.; Li, M.R.; et al. Selective conversion of syngas to light olefins. Science 2016, 351, 1065–1068. [Google Scholar] [CrossRef]
- Daté, M.; Okumura, M.; Tsubota, S.; Haruta, M. Vital role of moisture in the catalytic activity of supported gold nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 2129–2132. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Marx, D.; Dulub, O.; Diebold, U.; Kunat, M.; Langenberg, D.; Wöll, C. Partial dissociation of water leads to stable superstructures on the surface of zinc oxide. Angew. Chem. Int. Ed. 2004, 43, 6641–6645. [Google Scholar] [CrossRef]
- Dulub, O.; Meyer, B.; Diebold, U. Observation of the dynamical change in a water monolayer adsorbed on a ZnO surface. Phys. Rev. Lett. 2005, 95, 136101. [Google Scholar] [CrossRef]
- Dulub, O.; Diebold, U.; Kresse, G. Novel stabilization mechanism on polar surfaces: ZnO(0001)-Zn. Phys. Rev. Lett. 2003, 90, 016102. [Google Scholar] [CrossRef]
- Önsten, A.; Stoltz, D.; Palmgren, P.; Yu, S.; Göthelid, M.; Karlsson, U.O. Water adsorption on ZnO(0001): Transition from triangular surface structures to a disordered hydroxyl terminated phase. J. Phys. Chem. C 2010, 114, 11157–11161. [Google Scholar] [CrossRef]
- Song, B.T.; Li, Y.H.; Wu, X.-P.; Wang, F.; Lin, M.; Sun, Y.H.; Jia, A.-P.; Ning, X.; Jin, L.; Ke, X.K.; et al. Unveiling the surface structure of ZnO nanorods and H2 activation mechanisms with 17O NMR spectroscopy. J. Am. Chem. Soc. 2022, 144, 23340–23351. [Google Scholar] [CrossRef]
- Liao, F.L.; Huang, Y.Q.; Ge, J.W.; Zheng, W.R.; Tedsree, K.; Collier, P.; Hong, X.L.; Tsang, S.C. Morphology-dependent interactions of ZnO with Cu nanoparticles at the materials’ interface in selective hydrogenation of CO2 to CH3OH. Angew. Chem. Int. Ed. 2011, 50, 2162–2165. [Google Scholar] [CrossRef]
- Song, B.T.; Li, Y.H.; Sun, Y.H.; Peng, L.M.; Xie, L.-H. 17O solid-state NMR study on exposed facets of ZnO nanorods with different aspect ratios. Phys. Chem. Chem. Phys. 2024, 26, 7890–7895. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Turner, G.L.; Chung, S.E.; Oldfield, E. Solid-state oxygen-17 nuclear magnetic resonance spectroscopic study of the group 11 oxides. J. Magn. Reson. 1985, 64, 316–324. [Google Scholar] [CrossRef]
- Song, B.T.; Zhu, Q.; Xie, L.-H. Investigation of the water-induced polar facet stabilization mechanism in ZnO nanoplates with 1H NMR spectroscopy. Phys. Chem. Chem. Phys. 2025, 27, 14054–14059. [Google Scholar] [CrossRef] [PubMed]
- Iachella, M.; Cure, J.; Rouhani, M.D.; Chabal, Y.; Rossi, C.; Estève, A. Water dissociation and further hydroxylation of perfect and defective polar ZnO model surfaces. J. Phys. Chem. C 2018, 122, 21861–21873. [Google Scholar] [CrossRef]
- Noei, H.; Qiu, H.S.; Wang, Y.M.; Löffler, E.; Wöll, C.; Muhler, M. The identification of hydroxyl groups on ZnO nanoparticles by infrared spectroscopy. Phys. Chem. Chem. Phys. 2008, 10, 7092–7097. [Google Scholar] [CrossRef] [PubMed]
- Mora-Fonz, D.; Lazauskas, T.; Farrow, M.R.; Catlow, C.R.A.; Woodley, S.M.; Sokol, A.A. Why are polar surfaces of ZnO stable? Chem. Mater. 2017, 29, 5306–5320. [Google Scholar] [CrossRef]
- Ye, H.G.; Chen, G.D.; Niu, H.B.; Zhu, Y.Z.; Shao, L.; Qiao, Z.J. Structures and mechanisms of water adsorption on ZnO(0001) and GaN(0001) surface. J. Phys. Chem. C 2013, 117, 15976–15983. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Zhang, D.D.; Yan, W.S.; Luo, W.Y.; Zhao, C.P.; Liu, D. Optically induced rapid wetting transition on Zn-polar and O-polar zinc oxide. Langmuir 2019, 35, 14791–14796. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, B.; Xie, L. Revealing the Stabilization Mechanism of ZnO Polar Facets with 17O NMR Spectroscopy. Appl. Sci. 2025, 15, 11938. https://doi.org/10.3390/app152211938
Song B, Xie L. Revealing the Stabilization Mechanism of ZnO Polar Facets with 17O NMR Spectroscopy. Applied Sciences. 2025; 15(22):11938. https://doi.org/10.3390/app152211938
Chicago/Turabian StyleSong, Benteng, and Linghai Xie. 2025. "Revealing the Stabilization Mechanism of ZnO Polar Facets with 17O NMR Spectroscopy" Applied Sciences 15, no. 22: 11938. https://doi.org/10.3390/app152211938
APA StyleSong, B., & Xie, L. (2025). Revealing the Stabilization Mechanism of ZnO Polar Facets with 17O NMR Spectroscopy. Applied Sciences, 15(22), 11938. https://doi.org/10.3390/app152211938





