A Novel Chitosan Hydrochloride–Biosurfactant–Grape Seed Oil Nanoemulsion to Control Dental Carie: Antimicrobial, Antibiofilm Activity and Irritation Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation, Characterization and Stability of Nanoemulsion
2.3. Determination of Minimum Inhibitory Concentration (MIC) and Fraction Inhibitory Concentration (FIC)
- Final FIC ≤ 0.5: Synergistic effect;
- 0.5 < FIC < 1.0: Additive effect;
- 1.0 ≤ FIC < 4.0: Indifferent effect;
- Final FIC ≥ 4.0: Antagonistic effect.
2.4. Quantification of Biofilm Adhesion
2.5. Quantification of Exopolysaccharides (EPS) Produced by Oral Streptococcus Species in the Presence of Sucrose
2.6. Measurement of Bacteria Cell Wall Hydrophobicity
2.7. Determination of Irritation Potential
2.8. Statistical Analyzes
3. Results
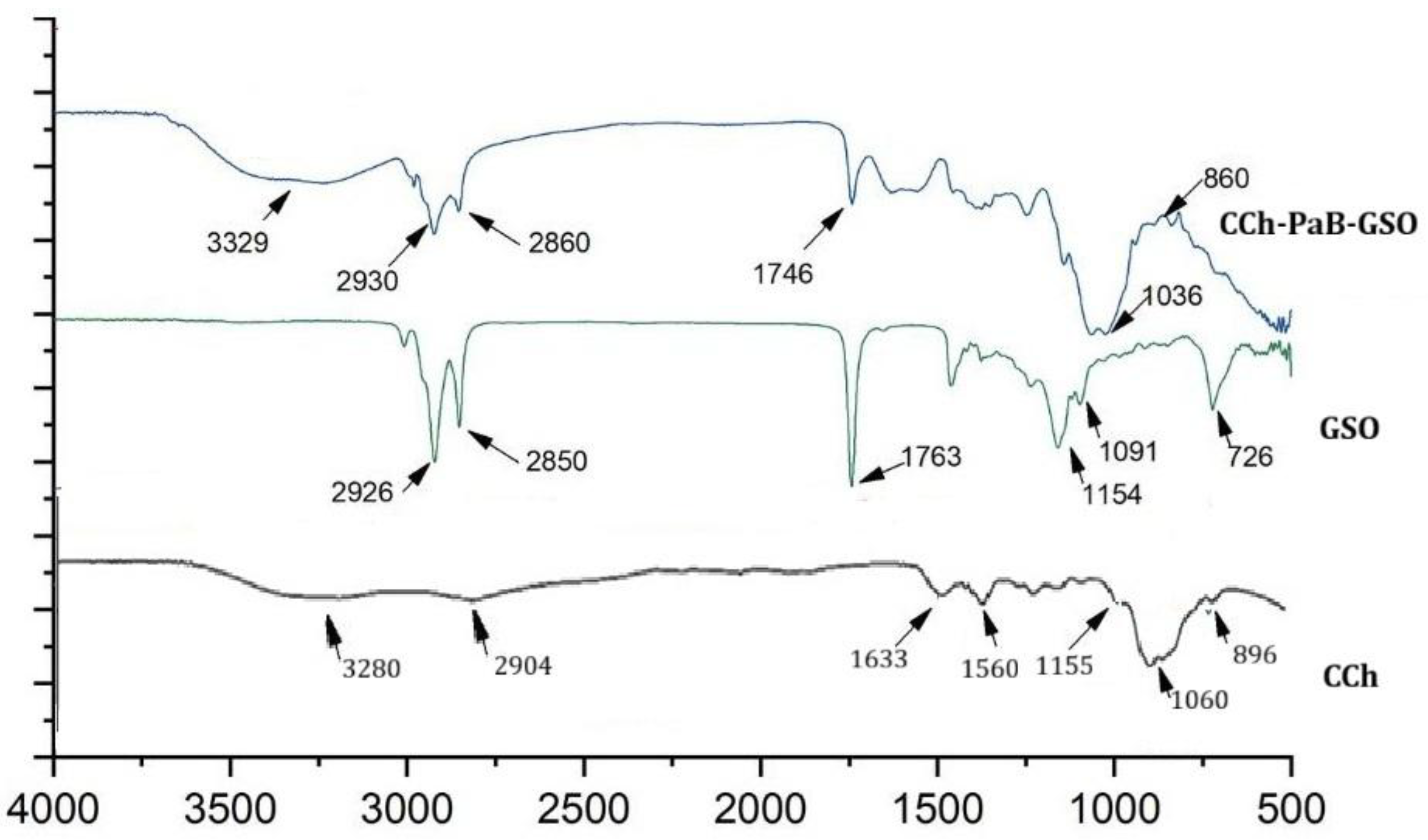
3.1. Characterization of CCh-PaB-GSO Nanoemulsion Using Fourier Transform Infrared Spectroscopy
3.2. Stability of Nanoemulsion
3.3. Determination of Minimum Inhibitory Concentration (MIC) and Fraction Inhibitory Concentration (FIC)
3.4. Quantification of Biofilm Adhesion, Exopolysaccharides (EPS) Production and Measurement of Bacteria Cell Wall Hydrophobicity
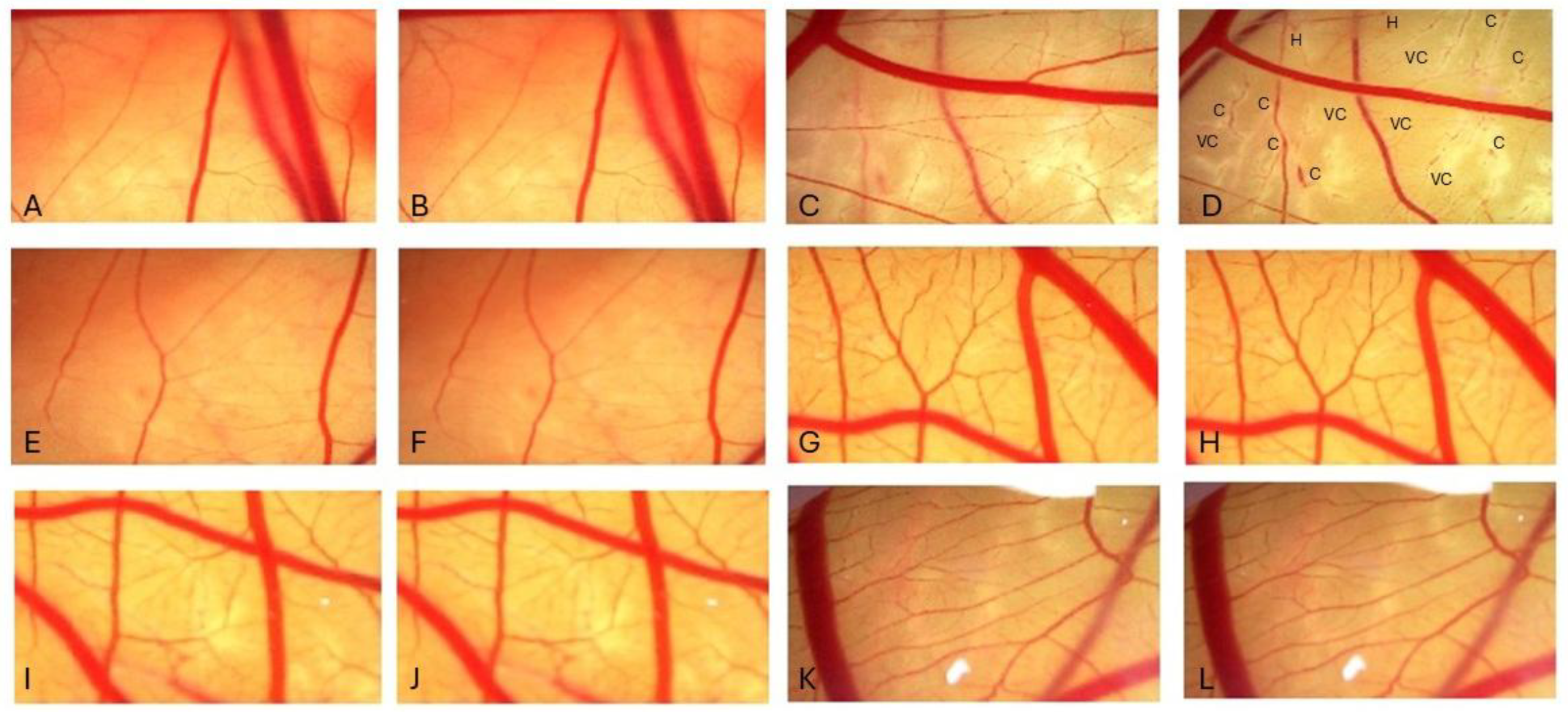
3.5. Determination of Irritation Potential: HET-CAM Assay
4. Discussion
- Inhibition of Initial Adhesion and EPS Synthesis: Chitosan Hydrochloride, a polycation, interferes with colonization by electrostatically neutralizing the anionic bacterial surface, preventing initial adhesion. Low molecular weight chitosan and their derivatives also interfere with extracellular glucan synthesis, analogous to the transcriptional repression of gtf and gbp genes observed with other natural compounds [5,28,42].
- Extracellular Matrix Disruption and Permeabilization: The P. aeruginosa biosurfactant reduces surface tension and intercalates into the extracellular matrix, disrupting the hydrophobic and ionic bonds of the EPS network. This action enhances the permeability of the biofilm and facilitates the diffusion of antimicrobial agents. PaB further modulates microbial communication, potentially repressing polymer synthesis genes [9,47].
- Membrane Lysis and Bioavailability Enhancement: Grape Seed Oil facilitates integration with the bacterial cell membranes due to its lipophilic nature, intensifying cell lysis and enhancing the overall antimicrobial effect of the nanoemulsion [18].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCh | Chitosan hydrochloride |
| PaB | Pseudomonas aeroginosa biosurfactant |
| GSO | Grape seed oil |
References
- Al-Marzooq, F.I.; Christidis, N. The interconnection of oral and systemic health. Sci. Rep. 2025, 15, 14931. [Google Scholar] [CrossRef]
- Murray, P.E.; Coffman, J.A.; Garcia-Godoy, F. Oral Pathogens Substantial Burden on cancer, Cardiovascular Diseases, Alzheimer’s, Diabetes, and Other Systemic Diseases: A Public Health Crisis—A Comprehensive Review. Pathogens 2024, 13, 1084. [Google Scholar] [CrossRef]
- Shen, Z.; Kuang, S.; Zhang, Y.; Chen, J.; Wang, S.; Xu, C.; Huang, Y.; Zhang, M.; Huang, S.; Wang, J.; et al. Restoring periodontal tissue homoeostasis prevents cognitive decline by reducing the number of Serpina3nhigh astrocytes in the hippocampus. Innovation 2024, 5, 100547. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, Y.; Ahmed, Z.; Qin, H.; Bhatti, I.A.; Cao, H. Calcium-dependent antimicrobials: Nature-inspired materials and designs. Exploration 2024, 4, 20230099. [Google Scholar] [CrossRef]
- Carvalho, M.M.S.G.; Stamford, T.C.M.; Santos, E.P.; Tenorio, P.; Sampaio, F. Chitosan as oral antimicrobial agent. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Mendez, A., Ed.; Formatex: Badajoz, Spain, 2011; pp. 542–550. [Google Scholar]
- Souza, I.R.; Bezerra, K.G.O.; Oliveira, C.L.; Meira, H.M.; Stamford, T.C.M.; Converti, A.; Sarubbo, L.A.; Rufino, R.D. Mouthwash Containing Plant-Derived Biosurfactant and Chitosan Hydrochloride: Assessment of Antimicrobial Activity, Antibiofilm Activity, and Genotoxicity. Appl. Sci. 2024, 14, 6711. [Google Scholar] [CrossRef]
- Wassel, M.O.; Khattab, M.A. Antibacterial activity against Streptococcus mutans and inhibition of bacterial induced enamel demineralization of propolis, miswak, and chitosan nanoparticles based dental varnishes. J. Adv. Res. 2017, 8, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chung, Y.C. Antibacterial effect of water-soluble chitosan on representative dental pathogens Streptococcus mutans and Lactobacilli brevis. J. Appl. Oral. Sci. 2012, 20, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Datta, M.; Chattopadhyay, I. Applications of microbial biosurfactants in human health and environmental sustainability: A narrative review. Discov. Med. 2024, 1, 160. [Google Scholar] [CrossRef]
- Resende, A.H.M.; Farias, J.M.; Silva, D.D.B.; Rufino, R.D.; Luna, J.M.; Stamford, T.C.M.; Sarubbo, L.A. Application of biosurfactants and chitosan in toothpaste formulation. Colloids Surf. B Biointerfaces. 2019, 181, 77–84. [Google Scholar] [CrossRef]
- Maj, M.; Tylkowski, B.; Konopka, P.; Woźniak-Budych, M.; Staszak, K.; Staszak, M.; Kaźmierski, Ł.; Bajek, A.; Jastrzab, R. Advancing oral health: Harnessing the potential of chitosan and polyphenols in innovative mouthwash formulation. Biomed. Pharmacother 2024, 175, 116654. [Google Scholar] [CrossRef]
- Yadav, A.A.; Mujawar, S.S.; Shinde, N.A.; Phalake, S.S.; Khot, V.M.; Kashte, S.B. A multifunctional tragacanth gum-chitosan hydrogel loaded with manganese ferrite nanoparticles for dental caries prevention and remineralization. Int. J. Biol. Macromol. 2025, 322, 146886, ISSN 0141-8130. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Wu, Z.Z.; Zhang, B.; Pan, Y.; Meng, R.; Chen, H.Q. Fabrication of chitosan hydrochloride and carboxymethyl starch complex nanogels as potential delivery vehicles for curcumin. Food Chem. 2019, 293, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Li, W.; Zhou, Y.; Guo, R.; Pei, X.; Xie, J. Chitosan and its functional derivatives for nutraceutical delivery: Focus on quaternized, hydrochloride, and carboxymethyl forms. Trends Food Sci. Technol. 2025, 164, 105237. [Google Scholar] [CrossRef]
- Joujou, F.M.; Darra, N.E.; Rajha, H.N.; Sokhn, E.S.; Alwan, N. Evaluation of synergistic/antagonistic antibacterial activities of fatty oils from apricot, date, grape, and black seeds. Sci. Rep. 2024, 14, 6532. [Google Scholar] [CrossRef] [PubMed]
- Delimont, N.M.; Carlson, B.N. Prevention of dental caries by grape seed extract supplementation: A systematic review. Nutr. Health. 2020, 26, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Xie, Q.; Bedran-Russo, A.K.; Pan, S.; Ling, J.; Wu, C.D. The preventive effect of grape seed extract on artificial enamel caries progression in a microbial biofilm-induced caries model. J. Dent. 2014, 42, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Khah, M.D.; Ghanbarzadeh, B.; Nezhad, L.R.; Ostadrahimi, A. Effects of virgin olive oil and grape seed oil on physicochemical and antimicrobial properties of pectin-gelatin blend emulsified films. Int. J. Biol. Macromol. 2021, 171, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Farias, J.M.; Stamford, C.M.; Resende, A.H.M.; Aguiar, J.S.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Mouthwash containing biosurfactant and chitosan: An eco-sustainable option for controlling cariogenic microorganisms. Int. J. Biol. Macromol. 2019, 129, 853–860. [Google Scholar] [CrossRef]
- Silva, E.J.; Correa, P.F.; Almeida, D.G.; Luna, J.M.; Rufino, R.D.; Sarubbo, L.A. Recovery of contaminated marine environments by biosurfactant-enhanced bioremediation. Colloids Surf. B Biointerfaces 2018, 172, 127–135. [Google Scholar] [CrossRef]
- Berger, L.R.R.; Stamford, T.C.M.; De Oliveira, K.A.R.; De Miranda, A.P.P.; Lima, M.A.B.; Pintado, M.M.E.; Câmara, M.P.S.; Franco, L.O.; Magnani, M.; Souza, E.L. Chitosan produced from Mucorales fungi using agroindustrial by-products and its efficacy to inhibit Colletotrichum species. Int. J. Biol. Macromol. 2018, 102, 635–641. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N.A.A.Q. Mycelia of Mucor rouxii as source of chitin and chitosan. Food Chem. 1997, 60, 605–610. [Google Scholar] [CrossRef]
- Araujo, A.S.; de Lima, G.S.; Nunes, I.S.; de Oliveira Farias de Aguiar, J.C.R.; Navarro, D.M.A.F.; Melo, N.F.C.B.; Magalhes, N.S.S.; Franca, R.; Carvalho, R.S.F.; Stamford, T.C.M. Chitosan hydrochloride-gum Arabic-passion fruit seed oil nanoparticle edible coating to control fungal infection and maintain quality parameters of strawberries. Food Control 2024, 161, 110360. [Google Scholar] [CrossRef]
- Tsai, M.L.; Chen, R.H.; Bai, S.W.; Chen, W.Y. The storage stability of chitosan/tripolyphosphate nanoparticles in a phosphate buffer. Carbohydr. Polym. 2011, 84, 756–761. [Google Scholar] [CrossRef]
- Driessche, F.V.; Rigole, P.; Brackman, G.; Coenye, T. Optimization of resazurin-based viability staining for quantification of microbial biofilms. J. Microbiol. Methods 2014, 98, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Doern, C.D. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar] [CrossRef]
- Huston, A.L.; Methe, B.; Deming, J.W. Purification, characterization and sequencing of an extracellular cold-active aminopeptidase produced by marine psychrophile Colwellia psychrerythraea strain 34H. Appl. Environ. Microbiol. 2004, 70, 3321–3328. [Google Scholar] [CrossRef]
- AlKanderi, S.; AlFreeh, M.; Bhardwaj, R.G.; Karched, M. Sugar Substitute Stevia Inhibits Biofilm Formation, Exopolysaccharide Production, and Downregulates the Expression of Streptococcal Genes Involved in Exopolysaccharide Synthesis. Dent. J. 2023, 11, 267. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method of determination of sugars and related substances. Anal. Chem. 1956, 18, 350–356. [Google Scholar] [CrossRef]
- Sano, H.; Shibasaki, R.; Matsukubo, T.; Takaesu, Y. Effect of molecular mass ans degree of deacetylation of chitosan on adsortption of Streptococcus sobrinus 6715 to saliva treated hydroxyapatite. Bull. Tokyo Dent. Coll. 2002, 48, 75–82. [Google Scholar] [CrossRef]
- Luepke, N.P. Hen’s egg chorioallantoic membrane test for irritation potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef]
- Steiling, W.; Bracher, M.; Coutellemont, P.; Silva, O. The HET-CAM, a useful in vitro assay for assessing the eye irritation properties of cosmetic formulations and ingredients. Toxicol. Vitr. 1999, 13, 375–384. [Google Scholar] [CrossRef]
- Freire, P.L.; Stamford, T.C.M.; Albuquerque, A.J.; Sampaio, F.; Cavalcante, H.M.M.; Macedo, R.O.; Galembeck, A.; Flores, M.A.P.; Rosenblat, A. Action of silver nanoparticles toward biological systems: Cytotoxicity evaluation using hen’s egg test and inhibition of Streptococcus mutans biofilm formation. Int. J. Antimicrob. Agents. 2015, 45, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Wackerly, D.; Mendenhall, W.; Scheaffer, R. Mathematical Statistics with Applications, 5th ed.; Duxbury Press: Boston, MA, USA, 1995; Volume 1, 798p. [Google Scholar]
- Gechev, B.; Zsivanovits, G.; Iliev, A.; Marudova, M. Chitosan/grapeseed oil multicomponent edible films-design and properties. J. Phys. Conf. Ser. 2023, 2436, 012029. [Google Scholar] [CrossRef]
- Ge, J.; Yue, P.; Chi, J.; Liang, J.; Gao, X. Formation and stability of anthocyanins-loaded nanocomplexes prepared with chitosan hydrochloride and carboxymethyl chitosan. Food Hydrocoll. 2018, 74, 23–31. [Google Scholar] [CrossRef]
- Vladimír, M.; Matwijczuk, A.P.; Niemczynowicz, A.; Kycia, R.A.; Karcz, D.; Gładyszewska, B.; Ślusarczyk, L.; Burg, P. Chemometric approach to characterization of the selected grape seed oils based on their fatty acids composition and FTIR spectroscopy. Sci. Rep. 2021, 11, 19256. [Google Scholar] [CrossRef]
- Mutlu, N. Effects of grape seed oil nanoemulsion on physicochemical and antibacterial properties of gelatin-sodium alginate film blends. Int. J. Biol. Macromol. 2023, 237, 124207. [Google Scholar] [CrossRef] [PubMed]
- Burr, S.J.; Williams, P.A.; Ratcliffe, I. Synthesis of cationic alkylated chitosans and an investigation of their rheological properties and interaction with anionic surfactant. Carbohydr. Polym. 2018, 201, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Chiappisi, L.; Gradzielski, M. Co-assembly in chitosan–surfactant mixtures: Thermodynamics, structures, interfacial properties and applications. Adv. Colloid. Int. Sci. 2015, 220, 92–107. [Google Scholar] [CrossRef]
- Senra, T.D.A.; Campana-Filho, S.P.; Desbrières, J. Surfactant-polysaccharide complexes based on quaternized chitosan. Characterization and application to emulsion stability. Eur. Polym. J. 2018, 104, 128–135. [Google Scholar] [CrossRef]
- Stamford, T.C.M.; Stamford-Arnaud, T.M.; Cavalcante, H.M.M.; Macedo, R.O.; Campos-Takaki, G.M. Microbiological Chitosan: Potential Application as Anticariogenic Agent. In Practical Applications in Biomedical Engineering; InTech: London, UK, 2013. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Madureira, A.R.; Cardelle-Cobas, A.; Tavaria, F.K.; Pintado, M.M. A comprehensive study into the impact of a chitosan mouthwash upon oral microorganism’s biofilm formation in vitro. Carbohydr. Polym. 2014, 101, 1081–1086. [Google Scholar] [CrossRef]
- Ahmed, S.A.A.; Mahsoub, F.; El Gamal, S.A.; Khamis, T.; Faroh, K.Y.; Abdelwarith, A.A.; Younis, E.M.; Saad, M.F.; Ali, H.S.; Davies, S.J.; et al. Chitosan-grape seed oil nanoemulsion enriched diet promotes performance, antioxidant-immune metrics and modifies immune- gene action and morphological architecture in Nile tilapia against Aeromonas veronii. Aquac. Rep. 2025, 41, 102697. [Google Scholar] [CrossRef]
- Govindarajan, D.K.; Mohanarangam, M.; Kadirvelu, L.; Sivaramalingam, S.S.; Jothivel, D.; Ravichandran, A.; Periasamy, S.; Kandaswamy, K. Biofilms and oral health: Nanotechnology for biofilm control. Discov. Nano 2025, 20, 114. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Jing, M.; Gong, T.; Yan, J.; Wang, X.; Xu, M.; Zhou, X.; Zeng, J.; Li, Y. Regulatory mechanisms of exopolysaccharide synthesis and biofilm formation in Streptococcus mutans. J. Oral. Microbiol. 2023, 15, 2225257. [Google Scholar] [CrossRef] [PubMed]
- Sultan, F.; Maji, D.; Phatake, R.S.; Kumar, K. Pharmaceutical applications of microbial biosurfactants. Int. J. Pharm. 2025, 681, 125887. [Google Scholar] [CrossRef]
- Vargas, A.; Zeisser-Labouèbe, M.; Lange, N.; Gurny, R.; Delie, F. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv. Drug Deliv. Rev. 2007, 59, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.L.; Heng, P.W.S.; Liew, C.V. Chick chorioallantoic membrane as an in situ biological membrane for pharmaceutical formulation development: A review. Drug Dev. Ind. Pharm. 2008, 34, 1168–1177. [Google Scholar] [CrossRef]
- Bagley, D.M.; Waters, D.; Kong, B.M. Development of a 10-day chorioallantoic membrane vascular assay as an alternative to the Draize rabbit eye irritation test. Food Chem. Toxicol. 1994, 32, 1155–1160. [Google Scholar] [CrossRef]

| Parameters | Time (Days) | |||
|---|---|---|---|---|
| 0 | 7 | 15 | 30 | |
| Avereage size (nm) | 169.5 ± 3.25 | 186.4 ± 1.7 | 188 ± 1.97 | 203.4 ± 0.99 |
| PDI | 0.241 | 0.264 | 0.268 | 0.271 |
| Zeta potential (mV) | +20.25 ± 2.42 | +25.4 ± 2.05 | +28.71 ± 1.85 | +31.94 ± 0.8 |
| pH | 7.27 ± 0.8 | 7.3 ± 0.15 | 7.34 ± 0.2 | 7.33 ± 0.18 |
| Microorganism | Test Substances Alone and in Combination | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCh | PaB | GSO | CCh + PaB | CCh + GSO | PaB + GSO | CCh-PaB-GSO | ||||||
| CCh | PaB | CCh | GSO | PaB | GSO | CCh | PaB | GSO | ||||
| Streptococcus mutans | 3.0 | 0.15 | 4.8 | 2.0 | 0.1 | 2.0 | 3.2 | 0.1 | 3.2 | 2.0 | 0.1 | 3.2 |
| Streptococcus sanguis | 3.0 | 0.15 | 4.8 | 2.0 | 0.1 | 2.0 | 3.2 | 0.1 | 3.2 | 2.0 | 0.1 | 3.2 |
| Streptococcus salivarius | 3.0 | 0.15 | 4.8 | 2.0 | 0.1 | 2.0 | 3.2 | 0.1 | 3.2 | 2.0 | 0.1 | 3.2 |
| Streptococcus mitis | 3.0 | 0.15 | 4.8 | 2.0 | 0.1 | 2.0 | 3.2 | 0.1 | 3.2 | 2.0 | 0.1 | 3.2 |
| CCh-PaB-GSO Nanoemulsion (mg/mL) | S. mutans | S. sanguis | S. salivarius | S. mitis | ||||
|---|---|---|---|---|---|---|---|---|
| 0% Sucrose | 5% Sucrose | 0% Sucrose | 5% Sucrose | 0% Sucrose | 5% Sucrose | 0% Sucrose | 5% Sucrose | |
| 0 | 100 (±0.00) | 100 (±0.00) | 100 (±0.00) | 100 (±0.00) | 100 (±0.00) | 100 (±0.00) | 100 (±0.00) | 100 (±0.00) |
| 0.25 | 62.73 (±0.38) | 91.00 (±1.00) | 61.10 (±0.17) | 88.00 (±1.73) | 51.33 (±1.53) | 87.33 (±0.58) | 52.00 (±1.73) | 86.00 (±1.00) |
| 0.50 | 20.33 (±0.58) | 48.00 (±1.73) | 18.57 (±0.40) | 46.00 (±1.00) | 18.00 (±1.73) | 43.00 (±1.00) | 18.67 (±1.15) | 44.33 (±0.58) |
| 1.00 | 14.9 (±0.85) | 41.57 (±0.51) | 17.17 (±0.76) | 43.10 (±1.01) | 11.00 (±1.73) | 39.33 (±0.58) | 16.00 (±1.73) | 39.07 (±0.90) |
| 1.50 | 11.73 (±0.64) | 36.00 (±1.73) | 13.00 (±1.00) | 36.33 (±0.58) | 11.00 (±1.00) | 34.60 (±0.53) | 15.00 (±1.00) | 35.33 (±0.58) |
| 2.00 | 7.57 (±0.51) | 31.73 (±1.55) | 8.53 (±0.50) | 37.00 (±0.00) | 10.00 (±1.00) | 28.67 (±1.15) | 8.00 (±0.00) | 31.13 (±1.03) |
| 2.50 | 5.00 (±0.00) | 26.33 (±1.15) | 7.00 (±0.00) | 22.00 (±2.00) | 7.00 (±0.00) | 21.97 (±1.00) | 7.00 (±0.00) | 22.37 (±0.55) |
| 3.00 | 4.00 (±0.00) | 17.97 (±0.95) | 4.33 (±0.58) | 12.57 (±1.43) | 7.00 (±0.00) | 14.23 (±0.68) | 4.67 (±0.58) | 11.77 (±0.68) |
| CCh-PaB-GSO Nanoemulsion (mg/mL) | S. mutans | S. sanguis | S. salivarius | S. mitis |
|---|---|---|---|---|
| 0 | 100 (±0.00) | 100 (±0.00) | 100 (±0.00) | 100 (±0.00) |
| 0.25 | 58.97 (±1.00) | 44.90 (±1.01) | 54.33 (±0.58) | 41.00 (±0.10) |
| 0.50 | 47.77 (±0.90) | 31.67 (±0.58) | 32.33 (±0.58) | 31.57 (±0.51) |
| 1.00 | 17.77 (±1.10) | 12.53 (±0.50) | 17.60 (±0.53) | 15.70 (±0.30) |
| 1.50 | 7.73 (±0.30) | 5.67 (±0.58) | 6.17 (±0.29) | 5.37 (±0.32) |
| CCh-PaB-GSO Nanoemulsion (mg/mL) | S. mutans | S. sanguis | S. salivarius | S. mitis |
|---|---|---|---|---|
| 0 | 100 (±0.00) | 100 (±0.00) | 100 (±0.00) | 100 (±0.00) |
| 0.25 | 97.87 (±0.20) | 91.90 (±1.01) | 78.87 (±1.03) | 88.33 (±0.58) |
| 0.50 | 59.13 (±1.00) | 56.20 (±0.80) | 46.27 (±1.10) | 51.57 (±0.59) |
| 1.00 | 42.33 (±1.50) | 36.73 (±0.61) | 30.90 (±1.01) | 28.80 (±0.72) |
| 1.50 | 12.43 (±0.50) | 19.90 (±0.17) | 15.47 (±061) | 20.90 (±1.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamford, T.C.M.; Sa, A.V.P.; Berger, L.R.R.; de Freitas Pontes Macedo, I.T.; Xavier-Júnior, F.H.; Rufino, R.D.; Sarubbo, L.A.; Diaz de Rienzo, M.A. A Novel Chitosan Hydrochloride–Biosurfactant–Grape Seed Oil Nanoemulsion to Control Dental Carie: Antimicrobial, Antibiofilm Activity and Irritation Potential. Appl. Sci. 2025, 15, 11773. https://doi.org/10.3390/app152111773
Stamford TCM, Sa AVP, Berger LRR, de Freitas Pontes Macedo IT, Xavier-Júnior FH, Rufino RD, Sarubbo LA, Diaz de Rienzo MA. A Novel Chitosan Hydrochloride–Biosurfactant–Grape Seed Oil Nanoemulsion to Control Dental Carie: Antimicrobial, Antibiofilm Activity and Irritation Potential. Applied Sciences. 2025; 15(21):11773. https://doi.org/10.3390/app152111773
Chicago/Turabian StyleStamford, Thayza Christina Montenegro, Antônio Vinicius Pinho Sa, Lúcia Raquel Ramos Berger, Isabella Teodora de Freitas Pontes Macedo, Francisco Humberto Xavier-Júnior, Raquel D. Rufino, Leonie A. Sarubbo, and Mayri Alejandra Diaz de Rienzo. 2025. "A Novel Chitosan Hydrochloride–Biosurfactant–Grape Seed Oil Nanoemulsion to Control Dental Carie: Antimicrobial, Antibiofilm Activity and Irritation Potential" Applied Sciences 15, no. 21: 11773. https://doi.org/10.3390/app152111773
APA StyleStamford, T. C. M., Sa, A. V. P., Berger, L. R. R., de Freitas Pontes Macedo, I. T., Xavier-Júnior, F. H., Rufino, R. D., Sarubbo, L. A., & Diaz de Rienzo, M. A. (2025). A Novel Chitosan Hydrochloride–Biosurfactant–Grape Seed Oil Nanoemulsion to Control Dental Carie: Antimicrobial, Antibiofilm Activity and Irritation Potential. Applied Sciences, 15(21), 11773. https://doi.org/10.3390/app152111773









