Changes in Electrical Properties of Graphite Coatings Annealed in Air and Nitrogen Environments
Abstract
1. Introduction
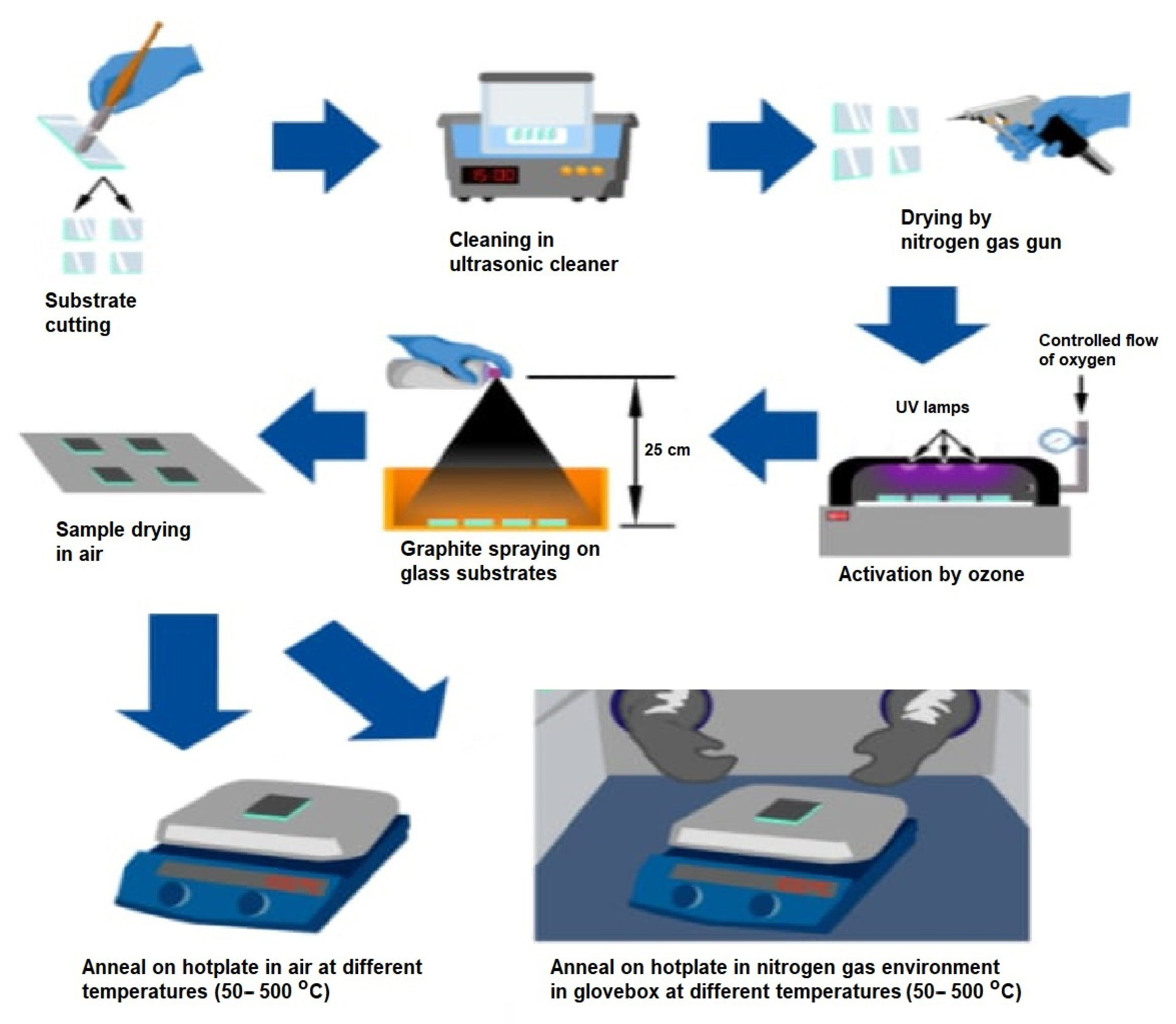
2. Materials and Methods
3. Results
3.1. SEM Morphology
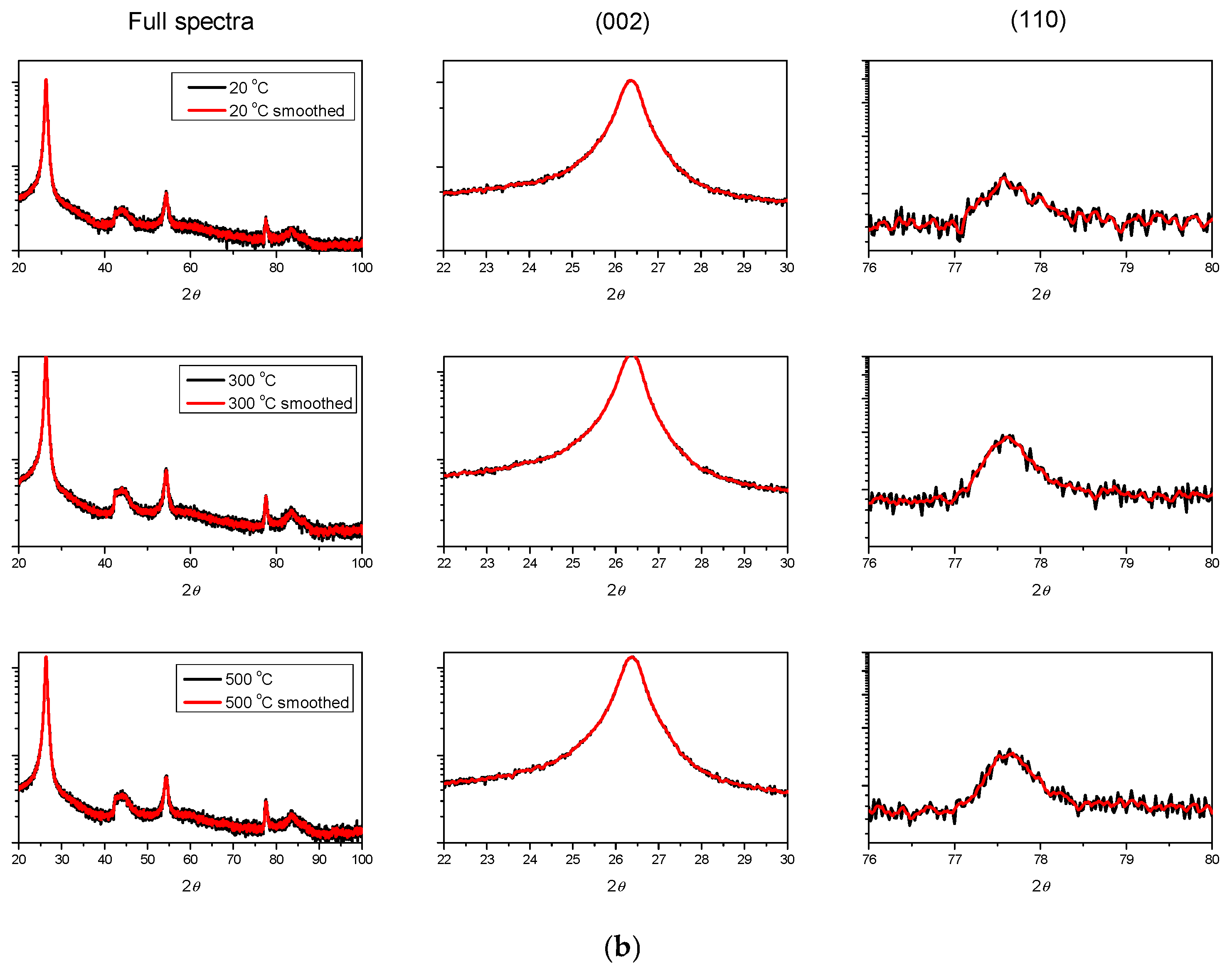
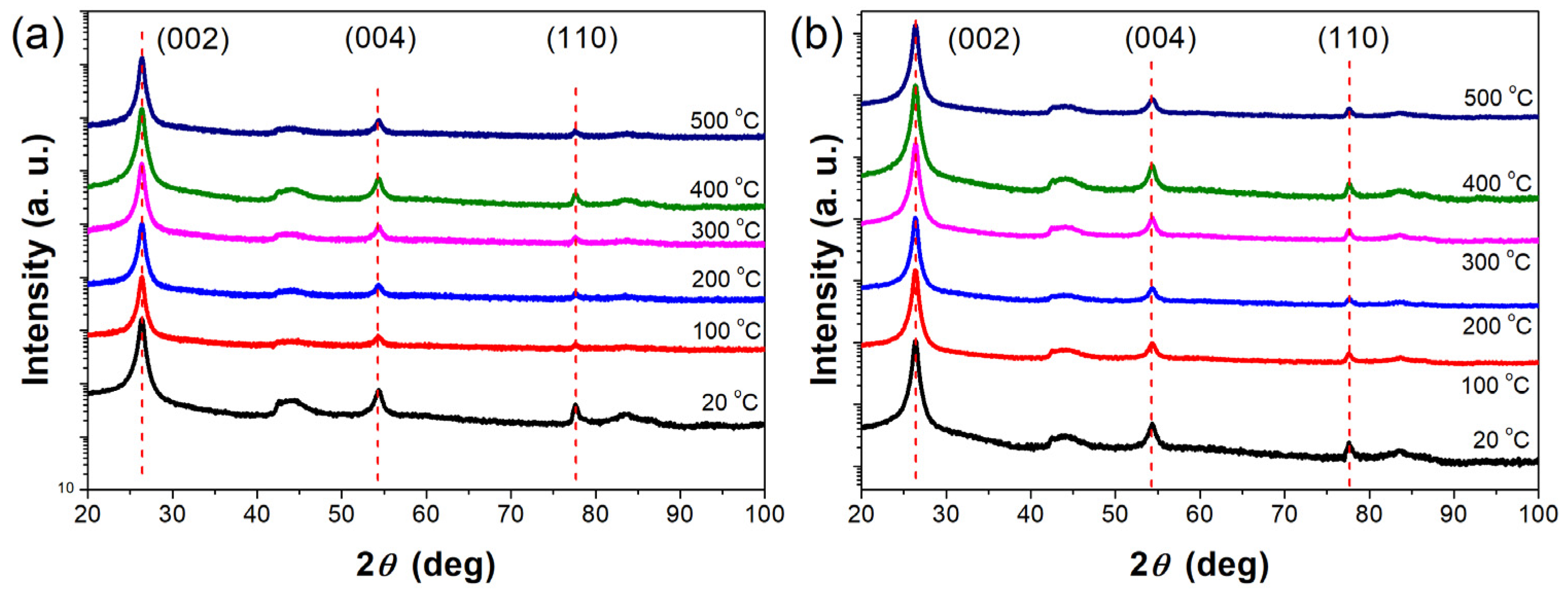
3.2. XRD Characterization
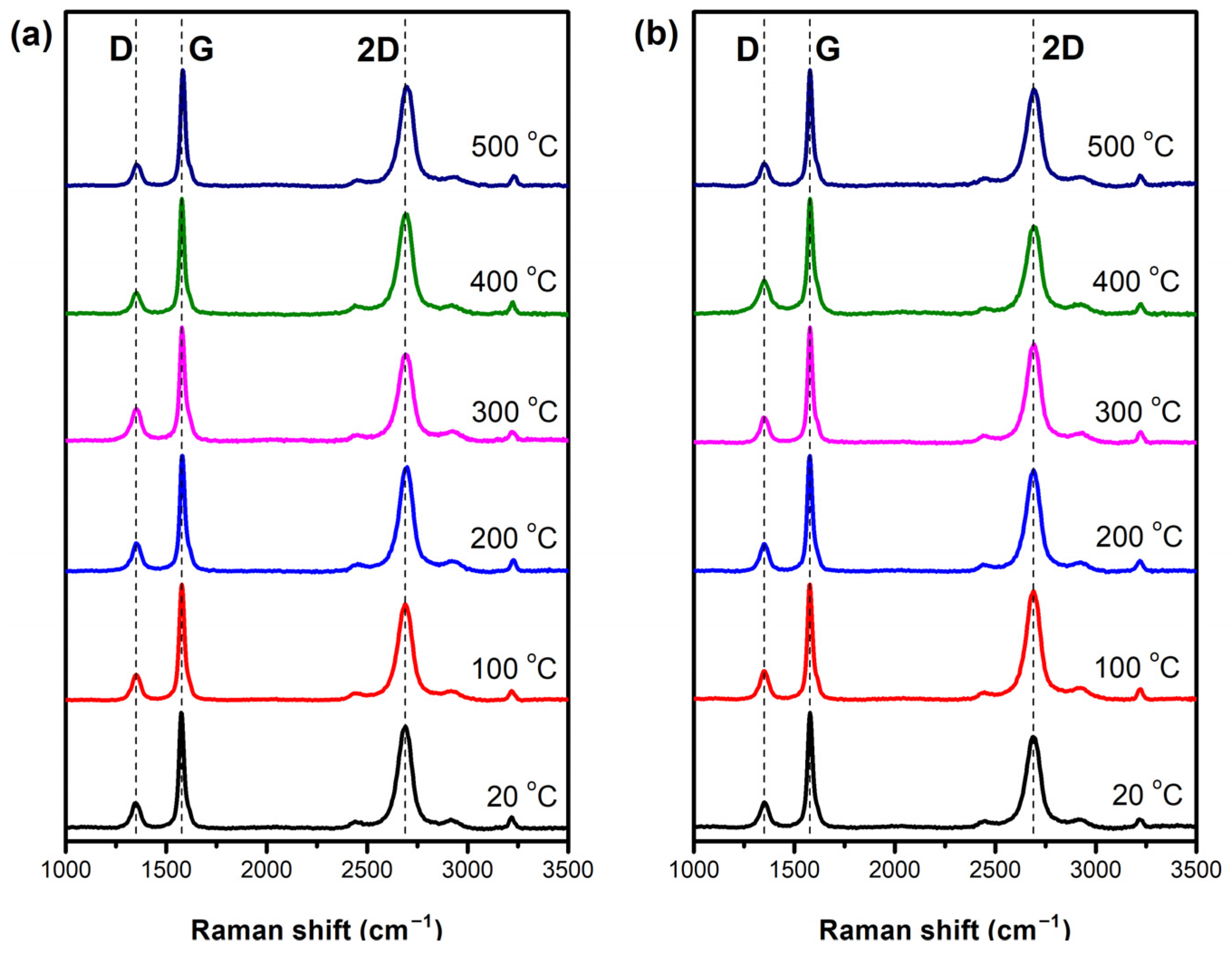
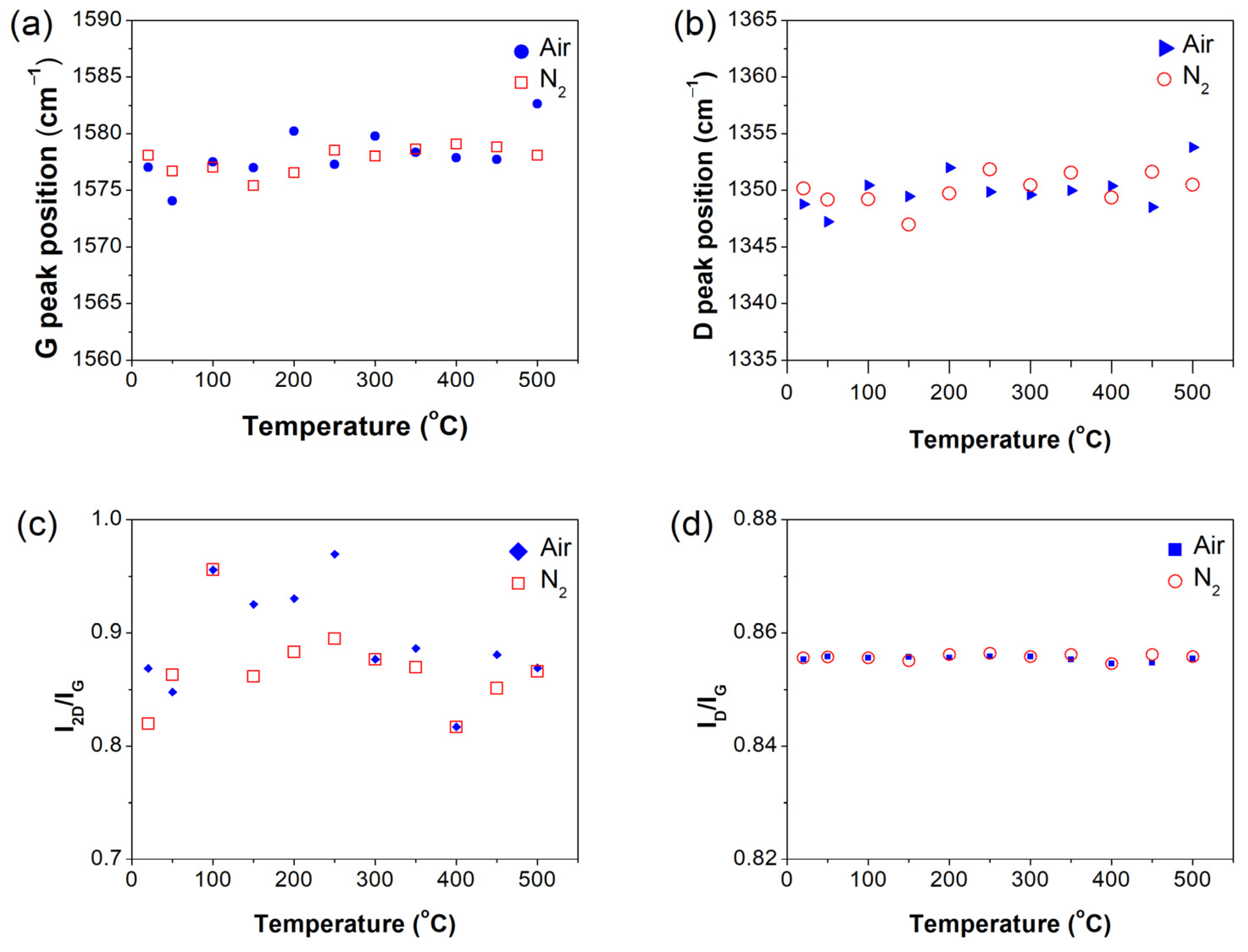
3.3. Raman Analysis
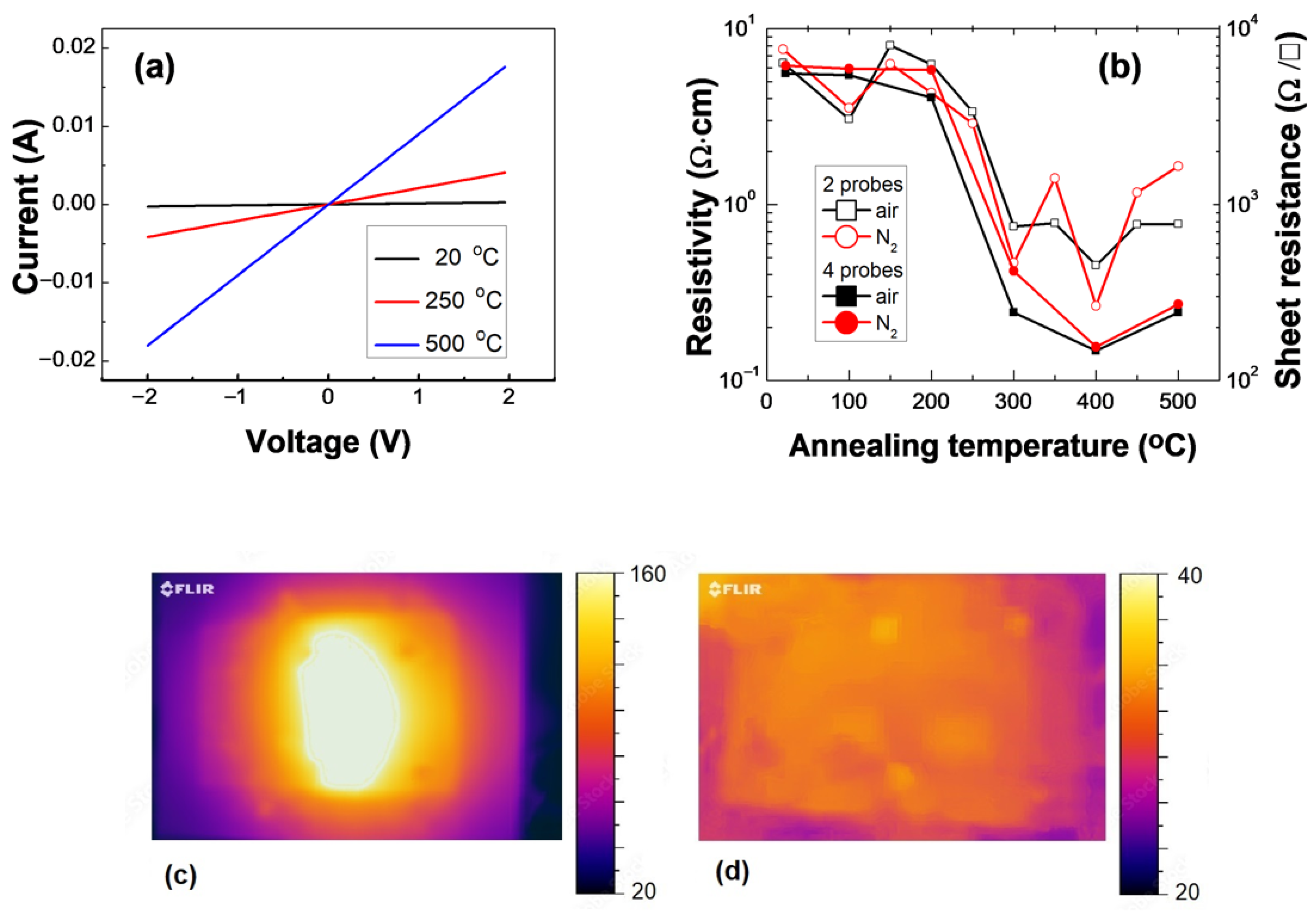
3.4. Conductivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SG | Savitzky–Golay |
| FWHM | Full Width at Half Maximum |
Appendix A
| Peak Location (cm−1) | FWHM (cm−1) | Intensity Ratio | |||||
|---|---|---|---|---|---|---|---|
| Temp. | D | G | 2D | D | G | ID/IG | I2D/IG |
| 20 | 1348.09 | 1575.70 | 2686.83 | 51.36 | 30.20 | 0.856 | 0.909 |
| 100 | 1350.43 | 1577.51 | 2687.84 | 46.22 | 30.46 | 0.856 | 0.835 |
| 200 | 1352.02 | 1580.20 | 2693.01 | 55.26 | 31.60 | 0.856 | 0.935 |
| 300 | 1349.61 | 1579.79 | 2690.41 | 73.70 | 35.24 | 0.854 | 0.813 |
| 400 | 1350.39 | 1577.90 | 2689.68 | 54.50 | 29.33 | 0.856 | 0.896 |
| 500 | 1353.724 | 1582.67 | 2696.10 | 47.48 | 27.97 | 0.855 | 0.870 |
| Peak Location (cm−1) | FWHM (cm−1) | Intensity Ratio | |||||
|---|---|---|---|---|---|---|---|
| Temp. | D | G | 2D | D | G | ID/IG | I2D/IG |
| 20 | 1350.15 | 1578.11 | 2688.119 | 57.79 | 31.71 | 0.856 | 0.820 |
| 100 | 1349.22 | 1577.03 | 2687.83 | 51.43 | 30.79 | 0.856 | 0.956 |
| 200 | 1349.72 | 1576.57 | 2687.65 | 49.26 | 30.44 | 0.856 | 0.883 |
| 300 | 1350.45 | 1578.03 | 2689.38 | 48.87 | 29.59 | 0.856 | 0.876 |
| 400 | 1349.35 | 1579.09 | 2690.02 | 79.30 | 36.75 | 0.854 | 0.817 |
| 500 | 1350.47 | 1578.10 | 2690.32 | 49.55 | 29.82 | 0.856 | 0.866 |
| Environment | Air | N2 Gas | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | 2θ (deg) | Average Layer per Domain | 2θ (deg) | Average Layer per Domain | ||||||||
| (002) | (110) | Da/Dc | Interplanar d(002) (Å) | Packing Density | (002) | (110) | Da/Dc | Interplanar d(002) (Å) | Packing Density | |||
| 20 | 26.36 | 77.68 | 2.53 | 41.63 | 3.38 | 2.26 | 26.36 | 77.64 | 3.82 | 42.16 | 3.38 | 2.26 |
| 100 | 26.36 | 77.69 | 2.37 | 42.50 | 3.38 | 2.26 | 26.36 | 77.66 | 2.38 | 41.64 | 3.38 | 2.26 |
| 200 | 26.37 | 77.69 | 2.63 | 41.68 | 3.38 | 2.26 | 26.36 | 77.69 | 2.05 | 42.75 | 3.38 | 2.26 |
| 300 | 26.36 | 77.67 | 2.21 | 42.17 | 3.38 | 2.26 | 26.36 | 77.65 | 2.54 | 41.86 | 3.38 | 2.26 |
| 400 | 26.36 | 77.68 | 2.46 | 41.64 | 3.38 | 2.26 | 26.36 | 77.68 | 2.47 | 41.58 | 3.38 | 2.26 |
| 500 | 26.37 | 77.66 | 2.27 | 43.06 | 3.38 | 2.26 | 26.36 | 77.68 | 2.55 | 41.13 | 3.38 | 2.26 |
Appendix B
| Phase Name | Formula | Figure of Merit | Phase Reg. Detail | DB Card Number |
| Silicon, syn | Si | 0.130 | ICDD (PDF – 4 + 2023) | 00-005-0565 |
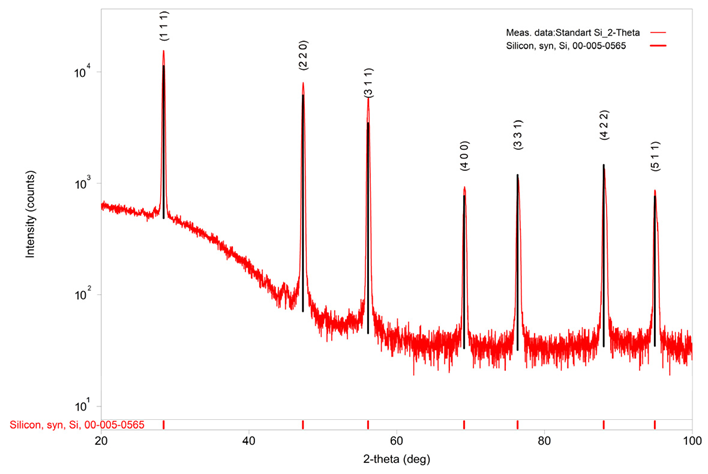
| Phase Name | a (A) | b (A) | c (A) | Alpha (deg) | Beta (deg) | Gamma (deg) | V (A^3) |
| Silicon, syn | 5.431668 | 5.431668 | 5.431668 | 90.000000 | 90.000000 | 90.000000 | 160.250575 |
Appendix C

References
- Kumar, A.; Gupta, R.K. 2D Semiconducting Materials for Electronic, Photonic, and Optoelectronic Devices, 1st ed.; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar] [CrossRef]
- Wei, J.; Sun, L.; Han, J.; Huang, W. MWCNTs/CB Waterborne Conductive Smart Coating for Damage Monitoring of Composites: Design, Fabrication, Characterization, and Verification. Prog. Org. Coat. 2022, 172, 107136. [Google Scholar] [CrossRef]
- Orts Mercadillo, V.; Chan, K.C.; Caironi, M.; Athanassiou, A.; Kinloch, I.A.; Bissett, M.; Cataldi, P. Electrically Conductive 2D Material Coatings for Flexible and Stretchable Electronics: A Comparative Review of Graphenes and MXenes. Adv. Funct. Mater. 2022, 32, 2204772. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Hang, C.-Z.; Zhao, X.-F.; Zhu, L.-Y.; Ma, R.-G.; Wang, J.-C.; Lu, H.-L.; Zhang, D.W. Advance on Flexible Pressure Sensors Based on Metal and Carbonaceous Nanomaterial. Nano Energy 2021, 87, 106181. [Google Scholar] [CrossRef]
- Shaker, L.M.; Abdulamie, A.A.; Al-Amiery, A.A. Graphene-Enabled Advancements in Solar Cell Technology. J. Alloys Compd. 2025, 1020, 179583. [Google Scholar] [CrossRef]
- Majeed, A.M.; Vaitkevičius, A.; Stanionytė, S.; Miasojedovas, S.; Kreiza, G.; Ščajev, P. Highly Stable Solution-Processed CsZnPbI3 Perovskite Distributed Feedback Lasers. Adv. Opt. Mater. 2025, e01896. [Google Scholar] [CrossRef]
- Chandrasekhar, P. Conducting Polymers, Fundamentals and Applications: Including Carbon Nanotubes and Graphene; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Guo, Y.; Zhong, M.; Fang, Z.; Wan, P.; Yu, G. A Wearable Transient Pressure Sensor Made with MXene Nanosheets for Sensitive Broad-Range Human–Machine Interfacing. Nano Lett. 2019, 19, 1143–1150. [Google Scholar] [CrossRef]
- Burchell, T.; Pavlov, T. Graphite: Properties and Characteristics. In Comprehensive Nuclear Materials; Elsevier: Amsterdam, The Netherlands, 2020; Volume 7, pp. 355–381. [Google Scholar]
- Malhotra, B.D. Graphene Based Biomolecular Electronic Devices; Micro and Nano Technologies Series; Elsevier: San Diego, CA, USA, 2023. [Google Scholar]
- Kumar, N.; Salehiyan, R.; Chauke, V.; Joseph Botlhoko, O.; Setshedi, K.; Scriba, M.; Masukume, M.; Sinha Ray, S. Top-down Synthesis of Graphene: A Comprehensive Review. FlatChem 2021, 27, 100224. [Google Scholar] [CrossRef]
- Yao, J.; Kim, C.; Nian, Q.; Kang, W. Copper-Graphene Composite (CGC) Conductors: Synthesis, Microstructure, and Electrical Performance. Small 2024, 20, e2403241. [Google Scholar] [CrossRef]
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; Khan, A.U. Graphene Synthesis, Characterization and Its Applications: A Review. Results Chem. 2021, 3, 100163. [Google Scholar] [CrossRef]
- Chung, D.D.L. A Perspective on Electromagnetic Interference Shielding Materials Comprising Exfoliated Graphite. Carbon 2024, 216, 118569. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Y.; Chen, M. Battery Thermal Management with a Modified Metal Alloy/Expanded Graphite/Paraffin Composite Phase Change Material. J. Energy Storage 2025, 113, 115652. [Google Scholar] [CrossRef]
- Zhao, H.; Zuo, H.; Wang, J.; Jiao, S. Practical Application of Graphite in Lithium-Ion Batteries: Modification, Composite, and Sustainable Recycling. J. Energy Storage 2024, 98, 113125. [Google Scholar] [CrossRef]
- Leng, Z.; Li, T.; Wang, X.; Zhang, S.; Zhou, J. Effect of Graphite Content on the Conductivity, Wear Behavior, and Corrosion Resistance of the Organic Layer on Magnesium Alloy MAO Coatings. Coatings 2022, 12, 434. [Google Scholar] [CrossRef]
- Abe, A.; Goss, J.A.; Zou, M. Exploring the Impact of Spray Process Parameters on Graphite Coatings: Morphology, Thickness, and Tribological Properties. Coatings 2024, 14, 714. [Google Scholar] [CrossRef]
- Żórawski, W.; Góral, A.; Makrenek, M.; Soboń, D.; Trelka, A.; Bara, M. Optimization of Mechanical Properties of Cr3C2-Ni20Cr/Graphite Cold Sprayed Coatings. Materials 2021, 14, 3458. [Google Scholar] [CrossRef]
- Zhou, L.; Zheng, Z.; Wang, Q.; Wu, F.; Hong, J.; Xie, S.; Ni, H.; Feng, Q.; Zhou, M.; Li, M.; et al. A Study on the Effect of Nickel-Plated Graphite Content on the Microstructure and Properties of AlZn/Nickel-Plated Graphite Composite Cold Spray Coatings. Materials 2025, 18, 388. [Google Scholar] [CrossRef]
- Di Bartolomeo, A.; Iemmo, L.; Urban, F.; Palomba, M.; Carotenuto, G.; Longo, A.; Sorrentino, A.; Giubileo, F.; Barucca, G.; Rovere, M.; et al. Graphite Platelet Films Deposited by Spray Technique on Low Density Polyethylene Substrates. Mater. Today Proc. 2020, 20, 87–90. [Google Scholar] [CrossRef]
- Crespi, A.E.; Nordet, G.; Peyre, P.; Ballage, C.; Hugon, M.-C.; Chapon, P.; Minea, T. The Use of Sacrificial Graphite-like Coating to Improve Fusion Efficiency of Copper in Selective Laser Melting. Materials 2023, 16, 2460. [Google Scholar] [CrossRef]
- Wu, G.-Y.; Lin, C.-H.; Zhan, K.-Y.; Tseng, C.-J. The Corrosion Characteristics of Graphite Coatings on Nickel Foam and Their Applications in High-Temperature Proton Exchange Membrane Fuel Cells. Int. J. Hydrog. Energy 2025, 137, 1000–1008. [Google Scholar] [CrossRef]
- Proskuryakov, V.; Rodionov, I.; Borodina, S. The Effect of Graphite Coating on the Composition, Structure and Microhardness of the Surface of Structural Chromiumnickel Steel during Laser Pulse Processing. J. Phys. Conf. Ser. 2020, 1695, 012069. [Google Scholar] [CrossRef]
- Abe, A.; Zou, M. Enhanced Scratch Resistance of Graphite Coating Using a Polydopamine Adhesive Underlayer. Coatings 2025, 15, 690. [Google Scholar] [CrossRef]
- Ersu, G.; Kuriakose, S.; Goldie, S.J.; Al-Enizi, A.M.; Nafady, A.; Munuera, C.; Backes, C.; Island, J.O.; Castellanos-Gomez, A. Improving the Conductivity of Graphite-Based Films by Rapid Laser Annealing. Nanoscale Adv. 2022, 4, 4724–4729. [Google Scholar] [CrossRef] [PubMed]
- Santhiago, M.; Strauss, M.; Pereira, M.P.; Chagas, A.S.; Bufon, C.C.B. Direct Drawing Method of Graphite onto Paper for High-Performance Flexible Electrochemical Sensors. ACS Appl. Mater. Interfaces 2017, 9, 11959–11966. [Google Scholar] [CrossRef] [PubMed]
- Levchenko, I.; Baranov, O.; Riccardi, C.; Roman, H.E.; Cvelbar, U.; Ivanova, E.P.; Mohandas, M.; Ščajev, P.; Malinauskas, T.; Xu, S.; et al. Nanoengineered Carbon-Based Interfaces for Advanced Energy and Photonics Applications: A Recent Progress and Innovations. Adv. Mater. Inter. 2023, 10, 2201739. [Google Scholar] [CrossRef]
- Ščajev, P.; Malinauskas, T.; Seniutinas, G.; Arnold, M.D.; Gentle, A.; Aharonovich, I.; Gervinskas, G.; Michaux, P.; Hartley, J.S.; Mayes, E.L.H.; et al. Light-Induced Reflectivity Transients in Black-Si Nanoneedles. Sol. Energy Mater. Sol. Cells 2016, 144, 221–227. [Google Scholar] [CrossRef]
- Rong, K.; Li, H.; Wang, X.; You, C.; Liu, H.; Ma, X.; Bai, L.; Hou, C.; Li, Z.; Zhou, Q.; et al. Constructing Robust Conductive Graphite with Local Sp3 Hybridization by High Pressure and High Temperature. J. Solid State Chem. 2025, 350, 125490. [Google Scholar] [CrossRef]
- Cao, K.-Z.; Ma, J.-H.; Dong, Y.-L.; Duan, Y.; Zheng, R.-T.; Bundhooa, D.; Liu, H.-Q.; Lei, Y. Graphitic Carbons: Preparation, Characterization, and Application on K-Ion Batteries. Rare Met. 2024, 43, 4056–4075. [Google Scholar] [CrossRef]
- Baca Arroyo, R. Graphite Intended for Green Engineering Developed by Noncontaminant Reverse Abrasion. Adv. Mater. Sci. Eng. 2016, 2016, 7016457. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Lee, Y.; Nguyen, P.Q.H.; Dera, P.; Yoon, S.-H.; Lee, W. Enhancing the Electrical Properties of Graphite Nanoflake through Gamma-Ray Irradiation. Sci. Rep. 2022, 12, 14824. [Google Scholar] [CrossRef]
- Ščajev, P.; Miasojedovas, S.; Mekys, A.; Kreiza, G.; Čeponkus, J.; Šablinskas, V.; Malinauskas, T.; Medvids, A. Oxidized Graphite Nanocrystals for White Light Emission. Crystals 2024, 14, 505. [Google Scholar] [CrossRef]
- Ščajev, P.; Durena, R.; Onufrijevs, P.; Miasojedovas, S.; Malinauskas, T.; Stanionyte, S.; Zarkov, A.; Zukuls, A.; Bite, I.; Smits, K. Morphological and Optical Property Study of Li Doped ZnO Produced by Microwave-Assisted Solvothermal Synthesis. Mater. Sci. Semicond. Process. 2021, 135, 106069. [Google Scholar] [CrossRef]
- Grivickas, V.; Ščajev, P.; Miasojedovas, S.; Voss, L.; Grivickas, P. Self-Trapped-Exciton Radiative Recombination in β–Ga2O3: Impact of Two Concurrent Nonradiative Auger Processes. ACS Appl. Electron. Mater. 2025, 7, 1829–1841. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Halley, J.M. Some Calculations of Temperature Profiles in Thin Films with Laser Heating. Appl. Phys. A 1987, 42, 279–285. [Google Scholar] [CrossRef]
- Smausz, T.; Kondász, B.; Gera, T.; Ajtai, T.; Utry, N.; Pintér, M.; Kiss-Albert, G.; Budai, J.; Bozóki, Z.; Szabó, G.; et al. Determination of UV–Visible–NIR Absorption Coefficient of Graphite Bulk Using Direct and Indirect Methods. Appl. Phys. A 2017, 123, 633. [Google Scholar] [CrossRef]
- Das, A.; Brown, A.K.; Mah, M.L.; Talghader, J.J. Photon Diffusion in Microscale Solids. J. Phys. Condens. Matter 2019, 31, 335703. [Google Scholar] [CrossRef]
- Liu, H.-N.; Cong, X.; Lin, M.-L.; Tan, P.-H. The Intrinsic Temperature-Dependent Raman Spectra of Graphite in the Temperature Range from 4K to 1000K. Carbon 2019, 152, 451–458. [Google Scholar] [CrossRef]
- Wang, C.; Su, Y.; Ouyang, Q.; Zhang, D. Effects of Graphite Flake Size on the Properties of Aligned Graphene Nanoplatelets Covered Graphite Flakes/Aluminum Composites. Diam. Relat. Mater. 2021, 116, 108381. [Google Scholar] [CrossRef]
- Li, Y.; Bi, Y.; Liu, X.; Dong, L.; Cai, W.; Lv, J.; Huang, L.; Duan, H.; Jia, Q.; Aygul Yeprem, H.; et al. Preparation and Properties of Alumina-Carbon Castables Using SiC Nanofiber Coated Graphite Flake. Appl. Surf. Sci. 2023, 615, 156275. [Google Scholar] [CrossRef]
- Hussain, S.A.; Ali, S.; Islam, Z.U.; Khan, M. Low-Temperature Synthesis of Graphite Flakes and Carbon-Based Nanomaterials from Banana Peels Using Hydrothermal Process for Photoelectrochemical Water-Splitting. Phys. E Low-Dimens. Syst. Nanostructures 2022, 141, 115231. [Google Scholar] [CrossRef]
- Kahraman, O.; Cevik, P.K.; Turunc, E.; Çelik, A.; Koyuncu, İ.; Binzet, R. Eco-Friendly Synthesis and Biomedical Assessment of Graphene Nanomaterials Using a Holoparasitic Plant Template. Diam. Relat. Mater. 2025, 157, 112551. [Google Scholar] [CrossRef]
- Silva Filho, J.C.; Venancio, E.C.; Silva, S.C.; Takiishi, H.; Martinez, L.G.; Antunes, R.A. A Thermal Method for Obtention of 2 to 3 Reduced Graphene Oxide Layers from Graphene Oxide. SN Appl. Sci. 2020, 2, 1450. [Google Scholar] [CrossRef]
- Milev, A.; Wilson, M.; Kannangara, G.S.; Tran, N. X-Ray Diffraction Line Profile Analysis of Nanocrystalline Graphite. Mater. Chem. Phys. 2008, 111, 346–350. [Google Scholar] [CrossRef]
- Zielinski, P.; Kühne, M.; Kärcher, D.; Paolucci, F.; Wochner, P.; Fecher, S.; Drnec, J.; Felici, R.; Smet, J.H. Probing Exfoliated Graphene Layers and Their Lithiation with Microfocused X-Rays. Nano Lett. 2019, 19, 3634–3640. [Google Scholar] [CrossRef]
- Popova, A.N. Crystallographic Analysis of Graphite by X-Ray Diffraction. Coke Chem. 2017, 60, 361–365. [Google Scholar] [CrossRef]
- Zólyomi, V.; Koltai, J.; Kürti, J. Resonance Raman Spectroscopy of Graphite and Graphene. Phys. Status Solidi 2011, 248, 2435–2444. [Google Scholar] [CrossRef]
- Vetrivendan, E.; Hareesh, R.; Ningshen, S. Synthesis and Characterization of Chemical Vapour Deposited Pyrolytic Graphite. Thin Solid. Film. 2022, 749, 139180. [Google Scholar] [CrossRef]
- Kaplas, T.; Jakstas, V.; Biciunas, A.; Luksa, A.; Setkus, A.; Niaura, G.; Kasalynas, I. Effect of High-Temperature Annealing on Graphene with Nickel Contacts. Condens. Matter 2019, 4, 21. [Google Scholar] [CrossRef]
- Chen, J.; Guo, X.; Tang, Q.; Zhuang, C.; Liu, J.; Wu, S.; Beake, B.D. Nanomechanical Properties of Graphene on Poly(Ethylene Terephthalate) Substrate. Carbon 2013, 55, 144–150. [Google Scholar] [CrossRef]
- Singh, S.B.; Dastgheib, S.A. Comparative Characteristics of Coal-Based and Graphite-Based Reduced Graphene Oxide Materials. Carbon Trends 2025, 20, 100511. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman Spectroscopy of Graphene and Graphite: Disorder, Electron–Phonon Coupling, Doping and Nonadiabatic Effects. Solid. State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Scardaci, V. Laser Synthesized Graphene and Its Applications. Appl. Sci. 2021, 11, 6304. [Google Scholar] [CrossRef]
- Kuliček, J.; Yamada, T.; Taniguchi, T.; Rezek, B. Visible-Frequency Plasmonic Enhancement at the Edge of Graphene/h-BN Heterostructures on Silicon Substrate. Carbon 2024, 219, 118836. [Google Scholar] [CrossRef]
- Ray, S.C. Applications of Graphene and Graphene-Oxide Based Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.; Xu, C.; Khatami, Y.; Banerjee, K. Synthesis of High-Quality Monolayer and Bilayer Graphene on Copper Using Chemical Vapor Deposition. Carbon 2011, 49, 4122–4130. [Google Scholar] [CrossRef]
- Lee, H.; Perumal, S.; Cheong, I. Amphiphilic Fluorinated Block Copolymer Synthesized by RAFT Polymerization for Graphene Dispersions. Polymers 2016, 8, 101. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Cui, X.; Hu, Y.; Lei, L.; Zhu, Y.; Jiang, W. Thermal Annealing Induced Enhancement of Electrical Properties of a Co-Continuous Polymer Blend Filled with Carbon Nanotubes. Compos. Sci. Technol. 2018, 167, 522–528. [Google Scholar] [CrossRef]

| Environment | Air | N2 Gas | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | 2θ (deg) | βobs, FWHM | Dc (nm) | Da (nm) | 2θ (deg) | βobs, FWHM | Dc (nm) | Da (nm) | ||||
| (002) | (110) | (002) | (110) | (002) | (110) | (002) | (110) | (002) | (110) | (002) | (110) | |
| 20 | 26.36 | 77.68 | 0.59 | 0.60 | 15.9 | 19.4 | 26.36 | 77.64 | 0.58 | 0.39 | 16.2 | 40.2 |
| 100 | 26.36 | 77.69 | 0.58 | 0.63 | 16.6 | 18.3 | 26.36 | 77.66 | 0.59 | 0.64 | 15.9 | 17.9 |
| 200 | 26.37 | 77.69 | 0.59 | 0.57 | 15.9 | 20.6 | 26.36 | 77.69 | 0.57 | 0.72 | 16.6 | 15.4 |
| 300 | 26.36 | 77.67 | 0.58 | 0.67 | 16.3 | 16.6 | 26.36 | 77.65 | 0.58 | 0.59 | 16.1 | 19.7 |
| 400 | 26.36 | 77.68 | 0.59 | 0.62 | 15.9 | 18.7 | 26.36 | 77.68 | 0.59 | 0.61 | 15.9 | 18.8 |
| 500 | 26.37 | 77.66 | 0.57 | 0.65 | 16.7 | 17.6 | 26.36 | 77.68 | 0.60 | 0.60 | 15.6 | 19.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamasali, Y.-d.; Majeed, A.M.; Stanionytė, S.; Šablinskas, V.; Kreiza, G.; Mekys, A.; Ščajev, P. Changes in Electrical Properties of Graphite Coatings Annealed in Air and Nitrogen Environments. Appl. Sci. 2025, 15, 11727. https://doi.org/10.3390/app152111727
Jamasali Y-d, Majeed AM, Stanionytė S, Šablinskas V, Kreiza G, Mekys A, Ščajev P. Changes in Electrical Properties of Graphite Coatings Annealed in Air and Nitrogen Environments. Applied Sciences. 2025; 15(21):11727. https://doi.org/10.3390/app152111727
Chicago/Turabian StyleJamasali, Yusof-den, Abdul Mannan Majeed, Sandra Stanionytė, Valdas Šablinskas, Gediminas Kreiza, Algirdas Mekys, and Patrik Ščajev. 2025. "Changes in Electrical Properties of Graphite Coatings Annealed in Air and Nitrogen Environments" Applied Sciences 15, no. 21: 11727. https://doi.org/10.3390/app152111727
APA StyleJamasali, Y.-d., Majeed, A. M., Stanionytė, S., Šablinskas, V., Kreiza, G., Mekys, A., & Ščajev, P. (2025). Changes in Electrical Properties of Graphite Coatings Annealed in Air and Nitrogen Environments. Applied Sciences, 15(21), 11727. https://doi.org/10.3390/app152111727






