Transition to Artificial Intelligence in Imaging and Laboratory Diagnostics in Rheumatology
Abstract
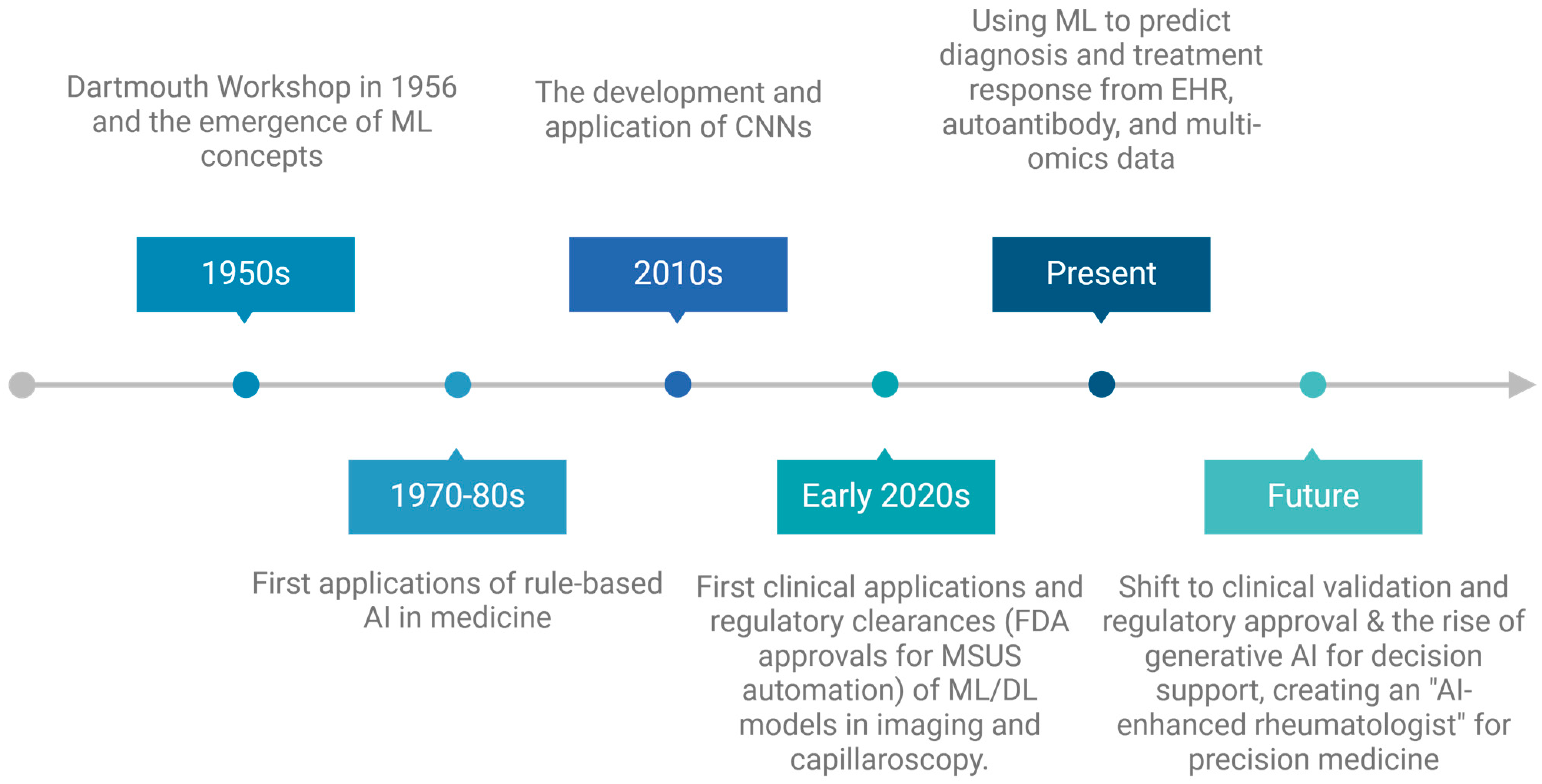
1. Introduction
2. Materials and Methods
2.1. Databases and Sources Consulted
2.2. Search Terms and Boolean Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Study Selection Flow
3. Overview of Artificial Intelligence in Medicine
3.1. Foundational Definitions: AI, ML, and DL
3.2. Current Applications in the Healthcare Ecosystem
3.3. Regulatory and Ethical Perspectives
4. AI in Imaging Diagnostics in Rheumatology
4.1. Musculoskeletal Ultrasound
4.2. Magnetic Resonance Imaging and Computed Tomography
4.3. Conventional Radiography
4.4. AI in Capillaroscopic Diagnostics
4.4.1. The Clinical Challenge: Subjectivity and Accessibility in Capillaroscopy
4.4.2. AI-Powered Quantitative Analysis: A Shift Towards Objectivity
4.4.3. Key AI Models and Their Performance
- CAPI-Score and CAPI-Detect: The CAPI-Score algorithm was an important early effort to standardize NVC interpretation using a set of simple, quantitative rules inspired by expert consensus [63]. Its successor, CAPI-Detect, represents a significant leap forward. It employs a machine learning model (CatBoost) trained on a large dataset of expert-annotated capillaroscopies and integrates 24 distinct quantitative variables related to capillary architecture [59]. CAPI-Detect significantly outperforms its predecessor, achieving accuracy rates exceeding 90% for distinguishing SSc from non-SSc patterns and over 92% for correctly staging SSc (early, active, or late), particularly when validated against cases with full expert consensus [59]. A key feature is its ability to provide probability scores for each potential pattern, offering a more nuanced output that reflects the model’s confidence [63].
- Deep Learning Systems (ResNet, ViT, and others): Other research groups have focused on deep learning architectures. A pilot study utilized a ResNet-34 deep residual neural network to classify NVC images as normal or pathological, reporting a sensitivity of 89.0% and a specificity of 86.9% on its validation set, along with a high precision of 96.48% for automated capillary counting [55]. Another fully automated system, developed at the University of Manchester, used deep learning networks to mimic the interpretation strategies of experts. It achieved an area under the receiver operating characteristic curve (AUC) of 97% for identifying SSc, a performance that exceeded the reported 82% sensitivity and 73% specificity of expert consensus [60]. This system was notably validated on images from both high-resolution systems and low-cost USB microscopes, demonstrating its robustness [64]. More recently, the Vision Transformer (ViT) architecture has been applied, showing strong performance in identifying specific microangiopathic changes like giant capillaries (AUC 92.6%) and enlarged capillaries (AUC 90.2%) [65].
- ARTIX (AI-based Raynaud’s Quantification Index): Moving beyond diagnosis, the ARTIX tool leverages AI to provide an objective, quantitative measure of Raynaud’s phenomenon (RP) severity directly from photographs taken with a standard mobile phone [62]. In a validation study comparing its output to thermography during a standardized cold challenge, ARTIX successfully discriminated between patients with RP and healthy controls (p < 0.001) and showed correlations with clinical features [62]. This innovation points toward a future of patient-centered, remote monitoring of disease activity.
| Model Name | AI Architecture | Primary Application | Key Performance Metrics | Source(s) |
|---|---|---|---|---|
| CAPI-DETECT | Machine Learning (CatBoost) | Classification of SSc vs. non-SSc patterns; Staging of SSc patterns (early, active, late) | Accuracy: >90% (SSc vs. non-SSc); >92% (SSc staging) on full consensus data. Provides probability scores. | [59] |
| RESNET-34 PILOT | Deep Learning (CNN) | Classification of NVC images as normal vs. pathological; Capillary counting. | Sensitivity: 89.0%, Specificity: 86.9% (validation); Precision for capillary count: 96.48%. | [55] |
| MANCHESTER SYSTEM | Deep Learning (CNNs) | Subject-level probability of SSc from multi-finger images. | AUC: 97% (high-res images), 95% (low-cost USB scope); Outperforms expert consensus (Sens 82%, Spec 73%). | [64] |
| VISION TRANSFORMER (VIT) | Deep Learning (Transformer) | Identification of specific microangiopathic changes (e.g., giant capillaries, capillary loss). | AUC: 81.8–84.5% for various changes; AUC: 92.6% for giant capillaries. Performance comparable to human assessors. | [65] |
| ARTIX | Machine Learning | Objective quantification of Raynaud’s phenomenon severity from mobile phone photos. | Successfully discriminated between RP patients and healthy controls during cold challenge (p < 0.001). | [62] |
5. AI in Laboratory Diagnostics and Biomarkers
5.1. Routine Tests
5.2. Molecular Diagnostics
5.3. Multi-Omics Integration
6. AI-Assisted Clinical Decision Support Systems
6.1. Disease Activity Prediction
6.2. Treatment Selection and Monitoring
6.3. Integration into Electronic Health Records (EHR)
7. Discussion: Challenges, Limitations, and Future Perspectives
7.1. Technical and Methodological Barriers
7.2. The Imperative of Validation and Generalizability
7.3. Ethical Dilemmas and Patient Data Protection
7.4. Barriers to Adoption: Clinician Acceptance and Trust
7.5. Defining Translational Pathways for Clinical Integration
7.6. The Crucial Role of Interdisciplinary Collaboration
7.7. Reforming Medical Education: Training AI-Fluent Rheumatologists
7.8. The Future Horizon: AI-Augmented Rheumatology
7.9. Limitations of the Review
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sequí-Sabater, J.M.; Benavent, D. Artificial Intelligence in Rheumatology Research: What Is It Good For? RMD Open 2025, 11, e004309. [Google Scholar] [CrossRef]
- Yaung, K.N.; Yeo, J.G.; Kumar, P.; Wasser, M.; Chew, M.; Ravelli, A.; Law, A.H.N.; Arkachaisri, T.; Martini, A.; Pisetsky, D.S.; et al. Artificial Intelligence and High-Dimensional Technologies in the Theragnosis of Systemic Lupus Erythematosus. Lancet Rheumatol. 2023, 5, e151–e165. [Google Scholar] [CrossRef] [PubMed]
- Kingsmore, K.M.; Puglisi, C.E.; Grammer, A.C.; Lipsky, P.E. An Introduction to Machine Learning and Analysis of Its Use in Rheumatic Diseases. Nat. Rev. Rheumatol. 2021, 17, 710–730. [Google Scholar] [CrossRef]
- Venerito, V.; Gupta, L.; Mileto, S.; Iannone, F.; Bilgin, E. Ethical Challenges and Regulatory Pathways for Artificial Intelligence in Rheumatology. Rheumatol. Adv. Pract. 2025, 9, rkaf035. [Google Scholar] [CrossRef]
- Park, J.; Bai, B.; Ryu, D.; Liu, T.; Lee, C.; Luo, Y.; Lee, M.J.; Huang, L.; Shin, J.; Zhang, Y.; et al. Artificial Intelligence-Enabled Quantitative Phase Imaging Methods for Life Sciences. Nat. Methods 2023, 20, 1645–1660. [Google Scholar] [CrossRef]
- Khalifa, M.; Albadawy, M. AI in Diagnostic Imaging: Revolutionising Accuracy and Efficiency. Comput. Methods Programs Biomed. Update 2024, 5, 100146. [Google Scholar] [CrossRef]
- Ou, J.; Zhang, J.; Alswadeh, M.; Zhu, Z.; Tang, J.; Sang, H.; Lu, K. Advancing Osteoarthritis Research: The Role of AI in Clinical, Imaging and Omics Fields. Bone Res. 2025, 13, 48. [Google Scholar] [CrossRef]
- Quinn, L.; Tryposkiadis, K.; Deeks, J.; De Vet, H.C.W.; Mallett, S.; Mokkink, L.B.; Takwoingi, Y.; Taylor-Phillips, S.; Sitch, A. Interobserver Variability Studies in Diagnostic Imaging: A Methodological Systematic Review. Br. J. Radiol. 2023, 96, 20220972. [Google Scholar] [CrossRef]
- Khalighi, S.; Reddy, K.; Midya, A.; Pandav, K.B.; Madabhushi, A.; Abedalthagafi, M. Artificial Intelligence in Neuro-Oncology: Advances and Challenges in Brain Tumor Diagnosis, Prognosis, and Precision Treatment. NPJ Precis. Oncol. 2024, 8, 80. [Google Scholar] [CrossRef]
- Mann, M.; Kumar, C.; Zeng, W.-F.; Strauss, M.T. Artificial Intelligence for Proteomics and Biomarker Discovery. Cell Syst. 2021, 12, 759–770. [Google Scholar] [CrossRef]
- Gasparyan, A.Y.; Ayvazyan, L.; Blackmore, H.; Kitas, G.D. Writing a Narrative Biomedical Review: Considerations for Authors, Peer Reviewers, and Editors. Rheumatol. Int. 2011, 31, 1409–1417. [Google Scholar] [CrossRef]
- Alhejaily, A.-M.G. Artificial Intelligence in Healthcare (Review). Biomed. Rep. 2025, 22, 11. [Google Scholar] [CrossRef]
- Koo, T.H.; Zakaria, A.D.; Ng, J.K.; Leong, X.B. Systematic Review of the Application of Artificial Intelligence in Healthcare and Nursing Care. Malays. J. Med. Sci. 2024, 31, 135–142. [Google Scholar] [CrossRef]
- Rahmani, A.M.; Yousefpoor, E.; Yousefpoor, M.S.; Mehmood, Z.; Haider, A.; Hosseinzadeh, M.; Ali Naqvi, R. Machine Learning (ML) in Medicine: Review, Applications, and Challenges. Mathematics 2021, 9, 2970. [Google Scholar] [CrossRef]
- Ahsan, M.M.; Luna, S.A.; Siddique, Z. Machine-Learning-Based Disease Diagnosis: A Comprehensive Review. Healthcare 2022, 10, 541. [Google Scholar] [CrossRef]
- Bottrighi, A.; Pennisi, M. Exploring the State of Machine Learning and Deep Learning in Medicine: A Survey of the Italian Research Community. Information 2023, 14, 513. [Google Scholar] [CrossRef]
- McMaster, C.; Bird, A.; Liew, D.F.L.; Buchanan, R.R.; Owen, C.E.; Chapman, W.W.; Pires, D.E.V. Artificial Intelligence and Deep Learning for Rheumatologists. Arthritis Rheumatol. 2022, 74, 1893–1905. [Google Scholar] [CrossRef]
- Amisha; Malik, P.; Pathania, M.; Rathaur, V. Overview of Artificial Intelligence in Medicine. J. Family Med. Prim. Care 2019, 8, 2328. [Google Scholar] [CrossRef]
- Jackson, B.R.; Rashidi, H.H.; Lennerz, J.K.; de Baca, M.E. Ethical and Regulatory Perspectives on Generative Artificial Intelligence in Pathology. Arch. Pathol. Lab. Med. 2025, 149, 123–129. [Google Scholar] [CrossRef]
- Shiferaw, K.B.; Roloff, M.; Balaur, I.; Welter, D.; Waltemath, D.; Zeleke, A.A. Guidelines and Standard Frameworks for Artificial Intelligence in Medicine: A Systematic Review. JAMIA Open 2024, 8, ooae155. [Google Scholar] [CrossRef]
- Harishbhai Tilala, M.; Kumar Chenchala, P.; Choppadandi, A.; Kaur, J.; Naguri, S.; Saoji, R.; Devaguptapu, B. Ethical Considerations in the Use of Artificial Intelligence and Machine Learning in Health Care: A Comprehensive Review. Cureus 2024, 16, e62443. [Google Scholar] [CrossRef]
- Buch, V.H.; Ahmed, I.; Maruthappu, M. Artificial Intelligence in Medicine: Current Trends and Future Possibilities. Br. J. Gen. Pract. 2018, 68, 143–144. [Google Scholar] [CrossRef]
- Dinescu, S.C.; Stoica, D.; Bita, C.E.; Nicoara, A.-I.; Cirstei, M.; Staiculesc, M.-A.; Vreju, F. Applications of Artificial Intelligence in Musculoskeletal Ultrasound: Narrative Review. Front. Med. 2023, 10, 1286085. [Google Scholar] [CrossRef]
- Shin, Y.; Yang, J.; Lee, Y.H.; Kim, S. Artificial Intelligence in Musculoskeletal Ultrasound Imaging. Ultrasonography 2021, 40, 30–44. [Google Scholar] [CrossRef]
- He, X.; Wang, M.; Zhao, C.; Wang, Q.; Zhang, R.; Liu, J.; Zhang, Y.; Qi, Z.; Su, N.; Wei, Y.; et al. Deep Learning-Based Automatic Scoring Models for the Disease Activity of Rheumatoid Arthritis Based on Multimodal Ultrasound Images. Rheumatology 2024, 63, 866–873. [Google Scholar] [CrossRef]
- Christensen, A.B.H.; Just, S.A.; Andersen, J.K.H.; Savarimuthu, T.R. Applying Cascaded Convolutional Neural Network Design Further Enhances Automatic Scoring of Arthritis Disease Activity on Ultrasound Images from Rheumatoid Arthritis Patients. Ann. Rheum. Dis. 2020, 79, 1189–1193. [Google Scholar] [CrossRef]
- Andersen, J.K.H.; Pedersen, J.S.; Laursen, M.S.; Holtz, K.; Grauslund, J.; Savarimuthu, T.R.; Just, S.A. Neural Networks for Automatic Scoring of Arthritis Disease Activity on Ultrasound Images. RMD Open 2019, 5, e000891. [Google Scholar] [CrossRef]
- D’Agostino, M.A.; Terslev, L.; Aegerter, P.; Backhaus, M.; Balint, P.; Bruyn, G.A.; Filippucci, E.; Grassi, W.; Iagnocco, A.; Jousse-Joulin, S.; et al. Scoring Ultrasound Synovitis in Rheumatoid Arthritis: A EULAR-OMERACT Ultrasound Taskforce—Part 1: Definition and Development of a Standardised, Consensus-Based Scoring System. RMD Open 2017, 3, e000428. [Google Scholar] [CrossRef]
- Mohammadi, S.; Salehi, M.A.; Jahanshahi, A.; Shahrabi Farahani, M.; Zakavi, S.S.; Behrouzieh, S.; Gouravani, M.; Guermazi, A. Artificial Intelligence in Osteoarthritis Detection: A Systematic Review and Meta-Analysis. Osteoarthr. Cartil. 2024, 32, 241–253. [Google Scholar] [CrossRef]
- Gandikota, G.; Fakuda, T.; Finzel, S. Computed Tomography in Rheumatology—From DECT to High-Resolution Peripheral Quantitative CT. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101641. [Google Scholar] [CrossRef]
- Lambert, R.G.W.; Østergaard, M.; Jaremko, J.L. Magnetic Resonance Imaging in Rheumatology. Magn. Reson. Imaging Clin. N. Am. 2018, 26, 599–613. [Google Scholar] [CrossRef]
- Hossain, M.B.; Shinde, R.K.; Oh, S.; Kwon, K.-C.; Kim, N. A Systematic Review and Identification of the Challenges of Deep Learning Techniques for Undersampled Magnetic Resonance Image Reconstruction. Sensors 2024, 24, 753. [Google Scholar] [CrossRef]
- Stoel, B. Use of Artificial Intelligence in Imaging in Rheumatology—Current Status and Future Perspectives. RMD Open 2020, 6, e001063. [Google Scholar] [CrossRef]
- Omar, M.; Watad, A.; McGonagle, D.; Soffer, S.; Glicksberg, B.S.; Nadkarni, G.N.; Klang, E. The Role of Deep Learning in Diagnostic Imaging of Spondyloarthropathies: A Systematic Review. Eur. Radiol. 2025, 35, 3661–3672. [Google Scholar] [CrossRef]
- Liu, W.-X.; Wu, H.; Cai, C.; Lai, Q.-Q.; Wang, Y.; Li, Y.-Z. Research on Automatic Recognition Radiomics Algorithm for Early Sacroiliac Arthritis Based on Sacroiliac MRI Imaging. J. Orthop. Surg. Res. 2024, 19, 96. [Google Scholar] [CrossRef]
- Jamaludin, A.; Windsor, R.; Ather, S.; Kadir, T.; Zisserman, A.; Braun, J.; Gensler, L.S.; Østergaard, M.; Poddubnyy, D.; Coroller, T.; et al. Automated Detection of Spinal Bone Marrow Oedema in Axial Spondyloarthritis: Training and Validation Using Two Large Phase 3 Trial Datasets. Rheumatology 2025, 64, 5446–5454. [Google Scholar] [CrossRef]
- Nicolaes, J.; Tselenti, E.; Aouad, T.; López-Medina, C.; Feydy, A.; Talbot, H.; Hoepken, B.; de Peyrecave, N.; Dougados, M. Performance Analysis of a Deep-Learning Algorithm to Detect the Presence of Inflammation in MRI of Sacroiliac Joints in Patients with Axial Spondyloarthritis. Ann. Rheum. Dis. 2024, 84, 60–67. [Google Scholar] [CrossRef]
- McDonald, S.M.; Felfeliyan, B.; Hassan, A.; Küpper, J.C.; El-Hajj, R.; Wichuk, S.; Aneja, A.; Kwok, C.; Zhang, C.X.Y.; Jans, L.; et al. Evaluating Potential for AI Automation of Quantitative and Semi-Quantitative MRI Scoring in Arthritis, Especially at the Knee: A Systematic Literature Review. Skeletal Radiol. 2025, 54, 2339–2349. [Google Scholar] [CrossRef]
- Wirth, W.; Maschek, S.; Wisser, A.; Eder, J.; Baumgartner, C.F.; Chaudhari, A.; Berenbaum, F.; Eckstein, F.; OA-BIO Consortium. Evaluation of an Automated Laminar Cartilage T2 Relaxation Time Analysis Method in an Early Osteoarthritis Model. Skelet. Radiol. 2025, 54, 571–584. [Google Scholar] [CrossRef]
- Hoffmann, T.; Teichgräber, U.; Brüheim, L.B.; Lassen-Schmidt, B.; Renz, D.; Weise, T.; Krämer, M.; Oelzner, P.; Böttcher, J.; Güttler, F.; et al. The Association of Symptoms, Pulmonary Function Test and Computed Tomography in Interstitial Lung Disease at the Onset of Connective Tissue Disease: An Observational Study with Artificial Intelligence Analysis of High-Resolution Computed Tomography. Rheumatol. Int. 2025, 45, 194. [Google Scholar] [CrossRef]
- Jia, J.; Hernández-Girón, I.; Schouffoer, A.A.; de Vries-Bouwstra, J.K.; Ninaber, M.K.; Korving, J.C.; Staring, M.; Kroft, L.J.M.; Stoel, B.C. Explainable Fully Automated CT Scoring of Interstitial Lung Disease for Patients Suspected of Systemic Sclerosis by Cascaded Regression Neural Networks and Its Comparison with Experts. Sci. Rep. 2024, 14, 26666. [Google Scholar] [CrossRef]
- Xie, Q.; Chen, Y.; Hu, Y.; Zeng, F.; Wang, P.; Xu, L.; Wu, J.; Li, J.; Zhu, J.; Xiang, M.; et al. Development and Validation of a Machine Learning-Derived Radiomics Model for Diagnosis of Osteoporosis and Osteopenia Using Quantitative Computed Tomography. BMC Med. Imaging 2022, 22, 140. [Google Scholar] [CrossRef]
- Nagawa, K.; Suzuki, M.; Yamamoto, Y.; Inoue, K.; Kozawa, E.; Mimura, T.; Nakamura, K.; Nagata, M.; Niitsu, M. Texture Analysis of Muscle MRI: Machine Learning-Based Classifications in Idiopathic Inflammatory Myopathies. Sci. Rep. 2021, 11, 9821. [Google Scholar] [CrossRef]
- Honda, S.; Yano, K.; Tanaka, E.; Ikari, K.; Harigai, M. Development of a Scoring Model for the Sharp/van Der Heijde Score Using Convolutional Neural Networks and Its Clinical Application. Rheumatology 2023, 62, 2272–2283. [Google Scholar] [CrossRef]
- Venäläinen, M.S.; Biehl, A.; Holstila, M.; Kuusalo, L.; Elo, L.L. Deep Learning Enables Automatic Detection of Joint Damage Progression in Rheumatoid Arthritis-Model Development and External Validation. Rheumatology 2025, 64, 1068–1076. [Google Scholar] [CrossRef]
- Moradmand, H.; Ren, L. Multistage Deep Learning Methods for Automating Radiographic Sharp Score Prediction in Rheumatoid Arthritis. Sci. Rep. 2025, 15, 3391. [Google Scholar] [CrossRef]
- Bird, A.; Oakden-Rayner, L.; Chakradeo, K.; Thomas, R.; Gupta, D.; Jain, S.; Jacob, R.; Ray, S.; Wechalekar, M.D.; Proudman, S.; et al. AI Automated Radiographic Scoring in Rheumatoid Arthritis: Shedding Light on Barriers to Implementation through Comprehensive Evaluation. Semin. Arthritis Rheum. 2025, 74, 152761. [Google Scholar] [CrossRef]
- Bo, Z.; Coates, L.C.; Papież, B.W. Deep Learning Models to Automate the Scoring of Hand Radiographs for Rheumatoid Arthritis. In Medical Image Understanding and Analysis; Springer Nature: Cham, Switzerland, 2024; pp. 398–413. ISBN 9783031669576. [Google Scholar]
- Fung, D.L.; Liu, Q.; Islam, S.; Lac, L.; O’Neil, L.; Hitchon, C.A.; Hu, P. Deep Learning-Based Joint Detection in Rheumatoid Arthritis Hand Radiographs. AMIA Jt. Summits Transl. Sci. Proc. 2023, 2023, 206–215. [Google Scholar]
- Lien, C.Y.; Wang, H.J.; Lu, C.K.; Hsu, T.H.; Chu, W.C.; Lai, C.C. Deep Learning with an Attention Mechanism for Enhancing Automated Modified Total Sharp/van der Heijde Scoring of Hand X-ray Images in Rheumatoid Arthritis. J. Med. Biol. Eng. 2025, 45, 298–306. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, J.; Chen, W. Artificial Intelligence in Rheumatoid Arthritis. Rheumatol. Autoimmun. 2025, 5, 88–100. [Google Scholar] [CrossRef]
- Wang, H.; Ou, Y.; Fang, W.; Ambalathankandy, P.; Goto, N.; Ota, G.; Okino, T.; Fukae, J.; Sutherland, K.; Ikebe, M.; et al. A Deep Registration Method for Accurate Quantification of Joint Space Narrowing Progression in Rheumatoid Arthritis. Comput. Med. Imaging Graph. 2023, 108, 102273. [Google Scholar] [CrossRef]
- Cutolo, M.; Smith, V. Detection of Microvascular Changes in Systemic Sclerosis and Other Rheumatic Diseases. Nat. Rev. Rheumatol. 2021, 17, 665–677. [Google Scholar] [CrossRef]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 Classification Criteria for Systemic Sclerosis: An American College of rheumatology/European League against Rheumatism Collaborative Initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef]
- Brzezińska, O.E.; Rychlicki-Kicior, K.A.; Makowska, J.S. Automatic Assessment of Nailfold Capillaroscopy Software: A Pilot Study. Reumatologia 2024, 62, 346–350. [Google Scholar] [CrossRef]
- Dinsdale, G.; Moore, T.; O’Leary, N.; Tresadern, P.; Berks, M.; Roberts, C.; Manning, J.; Allen, J.; Anderson, M.; Cutolo, M.; et al. Intra-and Inter-Observer Reliability of Nailfold Videocapillaroscopy—A Possible Outcome Measure for Systemic Sclerosis-Related Microangiopathy. Microvasc. Res. 2017, 112, 1–6. [Google Scholar] [CrossRef]
- Smith, V.; Beeckman, S.; Herrick, A.L.; Decuman, S.; Deschepper, E.; De Keyser, F.; Distler, O.; Foeldvari, I.; Ingegnoli, F.; Müller-Ladner, U.; et al. An EULAR Study Group Pilot Study on Reliability of Simple Capillaroscopic Definitions to Describe Capillary Morphology in Rheumatic Diseases. Rheumatology 2016, 55, 883–890. [Google Scholar] [CrossRef]
- Berks, M.; Dinsdale, G.; Marjanovic, E.; Murray, A.; Taylor, C.; Herrick, A.L. Comparison between Low Cost USB Nailfold Capillaroscopy and Videocapillaroscopy: A Pilot Study. Rheumatology 2021, 60, 3862–3867. [Google Scholar] [CrossRef]
- Lledó-Ibáñez, G.M.; Sáez Comet, L.; Freire Dapena, M.; Mesa Navas, M.; Martín Cascón, M.; Guillén del Castillo, A.; Simeon, C.P.; Martinez Robles, E.; Todolí Parra, J.; Varela, D.C.; et al. CAPI-Detect: Machine Learning in Capillaroscopy Reveals New Variables Influencing Diagnosis. Rheumatology 2025, 64, 3667–3675. [Google Scholar] [CrossRef]
- Bharathi, P.G.; Berks, M.; Dinsdale, G.; Murray, A.; Manning, J.; Wilkinson, S.; Cutolo, M.; Smith, V.; Herrick, A.L.; Taylor, C.J. A Deep Learning System for Quantitative Assessment of Microvascular Abnormalities in Nailfold Capillary Images. Rheumatology 2023, 62, 2325–2329. [Google Scholar] [CrossRef]
- Emam, O.S.; Ebadi Jalal, M.; Garcia-Zapirain, B.; Elmaghraby, A.S. Artificial Intelligence Algorithms in Nailfold Capillaroscopy Image Analysis: A Systematic Review. medRxiv 2024. [Google Scholar] [CrossRef]
- Di Battista, M.; Colak, S.; Howard, A.; Donadoni, F.; Owen-Smith, C.; Rindone, A.; Di Donato, S.; Hartley, C.; Bissell, L.-A.; Del Galdo, F. Artificial Intelligence-Based Raynaud’s Quantification Index (ARTIX): An Objective Mobile-Based Tool for Patient-Centered Assessment of Raynaud’s Phenomenon. Arthritis Res. Ther. 2025, 27, 120. [Google Scholar] [CrossRef]
- Gracia Tello, B.D.C.; Sáez Comet, L.; Lledó, G.; Freire Dapena, M.; Mesa, M.A.; Martín-Cascón, M.; Guillén Del Castillo, A.; Martínez Robles, E.; Simeón-Aznar, C.P.; Todolí Parra, J.A.; et al. Capi-Score: A Quantitative Algorithm for Identifying Disease Patterns in Nailfold Videocapillaroscopy. Rheumatology 2024, 63, 3315–3321. [Google Scholar] [CrossRef]
- Berks, M.; Gurunath Bharathi, P.; Murray, A.; Dinsdale, G.; Manning, J.; Herrick, A.L.; Taylor, C. POS0828 Automated analysis of nailfold capillaries using a low-cost usb microscope—A route to increasing uptake of nailfold capillaroscopy in the out-patient clinic? Ann. Rheum. Dis. 2024, 83, 1057–1058. [Google Scholar] [CrossRef]
- Garaiman, A.; Nooralahzadeh, F.; Mihai, C.; Gonzalez, N.P.; Gkikopoulos, N.; Becker, M.O.; Distler, O.; Krauthammer, M.; Maurer, B. Vision Transformer Assisting Rheumatologists in Screening for Capillaroscopy Changes in Systemic Sclerosis: An Artificial Intelligence Model. Rheumatology 2023, 62, 2492–2500. [Google Scholar] [CrossRef]
- Rahman, H.; Biswash, A.R.; Debnath, A.; Siddique, A.B.; Rahman, M.; Hasan, M.; Mou, M.A. The Future of AI in Laboratory Medicine: Advancing Diagnostics, Personalization, and Healthcare Innovation. J. Primeasia 2025, 6, 1–6. [Google Scholar] [CrossRef]
- Haymond, S.; McCudden, C. Rise of the Machines: Artificial Intelligence and the Clinical Laboratory. J. Appl. Lab. Med. 2021, 6, 1640–1654. [Google Scholar] [CrossRef]
- Srinivasu, P.N. Computational Intelligence and Machine Learning Approaches in Biomedical Engineering and Health Care Systems; Bentham Science Publishers: Sharjah, United Arab Emirates, 2022; ISBN 9781681089577. [Google Scholar]
- Ginghina, O.; Hudita, A.; Zamfir, M.; Spanu, A.; Mardare, M.; Bondoc, I.; Buburuzan, L.; Georgescu, S.E.; Costache, M.; Negrei, C.; et al. Liquid Biopsy and Artificial Intelligence as Tools to Detect Signatures of Colorectal Malignancies: A Modern Approach in Patient’s Stratification. Front. Oncol. 2022, 12, 856575. [Google Scholar] [CrossRef]
- Quan, T.; Yuan, Y.; Luo, Y.; Zhou, T.; Qin, J. Robust Exclusive Adaptive Sparse Feature Selection for Biomarker Discovery and Early Diagnosis of Neuropsychiatric Systemic Lupus Erythematosus. In Proceedings of the 26th International Conference on Medical Image Computing and Computer Assisted Intervention—MICCAI 2023, Vancouver, BC, Canada, 8–12 October 2023. [Google Scholar]
- Tiwari, A.; Mishra, S.; Kuo, T.-R. Current AI Technologies in Cancer Diagnostics and Treatment. Mol. Cancer 2025, 24, 159. [Google Scholar] [CrossRef]
- Calvino, G.; Farro, J.; Zampatti, S.; Peconi, C.; Megalizzi, D.; Trastulli, G.; Andreucci, S.; Cascella, R.; Strafella, C.; Caltagirone, C.; et al. From Genomics to AI: Revolutionizing Precision Medicine in Oncology. Appl. Sci. 2025, 15, 6578. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Liao, J.; Li, X.; Gan, Y.; Han, S.; Rong, P.; Wang, W.; Li, W.; Zhou, L. Artificial Intelligence Assists Precision Medicine in Cancer Treatment. Front. Oncol. 2022, 12, 998222. [Google Scholar] [CrossRef]
- Chang, L.; Liu, J.; Zhu, J.; Guo, S.; Wang, Y.; Zhou, Z.; Wei, X. Advancing Precision Medicine: The Transformative Role of Artificial Intelligence in Immunogenomics, Radiomics, and Pathomics for Biomarker Discovery and Immunotherapy Optimization. Cancer Biol. Med. 2025, 22, 33–47. [Google Scholar] [CrossRef]
- Yasar, S.; Yagin, F.H.; Melekoglu, R.; Ardigò, L.P. Integrating Proteomics and Explainable Artificial Intelligence: A Comprehensive Analysis of Protein Biomarkers for Endometrial Cancer Diagnosis and Prognosis. Front. Mol. Biosci. 2024, 11, 1389325. [Google Scholar] [CrossRef]
- Yetgin, A. Revolutionizing Multi-omics Analysis with Artificial Intelligence and Data Processing. Quant. Biol. 2025, 13, e70002. [Google Scholar] [CrossRef]
- Alum, E.U. AI-Driven Biomarker Discovery: Enhancing Precision in Cancer Diagnosis and Prognosis. Discov. Oncol. 2025, 16, 313. [Google Scholar] [CrossRef]
- Elemento, O.; Leslie, C.; Lundin, J.; Tourassi, G. Artificial Intelligence in Cancer Research, Diagnosis and Therapy. Nat. Rev. Cancer 2021, 21, 747–752. [Google Scholar] [CrossRef]
- Toussaint, P.A.; Leiser, F.; Thiebes, S.; Schlesner, M.; Brors, B.; Sunyaev, A. Explainable artificial intelligence for omics data: A systematic mapping study. Brief. Bioinform. 2024, 25, bbad453. [Google Scholar] [CrossRef]
- Tan, K.; Huang, W.; Liu, X.; Hu, J.; Dong, S. A Multi-Modal Fusion Framework Based on Multi-Task Correlation Learning for Cancer Prognosis Prediction. Artif. Intell. Med. 2022, 126, 102260. [Google Scholar] [CrossRef]
- Mondillo, G.; Colosimo, S.; Perrotta, A.; Frattolillo, V.; Gicchino, M.F. Unveiling Artificial Intelligence’s Power: Precision, Personalization, and Progress in Rheumatology. J. Clin. Med. 2024, 13, 6559. [Google Scholar] [CrossRef]
- Vlad, A.L.; Popazu, C.; Lescai, A.-M.; Voinescu, D.C.; Baltă, A.A. Ștefania The Role of Artificial Intelligence in the Diagnosis and Management of Rheumatoid Arthritis. Medicina 2025, 61, 689. [Google Scholar] [CrossRef]
- Salehi, F.; Zarifi, S.; Bayat, S.; Habibpour, M.; Asemanrafat, A.; Kleyer, A.; Schett, G.; Fritsch-Stork, R.; Eskofier, B.M. Predicting Disease Activity Score in Rheumatoid Arthritis Patients Treated with Biologic Disease-Modifying Antirheumatic Drugs Using Machine Learning Models. Technologies 2025, 13, 350. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Chen, Y.; Luo, D.; Xu, K.; Zhang, L. Artificial Intelligence for Predicting Treatment Responses in Autoimmune Rheumatic Diseases: Advancements, Challenges, and Future Perspectives. Front. Immunol. 2024, 15, 1477130. [Google Scholar] [CrossRef]
- Gorrepati, L.P. Integrating AI with Electronic Health Records (EHRs) to Enhance Patient Care. Int. J. Health Sci. 2024, 7, 38–50. [Google Scholar] [CrossRef]
- Joo, Y.B.; Baek, I.-W.; Park, Y.-J.; Park, K.-S.; Kim, K.-J. Machine Learning–Based Prediction of Radiographic Progression in Patients with Axial Spondyloarthritis. Clin. Rheumatol. 2020, 39, 983–991. [Google Scholar] [CrossRef]
- Hossain, E.; Rana, R.; Higgins, N.; Soar, J.; Barua, P.D.; Pisani, A.R.; Turner, K. Natural Language Processing in Electronic Health Records in Relation to Healthcare Decision-Making: A Systematic Review. Comput. Biol. Med. 2023, 155, 106649. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, R.; Panda, B.S.; Kumar, R.; Muften, N.F.; Abass, M.A.; Lozanović, J. Machine Learning-Powered Smart Healthcare Systems in the Era of Big Data: Applications, Diagnostic Insights, Challenges, and Ethical Implications. Diagnostics 2025, 15, 1914. [Google Scholar] [CrossRef]
- Khan, B.; Fatima, H.; Qureshi, A.; Kumar, S.; Hanan, A.; Hussain, J.; Abdullah, S. Drawbacks of Artificial Intelligence and Their Potential Solutions in the Healthcare Sector. Biomed. Mater. Devices 2023, 1, 731–738. [Google Scholar] [CrossRef]
- Gilvaz, V.J.; Sudheer, A.; Reginato, A.M. Emerging Artificial Intelligence Innovations in Rheumatoid Arthritis and Challenges to Clinical Adoption. Curr. Rheumatol. Rep. 2025, 27, 28. [Google Scholar] [CrossRef]
- El Arab, R.A.; Abu-Mahfouz, M.S.; Abuadas, F.H.; Alzghoul, H.; Almari, M.; Ghannam, A.; Seweid, M.M. Bridging the Gap: From AI Success in Clinical Trials to Real-World Healthcare implementation—A Narrative Review. Healthcare 2025, 13, 701. [Google Scholar] [CrossRef]
- Tang, L.; Li, J.; Fantus, S. Medical Artificial Intelligence Ethics: A Systematic Review of Empirical Studies. Digit. Health 2023, 9, 20552076231186064. [Google Scholar] [CrossRef]
- Al Zo’ubi, M. Review of 2024 Publications on the Applications of Artificial Intelligence in Rheumatology. Clin. Rheumatol. 2025, 44, 1427–1438. [Google Scholar] [CrossRef]
- Stafie, C.S.; Sufaru, I.-G.; Ghiciuc, C.M.; Stafie, I.-I.; Sufaru, E.-C.; Solomon, S.M.; Hancianu, M. Exploring the Intersection of Artificial Intelligence and Clinical Healthcare: A Multidisciplinary Review. Diagnostics 2023, 13, 1995. [Google Scholar] [CrossRef]
- Bobak, C.A.; Svoboda, M.; Giffin, K.A.; Wall, D.P.; Moore, J. Raising the Stakeholders: Improving Patient Outcomes through Interprofessional Collaborations in AI for Healthcare. Pac. Symp. Biocomput. 2021, 26, 351–355. [Google Scholar]
- Yan, A.P.; Guo, L.L.; Inoue, J.; Arciniegas, S.E.; Vettese, E.; Wolochacz, A.; Crellin-Parsons, N.; Purves, B.; Wallace, S.; Patel, A.; et al. A Roadmap to Implementing Machine Learning in Healthcare: From Concept to Practice. Front. Digit. Health 2025, 7, 1462751. [Google Scholar] [CrossRef]
- Venerito, V. Artificial Intelligence in Rheumatology: Days of a Future Past. Rheumatol. Adv. Pract. 2025, 9, rkaf022. [Google Scholar] [CrossRef]
- Purohit, R.; Saineni, S.; Chalise, S.; Mathai, R.; Sambandam, R.; Medina-Perez, R.; Bhanusali, N. Artificial Intelligence in Rheumatology: Perspectives and Insights from a Nationwide Survey of U.S. Rheumatology Fellows. Rheumatol. Int. 2024, 44, 3053–3061. [Google Scholar] [CrossRef]
- Holzer, M.T.; Meinecke, A.; Mueller, F.; Haase, I.; Morf, H.; Witte, T.; Labinsky, H.; Klemm, P.; Knitza, J.; Krusche, M. AB1347 Perspectives of Artificial Intelligence in Rheumatology- Results from a Prospective Survey in Germany. Ann. Rheum. Dis. 2024, 83 (Suppl. S1), 2025. [Google Scholar] [CrossRef]
- EULAR School of Rheumatology. Course: Machine Learning in Rheumatology. 2024. Available online: https://esor.eular.org/course/view.php?id=799 (accessed on 27 October 2025).
| COMPANY/PROJECT | REGION | APPLICATION/DESCRIPTION |
|---|---|---|
| SIEMENS, GE, PHILIPS | EU and US | Automation and quality in laboratory diagnostics |
| EMPAIA | EU | Standardization and integration of AI in digital pathology |
| ABIONIC | EU/US | Rapid sepsis diagnosis via PSP |
| DXCOVER | EU/US | Liquid biopsy for early cancer detection |
| OWKIN | EU/US | Pre-screening and prognosis in oncology |
| SOPHIA GENETICS | Global | Genomic and multi-omics data analysis |
| APPLIED SPECTRAL IMAGING | US | Automated microscopy and analysis |
| DXPLAIN | US | Decision support based on symptoms and laboratory data |
| ML MODELS (CRP AND OTHERS) | EU (Slovenia), others | Differentiation between viral/bacterial infection using routine markers |
| CLINLABOMICS AND AI ANALYSIS | Global | AI across all phases of the laboratory process |
| Field | Examples of AI Applications |
|---|---|
| Genomics | DeepVariant, DeepSEA, AlphaFold, biobank AI for proteomics and subtyping |
| Transcriptomics | Diagnosis and prognosis via RNA-Seq (AML, TNBC), immune microenvironment (CIBERSORT, etc.) |
| Proteomics | Discovery of prognostic protein markers, XAI models for early diagnosis |
| Multi-omics | Multimodal models (DL + ML), PandaOmics, integration of omics and clinical data |
| Explainability (xai) | Improving the reliability and interpretation of AI results |
| Images + data | Virchow2 (H & E slides to genomics), Pathomic Fusion (images + genomics) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrov, S.; Bogdanova, S.; Apostolova, Z.; Kasapska, B.; Kabakchieva, P.; Georgiev, T. Transition to Artificial Intelligence in Imaging and Laboratory Diagnostics in Rheumatology. Appl. Sci. 2025, 15, 11666. https://doi.org/10.3390/app152111666
Dimitrov S, Bogdanova S, Apostolova Z, Kasapska B, Kabakchieva P, Georgiev T. Transition to Artificial Intelligence in Imaging and Laboratory Diagnostics in Rheumatology. Applied Sciences. 2025; 15(21):11666. https://doi.org/10.3390/app152111666
Chicago/Turabian StyleDimitrov, Stoimen, Simona Bogdanova, Zhaklin Apostolova, Boryana Kasapska, Plamena Kabakchieva, and Tsvetoslav Georgiev. 2025. "Transition to Artificial Intelligence in Imaging and Laboratory Diagnostics in Rheumatology" Applied Sciences 15, no. 21: 11666. https://doi.org/10.3390/app152111666
APA StyleDimitrov, S., Bogdanova, S., Apostolova, Z., Kasapska, B., Kabakchieva, P., & Georgiev, T. (2025). Transition to Artificial Intelligence in Imaging and Laboratory Diagnostics in Rheumatology. Applied Sciences, 15(21), 11666. https://doi.org/10.3390/app152111666








