Integration of Active Personal Dosimeters, Videos from In-Room Monitors, and Videos from the Surgeon’s Main Panel Reveal Pitfalls in Radiation Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
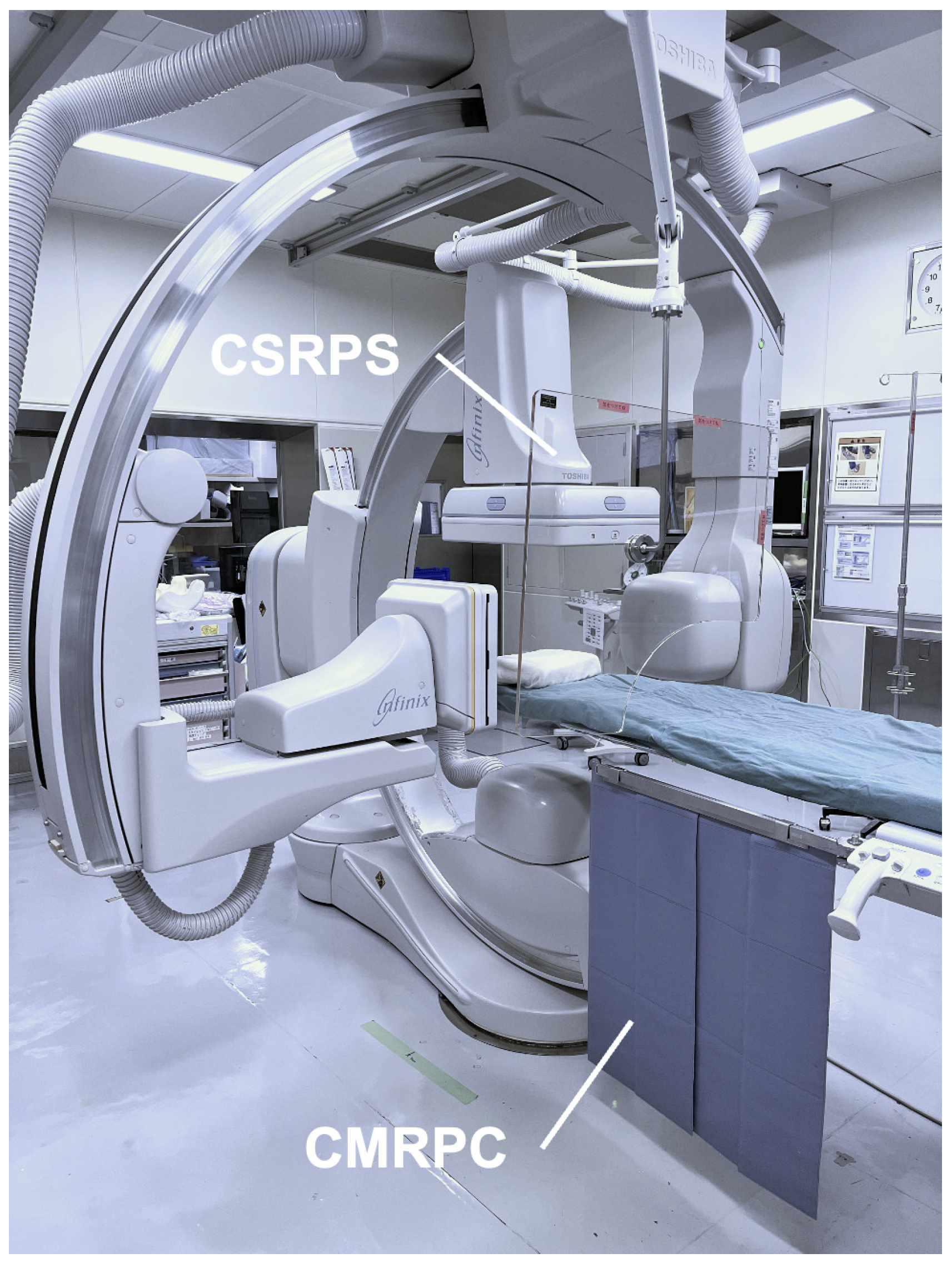
2.2. Angiography System
2.3. Active Personal Dosimeter System
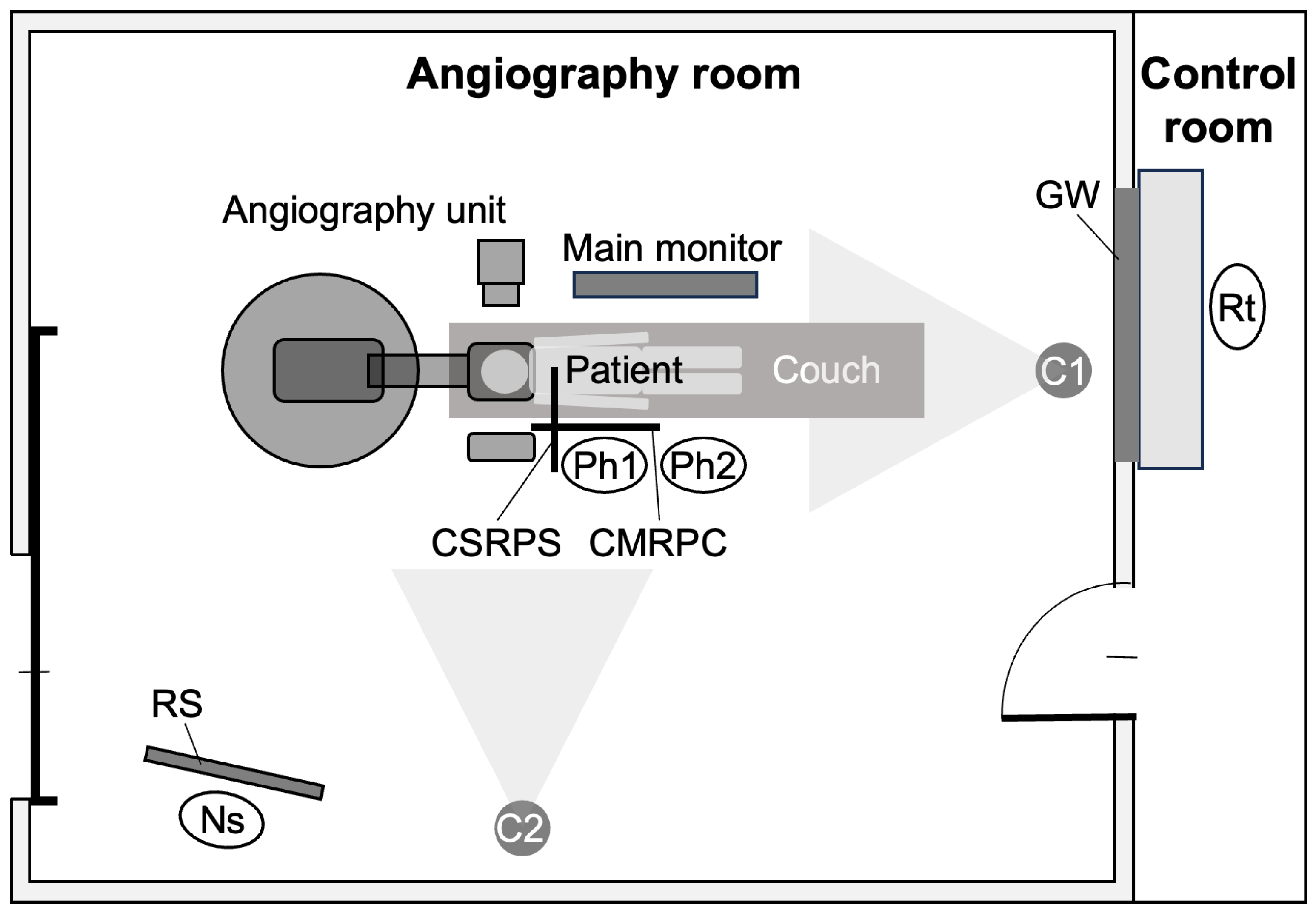
2.4. Placement of Staff and In-Room Monitoring Cameras
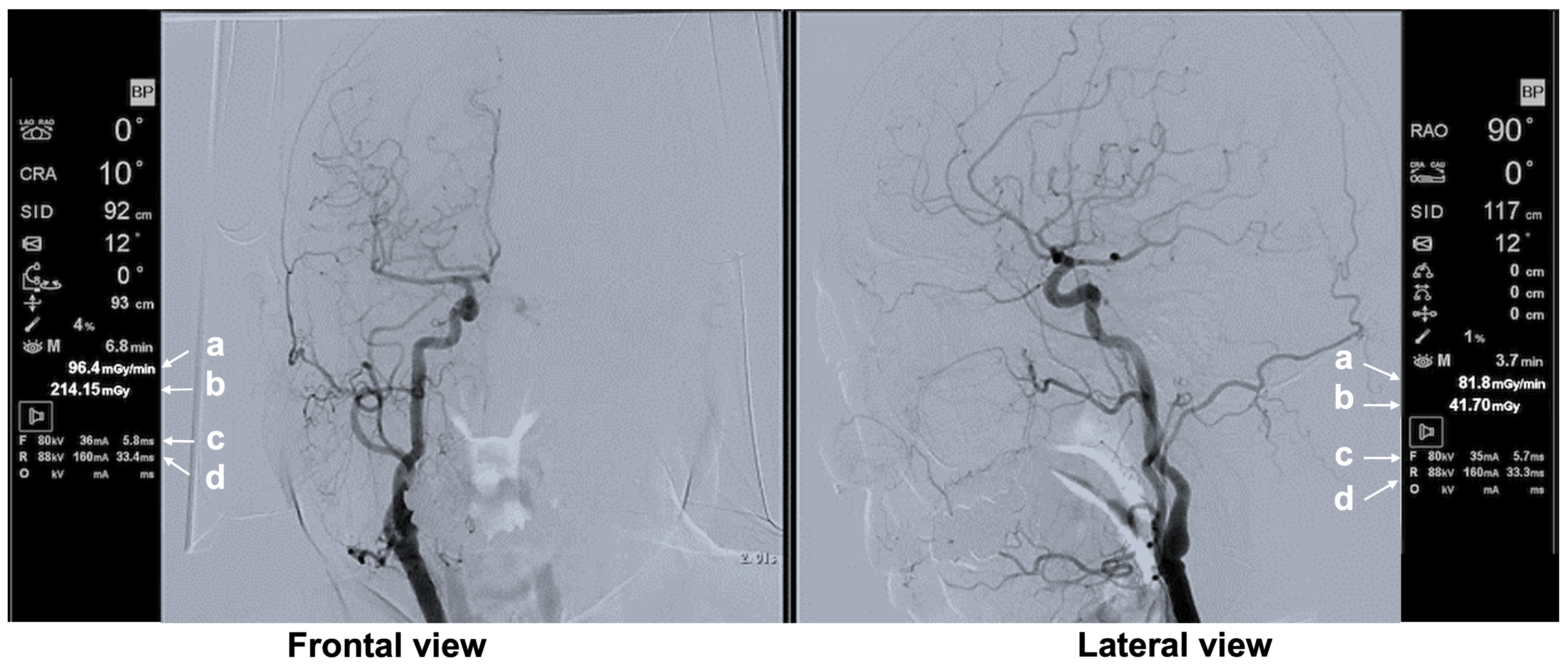
2.5. Recording of the Operator’s Main Monitor Screen
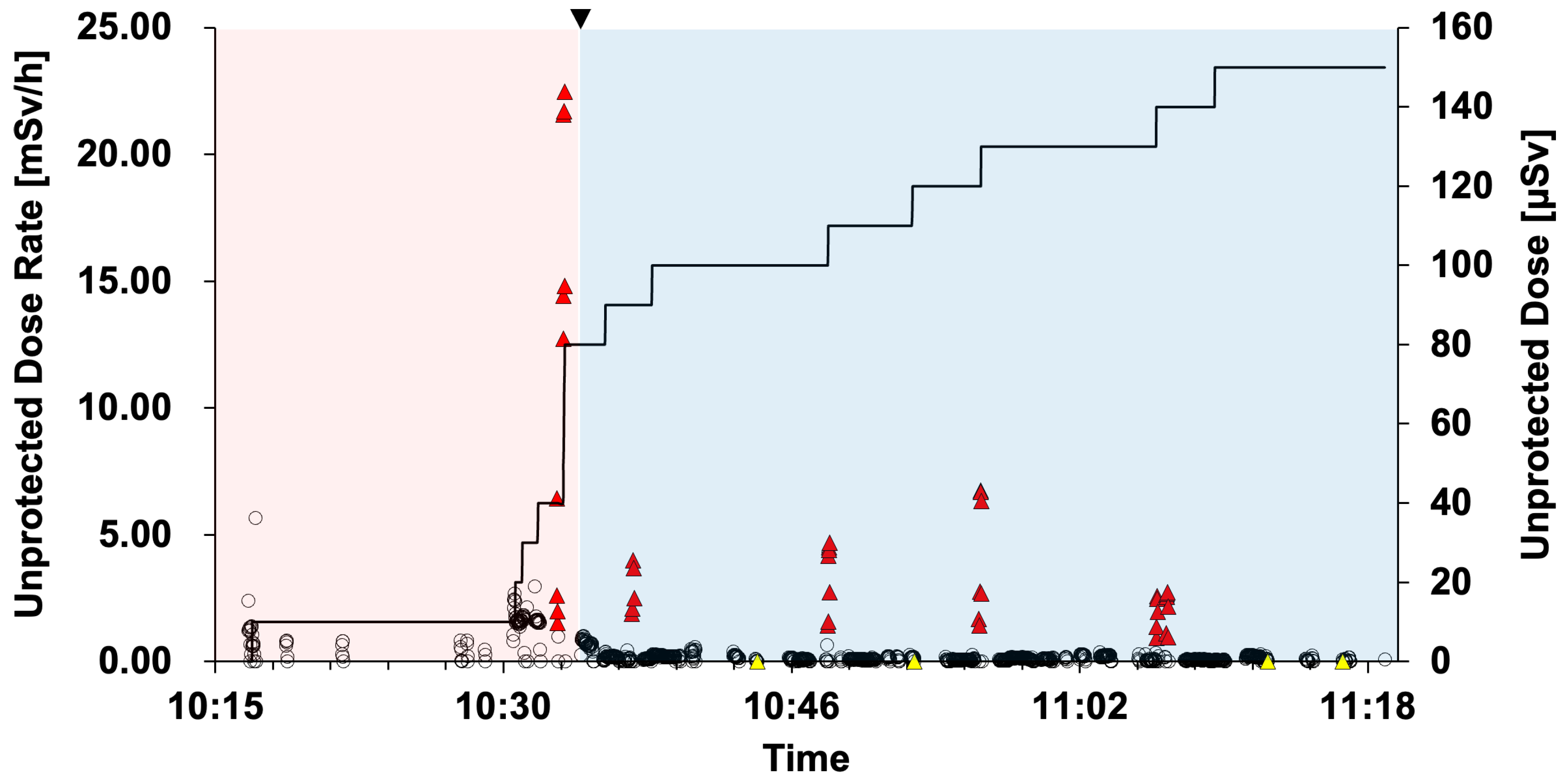
2.6. Analysis of Operator Unprotected Dose
2.7. Analysis of Factors Affecting Operator Unprotected Dose Rate
2.8. Statistical Analysis
3. Results
3.1. Operator Unprotected Dose Rate and Unprotected Dose
3.2. Effect of CSRPS Placement on Operator Unprotected Dose Rate
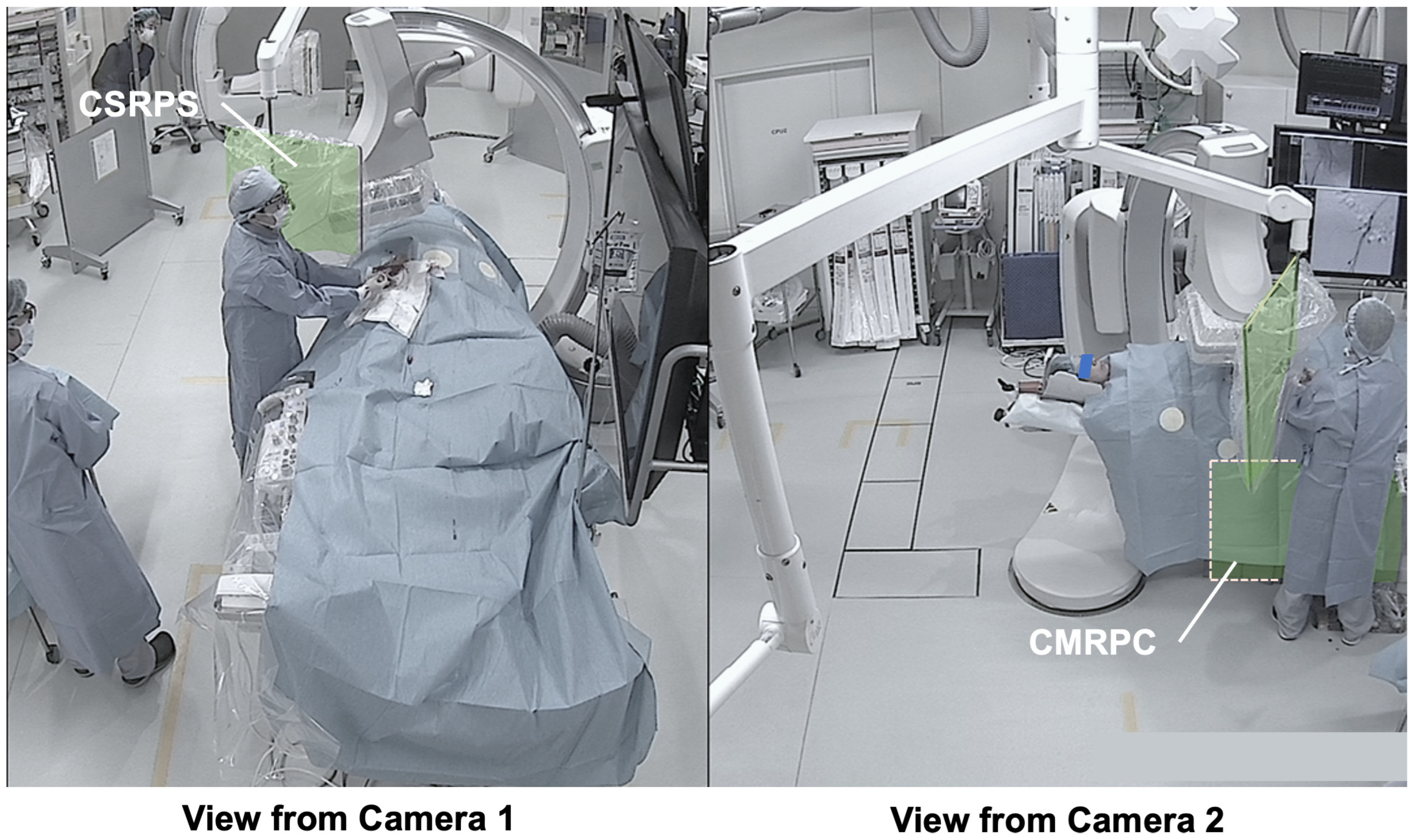
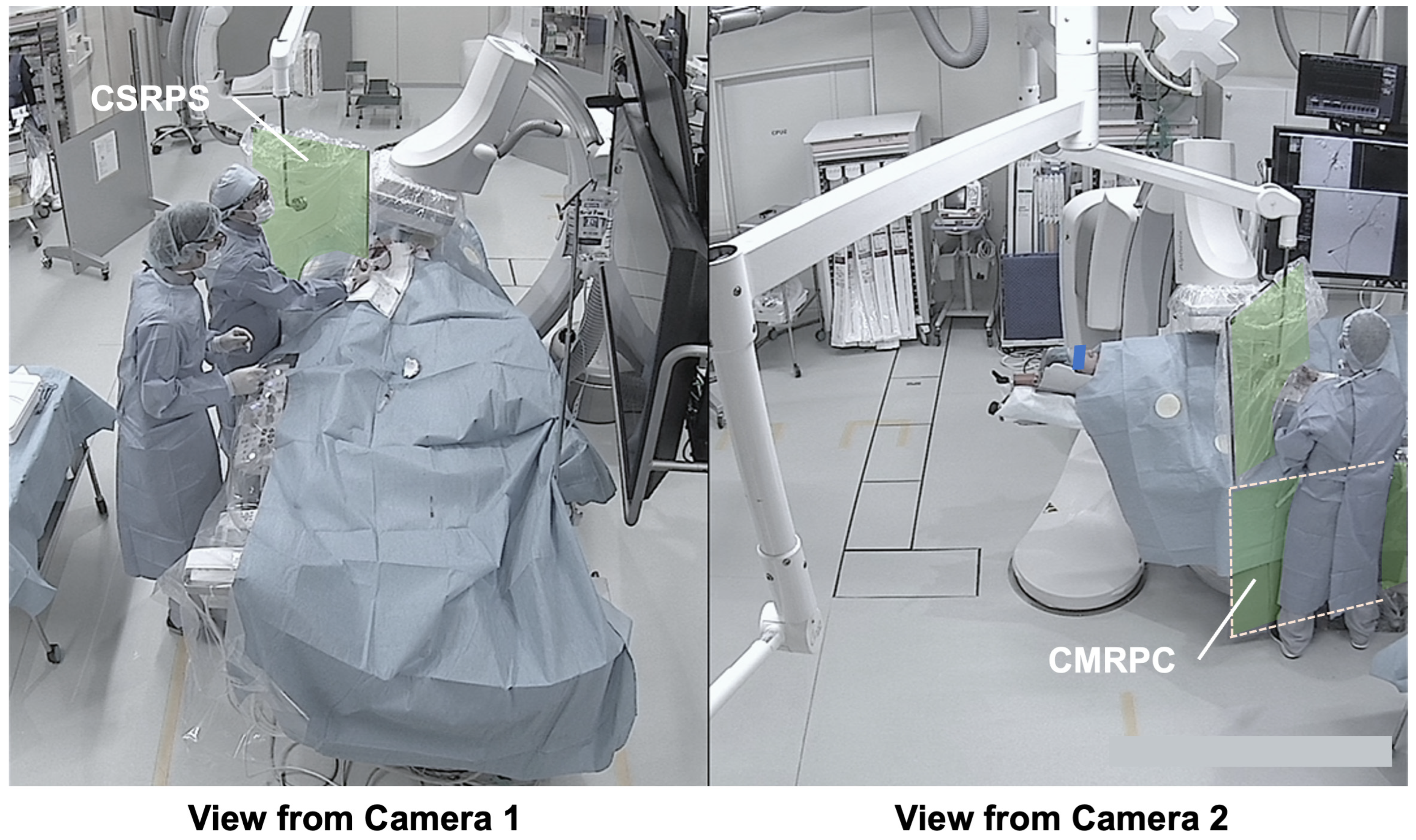
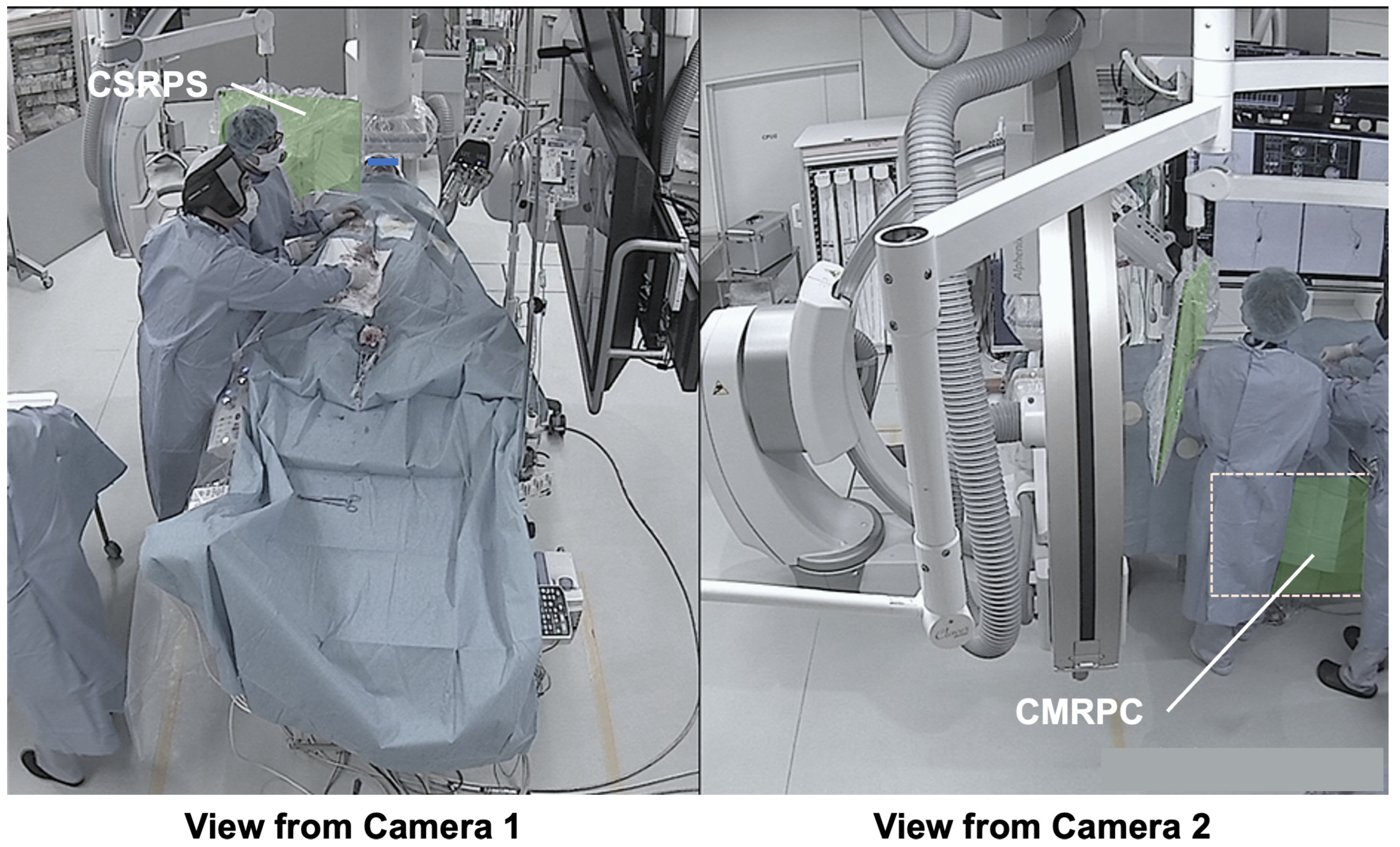
3.3. Presentation of Typical Scenes
3.3.1. Typical Scene 1 (Case 5)
3.3.2. Typical Scene 2 (Case 5)
3.3.3. Typical Scene 3 (Case 3)
3.3.4. Typical Scene 4 (Case 3)
3.3.5. Typical Scene 5 (Case 4)
4. Discussion
- Dual-angle in-room monitoring cameras provided good depth perception, making it easier to assess the appropriateness of CSRPS placement;
- The added camera on the patient’s right side allowed confirmation of the distance between the CSRPS and the operator;
- The additional camera on the patient’s right side allowed confirmation of the CMRPC placement status under the patient bed;
- Time-synchronized main monitor information enabled verification of the specific operator actions and irradiation conditions during exposure.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APD | active personal dosimeter |
| CSRPS | ceiling-suspended radiation protective shield |
| CMRPC | couch-mounted radiation protective curtain |
| C-CAG | common carotid artery angiography |
| SAG | subclavian artery angiography |
| DSA | digital subtraction angiography |
| RAO | right anterior oblique |
| LAO | left anterior oblique |
References
- ICRP. Statement on Tissue Reactions; ICRP: Ottawa, ON, Canada, 2011; ref 4825-3093-1464. [Google Scholar]
- ICRP. ICRP Statement on Tissue Reactions/Early and Late Effects of Radiation in Normal Tissues and Organs—Threshold Doses for Tissue Reactions in a Radiation Protection Context. ICRP publication 118. Ann. ICRP 2012, 41, 1–322. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare Ordinance Eighty-Second Number. Available online: https://www.mhlw.go.jp/content/001080975.pdf (accessed on 1 September 2025).
- Kato, T.; Fujisawa, M.; Hattori, K.; Yamada, A.; Haga, Y.; Kaga, Y.; Abe, M.; Inaba, Y.; Chida, K. Initial considerations for real-time measurement of lens dose in PCI surgeons. Nippon Hoshasen Anzen Kanri Gakkai-Shi 2023, 22, 10–18. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Moritake, T.; Morota, K.; Nagamoto, K.; Nakagami, K.; Kuriyama, T.; Kunugita, N. Development and assessment of an educational application for the proper use of ceiling-suspended radiation shielding screens in angiography rooms using augmented reality technology. Eur. J. Radiol. 2021, 143, 109925. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Moritake, T.; Sun, L.; Kobayashi, I.; Kawauchi, S.; Abe, T.; Tsukamoto, A.; Morimoto, Y.; Daida, H.; Matsumaru, Y. Monitoring and protection against radiation dose to eyes of operators performing neuroendovascular procedures: A nationwide study in Japan. J. Neuroendovasc. Ther. 2022, 16, 354–360. [Google Scholar] [CrossRef]
- Sumi, S.; Yasuda, M.; Ohtani, H.; Ishimoto, Y.; Wakabayashi, K.; Sai, S.; Sato, H.; Kato, K. The effect of radiation protection education for the operators’ ocular lens in cardiac catheterization. Nihon Hoshasen Gijutsu Gakkai Zasshi 2021, 77, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Chida, K. What are useful methods to reduce occupational radiation exposure among radiological medical workers, especially for interventional radiology personnel? Radiol. Phys. Technol. 2022, 15, 101–115. [Google Scholar] [CrossRef]
- Nakagami, K.; Moritake, T.; Nagamoto, K.; Morota, K.; Matsuzaki, S.; Kuriyama, T.; Kunugita, N. Strategy to reduce the collective equivalent dose for the lens of the physician’s eye using short radiation protection curtains to prevent cataracts. Diagnostics 2021, 11, 1415. [Google Scholar] [CrossRef]
- Akahane, M.; Yoshioka, N.; Kiryu, S. Radiation protection of the eye lens in fluoroscopy-guided interventional procedures. Interv. Radiol. 2022, 7, 44–48. [Google Scholar] [CrossRef]
- Haga, Y.; Chida, K.; Kaga, Y.; Sota, M.; Meguro, T.; Zuguchi, M. Occupational eye dose in interventional cardiology procedures. Sci. Rep. 2017, 7, 569. [Google Scholar] [CrossRef]
- Kato, M.; Chida, K.; Ishida, T.; Toyoshima, H.; Yoshida, Y.; Yoshioka, S.; Moroi, J.; Kinoshita, T. Occupational radiation exposure of the eye in neurovascular interventional physician. Radiat. Prot. Dosim. 2019, 185, 151–156. [Google Scholar] [CrossRef]
- Kato, M.; Chida, K.; Munehisa, M.; Sato, T.; Inaba, Y.; Suzuki, M.; Zuguchi, M. Non-lead protective aprons for the protection of interventional radiology physicians from radiation exposure in clinical settings: An initial study. Diagnostics 2021, 11, 1613. [Google Scholar] [CrossRef]
- Sato, T.; Eguchi, Y.; Yamazaki, C.; Hino, T.; Saida, T.; Chida, K. Development of a new radiation shield for the face and neck of IVR physicians. Bioengineering 2022, 9, 354. [Google Scholar] [CrossRef]
- Endo, M.; Haga, Y.; Sota, M.; Tanaka, A.; Otomo, K.; Murabayashi, Y.; Abe, M.; Kaga, Y.; Inaba, Y.; Suzuki, M.; et al. Evaluation of novel X-ray protective eyewear in reducing the eye dose to interventional radiology physicians. J. Radiat. Res. 2021, 62, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Fujibuchi, T.; Fujita, K.; Igarashi, T.; Nishimaru, E.; Horita, S.; Sakurai, R.; Ono, K. Proposal for reduction measures of eye lens exposure based on actual exposure management in radiation-exposed medical staff. Nihon Hoshasen Gijutsu Gakkai Zasshi 2021, 77, 160–171. [Google Scholar] [CrossRef]
- Hamada, N.; Fujimichi, Y. Classification of radiation effects for dose limitation purposes: History, current situation and future prospects. J. Radiat. Res. 2014, 55, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N.; Fujimichi, Y.; Iwasaki, T.; Fujii, N.; Furuhashi, M.; Kubo, E.; Minamino, T.; Nomura, T.; Sato, H. Emerging issues in radiogenic cataracts and cardiovascular disease. J. Radiat. Res. 2014, 55, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Chida, K.; Satsurai, K.; Haga, Y.; Kaga, Y.; Abe, M.; Inaba, Y.; Zuguchi, M. A phantom study to determine the optimal placement of eye dosemeters on interventional cardiology staff. Radiat. Prot. Dosim. 2019, 185, 409–413. [Google Scholar] [CrossRef]
- Ishii, H.; Chida, K.; Satsurai, K.; Haga, Y.; Kaga, Y.; Abe, M.; Inaba, Y.; Zuguchi, M. Occupational eye dose correlation with neck dose and patient-related quantities in interventional cardiology procedures. Radiol. Phys. Technol. 2022, 15, 54–62. [Google Scholar] [CrossRef]
- Kuriyama, T.; Moritake, T.; Nakagami, K.; Morota, K.; Hitomi, G.; Kitamura, H. Background factors affecting the radiation exposure of the lens of the eye among nurses in interventional radiology: A quantitative observational study. Nurs. Rep. 2024, 14, 413–427. [Google Scholar] [CrossRef]
- Ohno, S.; Konta, S.; Shindo, R.; Yamamoto, K.; Isobe, R.; Inaba, Y.; Suzuki, M.; Zuguchi, M.; Chida, K. Effect of backscatter radiation on the occupational eye-lens dose. J. Radiat. Res. 2024, 65, 450–458. [Google Scholar] [CrossRef]
- Yamada, A.; Haga, Y.; Sota, M.; Abe, M.; Kaga, Y.; Inaba, Y.; Suzuki, M.; Tada, N.; Zuguchi, M.; Chida, K. Eye lens radiation dose to nurses during cardiac interventional radiology: An initial study. Diagnostics 2023, 13, 3003. [Google Scholar] [CrossRef]
- Moritake, T.; Matsumaru, Y.; Takigawa, T.; Nishizawa, K.; Matsumura, A.; Tsuboi, K. Dose measurement on both patients and operators during neurointerventional procedures using photoluminescence glass dosimeters. AJNR Am. J. Neuroradiol. 2008, 29, 1910–1917. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Moritake, T.; Sun, L.; Morota, K.; Nagamoto, K.; Nakagami, K.; Kuriyama, T.; Hitomi, G.; Kajiki, S.; Kunugita, N. The Effect of Pre-Operative Verbal Confirmation for Interventional Radiology Physicians on Their Use of Personal Dosimeters and Personal Protective Equipment. Int. J. Environ. Res. Public Health 2022, 19, 16825. [Google Scholar] [CrossRef]
- Hattori, K.; Inaba, Y.; Kato, T.; Fujisawa, M.; Yasuno, H.; Yamada, A.; Haga, Y.; Suzuki, M.; Zuguchi, M.; Chida, K. Evaluation of a new real-time dosimeter sensor for interventional radiology staff. Sensors 2023, 23, 512. [Google Scholar] [CrossRef]
- Inaba, Y.; Chida, K.; Kobayashi, R. Usefulness of new personal dosimeter systems in the percutaneous coronary intervention procedures. Heart 2015, 47, 679–686. [Google Scholar] [CrossRef]
- James, R.F.; Wainwright, K.J.; Kanaan, H.A.; Hudson, S.; Wainwright, M.E.; Hightower, J.H.; Delaney, J.J. Analysis of occupational radiation exposure during cerebral angiography utilizing a new real time radiation dose monitoring system. J. Neurointerv. Surg. 2015, 7, 503–508. [Google Scholar] [CrossRef]
- Koch, V.; Conrades, L.M.; Gruenewald, L.D.; Eichler, K.; Martin, S.S.; Booz, C.; D’Angelo, T.; Yel, I.; Bernatz, S.; Mahmoudi, S.; et al. Reduction of radiation dose using real-time visual feedback dosimetry during angiographic interventions. J. Appl. Clin. Med. Phys. 2023, 24, e13860. [Google Scholar] [CrossRef]
- Murat, D.; Wilken-Tergau, C.; Gottwald, U.; Nemitz, O.; Uher, T.; Schulz, E. Effects of real-time dosimetry on staff radiation exposure in the cardiac catheterization laboratory. J. Invasive Cardiol. 2021, 33, E337–E341. [Google Scholar] [CrossRef]
- Olschewski, M.; Ullrich, H.; Brandt, M.; Steven, S.; Ahoopai, M.; Blessing, R.; Petrescu, A.; Wenzel, P.; Munzel, T.; Gori, T. Effectiveness of a real-time X-ray dosimetry monitor in reducing radiation exposure in coronary procedures: The ESPRESSO-Raysafe randomized trial. J. Clin. Med. 2021, 10, 5350. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Miller, C.; Kendrick, D.; Shevitz, A.; Kim, A.; Baele, H.; Jordan, D.; Kashyap, V.S. Evaluating strategies for reducing scattered radiation in fixed-imaging hybrid operating suites. J. Vasc. Surg. 2018, 67, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Castle, E.V.; Rathod, K.S.; Guttmann, O.P.; Jenkins, A.M.; McCarthy, C.D.; Knight, C.J.; O’Mahony, C.; Mathur, A.; Smith, E.J.; Weerackody, R.; et al. Routine use of fluoroscopic guidance and up-front femoral angiography results in reduced femoral complications in patients undergoing coronary angiographic procedures: An observational study using an Interrupted Time-Series analysis. Heart Vessel. 2019, 34, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Stewart, K.; Fontanarosa, D.; Malacova, E.; Gett, S.; Kruger, A.; Trapp, J.V. Occupational and patient radiation dose and quality implications of femoral access imaging during coronary angiography. J. Multidiscip. Healthc. 2021, 14, 1807–1818. [Google Scholar] [CrossRef]
- Jia, Q.; Chen, Z.; Jiang, X.; Zhao, Z.; Huang, M.; Li, J.; Zhuang, J.; Liu, X.; Hu, T.; Liang, W. Operator radiation and the efficacy of ceiling-suspended lead screen shielding during coronary angiography: An anthropomorphic phantom study using real-time dosimeters. Sci. Rep. 2017, 7, 42077. [Google Scholar] [CrossRef] [PubMed]




| Case No. | Procedure | Physician | Operator Qualification | Pre-CSRPS | Entire Procedure | ||
|---|---|---|---|---|---|---|---|
| Cervical Unprotected Dose (µSv) | Chest Unprotected Dose (µSv) | Cervical Unprotected Dose (µSv) | Chest Unprotected Dose (µSv) | ||||
| 1 | Post-procedural diagnostic imaging | B | Specialist | 3.96 | 4.30 | 7.63 | 7.53 |
| E | Resident | 19.25 | 34.88 | 29.31 | 47.34 | ||
| 2 | Pre-procedural imaging | C | Specialist | 14.78 | 39.45 | 29.05 | 144.36 |
| F | Resident | 84.03 | 140.57 | 148.98 | 175.36 | ||
| 3 | Diagnostic imaging | A | Specialist | 9.93 | 16.26 | 33.55 | 58.46 |
| G | Resident | 18.76 | 55.06 | 49.35 | 96.21 | ||
| 4 | Pre-procedural imaging | C | Specialist | 24.83 | 35.21 | 85.28 | 106.25 |
| E | Resident | 94.90 | 97.01 | 164.88 | 279.50 | ||
| 5 | Pre-procedural imaging | A | Specialist | 71.47 | 167.40 | 89.02 | 198.85 |
| G | Resident | 31.71 | 42.24 | 58.94 | 94.76 | ||
| 6 | Pre-procedural imaging | A | Specialist | 11.28 | 17.96 | 21.00 | 32.87 |
| E | Resident | 38.22 | 81.75 | 49.10 | 121.77 | ||
| 7 | Pre-procedural imaging | B | Specialist | 36.23 | 51.17 | 60.95 | 69.47 |
| D | Resident | 47.40 | 89.06 | 73.15 | 205.34 | ||
| 8 | Pre-procedural imaging | D | Resident | 62.95 | 85.35 | 72.93 | 96.82 |
| F | Resident | 19.87 | 73.62 | 43.74 | 105.20 | ||
| 9 | Pre-procedural imaging | A | Specialist | 20.27 | 21.96 | 31.64 | 35.76 |
| F | Resident | 77.57 | 145.54 | 102.22 | 199.34 | ||
| 10 | Pre-procedural imaging | C | Specialist | 5.64 | 3.38 | 10.80 | 7.28 |
| H | Resident | 24.82 | 46.45 | 39.06 | 68.08 | ||
| 11 | Post-procedural diagnostic imaging | B | Specialist | 2.94 | 4.11 | 17.80 | 58.75 |
| H | Resident | 22.96 | 44.74 | 47.09 | 70.67 | ||
| 12 | Post-procedural diagnostic imaging | A | Specialist | 15.58 | 17.53 | 75.44 | 85.34 |
| F | Resident | 46.72 | 121.59 | 62.60 | 149.10 | ||
| mean ± SD | 33.59 ± 26.64 | 59.86 ± 47.33 | 58.48 ± 39.39 | 104.77 ± 68.55 | |||
| median [IQR] | 23.89 [15.38–46.89] | 45.60 [20.96–86.28] | 49.23 [31.06–73.72] | 95.49 [58.68–145.55] | |||
| range | 2.94–94.90 | 3.38–167.40 | 7.63–164.88 | 7.28–279.50 | |||
| p-value (Mann–Whitney’s U-test) | p = 0.053 | p < 0.01 | |||||
| Case No. | Physician | Cervical Unprotected Dose (µSv) | Chest Unprotected Dose (µSv) | ||
|---|---|---|---|---|---|
| 1st Position #1 | 2nd Position #2 | 1st Position #1 | 2nd Position #2 | ||
| 1 | B | - | 7.63 | - | 7.53 |
| E | 29.31 | - | 47.34 | - | |
| 2 | C | 2.98 | 26.07 | 64.56 | 79.80 |
| F | 120.26 | 28.72 | 163.83 | 11.53 | |
| 3 | A | 10.92 | 22.63 | 30.17 | 28.29 |
| G | 34.06 | 15.29 | 84.68 | 11.53 | |
| 4 | C | 25.15 | 60.13 | 52.96 | 53.29 |
| E | 136.43 | 28.45 | 251.62 | 27.88 | |
| 5 | A | 70.17 | 18.85 | 172.65 | 26.20 |
| G | 39.36 | 19.58 | 66.28 | 28.48 | |
| 6 | A | - | 21.44 | - | 32.87 |
| E | 49.10 | - | 121.77 | - | |
| 7 | B | 33.16 | 27.79 | 35.81 | 33.66 |
| D | 51.46 | 21.69 | 200.82 | 4.52 | |
| 8 | D | 53.43 | 19.50 | 73.73 | 23.09 |
| F | 41.14 | 2.60 | 102.34 | 2.86 | |
| 9 | A | 5.69 | 25.95 | 4.76 | 31.00 |
| F | 100.70 | 1.52 | 198.07 | 1.27 | |
| 10 | C | - | 10.80 | - | 7.28 |
| H | 39.06 | - | 68.08 | - | |
| 11 | B | 10.83 | 6.97 | 23.99 | 34.76 |
| H | 47.09 | - | 70.67 | - | |
| 12 | A | 56.33 | 19.11 | 63.88 | 21.46 |
| F | 55.46 | 7.14 | 141.34 | 7.76 | |
| mean ± SD | 48.19 ± 35.03 | 19.59 ± 12.86 | 97.11 ± 66.88 | 23.75 ± 18.98 | |
| median [IQR] | 41.14 [29.31–55.46] | 19.54 [10.01–25.98] | 70.67 [52.96–141.34] | 24.65 [7.70–31.47] | |
| range | 2.98–136.43 | 1.52–60.13 | 4.76–251.62 | 1.27–79.80 | |
| p-value (Mann–Whitney’s U-test) | p < 0.01 | p < 0.01 | |||
| RaySafei3 Placement | CSRPS Placement | No. of Scenes | Operator Unprotected Dose Rate (mSv/h) | p-Value (Mann–Whitney U Test) | |
|---|---|---|---|---|---|
| Median [IQR] | Range | ||||
| Cervical | Appropriate | 4 | 0.65 [0.53–0.79] | 0.48–0.87 | p < 0.01 |
| Inappropriate | 39 | 2.20 [1.54–2.88] | 0.41–4.69 | ||
| Chest | Appropriate | 4 | 2.05 [1.60–2.54] | 1.45–2.80 | p = 0.917 |
| Inappropriate | 39 | 2.04 [1.49–2.66] | 0.70–5.08 | ||
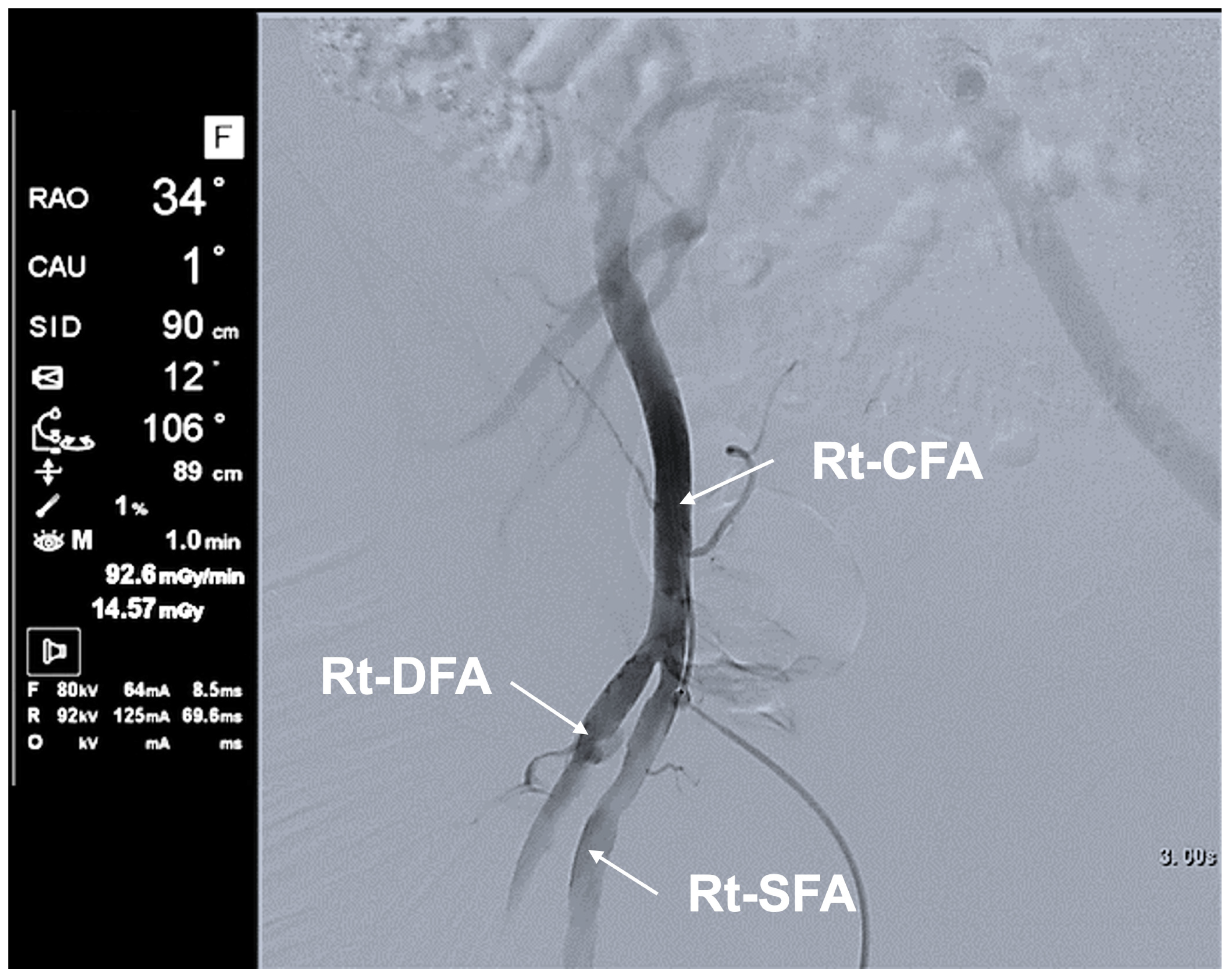
| Typical Scene No. | Case No. | DSA Site | X-Ray Projection | FPD Size (inch) | SID (cm) | Ka,r (mGy) | Table Height (cm) | Cervical Unprotected Dose Rate (mSv/h) | Chest Unprotected Dose Rate (mSv/h) | Cervical/Chest Unprotected Dose Rate Ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | Rt FAG | Frontal—RAO 16°, Caudal 1° | 12 | 90 | 62.8 | 79 | 5.16 | 11.75 | 0.44 |
| 2 | 5 | Rt FAG | Frontal—LAO 20°, Cranial 0° | 12 | 90 | 70.4 | 87 | 11.29 | 35.27 | 0.32 |
| 3 | 3 | Lt C-CAG | Frontal—LAO 0°, Cranial 1° | 12 | 90 | 80.06 | 93 | 0.48 | 1.65 | 0.29 |
| Lateral—RAO 90°, Cranial 0° | 12 | 111 | 100.1 | |||||||
| 4 | 3 | Lt SAG | Frontal—LAO 0°, Cranial 0° | 12 | 91 | 93.2 | 94 | 1.01 | 3.20 | 0.32 |
| Lateral—RAO 90°, Cranial 0° | 12 | 112 | 107 | |||||||
| 5 | 4 | Lt C-CAG | Frontal—LAO 0°, Caudal 2° | 12 | 96 | 53 | 98 | 1.17 | 7.64 | 0.15 |
| Lateral—RAO 90°, Cranial 0° | 12 | 119 | 60.8 |
| 1 | Place CSRPS between the scatter source and the operator’s eyes |
| 2 | Position CSRPS as close to the operator as possible |
| 3 | Eliminate gaps between the lower edge of the CSRPS and the patient’s trunk |
| 4 | Place CMRPC between the scatter source and the operator’s lower body |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hitomi, G.; Moritake, T.; Tanaka, Y.; Kurokawa, T.; Nakagami, K.; Kuriyama, T.; Morota, K.; Matsuzaki, S.; Ishidao, T. Integration of Active Personal Dosimeters, Videos from In-Room Monitors, and Videos from the Surgeon’s Main Panel Reveal Pitfalls in Radiation Protection. Appl. Sci. 2025, 15, 11584. https://doi.org/10.3390/app152111584
Hitomi G, Moritake T, Tanaka Y, Kurokawa T, Nakagami K, Kuriyama T, Morota K, Matsuzaki S, Ishidao T. Integration of Active Personal Dosimeters, Videos from In-Room Monitors, and Videos from the Surgeon’s Main Panel Reveal Pitfalls in Radiation Protection. Applied Sciences. 2025; 15(21):11584. https://doi.org/10.3390/app152111584
Chicago/Turabian StyleHitomi, Go, Takashi Moritake, Yuko Tanaka, Toru Kurokawa, Koichi Nakagami, Tomoko Kuriyama, Koichi Morota, Satoru Matsuzaki, and Toru Ishidao. 2025. "Integration of Active Personal Dosimeters, Videos from In-Room Monitors, and Videos from the Surgeon’s Main Panel Reveal Pitfalls in Radiation Protection" Applied Sciences 15, no. 21: 11584. https://doi.org/10.3390/app152111584
APA StyleHitomi, G., Moritake, T., Tanaka, Y., Kurokawa, T., Nakagami, K., Kuriyama, T., Morota, K., Matsuzaki, S., & Ishidao, T. (2025). Integration of Active Personal Dosimeters, Videos from In-Room Monitors, and Videos from the Surgeon’s Main Panel Reveal Pitfalls in Radiation Protection. Applied Sciences, 15(21), 11584. https://doi.org/10.3390/app152111584






