Abstract
Background/Objectives: The use of dentin grafts in bone regeneration has gained increasing attention as an alternative to conventional grafting materials. Mesenchymal stem cells (MSCs), known for their osteogenic potential, have been combined with various biomaterials to enhance regenerative outcomes. This study aimed to evaluate the regenerative potential of dentin grafts and bovine-derived xenografts, with or without MSCs, in experimentally created bone defects in a rat model. Methods: A total of 25 male rats were randomly assigned to five groups: control, dentin graft, dentin graft and MSC, xenograft, and xenograft and MSC. Standardized 2-mm cortical defects were created bilaterally in the femoral shafts. Histological and immunohistochemical analyses were performed after a 90-day healing period. Statistical evaluation was carried out using the Kruskal–Wallis H test and Bonferroni-adjusted pairwise comparisons. Results: Complete healing was achieved in all groups without evidence of complications or inflammatory reactions. Immunohistochemical staining demonstrated no positive vascular endothelial growth factor (VEGF), collagen type I (COL1), or osteopontin (OPN) reactions in defect areas, consistent with complete maturation, although collagen type 3 (COL3) positivity was observed in residual xenograft material. Quantitative analysis showed that the dentin graft and MSC group achieved the highest degree of new bone formation (M = 92.88%, SD = 6.09), significantly greater than the control (p = 0.002) and xenograft groups (p = 0.013). Conclusions: Both dentin grafts and xenografts demonstrated enhanced bone defect healing when combined with MSCs. Nevertheless, dentin grafts in conjunction with MSCs yielded the most favorable regenerative outcomes, suggesting their clinical superiority over conventional xenografts.
1. Introduction
Autologous bone grafts are still regarded as the gold standard in bone regeneration due to their osteogenic, osteoinductive, and osteoconductive properties; however, their clinical use is limited by drawbacks such as donor-site morbidity, limited availability, and the risk of complications. To address these limitations, recent research has increasingly focused on developing graft materials enriched with bioactive molecules that can enhance regenerative outcomes [1].
The enhancement of bone regeneration, particularly in defects that cannot heal spontaneously and require advanced treatment, remains an area of active research. Within this broader context, dentin grafts have gained increasing attention as a low-morbidity method that combines both biological relevance and clinical practicality. Dentin grafts may represent an effective alternative grafting material, especially when a tooth scheduled for extraction can be utilized as an autogenous source, thereby avoiding additional donor site complications. The success rate of dentin graft procedures appears to be high in the literature; however, the long-term outcomes remain uncertain. As a grafting method with low morbidity, it has been gaining both relevance and popularity. The clinical use of dentin grafts was first reported by Murata et al. in 2002, and this case is recognized as the first human application of a demineralized dentin matrix autograft [2]. The modern concept of particulate dentin/tooth grafts as a clinically applicable technique was established by Kim et al. [3] in 2010 through the introduction of a standardized preparation protocol. Subsequently, Jeong et al. [4] published one of the earliest clinical series in 2011, demonstrating the efficacy of dentin grafts in maxillary sinus augmentation. Utilizing extracted or impacted teeth as graft material for defect regeneration eliminates the morbidity associated with donor sites. Moreover, dentin and bone share several chemical and structural properties, including the distribution of inorganic and organic components [5,6].
Dentin has been shown to contain numerous factors commonly involved in osteogenesis, such as transforming growth factor-β, fibroblast growth factor, VEGF, bone morphogenetic proteins (BMPs), insulin-like growth factor, platelet-derived growth factor and OPN [7,8]. These characteristics suggest that dentin harvested from extracted teeth may serve as a suitable alternative for alveolar bone preservation. The osteoconductive and osteoinductive properties of dentin, which can stimulate bone formation in grafted defects, have been demonstrated in both animal and clinical studies [9,10,11]. In recent years, the use of dentin grafts has become increasingly widespread in both experimental and clinical settings. Furthermore, histological studies have demonstrated that dentin grafts participate in bone remodeling processes and allow for adequate osseointegration of dental implants [12,13].
To improve the effectiveness of grafts within defect areas, the incorporation of cellular or acellular components has been one of the most strategically preferred approaches to enhance bone regeneration. MSCs, in particular, have attracted great attention due to their multilineage differentiation potential and excellent osteogenic capacity, which can be used to increase and accelerate the regenerative capability of graft materials [14]. MSCs are tissue-specific, clonogenic cells capable of differentiating into various mesodermal cell types, including osteoblasts, chondrocytes, and adipocytes. Initially identified in bone marrow, MSCs can now be obtained from a variety of tissues, such as peripheral blood, umbilical cord blood, adipose tissue, and dental pulp. In addition to secreting molecules that initiate or support tissue regeneration and repair, MSCs are characterized by their self-renewal and expansion capacity, multipotency, anti-inflammatory properties, and immunomodulatory effects. They have been utilized in clinical applications for over two decades to enhance bone healing potential. Owing to their ability to differentiate into osteoblasts, MSCs have attracted particular attention for their capacity to accelerate regeneration in the clinical management of bone defects.
Several studies have evaluated the effects of combining xenografts with MSCs on the osteoinductive and osteoconductive properties of bone grafts. In these investigations, MSCs are typically applied in combination with carrier biomaterials, demonstrating significant efficacy in accelerating bone tissue regeneration [15,16,17]. The clinical use of dentin grafts has intensified particularly over the last six years, with animal and human studies reporting favorable outcomes compared with conventional grafting materials. Despite the large body of evidence confirming the beneficial effects of MSCs on bone healing, studies investigating their combined use with dentin grafts remain limited. In experimentally created bone defects, no studies could be found in the English-language literature evaluating the effects of mesenchymal stem cells in combination with xenografts and dentin grafts. The aim of this study is to evaluate the effects of MSC-assisted grafting, dentin grafts, and xenografts on bone regeneration.
2. Methods
This study was conducted between September 2024 and June 2025 at Kobay Experimental Animals Laboratory Inc. (Ankara, Turkey), using 25 Wistar albino rats. Ethical approval was obtained from the Local Ethics Committee of Kobay Experimental Animals Laboratory Inc. (Protocol no: 735, Date: 13 December 2024).
Male Wistar albino rats weighing 250–300 g were selected for the experiment. The animals were housed in air-filtered, humidity- and temperature-controlled (23 ± 1 °C) microisolators under a 12-h light/dark cycle, with an illumination of 300 lux in the room and 60 lux within the cages. A maximum of four rats were housed per cage under standard laboratory conditions, with ad libitum access to food and filtered water. Cages were routinely cleaned and replaced to ensure proper hygienic conditions and animal welfare throughout the experimental period. After the animals had acclimatized to the environment, the experimental procedures were initiated.
2.1. Dentin Graft Preparation Protocol
The dentin grafts used in this study were obtained from maxillary and mandibular central incisors extracted from rats that were sacrificed for other experiments. This selection was made because the central incisors in rats are extremely long and extraction results in impaired feeding, rendering the animals unsuitable for use in the experimental protocol.
Particulate dentin grafts were prepared with the Smart Dentin Grinder™ system (KometaBio, Tenafly, NJ, USA) using the manufacturer’s standardized protocol and accompanying kit. After grinding with the Smart Dentin Grinder™, the dentin particles were immersed in a sterilization/decontamination solution containing 70% ethanol and 0.1 M NaOH for 10 min to reduce bacterial load while preserving the biologically active proteins and were subsequently washed thoroughly with sterile distilled water [18,19]. In the first step, enamel, cementum and organic debris were removed from the extracted teeth until the dentin tissue was exposed. The pulp was also removed to obtain pure dentin tissue. The prepared teeth were then placed into the grinder chamber, and once ground, the particulate dentin grafts were collected in a sterile container provided with the system.
Subsequently, Dentin Cleanser, supplied with the kit, was added to cover the particulate dentin grafts. The material was left at room temperature for 10 min, after which the solution was removed using sterile gauze. Phosphate-buffered saline (PBS) was then added to completely cover the grafts and gently mixed with a sterile instrument. After 3 min of incubation, the solution was removed with sterile gauze [9]. This process was repeated to ensure that the grafts reached the appropriate pH level.
2.2. MSC Preparation Protocol
Bone marrow-derived MSCs (ATCC PCS-500-012; obtained through LGC Promochem, Teddington, UK) were commercially sourced from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were expanded and cryopreserved at passages 5 and 6, and these passages were used for subsequent experiments. The cells were cultured in Dulbecco’s Modified Eagle Medium (low-glucose formulation) supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin, and 1% L-glutamine. After approximately 7–10 days, when the culture reached 80% confluence, the medium was aspirated, and the cells were washed with PBS. Passaging was performed by treating the cells with trypsin/ethylenediaminetetraacetic acid (EDTA) for 5 min in a 37 °C incubator, followed by transfer into new T75 flasks at a seeding density of 3000 cells/cm2. Cell viability was assessed using the Trypan Blue exclusion assay, and viable cells were counted under a microscope. The culture medium was replaced twice per week. Cell viability was determined using the Trypan Blue exclusion assay and was consistently in the range of 94–96%; prior to application, the suspension contained approximately 2.0 × 106 viable cells in 2 mL (1.0 × 106 cells/mL). For all experimental applications, early-passage cells (P2–P4) were used to maintain proliferative and differentiation capacity. Phenotypic characterization confirmed that the cells met the minimal criteria of the International Society for Cellular Therapy (ISCT), showing positive expression of CD73, CD90, and CD105 and negative expression of CD34, CD45, CD14/CD19, and HLA-DR, while trilineage differentiation potential was verified by osteogenic (Alizarin Red S), adipogenic (Oil Red O), and chondrogenic (Alcian Blue) assays.
2.3. Experimental Protocol
A total of 25 male Wistar albino rats were randomly allocated into five experimental groups, each comprising five animals. The groups consisted of a control group, a dentin graft group, a dentin graft combined with mesenchymal stem cells group, a xenograft group, and a xenograft combined with mesenchymal stem cells group. As both the right and left femora of each animal were utilized, a total of 10 samples were obtained for analysis in each group. The optimal sample size was calculated through power analysis to ensure adequate statistical power for the study. In addition, potential animal loss was taken into consideration, and it was decided to include 10 subjects in each group.
General anesthesia was induced in all animals with ketamine HCl (50–100 mg/kg) and xylazine HCl (5–10 mg/kg). Following shaving of the hind limbs, an incision was made, and the skin and fascia were dissected [20]. The femur was accessed between the quadriceps and biceps femoris muscles. To standardize the procedure, a defect was created in the mid-diaphyseal region corresponding to approximately 40–45% of the total femoral length measured from the proximal metaphysis toward the distal end. Using a piezo surgical instrument and fissure bur at 40.000 rpm under sterile saline irrigation, a 2-mm circular cortical defect was prepared. A defect size of 2 mm was selected to minimize the risk of impaired bone integrity and pathological fracture, which significantly increases with larger defects, while also ensuring animal welfare and experimental safety.
In the control group, defects were created but left empty, and the soft tissues were closed in layers without biomaterial placement. In dentin group, the defects were filled with dentin grafts. In the dentin graft and MSC group, 0.1 mL of MSC suspension, containing approximately 100.000 viable cells, was applied to the defect prior to dentin graft placement, followed by an additional 0.1 mL of the same MSC suspension after graft insertion (Figure 1). This two-step method was used to prevent possible displacement of the graft material by the MSC suspension if applied only after grafting. In the xenograft group, the defects were filled with bovine-derived xenograft (Ubgen, Italy), whereas in the xenograft plus MSC group, the xenograft was combined with MSCs, using the same protocol as in the dentin graft group, with matched cell concentration.
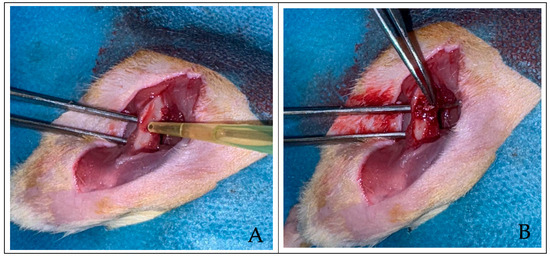
Figure 1.
(A) Two views of the rat femur after the defect was created, followed by the application of 0.1 mL MSCs and (B) xenograft to the created defect area.
To eliminate confounding variables and maintain consistency, all surgical procedures were performed on the same day by a team consisting of one veterinarian and two oral and maxillofacial surgeons. Dissection and wound closure were carried out by the veterinarian, while defect preparation and grafting procedures were performed by the surgeons. All surgical sites were closed primarily, and the animals were allowed a 90-day healing period to ensure complete bone regeneration. Postoperatively, the animals were housed individually in air-filtered microisolators under controlled conditions of temperature (23 ± 1 °C) and humidity, with a 12-h light/dark cycle (300 lux ambient room light and 60 lux inside cages). Food and filtered water were provided ad libitum, and cages were routinely cleaned and replaced throughout the 90-day observation period to ensure proper hygienic conditions and animal welfare.
A total of 25 animals were used in the experiment, and both femora were evaluated to establish five groups, each comprising ten samples. At 1.5 months postoperatively, one animal in the control group was lost before the study was completed and was excluded from the evaluation. In addition, one specimen from the xenograft and MSC group was excluded due to tissue deformation during sectioning. Accordingly, eight samples from the control group, ten from the dentin graft group, ten from the dentin and MSC group, ten from the xenograft group, and nine from the xenograft and MSC group were evaluated. The study proceeded as planned without any additional animal loss or unexpected complications, such as feeding difficulties. Ninety days after the surgical procedures, all animals were sacrificed by cervical dislocation. The hind limbs were dissected, the femora were exposed, and samples were collected from the defect sites.
2.4. Tissue Preparation
Bone tissue samples obtained from the defect sites were fixed in 10% neutral buffered formalin for 24 h. Decalcification was performed to remove calcium deposits, after which the tissues were washed in distilled water for 4 h. Tissue processing was carried out using an automated tissue processor (Leica TP1020). Samples were sequentially dehydrated in graded ethanol series: 50% (50 min), 70% (1 h), 80% (1 h), 96% (1 h), and 100% ethanol (2 × 75 min). Tissues were then cleared in two xylene baths (30 min each) and infiltrated with paraffin wax at 61 °C. The paraffin-embedded tissues were blocked using a paraffin embedding system (Leica EG1120, Leica Microsystems GmbH, Wetzlar, Germany), and 4 µm sections were obtained with a microtome (Thermo Shandon Finesse ME, Thermo Fisher Scientific Inc., Waltham, MA, USA).
2.5. Histochemical Staining
For histological evaluation, hematoxylin and eosin staining was performed. Sections were incubated at 60–65 °C for 2 h, deparaffinized in xylene (3 × 10 min), and rehydrated through graded alcohols (100%, 96%, 80%, 70%) before immersion in distilled water. Slides were stained with hematoxylin (Sigma-Aldrich, Darmstadt, Germany) for 3 min, rinsed, then counterstained with eosin (Besolab, Ankara, Turkey) for 3 min. Following dehydration in ascending ethanol concentrations and clearing in xylene, slides were mounted with Entellan (Merck, Darmstadt, Germany) and examined under a light microscope.
2.6. Immunohistochemical Analysis
Sections (4 µm) were incubated at 60 °C for 1 h and deparaffinized in xylene (3 × 5 min), followed by rehydration in decreasing ethanol concentrations (100%, 96%, 80%, 70%) and rinsing in distilled water. Antigen retrieval was performed using 1:10 diluted citrate buffer (pH 6.0; Thermo Fisher Scientific, AP-9003-999, Waltham, MA, USA) in a PT module (LabVision A80400012, Bucharest, Romania). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide (Thermo Scientific, TA-125-HP) for 10 min, followed by blocking with Protein Block (Thermo Scientific, TA-125-PBQ) for 10 min at room temperature.
Sections were then incubated for 2 h at room temperature with the following primary antibodies:
- COL I (orb241216, Biorbyt; dilution 1:50)
- COL III (orb304041, Biorbyt; dilution 1:100)
- VEGF (bs-0279R, Bioss; dilution 1:100)
- OPN (NB110-89062, Novus Biologicals; dilution 1:100)
After washing with phosphate-buffered saline (PBS), slides were incubated with Amplifier Quanto (Thermo Scientific, TL-125-QPB) for 20 min and horseradish peroxidase (HRP) Polymer Quanto (Thermo Scientific, TL-125-QPH) for 30 min. Immunoreactivity was visualized using 3.3’-diaminobenzidine (DAB) chromogen (Thermo Scientific, TA-125-HA), and nuclei were counterstained with hematoxylin (Sigma-Aldrich, HHS32) for 30 s. Finally, slides were dehydrated through graded ethanol series, cleared in xylene, mounted with Entellan (Merck), and examined microscopically.
2.7. Statistics
For statistical analysis, the percentage of newly formed bone was calculated by measuring the surface area of new bone relative to the total defect area on histological sections using the Optika ProView digital image analysis software (version 4.8; Optika Microscopes S.r.l., Ponteranica, Italy). A calibration image is integrated into the software to ensure accurate standardization, enabling reliable comparison of histological section images. Subsequently, the histological images of the samples are uploaded, and the areas of newly formed bone within the defect regions are delineated using the program interface. This approach allows for volumetric comparison across histological images, yielding the percentage of newly formed bone. Thus, the samples can be quantitatively evaluated to compare new bone formation rates. The data were analyzed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). Since the data did not meet parametric assumptions, the non-parametric Kruskal–Wallis H test was applied to evaluate differences among groups. When a significant difference was detected, pairwise comparisons with Bonferroni correction were performed to identify the groups responsible for the difference.
3. Results
Histological evaluation revealed that defect areas had healed in all specimens after the 90-day period. The examination demonstrated complete bone formation without evidence of inflammation, granulation tissue, or other complications (Figure 2). Surface bone formation, osteocytes, bone trabeculae, and vascular structures appeared normal. Morphologically, no abnormalities were observed in the experimental groups when compared with the control group or normal bone morphology. In some specimens from the xenograft (Figure 2C) and xenograft and MSC (Figure 2D) groups, graft particles surrounded by bone tissue were observed without evidence of active inflammatory reaction.
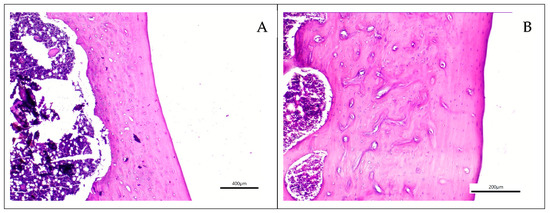
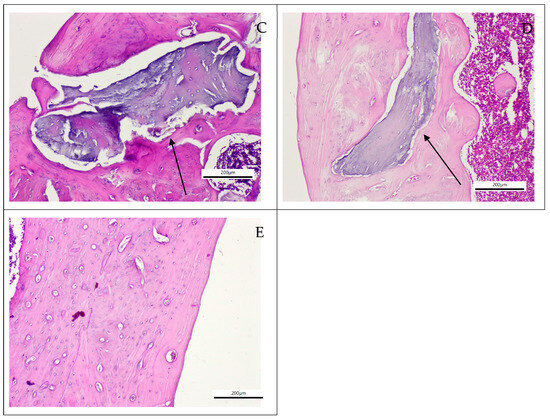
Figure 2.
Histological sections of the grafted groups stained with Hematoxylin and Eosin. (A) Control group (×40 magnification), (B) Dentin group (×100 magnification), (C) Xenograft material (black arrow) embedded within bone tissue in the xenograft group (×100 magnification) (D) Xenograft material (black arrow) embedded within bone tissue the xenograft and MSC group (×100 magnification), (E) Dentin and MSC Group (×100 magnification).
Immunohistochemical staining with OPN revealed similar results across all groups, with no positive reactions observed for osteoblastic, fibroblastic, or bone remodeling activity. COL1 staining likewise demonstrated no positive reactions, as there were no active healing areas detected in the groups. Overall, the immunohistochemical responses were found to be at comparable levels in all groups. VEGF staining showed negative reactions in the healed defect areas, while positivity was observed in megakaryocytes of the bone marrow and in immature myeloid cells. COL3 staining demonstrated positive reactions in only two specimens within the examined defect areas (Figure 3). These positive findings were associated with residual, non-resorbed xenograft material in samples from the xenograft group.
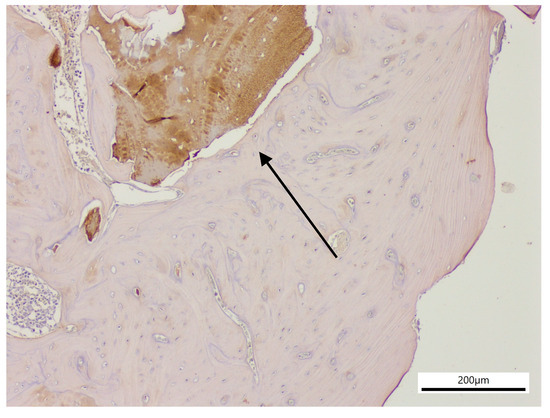
Figure 3.
Experimental site from the xenograft group (×100), showing graft material with positive reaction (black arrow) in a histological section stained for COL3.
The lowest measurement value was observed in the xenograft group (Min = 52.30%), whereas the highest was recorded in the dentin and MSC group (Max = 100.99%). In terms of mean scores, the dentin and MSC group demonstrated the highest average (M = 92.88, SD = 6.09), followed by the xenograft and MSC group (M = 85.08, SD = 11.70) and the dentin group (M = 81.93, SD = 9.65). The control group (M = 74.40, SD = 9.33) and the xenograft group (M = 77.03, SD = 11.26) presented relatively lower mean scores. When median values were examined, the dentin and MSC (Median = 93.00) and xenograft and MSC (Median = 85.30) groups showed the highest medians, consistent with their mean ranks, while the control group (Median = 72.50) had the lowest. The ranges between minimum and maximum values provided further insight into variability across groups. The narrowest range was found in the dentin and MSC group (82.00–100.99%), indicating greater homogeneity compared to the other groups. In contrast, the widest range was observed in the xenograft group (52.30–95.00%), suggesting a more heterogeneous distribution of values in this group (Table 1, Figure 4).

Table 1.
Descriptive statistics of the applied treatment methods.
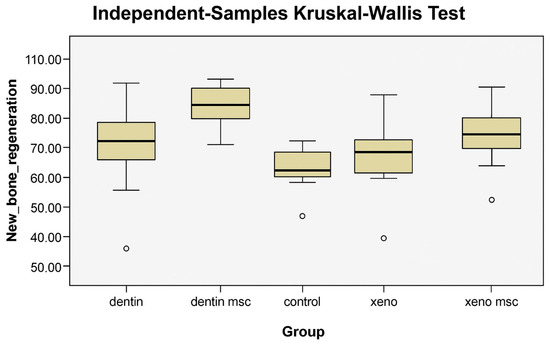
Figure 4.
Box plots showing the distribution of new bone regeneration values across experimental groups (control, dentin, dentin and MSC, xenograft, and xenograft and MSC). The dentin and MSC group demonstrated the highest median and the narrowest range, indicating greater homogeneity, whereas the xenograft group exhibited the widest range, reflecting more heterogeneous outcomes. Circles denote statistical outliers that lie outside the overall data distribution.
According to the results of the Kruskal–Wallis H test, a statistically significant difference was found among the groups in terms of new bone formation (p = 0.002). This finding indicates that the level of new bone formation differed significantly among the groups.
Post-hoc pairwise comparisons with Bonferroni correction revealed that the dentin and MSC group exhibited significantly greater new bone formation compared with both the control group (p = 0.002) and the xenograft group (p = 0.013). No statistically significant differences were observed among the other groups (Table 2).

Table 2.
Results of Bonferroni-adjusted pairwise comparisons among groups.
As a result, after a 90-day period following the creation of bone defects, it was observed that the control, dentin, xenograft, dentin and MSC, and xenograft and MSC groups all exhibited complete bone remodeling and healing, with no evidence of the tissue being in an intermediate stage of repair. The dentin and MSC group showed significantly greater new bone formation compared with both the control group (p = 0.002) and the xenograft group (p = 0.013).
4. Discussion
In this study, the effects of bovine-derived xenograft and dentin graft, with or without MSCs, on bone regeneration in Wistar rats were investigated and compared with the control group using light microscopy and immunohistochemical analysis.
Dentin has the same chemical composition as bone and may therefore serve as a suitable grafting material capable of replacing bone. Both bone and dentin undergo mineralization through similar processes [19,20,21]. The application of dentin grafts in bone defect management continues to attract attention in current research in the literature. Schwarz et al. [22] demonstrated that even when dentin grafts derived from teeth in contact with the oral cavity were used, there was no histological evidence of increased inflammation, nor was there an elevated incidence of wound infection or graft loss [23]. Similarly, in this study, no infections were observed in the surgical sites of the rats. In a meta-analysis conducted by Mahendra et al. [11], the effectiveness of dentin grafts for alveolar augmentation was evaluated, and in some studies, dentin grafts provided greater new bone formation and vertical/horizontal bone gain compared with bone-derived grafts such as bovine xenografts. However, other studies have reported that the use of demineralized dentin matrix resulted in lower amounts of new bone formation compared with xenograft. In this study, the dentin and MSC group showed significantly greater new bone formation compared with both the control (p = 0.002) and the xenograft group (p = 0.013).
MSCs have been used in combination with many different carrier materials, including bovine-derived grafts [24,25,26]. Piattelli et al. [27] performed a histological analysis of bone healing in 20 patients treated with xenografts and reported favorable long-term outcomes, with inorganic bovine bone proving to be osteoconductive and supporting successful grafting results for up to four years. Similarly, a six-year randomized controlled clinical trial by Stavropoulos and Karring [28] demonstrated bone healing in intrabony defects using radiographic and clinical parameters, confirming the long-term efficacy of bovine bone material. The biological performance of deproteinized bovine bone grafts has been widely investigated in preclinical studies involving healthy animals [29,30,31,32], showing that bovine bone particles enhance bone regeneration and integrate well with newly formed bone. Histological studies have consistently demonstrated osteoblasts and osteoclasts adjacent to both newly formed bone and bovine bone particles, as well as close contact between the implanted material and the new bone. For instance, Van Houdt et al. [29] examined bovine bone implanted into femoral condylar defects in rats, and by week 12, newly formed bone was observed in direct contact with xenograft particles, which were fully covered by new bone.
MSCs are tissue-specific, clonogenic cells with the ability to differentiate into various mesodermal lineages such as osteoblasts, chondrocytes, and adipocytes. Several studies have assessed the changes in osteoinductive and osteoconductive properties of xenografts, including bovine bone, when combined with MSCs in the treatment of bone defects caused by trauma, cysts, tumors. In the present study, dentin grafts were compared with xenografts, and the influence of MSCs on each graft type was evaluated independently. Gutwald et al. [33], in a bovine model of sinus augmentation, compared the osteogenic potential of mononuclear cells containing MSCs from the iliac crest combined with bovine bone mineral to that of autologous cancellous bone alone. They concluded that bovine-derived xenografts loaded with MSCs produced bone formation kinetics comparable to those of regions treated with autologous bone alone. Pieri et al. [34], in a bilateral sinus augmentation study in pigs, reported that the combination of MSCs, platelet-rich plasma, and fluorohydroxyapatite carriers significantly increased bone formation compared with fluorohydroxyapatite alone. D’Aquino et al. [35] demonstrated that the application of collagen sponges containing MSCs in mandibular third molar extraction sockets led to faster regeneration and greater mature bone formation at three months compared with collagen sponges without MSCs. Similarly, another study showed that in rabbits, MSC-loaded hydroxyapatite scaffolds produced greater bone volume than scaffolds alone [36].
Rickert et al. [37], in a randomized–controlled split-mouth study on atrophic maxilla reconstruction, reported that xenogeneic bovine bone grafts supplemented with bone marrow aspirate concentrate after sinus augmentation resulted in greater bone formation compared with bovine bone grafts mixed with autogenous bone; this finding was also confirmed by Sauerbier et al. [38]. In the present study, although the difference did not reach statistical significance, it was observed that xenografts combined with MSCs provided proportionally better outcomes compared with both the control and the xenograft-only groups. Future studies with larger sample sizes may reveal statistical significance for this finding Smith et al. [39] demonstrated in a sheep model that some xenograft particles used for maxillary sinus grafting remained unresorbed at 4, 6, and 12 weeks and were surrounded by lamellar bone. Similarly, Addis et al. [40] showed in an animal model that even after 90 days, xenograft particles persisted despite the formation of lamellar bone islands. Additional reports have indicated that such xenograft materials can persist for up to six months [41] and even several years after grafting, either within bone or in the surrounding connective tissue [42]. These findings are consistent with the present study, in which xenograft and xenograft and MSC groups showed graft particles embedded within bone tissue, without any evidence of active inflammatory reaction (Figure 3). Bareiro et al. [43] reported that the xenograft group supported less bone formation compared with the dentin/MSC group. Moreover, the dentin/MSC group demonstrated greater bone formation compared with both the dentin-only and MSC-only groups. This finding suggests that the regenerative effect of dentin grafts is enhanced by MSCs, which migrate to the defect area, differentiate into osteoblasts, and promote bone regeneration. In the present study, this was further supported by the significantly greater regeneration observed in the dentin and MSC group.
COL1 is the principal collagen protein of the bone matrix, whereas OPN is one of the major non-collagenous proteins. Together, these proteins represent osteogenic activity, which also requires the upregulation of VEGF [44,45]. In the study by Barreiro et al. [43], specimens harvested on day 35 demonstrated higher OPN expression in the dentin, MSC, and dentin/MSC groups, along with higher VEGF expression in the MSC group. In line with our findings, after 90 days, the bone tissue was observed to be fully remodeled and healed, with no evidence of the tissue being in an intermediate stage of repair. Consequently, immunohistochemical staining with VEGF revealed negative reactions in the defect areas, as there was no active angiogenesis, bone formation, or bone defects present. Positivity was observed in megakaryocytes of the bone marrow and in immature myeloid cells, which is considered a normal finding of VEGF expression in bone marrow. Furthermore, since bone modeling was complete at 90 days, no active healing areas were detected, and immunohistochemical staining for COL1 and OPN showed no positive reactions in the specimens from any group. The immunohistochemical response was comparable in intensity across all groups, reflecting the natural outcome of complete bone maturation. COL3, by contrast, is found in healing tissues where bone is present and is typically expressed by osteoblasts during embryonic stages, but its expression decreases during the healing process (approximately days 21–28). In the specimens examined, no positive COL3 reactions were observed in the defect areas; however, positivity was detected in the xenograft material (Figure 3).
This study also has certain limitations. The number of samples was kept at the minimum level required for analysis in accordance with ethical considerations. As a result, evaluations could not be performed at multiple time points due to the inability to achieve sufficient sample size. Moreover, instead of harvesting dentin grafts from the rats themselves—which would have required the creation of a second donor site—dentin grafts were obtained from rats previously sacrificed in other experiments.
5. Conclusions
Dentin grafts and xenografts exhibited highly favorable outcomes in promoting new bone formation, primarily through the osteogenic and regenerative effects of MSCs. Among all the experimental groups, the dentin graft–MSC combination yielded the most pronounced regenerative response. The utilization of autogenous dentin grafts derived from extracted teeth, in conjunction with MSCs, represents a promising strategy for enhancing and accelerating bone regeneration, particularly for critical-sized defects where rapid healing is essential. Further investigations with larger cohorts are essential to substantiate these findings and to provide deeper insights into the mechanisms of dentin graft with MSC applications.
Author Contributions
Conceptualization, S.Y.E.; methodology, S.Y.E.; validation, S.Y.E. and H.T.B.; formal analysis, S.Y.E. and H.T.B.; investigation S.Y.E.; resources, H.T.B.; data curation, S.Y.E. and H.T.B.; writing—original draft preparation, S.Y.E. and H.T.B.; writing—review and editing, S.Y.E.; visualization, S.Y.E.; supervision, S.Y.E.; project administration, S.Y.E.; funding acquisition, S.Y.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ankara Yildirim Beyazit University Scientific Research Projects Coordination Unit, grant number TSA-2024-2723.
Institutional Review Board Statement
The animal study protocol was approved, and ethical approval was obtained in from the Local Ethics Committee of Kobay Experimental Animals Laboratory Inc. (Protocol no: 735–13 December 2024).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This research was supported by the Scientific Research Projects Coordination Unit of Ankara Yildirim Beyazit University (Project Code: TSA-2024-2723).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MSC | Mesenchymal stem cells |
| VEGF | Vascular endothelial growth factor |
| BMP | Bone morphogenetic protein |
| OPN | Osteopontin |
| COL1 | Collagen type 1 |
| COL3 | Collagen type 3 |
References
- Mitić, D.; Čarkić, J.; Jaćimović, J.; Lazarević, M.; Jakšić Karišik, M.; Toljić, B.; Milašin, J. The impact of nano-hydroxyapatite scaffold enrichment on bone regeneration in vivo—A systematic review. Biomimetics 2024, 9, 386. [Google Scholar] [CrossRef]
- Murata, M.; Hirose, Y.; Ochi, M.; Tazaki, J.; Okubo, N.; Akazawa, T. Twenty years-passed case of demineralized dentin matrix autograft for sinus bone augmentation—A first case of dentin graft in human. J. Clin. Exp. Dent. 2023, 15, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kim, S.G.; Byeon, J.H.; Lee, H.J.; Um, I.U.; Lim, S.C.; Kim, S.Y. Development of a novel bone grafting material using autogenous teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.I.; Kim, S.G.; Kim, Y.K.; Oh, J.S.; You, J.S.; Kim, J.S. Clinical study of graft materials using autogenous teeth in maxillary sinus augmentation. Implant Dent. 2011, 20, 471–479. [Google Scholar] [CrossRef]
- Sapoznikov, L.; Humphrey, M. Progress in dentin-derived bone graft materials: A new xenogeneic dentin-derived material with retained organic component allows for broader and easier application. Cells 2024, 13, 1806. [Google Scholar] [CrossRef]
- Fontana, T.P.; Corazza, P.H.; Castro, D.M.M.; Bassani Deconto, A.; Dogenski, L.C.; Souza, M.A.; Bervian, J.; Rovani, G.; Trentin, M.S.; De Carli, J.P. Clinical, tomographic, and histological analysis of post-extraction dental sockets filled with particulate dentin or blood clot: Pilot study of a randomized clinical trial. Clin. Oral Investig. 2025, 29, 300. [Google Scholar] [CrossRef]
- Nampo, T.; Watahiki, J.; Enomoto, A.; Taguchi, T.; Ono, M.; Nakano, H.; Yamamoto, G.; Irie, T.; Tachikawa, T.; Maki, K. A new method for alveolar bone repair using extracted teeth for the graft material. J. Periodontol. 2010, 81, 1264–1272. [Google Scholar] [CrossRef]
- Avery, S.J.; Sadaghiani, L.; Sloan, A.J.; Waddington, R.J. Analysing the bioactive makeup of demineralised dentine matrix on bone marrow mesenchymal stem cells for enhanced bone repair. Eur. Cell Mater. 2017, 21, 1–17. [Google Scholar] [CrossRef]
- Minetti, E.; Taschieri, S.; Berardini, M.; Corbella, S. New classification of autologous tooth-derived grafting materials: Fundamental concepts. Int. J. Dent. 2025, 2025, 6646405. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Yun, P.Y.; Um, I.W.; Lee, H.J.; Yi, Y.J.; Bae, J.H.; Lee, J. Alveolar ridge preservation of an extraction socket using autogenous tooth bone graft material for implant site development: A prospective case series. J. Adv. Prosthodont. 2014, 6, 521–527. [Google Scholar] [CrossRef]
- Mahendra, D.A.; Bilbalqish, K.; Nugraha, A.P.; Cahyanto, A.; Sengupta, K.; Hanna, K.; Meizarini, A.; Hariyani, N. Dentin-derived alveolar bone graft for alveolar augmentation: A systematic review. J. Oral Biol. Craniofac. Res. 2024, 14, 395–406. [Google Scholar] [CrossRef]
- Schwarz, F.; Golubovic, V.; Becker, K.; Mihatovic, I. Extracted tooth roots used for lateral alveolar ridge augmentation: A proof-of-concept study. J. Clin. Periodontol. 2016, 43, 345–353. [Google Scholar] [CrossRef]
- Schwarz, F.; Mihatovic, I.; Golubovic, V.; Becker, J. Dentointegration of a titanium implant: A case report. Oral Maxillofac. Surg. 2013, 17, 235–241. [Google Scholar] [CrossRef]
- Namjoynik, A.; Islam, M.A.; Islam, M. Evaluating the efficacy of human dental pulp stem cells and scaffold combination for bone regeneration in animal models: A systematic review and meta-analysis. Stem Cell Res. Ther. 2023, 14, 132. [Google Scholar] [CrossRef]
- Fan, S.; Li, J.; Zheng, G.; Ma, Z.; Peng, X.; Xie, Z.; Liu, W.; Yu, W.; Lin, J.; Su, Z.; et al. Wac facilitates mitophagy-mediated MSC osteogenesis and new bone formation via protecting pink1 from ubiquitination-dependent degradation. Adv. Sci. 2025, 12, 2404107. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Boonyuen, S.; Apinyauppatham, K.; Pripatnanont, P. Effects of oral cavity stem cell sources and serum-free cell culture on hydrogel encapsulation of mesenchymal stem cells for bone regeneration: An in vitro investigation. Bioengineering 2024, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Chen, C.H.; Tsai, C.L.; Lin, I.H.; Hsiue, G.H. Heterobifunctional poly(ethylene glycol)-tethered bone morphogenetic protein-2-stimulated bone marrow mesenchymal stromal cell differentiation and osteogenesis. Tissue Eng. 2007, 13, 1113–1124. [Google Scholar] [CrossRef]
- Olchowy, A.; Olchowy, C.; Zawiślak, I.; Matys, J.; Dobrzyński, M. Revolutionizing Bone Regeneration with Grinder-Based Dentin Biomaterial: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 9583. [Google Scholar] [CrossRef]
- Desarda, H.M.; Shetgar, S.S.; Chaudhari, S.U.; Chaudhari, R.K.; Chandrahas, B.; Burli, V.V.A. Evaluation of human tooth properties to use as an autogenous graft—An in vitro study. J. Pharm. Bioallied Sci. 2025, 17 (Suppl. S1), 878–880. [Google Scholar] [CrossRef] [PubMed]
- Yasser, S.; Mohammed, A.A.A.R.; El-Safty, S.; Shon, A.; Al-Gabri, R.S.; Alqutaibi, A.Y.; Fouad, H.; Saleh, R.G. Comparing the effect of using calcified autogenous nano dentin particles versus micro dentin particles in the healing of mandibular bony defects in New Zealand rabbits. BMC Res. Notes 2025, 18, 125. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, composition and mineralization. Front. Biosci. (Elite Ed) 2011, 1, 711–735. [Google Scholar] [CrossRef]
- Schwarz, F.; Golubovic, V.; Mihatovic, I.; Becker, J. Periodontally diseased tooth roots used for lateral alveolar ridge augmentation: A proof-of-concept study. J. Clin. Periodontol. 2016, 43, 797–803. [Google Scholar] [CrossRef]
- Schwarz, F.; Sahin, D.; Becker, K.; Sader, R.; Becker, J. Autogenous tooth roots for lateral extraction socket augmentation and staged implant placement: A prospective observational study. Clin. Oral Implants Res. 2019, 30, 439–446. [Google Scholar] [CrossRef]
- Castillo-Cardiel, G.; López-Echaury, A.C.; Saucedo-Ortiz, J.A.; Fuentes-Orozco, C.; Michel-Espinoza, L.R.; Irusteta-Jiménez, L.; Salazar-Parra, M.; González-Ojeda, A. Bone regeneration in mandibular fractures after the application of autologous mesenchymal stem cells: A randomized clinical trial. Dent. Traumatol. 2017, 33, 38–44. [Google Scholar] [CrossRef]
- Liu, X.; Liao, X.; Luo, E.; Chen, W.; Bao, C.; Xu, H.H. Mesenchymal stem cells systemically injected into femoral marrow of dogs home to mandibular defects to enhance new bone formation. Tissue Eng. Part A 2014, 20, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Roato, I.; Perale, G.; Rossi, F.; Genova, T.; Mussano, F.; Ferracini, R.; Sorlini, M.; Torre, M.L.; Perteghella, S. Biohybrid bovine bone matrix for controlled release of mesenchymal stem/stromal cell lyosecretome: A device for bone regeneration. Int. J. Mol. Sci. 2021, 22, 4064. [Google Scholar] [CrossRef] [PubMed]
- Piattelli, M.; Favero, G.A.; Scarano, A.; Orsini, G.; Piattelli, A. Bone reactions to anorganic bovine bone (Bio-Oss) used in sinus augmentation procedures: A histologic long-term report of 20 cases in humans. Int. J. Oral Maxillofac. Implants. 1999, 14, 835–840. [Google Scholar] [PubMed]
- Stavropoulos, A.; Karring, T. Guided tissue regeneration combined with a deproteinized bovine bone mineral (Bio-Oss®) in the treatment of intrabony periodontal defects: 6-year results from a randomized-controlled clinical trial. J. Clin. Periodontol. 2010, 37, 200–210. [Google Scholar] [CrossRef]
- Van Houdt, C.I.A.; Ulrich, D.J.O.; Jansen, J.A.; van den Beucken, J.J.J.P. The performance of CPC/PLGA and Bio-Oss® for bone regeneration in healthy and osteoporotic rats. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 131–142. [Google Scholar] [CrossRef]
- Van Houdt, C.I.; Tim, C.R.; Crovace, M.C.; Zanotto, E.D.; Peitl, O.; Ulrich, D.J.; Jansen, J.A.; Parizotto, N.A.; Renno, A.C.; van den Beucken, J.J. Bone regeneration and gene expression in bone defects under healthy and osteoporotic bone conditions using two commercially available bone graft substitutes. Biomed. Mater. 2015, 10, 035003. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J. Healing around implants placed in bone defects treated with Bio-Oss: An experimental study in the dog. Clin. Oral Implants Res. 1997, 8, 117–124. [Google Scholar] [CrossRef]
- Tapety, F.I.; Amizuka, N.; Uoshima, K.; Nomura, S.; Maeda, T. A histological evaluation of the involvement of Bio-Oss® in osteoblastic differentiation and matrix synthesis. Clin. Oral Implants Res. 2004, 15, 315–324. [Google Scholar] [CrossRef]
- Gutwald, R.; Haberstroh, J.; Kuschnierz, J.; Kister, C.; Lysek, D.A.; Maglione, M.; Xavier, S.P.; Oshima, T.; Schmelzeisen, R.; Sauerbier, S. Mesenchymal stem cells and inorganic bovine bone mineral in sinus augmentation: Comparison with augmentation by autologous bone in adult sheep. Br. J. Oral Maxillofac. Surg. 2010, 48, 285–290. [Google Scholar] [CrossRef]
- Pieri, F.; Lucarelli, E.; Corinaldesi, G.; Iezzi, G.; Piattelli, A.; Giardino, R.; Bassi, M.; Donati, D.; Marchetti, C. Mesenchymal stem cells and platelet-rich plasma enhance bone formation in sinus grafting: A histomorphometric study in minipigs. J. Clin. Periodontol. 2008, 35, 539–546. [Google Scholar] [CrossRef]
- D’Aquino, R.; De Rosa, A.; Lanza, V.; Tirino, V.; Laino, L.; Graziano, A.; Desiderio, V.; Laino, G.; Papaccio, G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur. Cell Mater. 2009, 18, 75–83. [Google Scholar] [CrossRef]
- Al-Qadhi, G.; Soliman, M.; Abou-Shady, I.; Rashed, L. Gingival mesenchymal stem cells as an alternative source to bone marrow mesenchymal stem cells in regeneration of bone defects: In vivo study. Tissue Cell 2020, 63, 101325. [Google Scholar] [CrossRef]
- Rickert, D.; Sauerbier, S.; Nagursky, H.; Menne, D.; Vissink, A.; Raghoebar, G.M. Maxillary sinus floor elevation with bovine bone mineral combined with either autogenous bone or autogenous stem cells: A prospective randomized clinical trial. Clin. Oral Implants Res. 2011, 22, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Sauerbier, S.; Stricker, A.; Kuschnierz, J.; Bühler, F.; Oshima, T.; Xavier, S.P.; Schmelzeisen, R.; Gutwald, R. In vivo comparison of hard tissue regeneration with human mesenchymal stem cells processed with either the FICOLL method or the BMAC method. Tissue Eng. Part C Methods 2010, 16, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.M.; Duncan, W.J.; Coates, D.E. Attributes of Bio-Oss® and Moa-Bone® graft materials in a pilot study using the sheep maxillary sinus model. J. Periodontal Res. 2018, 53, 80–90. [Google Scholar] [CrossRef]
- Addis, A.; Canciani, E.; Campagnol, M.; Colombo, M.; Frigerio, C.; Recupero, D.; Dellavia, C.; Morroni, M. A new anorganic equine bone substitute for oral surgery: Structural characterization and regenerative potential. Materials 2022, 15, 1031. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Cecchinato, D.; Donati, M.; Tomasi, C.; Liljenberg, B. Ridge preservation with the use of deproteinized bovine bone mineral. Clin. Oral Implants Res. 2014, 25, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Traini, T.; Valentini, P.; Iezzi, G.; Piattelli, A. A histologic and histomorphometric evaluation of anorganic bovine bone retrieved 9 years after a sinus augmentation procedure. J. Periodontol. 2007, 78, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, B.O.B.; Koth, V.S.; Sesterheim, P.; Salum, F.G.; Rübensam, G.; Augustin, A.H.; Cherubini, K. Autogenous dentin combined with mesenchymal stromal cells as an alternative alveolar bone graft: An in vivo study. Clin. Oral Investig. 2023, 27, 1907–1922. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.D.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Asakura, T.; Diep, T.T.T.; Ueda, Y.; Yamada, A.; Tsuzuno, T.; Takahashi, N.; Miyata, M.; Tabeta, K.; Nagata, M.; Matsuda, K. Analysis of the effect of human type i collagen-derived peptide on bone regenerative capacity and comparison with various collagen materials in vivo. Medicina 2025, 61, 57. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).