Abstract
Ammonia, due to its favorable physicochemical properties, is considered an effective hydrogen carrier, enabling the storage of surplus energy generated from renewable sources. Large-scale implementation of this concept requires the safe transport of ammonia over long distances, commonly achieved through pipeline systems—a practice with global experience dating back to the 1960s. However, operational history demonstrates that failures in such infrastructures remain inevitable, often leading to severe environmental consequences. This article reviews both passive and active methods for preventing and mitigating incidents in ammonia pipeline systems. Passive measures include the assessment of material compatibility with ammonia and the designation of adequate buffer zones. Active methods focus on leak detection techniques, such as balance-based systems, acoustic monitoring, and ammonia-specific sensors. Additionally, the article highlights the potential environmental risks associated with ammonia release, emphasizing its contribution to the greenhouse effect, as well as its adverse impacts on soil, surface and groundwater, and human health. By integrating historical lessons with modern safety technologies, the article contributes to the development of reliable ammonia transport infrastructure for the hydrogen economy.
1. Introduction
The volatility of renewable energy sources and the storage of generated energy represent two major challenges in the transition to a sustainable energy system. Renewable energy sources, such as solar and wind power, are inherently volatile because they depend on weather conditions. This creates a number of problems related to the unpredictability of energy production in RES and interruptions in access to a given energy source. These difficulties arise from the specific characteristics of each energy source. Solar energy generation fluctuates due to cloud cover and seasonal and daily variations. In the case of wind energy, balancing difficulties arise from the unpredictable variability of wind speed over time. Even for hydroelectric energy, seasonal changes in precipitation and droughts can affect the availability of water, and thus the ability to generate energy. Excess electricity production derived from RES should be effectively stored to balance the system in case of shortages in renewable energy production. Direct storage of electricity in various types of batteries is considered inefficient. In addition, the production of such devices requires access to critical raw materials, the extraction of which significantly impacts the environment. For this reason, research and energy storage efforts have focused on storing energy in the form of chemical carriers such as hydrogen. In this approach, energy storage is based on the electrolysis of water to produce green hydrogen from excess energy, and the use of fuel cells to generate electricity during periods of lower supply. With the global drive towards decarbonization and the development of the hydrogen economy, ammonia is increasingly being considered a strategic vector for large-scale hydrogen storage and transport.
Hydrogen, due to its unique properties, is a promising energy storage source, but the transportation of hydrogen presents significant challenges. Hydrogen has a low energy density per unit volume compared to other fuels, which requires larger storage volumes or higher pressures to transport the same amount of energy. In addition, liquefying hydrogen is not a simple process, as it requires hydrogen to be cooled to −253 °C. As a result, other forms of chemical energy storage are being explored. Moreover, hydrogen transport poses many challenges, including problems related to hydrogen embrittlement. This phenomenon occurs when hydrogen atoms diffuse into a metal structure, making it brittle [1]. Scaling up a hydrogen economy will inevitably require effective hydrogen carriers, yet this does not diminish the simple fact that the most direct use of molecules remains the most efficient. When we move from electrons to molecules, direct utilization is inherently optimal—and this principle applies equally to ammonia. It is clear that the hydrogen economy cannot be realized without ammonia: the two are intrinsically linked. Large-scale hydrogen production and transport are feasible only if ammonia is part of the equation, thanks to its exceptional energy density and ability to store and transport hydrogen effectively.
However, direct use of ammonia energy depends on reliable transport from production or import sites to end-users. This underscores the urgent need for ammonia pipelines as a safe, efficient, and scalable alternative to road, rail, or maritime transport. Just as today’s energy infrastructure relies on national gas grids to move large volumes of energy, ammonia pipelines are the logical solution for enabling a hydrogen-powered future.
Transporting ammonia also requires compliance with stringent international regulations for hazardous materials, which significantly influence infrastructure design, operational procedures, and overall costs.
Increased permeation through the walls of polymer gas has been observed when transporting hydrogen compared to conventional fuels [2,3,4,5]. In addition, hydrogen is highly flammable and explosive, which can lead to potential accidents during transportation. The use of high-pressure containers and pipelines, if not properly maintained and monitored, further exacerbates these safety risks. The problems associated with hydrogen storage and transportation are related to the need for high pressures and safety considerations that can be solved by using chemical hydrogen carriers, such as methanol or ammonia. Ammonia has a high hydrogen content (17.8% m/m and volumetric density of 121 kg H2/m3 at 10 bar and can be liquefied at low pressure and temperature, 8.6 bar at 20 °C) [6], so its transportation and storage are relatively easy and require little energy input [7]. The choice of transportation method for ammonia is determined by a range of technical and economic factors, among which the most critical are the following:
- Quantity of transported gas—the volume of ammonia to be shipped directly influences the selection of an appropriate transportation mode.
- Transport distance—the distance between the production site and the destination (storage or consumption facility) is a decisive factor in the economic viability of specific transport modes.
- Locational considerations of the manufacturing and storage site (including access to appropriate infrastructure—road and rail networks, location in relation to the river network, ports, etc.).
- Transport safety—ammonia is a hazardous substance, so issues related to the risk of spills, fires, or explosions are present.
- Transportation costs—these vary depending on the selected mode of transport (road, rail, waterway, pipeline) and include infrastructure, logistics operations, and compliance with safety regulations.
- Phase of ammonia—ammonia can be transported in either liquid or gaseous form. The choice of the form of transport depends on the temperature and pressure at which the ammonia is stored.
- Transport and storage time—optimization of transport processes may also depend on how quickly the ammonia must reach its destination. Longer transport durations may require ammonia to be stored under controlled conditions, which can generate additional costs.
- Environmental factors, such as greenhouse gas emissions and potential impacts on local ecosystems, may also influence the selection of the transportation mode.
- Regulatory and legal perspectives—compliance with national and international regulations for the transport of hazardous materials can further constrain or dictate the choice of transportation method.
In most cases, ammonia distribution relies on a combination of transportation modes to deliver the product from the production site to its final destination. In the United States, the modal share of ammonia transportation in 2017 is presented in Figure 1.
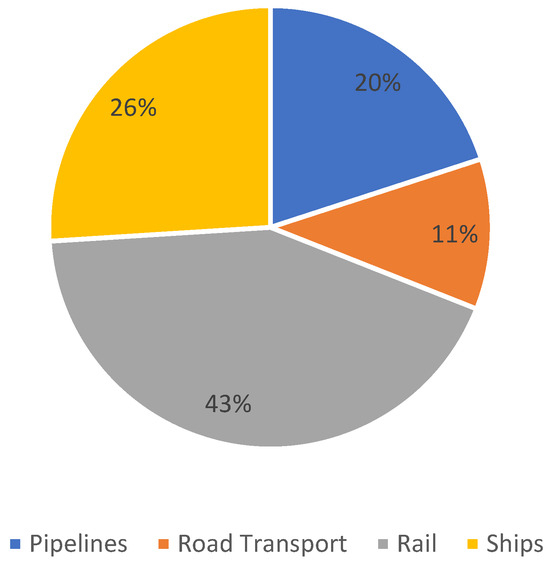
Figure 1.
Distribution of transportation modes for ammonia in the United States [8].
Transportation of ammonia by pipelines, alongside road transport using wheeled vehicles, is among the least commonly employed methods for moving this raw material. In contrast, ammonia transported by rail or maritime routes typically covers substantially greater distances on average compared to pipeline or road-based methods [8]. The ultimate choice of transportation mode is primarily influenced by economic factors, including the distance over which ammonia must be shipped and the volume of the material to be transported [9,10]. Despite the long history of ammonia transport, it is essential to remain aware of the inherent risks associated with handling this substance. Workers must be informed of potential hazards related to ammonia exposure, and appropriate safety measures, including the use of personal protective equipment, should be rigorously implemented. Future research and technological development should therefore focus on enhancing the safety, efficiency, and sustainability of ammonia transport systems, thereby supporting the broader transition to a low-carbon energy economy.
2. Materials and Methods
This review focuses on examining global experience in ammonia transport via pipeline systems, with particular attention to identifying associated risks and potential mitigation strategies.
To conduct the review, the authors employed a comprehensive search strategy, using Google Scholar as the primary database. Google Scholar was selected for its extensive coverage of academic sources, including peer-reviewed journals, conference proceedings, theses, books, and patents. This platform offers a broad and up-to-date overview of the available literature, which is essential for ensuring the inclusion of the most recent and relevant studies. Google Scholar was chosen as the primary database for this review due to several key advantages. First, it provides extensive coverage of a wide range of academic sources, including peer-reviewed journals, conference proceedings, theses, books, and patents, which ensures a comprehensive overview of the topic. Second, it is regularly updated, allowing access to the most recent publications, which is critical for rapidly evolving research areas such as ammonia transport and hydrogen energy. Third, Google Scholar indexes literature from multiple disciplines and publishers, increasing the likelihood of identifying relevant studies that may not be included in more specialized databases. Finally, its accessibility and search functionalities, including keyword and citation tracking, facilitate a systematic and efficient literature search, supporting the rigor and transparency required in a scientific review. Additionally, the platform provides citation metrics, facilitating the assessment of the impact and relevance of publications. This combination of breadth, accessibility, and evaluative tools makes Google Scholar a reliable and efficient resource for conducting thorough literature reviews. What’s more, it provides free and easy access to citation metrics, making it more accessible for comprehensive literature searches.
The search queries were constructed using a combination of key terms related to the topic of interest. These terms included “pipeline system in ammonia transportation, ”ammonia transport risk”, “accident in ammonia transport”, “methods of ammonia distribution”, and “environmental risks in ammonia pipeline systems”. Boolean operators (AND, OR) were used to refine and focus the search results.
To ensure the selection of high-quality and relevant documents, the authors applied specific inclusion and exclusion criteria. The inclusion criteria encompassed peer-reviewed articles published in the last ten years, studies focusing on ammonia transportation technologies, articles available in English, and papers providing detailed methodological descriptions. The exclusion criteria ruled out non-peer-reviewed articles and grey literature, studies published more than ten years ago (unless they are seminal works), articles not available in full text, and documents not directly related to the topic.
The initial search yielded a large number of results. Each title and abstract was screened for relevance, and full texts of potentially relevant articles were retrieved for further assessment. Duplicate records were removed, and the remaining articles were reviewed in detail to ensure they met the inclusion criteria.
To gain an understanding of the aspects of global experience in ammonia transport via pipeline systems, a thorough review of relevant literature was conducted. Then, research articles, reports, and data from sources that covered aspects such as global experience in ammonia pipeline system transportation, existing ammonia transport pipelines, ammonia pipeline incidents, various causes of failures in ammonia pipelines, accident prevention methods in ammonia pipeline transport, environmental impact, and environmental risks associated with ammonia pipeline systems, as well as the information about methods which can be helpful in minimizing risks associated with ammonia transport via pipelines were carefully gathered. After gathering the data, we analyzed it meticulously to extract insights and identify trends related to threats and challenges associated with ammonia transport via pipeline systems.
Figure 2 illustrates the methodology phases for this research. It is crucial to note that this research heavily depended on existing literature and data sources, which may introduce biases or gaps. Furthermore, since alternative energy technologies are constantly evolving, some data points may become outdated or subject to change.
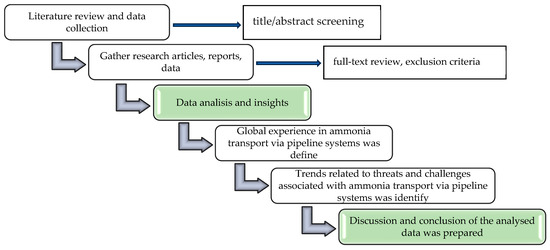
Figure 2.
The Methodology flowchart.
3. Results
3.1. Global Experience in Ammonia Pipeline System Transportation
Among the various methods of ammonia transport, pipelines represent a key option due to their ability to efficiently and continuously handle large volumes. Pipeline transportation is a mature technology that has been in use for decades and is generally considered safe and low-risk. As of today, transport of ammonia by pipelines is used in two extremely different cases. On the one hand, pipelines are used to transport large quantities of ammonia over long distances (more than 500 km); on the other hand, pipelines are also used to transport ammonia on the premises of production plants, when ammonia is transported through process pipelines. The disadvantage of this type of transport is the lack of flexibility compared to transport by road, for example. Ammonia can be transported via pipelines in both gaseous and liquid phases. However, in the case of pipeline transportation other than intra-plant transport, the ammonia should be in the liquid phase, not in the gaseous phase, in order for the transport to be profitable. Transportation of ammonia in the gaseous phase is not economically feasible due to the low density of this gas. Numerous ammonia pipelines of varying lengths and specifications exist worldwide, examples of which are presented in Table 1.

Table 1.
Overview of existing ammonia transport pipelines worldwide [11,12,13,14,15,16].
Ammonia transport via pipeline systems has been successfully implemented since the 1960s, reflecting decades of experience in handling this hazardous but industrially important chemical. Analysis of the available data indicates that the lengths, diameters, and materials of existing ammonia pipelines vary considerably, depending on the intended capacity, operational pressure, and regional conditions. Nevertheless, ammonia pipelines are widely utilized worldwide, demonstrating their reliability and efficiency as a transport method. Long-distance pipelines, typically exceeding 1000 km, are primarily employed for ammonia transport in the United States and Russia, where they play a crucial role in connecting large-scale production facilities with distribution centers. These pipelines offer significant economic advantages by enabling continuous, high-volume transport, reducing reliance on road or rail transport, and lowering operational costs over long distances.
In most countries, the design and construction of ammonia transport pipelines are based on established technical standards and norms [17,18,19]. These documents define the requirements for materials, design procedures, and safety measures to ensure reliable and leak-free operation [20,21]. Table 2 provides an overview of selected series of technical documents and standards dedicated to ammonia transport [22,23]. The application of standardized technical guidelines in pipeline construction is particularly important, given that pipeline materials, including those used for ammonia transport, are subject to degradation over their operational lifespan due to processes such as corrosion, fatigue, and chemical interactions. Addressing this degradation effectively requires an integrated approach combining material selection, protective measures, systematic inspections, and predictive modeling to ensure structural integrity and mitigate associated operational risks [16].

Table 2.
Overview of selected series of technical documents and standards dedicated to ammonia transport pipelines [11,16,17,18,19,20,21,22,23].
Despite more than fifty years of operational experience with ammonia pipelines, it has not been possible to completely eliminate incidents and accidents, some of which have resulted in serious environmental consequences. This underscores the ongoing need for rigorous safety measures, effective monitoring systems, and continuous improvements in pipeline design and operation. Table 3 presents the location, year of occurrence, cause of failure, and recommended preventive measures for ammonia pipeline incidents.

Table 3.
Overview of ammonia pipeline incidents, including location, year of occurrence, identified causes, and recommended preventive measures [11,14,24].
Failures in ammonia pipelines can arise from various causes, with the most commonly reported factors including corrosion (3 incidents), third-party actions or acts of war (3 incidents), failures during repair and maintenance work (2 incidents), mechanical damage (2 incidents), weld failure (1 incident), and freeze–thaw cycles (1 incident). Although such failures are relatively rare, their consequences can be severe due to the uncontrolled release of ammonia into the atmosphere. Ammonia emissions can pose significant environmental hazards, including toxicity to humans, contamination of soil and water, and contribution to atmospheric nitrogen loading. These risks underscore the importance of rigorous monitoring, preventive maintenance, and the implementation of advanced safety measures in ammonia pipeline systems. The following subsections provide a detailed discussion of the potential environmental impacts associated with ammonia emissions from pipeline networks, highlighting both acute and long-term hazards [11,14,24].
3.2. Accident Prevention in Ammonia Pipeline Transport
Safe transport of ammonia via pipeline systems, first of all, requires the construction of pipelines from materials compatible with ammonia.
Table 4 presents a comprehensive overview of the parameters for assessing the durability of ammonia pipelines. Systematic evaluation and continuous monitoring of these parameters during pipeline operation are critical for effective risk management and for ensuring the safe transport of ammonia, thereby minimizing potential hazards and operational failures.

Table 4.
Parameters for assessing the durability of ammonia pipelines [17,18,19,20,21,22,23].
The durability of ammonia pipelines depends on material properties, operating conditions, corrosion and mechanical factors, and regular inspection and maintenance. Key parameters include material strength and toughness, pressure and temperature variations, corrosion susceptibility, wall thickness, and environmental influences such as soil and vibration. Ensuring long-term safe operation requires an integrated approach combining appropriate materials, controlled conditions, monitoring, and adherence to standards.
Therefore, when designing and constructing pipelines to transport ammonia, it is necessary to ensure the material compatibility not only of the pipes themselves, but also of the fittings and joints present on the pipelines. There are a number of published assessments of the corrosivity of ammonia to structural steel materials used in natural gas pipelines [25,26,27,28]. These evaluations show that at typical transport pipeline temperatures, carbon and stainless 316 steels have excellent corrosion resistance (<0.051 mm/year) in anhydrous ammonia. However, carbon and low-alloy steels are prone to stress cracking if impurities, particularly oxygen, are present in the ammonia. Interestingly, the addition of a small amount of water to ammonia [0.2% is usually used] is known to inhibit the cracking mechanism [19].
Copper-containing alloys, such as brass and bronze, are generally suitable for use in high-purity anhydrous ammonia environments, but are susceptible to stress corrosion cracking if even small amounts of impurities, such as water, are present in the ammonia; therefore, these alloys are generally not recommended for use in ammonia environments. Due to its properties, ammonia causes accelerated corrosion of copper, aluminum, and zinc (and their alloys), while it has no negative effect on carbon steel [29], so it is usually transported through carbon steel pipelines in a liquid state at a pressure of about 17 bar. In doing so, it should be noted that valves are made of brass. Such valves will not be compatible with ammonia-containing water as an impurity [30].
The compatibility of ammonia with polymeric materials is well known. There are ammonia polymer pipelines used for agricultural applications that can provide good experimental material for handling and transporting ammonia. The compatibility of ammonia with polymers varies considerably depending on the polymer type, but as a rule, ammonia also shows good compatibility with polymers [27,28,31]. Above all, ammonia has a negligibly low permeation rate through polyethylene compared to hydrogen or methane (Table 5).

Table 5.
Comparison of methane, hydrogen, and ammonia permeation factors through polyethylene [2,4,32].
Ammonia permeates through polyethylene approximately 12 times more slowly than methane and over 300 times more slowly than hydrogen. Nevertheless, this property alone does not preclude the use of polyethylene for ammonia transport. An important criterion here is the possible damage that can be caused by contact between the polymer and ammonia. Damage to polymers is usually based on swelling and changes in permeability. Changes in properties often accompany swelling, which includes reductions in strength and hardness. In turn, these property changes affect the permanent deformation of polymer seals and sealing ability. Structural changes related to crosslinking, chemical bonding, and composition (such as fluid penetration and extraction of additives) will potentially change the glass transition temperature, making the polymer more brittle or rubbery during operation, which can affect design overruns [33]. Based on an analysis of ammonia compatibility with various polymers, the following conclusions can be drawn:
- ▪
- Polyethylene, epoxies, and certain elastomers: Nitrile Butadiene Rubber (NBR), Ethylene Propylene Diene Monomer (EPDM), neoprene or Chloroprene Rubber (CR), and Perfluoroelastomer (FFKM) are suitable for use with ammonia.
- ▪
- Polyurethane, epichlorohydrin rubber (ECO), and fluorosilicone should not come into contact with ammonia.
- ▪
- Polytetrafluoroethylene (PTFE), nylons, and elastomers such as certain fluorocarbons, PTFE, and SBR are highly incompatible with ammonia [28].
A passive way to mitigate the negative effects of pipeline failures, in addition to receiving the right materials for pipeline construction, is to maintain adequate buffer areas. Ensuring adequate distance can effectively mitigate the hazards posed by ammonia leakage in pipelines. Ongoing CFD simulations show that the range of toxic and explosion hazards associated with ammonia leakage, depending on weather conditions, can extend to about 500–1000 m. Factors such as wind direction and speed, temperature, and humidity significantly influence the dispersion of ammonia. These results highlight that, in the event of a release, ammonia can pose a serious risk within a radius of up to one kilometer, which should be considered when planning safety zones and emergency response measures [24,34]. Therefore, strategically locating pipelines away from residential or densely populated areas has proven to be an effective means of reducing potential public safety risks. However, it should be emphasized that this does not reduce environmental risks.
Among the passive methods to reduce the negative impact of ammonia pipeline failures, issues related to the placement of pipelines underwater or underground are also mentioned. In such cases, the high solubility of ammonia in water will cause much of it to be retained, preventing it from entering the atmosphere. However, this approach is somewhat controversial due to the strong effects ammonia has on both soil and water. In addition, if ammonia enters groundwater or surface water, it can cause the ammonia transported with water to contaminate a much larger area than if it enters the atmosphere directly.
Minimizing risks associated with ammonia transport via pipelines can be implemented on many levels using both passive and active methods. Active methods are mainly based on pipeline condition monitoring systems and use various types of methods to quickly detect ammonia leaks from damaged or leaking pipelines. Recent advances in optoelectronic materials and sensing technologies have enabled the development of a wide range of sensors for monitoring ammonia in the air, including electrochemical, photoacoustic, infrared, and ammonia-specific chemical sensors. These devices provide real-time detection and early warning, allowing for immediate intervention to mitigate the impact of leaks and enhance overall pipeline safety.
Among these, semiconductor sensors, also known as Metal Oxide Semiconductor (MOS) sensors, operate based on changes in electrical conductivity caused by gas adsorption onto the surface of the semiconductor [35]. Typically, a thin layer of metal oxide is deposited on a silicon substrate. The detection process involves reversible gas adsorption onto the heated metal oxide surface, followed by catalytic oxidation of the adsorbed gas. This reaction induces a change in the electrical resistance of the oxide material, which is proportional to the concentration of the target gas in the mixture [36,37]. MOS sensors are widely used due to their simplicity and low cost; however, they also present certain limitations, including relatively long response times and low selectivity for ammonia in gas mixtures. Most solid-state sensors have detection limits above 5 ppm and response times ranging from 4 s to over one minute, which can limit their suitability for continuous monitoring of ammonia in the air [38,39].
In addition to semiconductor sensors, electrochemical sensors are widely employed for ammonia detection due to their high sensitivity and selectivity. These sensors operate based on redox reactions at the electrodes, providing an alternative approach to monitoring ammonia concentrations in air. Electrochemical sensors determine the concentration of a target gas by measuring its oxidation or reduction reaction at the electrode, depending on the type of gas. For example, carbon monoxide can be oxidized to carbon dioxide, while oxygen is reduced to water. The oxidation reaction causes electrons to flow from the sensing electrode to the counter-electrode through an external circuit, whereas in the case of reduction, the flow of electrons is reversed. The resulting current is proportional to the concentration of the target gas in the mixture [40]. Electrochemical sensors are generally sensitive, selective, and inexpensive, making them suitable for leak detection, emissions monitoring, and fire safety applications; however, they require frequent calibration to maintain accuracy [41].
Beyond semiconductor and electrochemical sensors, optical sensors offer a highly sensitive and selective approach for ammonia detection. These sensors operate based on absorption and luminescence principles, providing precise measurements even at very low concentrations. Optical sensors can detect ammonia concentrations from about 1 ppm (non-dispersive infrared spectroscopy, NDIR [42]) to about 1 ppb (cavity ring-down spectroscopy, CRDS [42]) and tunable diode laser absorption spectroscopy, TDLAS [39], with higher selectivity and slightly faster response times. However, these systems are relatively expensive, and the miniaturization of these sensors is challenging.
In addition to individual sensor technologies, integrated monitoring approaches can be employed to enhance the safety of ammonia transport systems. Various types of sensors can be incorporated into remote sensing systems to monitor confined spaces exposed to ammonia emissions, ensuring rapid detection of leaks and minimizing potential hazards. In the context of ammonia pipelines, these sensors can be installed at locations where ammonia volume is measured or where pumping support systems are present. Moreover, the aforementioned sensors can be integrated into portable leak detection devices, commonly referred to as “sniffers,” which are used by personnel responsible for the safe operation of pipelines and related installations. Beyond point detection, the pipelines themselves or the overall transport system can be monitored using various balance-based methods that rely on mass and/or volume measurements to identify leaks. The methods used for this purpose are described below.
The basic method is based on mass or volume balance at the entrance and exit of the pipeline section under examination. A discrepancy between the amount of gas entering the system and the amount measured at the exit may indicate the presence of a leak in the pipeline. The accuracy of this method depends on the accuracy of the measuring instruments. In addition, this method gives much better results for time-constant gas flows. If the gas reception at the exit points varies over time, this can lead to false alarms or increase the leak detection time [43,44,45].
An improvement of the method based on mass flow rate or volume balance is referred to as RTTM (real-time transient model method). This method, unlike methods based on mass flow rate or volume balance, allows compensation for dynamic changes occurring in the pipeline [43,45]. A real-time transient model detects anomalies in a flowing fluid or gas using mathematical simulations governed by fundamental laws of physics, such as conservation of mass, conservation of momentum, and conservation of energy. Pipeline operating parameters, such as flow, pressure, and temperature, are extracted by the SCADA system.
The negative pressure wave propagation method is a technique for locating leaks and defects in pipelines. The amplitude of negative pressure waves can be used to obtain information about the flow rate of the leak and the leak area. A sudden change in density at the leak site causes a drop in pressure, generating a negative pressure wave that propagates both upstream and downstream, where it is detected by a pressure sensor. Time-of-flight analysis reveals the origin of the pressure wave and thus the location of the spill [46,47].
Another calculation method is point pressure analysis. This method is based on the assumption that if there is a leak in the pipeline, the gas pressure in the pipeline decreases [43,44]. This method requires continuous pressure measurements at various points along the pipeline [45,46,47,48]. Pressure sensors can be spaced far apart from each other, unless the location of the gas pipeline forces a denser placement of sensors. To detect a leak in a gas pipeline, a comparison of the received results of gas pressure measurements in the pipeline with the average value is used. If the measurement result is lower than the average value by a certain threshold value, it should be considered that the section of the monitored gas pipeline under study is leaking [43,44,45]. However, this method has numerous drawbacks. One of them is that it does not provide reliable results for gas flows that are unstable over time.
The last group of calculation methods is statistical methods. Statistical analysis is the simplest way to detect gas pipeline leaks without using a mathematical model. In this method, an analysis of parameters measured at multiple locations along the pipeline, such as pressure and gas flow, is carried out. An alarm indicating the presence of a leak is generated only when the measured values deviate in a statistically significant way from the assumed values [43,44,45].
Another way to detect leaks in gas pipelines is through acoustic systems. Leak detection using acoustic emission (AE) sensors is a technology capable of detecting and locating gas leaks, even from small cracks or perforations in gas networks [49,50]. Acoustic emissions associated with leaks in gas infrastructure are caused by the turbulent outflow of a high-pressure gas stream through the hole representing the leak. For underground infrastructure (gas pipelines), acoustic sensors placed along pipelines are used to detect leaks. In the acoustic leak localization method, acoustic sensors are key components of the system, so the choice of sensors should be based on the frequency range and propagation characteristics of the signal generated by the leak. Basically, the choice of acoustic sensors is a choice between high-frequency and low-frequency sensors. High-frequency sensors have the distinct advantage of being immune to ambient noise, which tends to propagate in the low-frequency range. However, since high-frequency signals are strongly attenuated during propagation, monitoring gas pipelines of considerable length using them is difficult [49,51].
Another type of system used for ammonia leak detection is seismic systems [15]. This type of system consists of seismic sensors placed along and near the pipeline. These wireless sensors detect any activity that generates a seismic signal in the ground. The sensor or sensors then transmit the detected signal to a processing unit, which analyzes the data to determine the nature of the event that generated that seismic signal. The system can distinguish between different events, such as a person walking, a vehicle driving, heavy machinery, digging, hammering, pipeline drilling, leaks, etc. The processed data is sent to the pipeline operator’s control center. A real-time warning is displayed to the operator, which includes the location and classification of the event. These two parameters (location and classification) enable the operator to take appropriate action. The system can be used in both above-ground and underground pipelines. Systems of this type have been applied to pipelines transporting water and oil in Asia and Latin America, but so far they have not been used for pipelines transporting ammonia [15]. The seismic monitoring systems, widely used for oil and gas pipelines, have not yet been applied to ammonia pipelines. While these systems hold potential for leak detection, their direct application is challenging because ammonia exhibits different physical and chemical signatures compared to oil or gas. Adapting seismic systems to ammonia transport would therefore require careful calibration and potentially new detection strategies to ensure reliable monitoring. Ensuring the safe and reliable operation of ammonia pipeline systems requires continuous monitoring of corrosion and overall pipeline integrity. Corrosion can compromise the structural components of pipelines, potentially leading to leaks, ruptures, and serious environmental and safety hazards. To address these risks, integrity management programs commonly incorporate regular inspections, wall thickness measurements, and non-destructive testing techniques, such as ultrasonic testing, radiography, or magnetic flux leakage. Early detection of material degradation or mechanical damage enables operators to implement timely maintenance and preventive measures, thereby safeguarding both the longevity of the pipeline and the well-being of surrounding communities and ecosystems.
In parallel, recent advances in automation and artificial intelligence (AI) offer significant opportunities to enhance pipeline monitoring and management. AI-driven analytics can process large volumes of real-time sensor data, facilitating the rapid detection of anomalies, the prediction of potential failures, and the optimization of maintenance schedules. When combined with predictive maintenance systems and comprehensive risk analysis models, these technologies allow operators to proactively identify and mitigate issues before they result in leaks or accidents. Collectively, such innovations not only improve operational efficiency but also markedly enhance the safety, reliability, and environmental performance of ammonia pipeline transport systems.
3.3. Environmental Risks Associated with Ammonia Pipeline Systems
Ammonia pipeline systems, while essential for the large-scale transport of this chemical, pose significant environmental risks in the event of leaks or accidental releases. The release of ammonia into the atmosphere, water, or soil due to potential pipeline failures can impact various elements of the environment in multiple ways. Such incidents not only threaten ecosystems but also pose serious risks to public safety by affecting human health and life, and by contributing to the formation of an explosive atmosphere. Understanding these risks is crucial for designing effective safety measures and minimizing the impact on air, water, and soil quality.
The primary danger associated with the release of ammonia into the atmosphere is its negative effects on human health. Ammonia is a powerful irritant, and contact with its vapors can lead to irritation of the respiratory tract, eyes, and skin [52]. Prolonged exposure to airborne ammonia can lead to serious health problems, such as lung damage, asthma, and other respiratory diseases. For this reason, the release of ammonia into the atmosphere as a result of an accident poses a direct threat to the health of those in the vicinity of the emission source. Ammonia can have harmful effects on humans through inhalation, ingestion, or skin or eye contact. Its toxic effects depend on the concentration and duration of exposure. Ammonia has a toxic effect on the respiratory system. Inhalation of ammonia fumes can lead to the following:
- As a result of short-term exposure: coughing, throat irritation, runny nose, and a feeling of tightness in the chest,
- As a result of exposure to high concentrations: severe irritation or damage to the respiratory tract leading to difficulty breathing, wheezing, and in extreme cases, pulmonary edema or respiratory failure [53,54].
In addition to affecting the respiratory system, ammonia has toxic effects on the eyes. Contact of ammonia with the mucous membrane of the eye can cause redness, tearing, a burning sensation, and severe discomfort. Prolonged exposure in this range can cause corneal damage and lead to loss of vision. In case of high and/or prolonged exposure, ammonia can lead to systemic poisoning. Symptoms of such poisoning can include headaches, dizziness, confusion, and fatigue. In very high concentrations, exposure to ammonia can lead to neurological damage and even death due to its toxic effects on the brain and nervous system. In contrast, chronic exposure to low levels of ammonia, such as in industrial settings, can lead to respiratory diseases such as asthma, bronchitis, and lung damage [55,56], as well as decreased lung function and increased risk of respiratory infections. For this reason, ammonia content in the air is limited by relevant laws. Health, Safety, and Environment (HSE) schemes set short-term and long-term exposure limits at 25 ppm and 35 ppm [57]. It is a threat to public safety if ammonia is released into the atmosphere due to the fact that ammonia is flammable in gaseous form when mixed with air or oxygen, and can be explosive under certain conditions—the explosive limit of ammonia–air mixtures is 15.5–26.6% by volume [58].
High levels of ammonia in the air can contribute to the formation of photochemical smog, especially in industrial areas, where reactions between ammonia and other air pollutants can lead to the formation of harmful substances that degrade air quality [59]. As a result of photochemical reactions occurring in the atmosphere, ammonia can also contribute to the formation of acid rain, even though ammonia is an alkaline substance. When ammonia is released into the air, it can react with nitrogen oxides and sulfur dioxide to form nitrogen and sulfur compounds, which then contribute to the formation of nitric (V) and sulfuric (VI) acid. These acids, combined with rainfall, can fall to the ground in the form of acid rain [60].
In addition to its effects on human health and air quality, ammonia released from pipeline systems can have significant environmental impacts on soil and water ecosystems. Due to its high solubility, ammonia can readily dissolve in rainwater or surface runoff, leading to contamination of rivers, lakes, and groundwater. Elevated ammonia concentrations in aquatic environments can disrupt the nitrogen balance, causing eutrophication, algal blooms, and oxygen depletion, which negatively affect fish and other aquatic organisms. In soil, ammonia can alter pH levels, disturb microbial communities, and negatively impact plant growth. Chronic contamination may result in long-term soil degradation and reduced fertility, which can have cascading effects on surrounding terrestrial ecosystems. These environmental consequences underscore the importance of effective monitoring, leak detection, and emergency response strategies for ammonia pipeline systems [61].
4. Discussion
Given that long-distance hydrogen transport entails a range of technical and logistical challenges, there is an increasing consensus that the development of the hydrogen economy should be facilitated through the use of ammonia as a hydrogen carrier. Nevertheless, the transportation of ammonia—similar to other gaseous fuels—necessitates a rigorous approach to safety assurance, accurate quantification of transported volumes, and thorough identification of environmental risks associated with such operations. This review article explores diverse aspects of ammonia transport safety and the corresponding technological capabilities. It examines international experience in ammonia transportation, addresses issues related to the compatibility of ammonia with common pipeline construction materials, and outlines the potential environmental risks associated with pipeline-based transport of ammonia, with particular emphasis on its possible impacts on various components of the natural environment. Ammonia is increasingly recognized as both a promising medium for chemical energy storage and an effective hydrogen carrier, with the potential to support the expansion of the hydrogen economy in the long term. Realizing this potential, however, requires the establishment of conditions that ensure the safe and efficient transport of ammonia, including its conveyance through dedicated pipeline systems.
The choice of transportation method for ammonia is determined by a variety of technical, economic, and regulatory factors. The most critical among these are the quantity of ammonia to be transported, transport distance, geographic and locational considerations, operational safety, transportation and storage costs, the physical state of ammonia during transport and storage, environmental impacts, and the applicable regulatory and legal framework. In addition, aspects such as infrastructure availability, energy efficiency, technological maturity of transport solutions, and public acceptance may also play a decisive role. Ultimately, the methods used for ammonia transport are determined by the availability of existing transport infrastructure, including roads, railways, ports, and pipeline networks, as well as access to natural transport routes such as inland waterways and seas. In most cases, ammonia distribution involves a combination of several modes of transport from the site of production to the final destination, including pipeline transport.
The literature review conducted in this area indicates the following:
- Pipeline transport of ammonia is already in operation, implemented at various scales and across different distances.
- Pipeline-based ammonia transport is less common compared to rail and maritime transport.
- Since the 1960s, ammonia pipelines have been subject to failures, caused both by material defects and by third-party activities.
- Experience gained during the construction and operation of ammonia pipelines has made it possible to identify materials that are compatible with ammonia (e.g., carbon steel) as well as those that are incompatible (e.g., copper alloys).
- The compatibility of polymeric materials is diverse and, according to the authors of the reviewed reports, requires further research.
- In the long term, it appears necessary to establish technical standards and regulatory frameworks dedicated specifically to ammonia pipeline transport, analogous to those already in place for natural gas and being developed for hydrogen.
- Existing monitoring and safety technologies used in gas pipelines may require adaptation to the specific conditions of ammonia transport, particularly with regard to leak detection and corrosion protection.
- The development of pipeline infrastructure for ammonia transport should take into account environmental and social aspects, including impacts on ecosystems and the social acceptance of new investments.
- Occupational safety considerations require the development of comprehensive emergency procedures, personnel training programs, and early warning systems to mitigate the risks associated with accidental releases of ammonia.
- Economic aspects need to be evaluated, including comparative cost analyses of pipeline construction and operation versus rail and maritime transport, as well as the costs associated with safety measures and potential accident mitigation.
- Integration with the emerging hydrogen economy should be considered, particularly the role of ammonia pipelines as part of future infrastructure for hydrogen storage and distribution, including options for on-site ammonia cracking to supply hydrogen.
It should be emphasized that failures of pipelines transporting ammonia can pose a significant threat to both the environment and public safety. Pipeline accidents may result in the release of toxic ammonia and associated impurities into the atmosphere, and in certain cases also into soil and water. Moreover, uncontrolled emissions of ammonia into the atmosphere as a consequence of pipeline failures involve a wide range of environmental risks. Ammonia leaks from pipelines represent a serious hazard to the environment, adversely affecting soil, water, air, and fauna. Ammonia itself, as well as typical impurities transported with it, can negatively impact individual components of the environment, including soil and water quality. The release of ammonia into the atmosphere following a pipeline failure may also pose a substantial risk to human health and life, while negatively influencing biodiversity within the affected ecosystems.
Another important issue associated with the growing scale of ammonia use and transport—and one that requires further investigation—is the impact of ammonia on climate change. According to the authors, it is essential to conduct detailed research and analyses on the kinetics of ammonia reactions in the atmosphere in order to comprehensively assess its role in climate change.
A related challenge requiring further research and development concerns the detection of ammonia pipeline leaks. Only the implementation of effective detection systems can minimize the risks associated with potential failures. At present, various types of sensors are available that can detect ammonia in the atmosphere; however, their application to monitoring large-scale pipeline networks appears to be of limited effectiveness. Furthermore, currently available leak detection systems based on balance and computational methods require calibration for ammonia-specific conditions, as well as the establishment of detection thresholds tailored to the measurement devices employed.
5. Conclusions
While ammonia transport via pipeline systems is a mature and efficient technology for large-scale distribution, it is associated with a range of technical, environmental, and safety challenges. Key threats include pipeline material degradation, corrosion, mechanical damage, and operational failures, which can result in leaks with serious consequences for human health, the environment, and public safety. The flammability and toxicity of ammonia further increase the potential hazards. Mitigating these risks requires a comprehensive approach that combines proper material selection, strategic pipeline routing, implementation of passive safety measures, and advanced active monitoring systems, including sensor networks and balance-based leak detection. Continuous research, technological innovation, and rigorous maintenance practices are essential to ensure the safe, reliable, and sustainable transport of ammonia in support of the growing hydrogen and chemical energy sectors.
For the safe and sustainable development of ammonia transport via pipeline systems, several recommendations can be considered for future practice and research. First, the implementation of advanced monitoring and leak detection systems, including hybrid sensor technologies such as optical, electrochemical, and acoustic sensors, can enable rapid detection and precise localization of leaks. Second, predictive maintenance and pipeline integrity management using real-time monitoring systems (e.g., SCADA, RTTM) and predictive modeling can help minimize the risks of corrosion, mechanical damage, and operational failures. Third, optimization of pipeline materials, including the use of ammonia-resistant steels, compatible polymers, and protective coatings, as well as the design of appropriate buffer zones, can enhance structural safety. Additionally, strategic pipeline routing away from densely populated or environmentally sensitive areas, combined with rigorous emergency response planning and personnel training, is essential. Finally, ongoing research and technological innovation should focus on improving the efficiency, environmental sustainability, and safety of ammonia transport systems to support the broader transition to low-carbon energy carriers, including hydrogen.
In summary, while ammonia transport offers significant potential as a cornerstone of the emerging hydrogen economy, its safe and sustainable deployment requires advances in materials compatibility, leak detection and monitoring technologies, predictive maintenance, and robust regulatory frameworks. Equally important are comprehensive environmental risk assessments and international cooperation to establish common technical standards and best practices. Addressing these challenges through research, innovation, and coordinated policy action will be essential to ensure that ammonia pipelines can operate reliably and contribute effectively to the broader transition toward low-carbon energy systems.
Author Contributions
Conceptualization, J.H.-R., A.W., T.K. and A.K.; methodology, J.H.-R., A.W., T.K. and A.K.; formal analysis, J.H.-R. and A.K.; writing—original draft preparation, J.H.-R., A.W. and T.K.; writing—review and editing, J.H.-R., A.K. and A.W.; visualization, T.K. and J.H.-R.; supervision, J.H.-R.; project administration, J.H.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding as part of the statutory work commissioned by the Ministry of Education and Science; order No. 0071/GE/24, archival number: DK-4100-60/24.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AE | Acoustic Emission |
| AI | Artificial Intelligence |
| CFD | Computational Fluid Dynamics |
| CR | Chloroprene Rubber |
| CRDS | Cavity Ring-Down Spectroscopy |
| ECO | Epichlorohydrin Rubber |
| EPDM | Ethylene Propylene Diene Monomer |
| FFKM | Perfluoroelastomer |
| HSE | Health and Safety Executive |
| MAPCO | Mid-America Pipeline Company |
| MOS | Metal Oxide Semiconductor |
| NBR | Nitrile Butadiene Rubber |
| NDIR | Non-Dispersive Infrared Spectroscopy |
| PTFE | Polytetrafluoroethylene |
| RES | Renewable Energy Sources |
| RTTM | Real-Time Transient Model |
| SBR | Styrene-Butadiene Rubber |
| SCADA | Supervisory Control and Data Acquisition |
| SIS | Safety instrumented systems |
| TDLAS | Tunable Diode Laser Absorption Spectroscopy |
References
- Calabrese, M.; Portarapillo, M.; Di Nardo, A.; Venezia, V.; Turco, M.; Luciani, G.; Di Benedetto, A. Hydrogen Safety Challenges: A Comprehensive Review on Production, Storage, Transport, Utilization, and CFD-Based Consequence and Risk Assessment. Energies 2024, 17, 1350. [Google Scholar] [CrossRef]
- CEN/TR 17797:2022; Gas Infrastructure—Consequences of Hydrogen in the Gas Infrastructure and Identification of Related Standardisation Need in the Scope of CEN/TC 234. BSI: London, UK, 2022.
- KIWA. Leak Tightness of PVC Fittings with Hydrogen, GT-210280. 2022. Available online: https://www.marcogaz.org/publications/assessment-of-methane-emissions-for-gas-transmission-distribution-system-operators/ (accessed on 24 August 2025).
- Marcogaz. Assessment of Methane Emissions for Gas Transmission and Distribution System Operators, WG-ME-485. 2019. Available online: https://www.marcogaz.org/wp-content/uploads/2021/04/WG_ME-485-Assessment-of-methane-emissions-for-gas-Transmission-and-Distribution-system-operator.pdf (accessed on 24 August 2025).
- PESTCE. Gas Permeability of HDPE Pipes, TI-610-3 Edition 0104. 2022. Available online: https://www.pes-tec.com/images/pestec-docs/products/PR-610-Standart-Solid-pipe/TI/TI-610-3-Gas-permeability-of-HDPE-Pipes.pdf (accessed on 24 August 2025).
- Lan, R.; Irvine, J.T.S.; Tao, S. Ammonia and Related Chemicals as Potential Indirect Hydrogen Storage Materials. Int. J. Hydrogen Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- Klerke, A.; Christensen, C.H.; Nørskov, J.K.; Vegge, T. Ammonia for Hydrogen Storage: Challenges and Opportunities. J. Mater. Chem. 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Elishav, O.; Lis, B.M.; Valera-Medina, A.; Grader, G.S. Storage and distribution of ammonia. In Techno-Economic Challenges of Green Ammonia as an Energy Vector; Academic Press: Cambridge, MA, USA, 2021; Volume 8. [Google Scholar] [CrossRef]
- Tchorek, G. Webinarium “Rewolucja wodorowa jak skorzystać?” Prezentacja PARP Grupa PFR 18 października 2022 r. Available online: https://www.youtube.com/watch?v=j4M-7Gg3HoE (accessed on 24 August 2025).
- Tchorek, G.; Targowski, F.; Mikusek, P.; Grzybowski, M. Raport “Łańcuch wartości gospodarki wodorowej w Polsce” Instytut Energetyki, Uniwersytet Warszawski, Wydział Zarządzania 2023.05.11. Available online: https://www.wz.uw.edu.pl/wp-content/uploads/2023/05/2023-04-Hydrogen-Conference-konferencja-raport_21-DRUK.pdf (accessed on 24 August 2025).
- van ‘t Noordende, H.; Tubben, G.; Rouwenhorst, K.; Fruytier, M. Clean Ammonia Roadmap Report: Bureau Lorient Communicatie January 2024. Available online: https://ris.utwente.nl/ws/portalfiles/portal/346613410/2024-ISPT-Clean-Ammonia-Roadmap-report_online-versie.pdf (accessed on 24 August 2025).
- Nayak-Luke, R.M.; Forbes, C.; Cesaro, Z.; Bañares-Alcántara, R.; Rouwenhorst, K.H.R. Chapter 8 Techno-Economic Aspects of Production, Storage and Distribution of Ammonia. In Techno-Economic Challenges of Green Ammonia as an Energy Vector; Academic Press: Cambridge, MA, USA, 2021; pp. 191–207. [Google Scholar]
- Szczęśniak, A. Zdaniem Szczęśniaka: Amoniakowe Turbulencje. Available online: https://www.kierunekchemia.pl/artykul,36762,zdaniem-szczesniaka-amoniakowe-turbulencje.html (accessed on 24 August 2025).
- Europe, EUROPA—eMARS Accidents Search—European Commission. Available online: https://emars.jrc.ec.europa.eu/en/emars/accident/search (accessed on 24 August 2025).
- Fertilizers Europe. Guidance for Inspection of and Leak Detection in Liquid Ammonia Pipeline. 2012. Available online: https://www.fertilizerseurope.com/publications/guidance-for-inspection-of-and-leak-detection-in-liquid-amonia-pipelines-copy-1/ (accessed on 24 August 2025).
- SCI4climate.NRW. Wasserstoffimporte, Bewertung der Realisierbarkeit von Wasserstoffimporten gemäß den Zielvorgaben der Nationalen Wasserstoffstrategie bis zum Jahr 2030, Gelsenkirchen. 2021. Available online: https://www.iwkoeln.de/studien/bewertung-der-realisierbarkeit-von-wasserstoffimporten-gemaess-den-zielvorgaben-der-nationalen-wasserstoffstrategie-bis-zum-jahr-2030.html (accessed on 21 October 2025).
- ISO 15649:2001; Petroleum and Natural Gas Industries—Piping. ISO: Geneva, Switzerland, 2001.
- EN 13480-1:2017; Metallic Industrial Piping—General Requirements. BSI: London, UK, 2017.
- ASME B31.5-2023; Refrigeration Piping and Heat Transfer Components. ASME: Washington, DC, USA, 2023.
- API 2510; Design and Construction of Ammonia and Liquefied Gas Installations. API Publishing Services: Washington, DC, USA, 2001.
- ISO 14113:2021; Gas Welding Equipment—Rubber and Plastics Hose Assemblies for Industrial Applications. ISO: Geneva, Switzerland, 2021.
- CEN. European Committee for Standardization Guidelines for Pressure Systems; CEN: Brussels, Belgium, 2020. [Google Scholar]
- EIGA. Ammonia Pipeline Systems: Safety and Design Considerations; EIGA: Brussels, Belgium, 2022. [Google Scholar]
- Li, X.; Sin, S.M.I.; Panikkar, S.B.; Loh, T.Y. Safe Transfer of Ammonia in Pipelines: An Analysis of Risk. Green Low-Carbon Econ. 2025, 3, 87–95. [Google Scholar] [CrossRef]
- Craig, B.D. Handbook of Corrosion Data; ASM International: Metals Park, OH, USA, 1990; ISBN 0871705184/9780871705181. [Google Scholar]
- Davies, M. Corrosion by Ammonia. In ASM Handbook, Volume 13C Corrosion: Environments and Industries; Cramer, S., Covino, B.S., Eds.; ASM International: Metals Park, OH, USA, 2006. [Google Scholar]
- Schweitzer, P.A. Corrosion Resistance Tables, Metals, Plastics, Nonmetals and Rubbers, 2nd ed.; Marcel Dekker: New York, NY, USA, 1986. [Google Scholar]
- Kass, M.D.; Keiser, J.R.; Liu, Y.; Moore, A.; Polsky, J. Assessing Compatibility of Natural Gas Pipeline Materials with Hydrogen, CO2, and Ammonia. J. Pipeline Syst. Eng. Pract. 2023, 14, 04023007. [Google Scholar] [CrossRef]
- Abramowska, E. Skroplony Amoniak jako Ładunek Luzem w Transporcie Morskim. Zeszyty Naukowe Wyższej Szkoły Morskiej w Szczecinie, 1988 nr 32. Available online: https://smp.pm.szczecin.pl/publication/201/edition/112/zeszyty-naukowe-wyzsza-szkola-morska-w-szczecinie-1988-nr-32?language=pl (accessed on 24 August 2025).
- Galimova, T.; Fasihi, M.; Bogdanov, D.; Breyer, C. Feasibility of green ammonia trading via pipelines and shipping: Cases of Europe, North Africa, and South America. J. Clean. Prod. 2023, 427, 139212. [Google Scholar] [CrossRef]
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as effective hydrogen storage: A review on production, storge and utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Lee, C.O.; Hart, G.K. The Permeability of Polyethylene to Ammonia. J. Appl. Polym. Sci. 1980, 25, 955–957. [Google Scholar] [CrossRef]
- Menon, N.; Walker, W.; Anderson, M.; Colgan, N. Compatibility of Polymers in Super-Critical Carbon Dioxide for Power Generation Systems: High Level Findings for Low Temperatures and Pressure Conditions; Sandia Rep. No. SAND2019-14400R; Sandia National Laboratory: Sandia, NM, USA, 2019. [CrossRef]
- Teng, L.; Wang, K.; Liu, B.; Li, W.; Yin, P.; Li, Z.; Huang, X.; Luo, Y.; Jiang, L. The consequence distance of liquid ammonia release from a pipeline in complex terrain. Process Saf. Environ. Prot. 2025, 196, 106921. [Google Scholar] [CrossRef]
- Grupa Wolff. 2021. Available online: https://www.grupa-wolff.eu/rodzaje-czujnikow-stacjonarne-systemy-detekcji-gazu-plomienia/ (accessed on 24 August 2025).
- Yunusa, Z.; Hamidon, M.N.; Kaiser, A.; Awang, Z. Gas Sensors: A Review. Sens. Transducers 2014, 168, 61–75. [Google Scholar]
- Bielecki, Z.; Stacewicz, T.; Smulko, J.; Wojtas, J. Ammonia Gas Sensors: Comparison of Solid-State and Optical Methods. Appl. Sci. 2020, 10, 5111. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef]
- Fischbacher, B.; Lechner, B.; Brandstätter, B. Ammonia Distribution Measurement on a Hot Gas Test Bench Applying Tomographical Optical Methods. Sensors 2019, 19, 896. [Google Scholar] [CrossRef]
- Mikołajczyk, J.; Bielecki, Z.; Stacewicz, T.; Smulko, J.; Wojtas j Szabra, D.; Lentka, Ł.; Prokopiuk, A.; Magryta, P. Detection of gaseous compounds with different techniques, Metrol. Meas. Syst. 2016, 23, 205–224. [Google Scholar] [CrossRef]
- Aldhafeeri, T.; Tran, M.-T.; Vrolyk, R.; Pope, M.; Fowler, M. A Review of Methane Gas Detection Sensors: Recent Developments and Future Perspectives. Inventions 2020, 5, 28. [Google Scholar] [CrossRef]
- Maithani, S.; Mandal, S.; Maity, A.; Pal, M.; Pradhan, M. High-resolution spectral analysis of ammonia near 6.2 μm using a cw EC-QCL coupled with cavity ring-down spectroscopy. Analyst 2018, 143, 2109–2114. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.C. Gas Leak Detection in Pipelines & Repairing System of Titas Gas. J. Appl. Eng. (JOAE) 2014, 2, 23–34. [Google Scholar]
- Murvay, P.S.; Silea, I. A survey on gas leak detection and localization techniques. J. Loss Prev. Process Ind. 2012, 25, 966–973. [Google Scholar] [CrossRef]
- Sekhavati, J.; Hashemabadi, S.H.; Soroush, M. Computational methods for pipeline leakage detection and localization: A review and comparative study. J. Loss Prev. Process Ind. 2022, 77, 104771. [Google Scholar] [CrossRef]
- Tian, C.H.; Yan, J.C.; Huang, J.; Wang, Y.; Kim, D.-S.; Yi, T. Negative pressure wave based pipeline Leak Detection: Challenges and algorithms. In Proceedings of the 2012 IEEE International Conference on Service Operations and Logistics, and Informatics, Suzhou, China, 8–10 July 2012; pp. 372–376. [Google Scholar] [CrossRef]
- Hinderdael, M.; Jardon, Z.; Guillaume, P. An analytical amplitude model for negative pressure waves in gaseous media. Mech. Syst. Signal Process. 2020, 144, 106800. [Google Scholar] [CrossRef]
- bin Md Akib, A.; bin Saad, N.; Asirvadam, V. Pressure point analysis for early detection system. In Proceedings of the IEEE 7th International Colloquium on Signal Processing and Its Applications, Penang, Malaysia, 4–6 March 2011; pp. 103–107. [Google Scholar] [CrossRef]
- Cui, X.; Yan, Y.; Ma, Y.; Ma, L.; Han, X. Localization of CO2 leakage from transportation pipelines through low frequency acoustic emission detection. Sens. Actuators A Phys. 2016, 237, 107–118. [Google Scholar] [CrossRef]
- Nicola, M.; Nicola, C.; Vintilă, A.; Hurezeanu, I.; Duță, M. Pipeline Leakage Detection by Means of Acoustic Emission Technique Using Cross-Correlation Functi. J. Mech. Eng. Autom. 2018, 8, 59–67. [Google Scholar] [CrossRef]
- Meng, L.; Yuxing, L.; Wuchang, W.; Juntao, F. Experimental study on leak detection and location for gas pipeline based on acoustic method. J. Loss Prev. Process Ind. 2012, 25, 90–102. [Google Scholar] [CrossRef]
- Sommer, S.G.; Hutchings, N.J. Ammonia emission from field applied manure and its reduction—Invited paper. Eur. J. Agron. 2001, 15, 1–15. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Toxicity of Per- and Polyfluoroalkyl Substances (PFAS); National Academies Press: Washington, DC, USA, 2022. [Google Scholar]
- Ubowska, A. Zagrożenie środowiska na skutek wypadku cysterny kolejowej transportującej amoniak. Zeszyty Naukowe SGSP 2018, 2, 51–63. [Google Scholar]
- An, Y.; Xing, H.; Zhang, Y.; Jia, P.; Gu, X.; Teng, X. The Evaluation of Potential Immunotoxicity Induced by Environmental Pollutant Ammonia in Broilers. Poult. Sci. 2019, 98, 3165–3175. [Google Scholar] [CrossRef]
- Li, Y.; Pan, L.; Zeng, X.; Zhang, R.; Li, X.; Li, J.; Xing, H.; Bao, J. Ammonia Exposure Causes the Imbalance of the Gut-Brain Axis by Altering Gene Networks Associated with Oxidative Metabolism, Inflammation and Apoptosis. Ecotoxicol. Environ. Saf. 2021, 224, 112668. [Google Scholar] [CrossRef] [PubMed]
- Health and Safety Executive. EH40/2005 Workplace Exposure Limits; The Stationery Office: London, UK, 2005. [Google Scholar]
- Wan, Z.; Tao, Y.; Shao, J.; Zhang, Y.; You, H. Ammonia as an effective hydrogen carrier and a clean fuel for solid oxide fuel cells. Energy Convers. Manag. 2021, 228, 113729. [Google Scholar] [CrossRef]
- Rathod, S.; Edwards, M.R.; Roy, C.; Warnecke, L.; Rafaj, P.; Kiesewetter, G.; Klimont, Z. Air quality and health effects of a transition to ammonia–fueled shipping in Singapore. Environ. Res. Health 2023, 1, 041002. [Google Scholar] [CrossRef]
- Sanderson, M.G.; Collins, W.J.; Johnson, C.E.; Derwent, R.G. Present and Future Acid Deposition to Ecosystems: The Effect of Climate Change. Atmos. Environ. 2006, 40, 1275–1283. [Google Scholar] [CrossRef]
- Edwards, T.M.; Puglis, H.J.; Kent, D.B.; López Durán, J.; Bradshaw, L.M.; Farag, A.M. Ammonia and aquatic ecosystems—A review of global sources, biogeochemical cycling, and effects on fish. Sci. Total Environ. 2024, 907, 167911. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).