Ge4+ Stabilizes Cu1+ Active Sites to Synergistically Regulate the Interfacial Microenvironment for Electrocatalytic CO2 Reduction to Ethanol
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
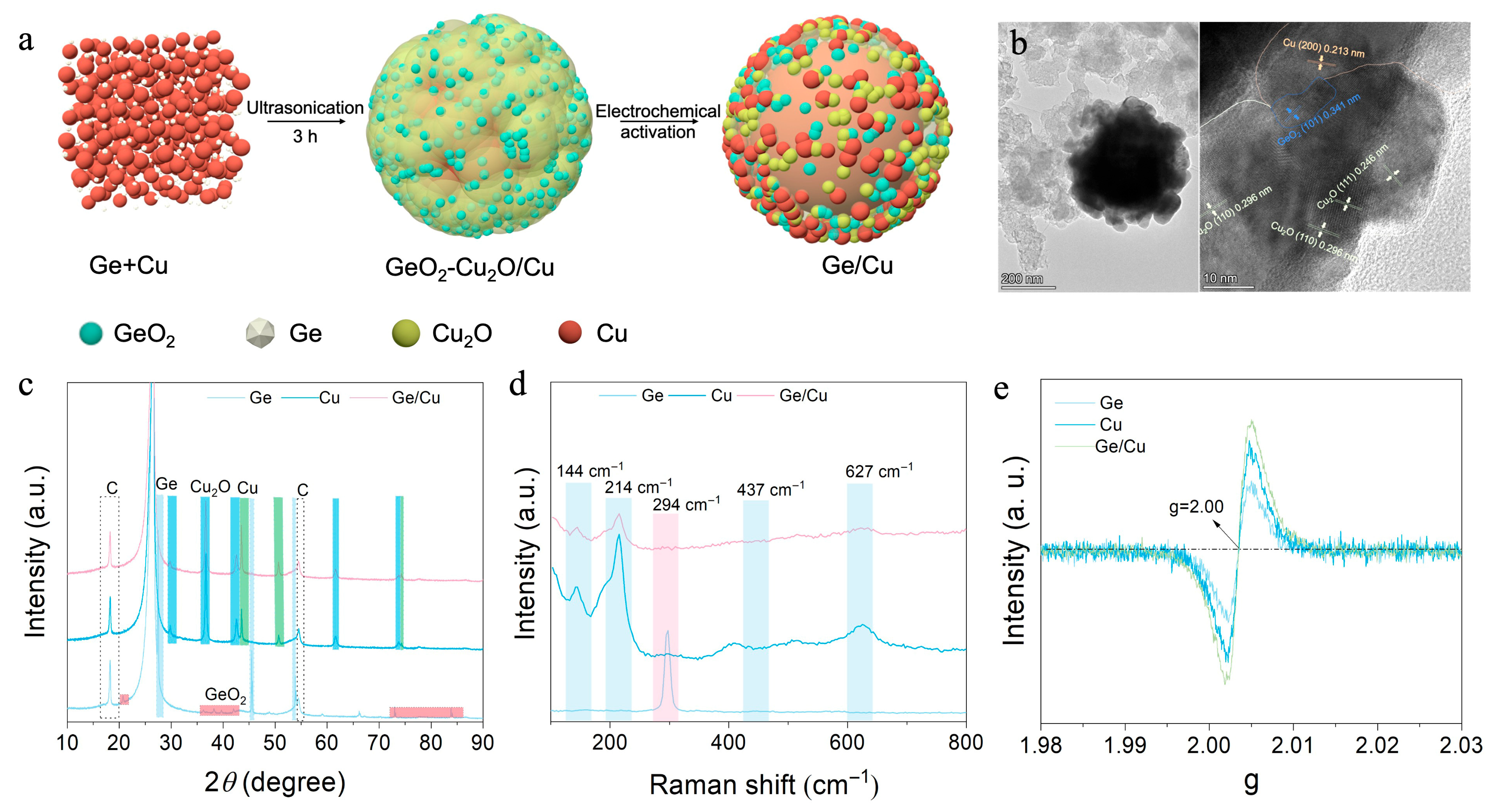
3.1. Synthesis and Characterization of Ge/Cu-Catalysts
3.2. Electrocatalytic CO2 Reduction to Ethanol over Ge/Cu-Catalyst
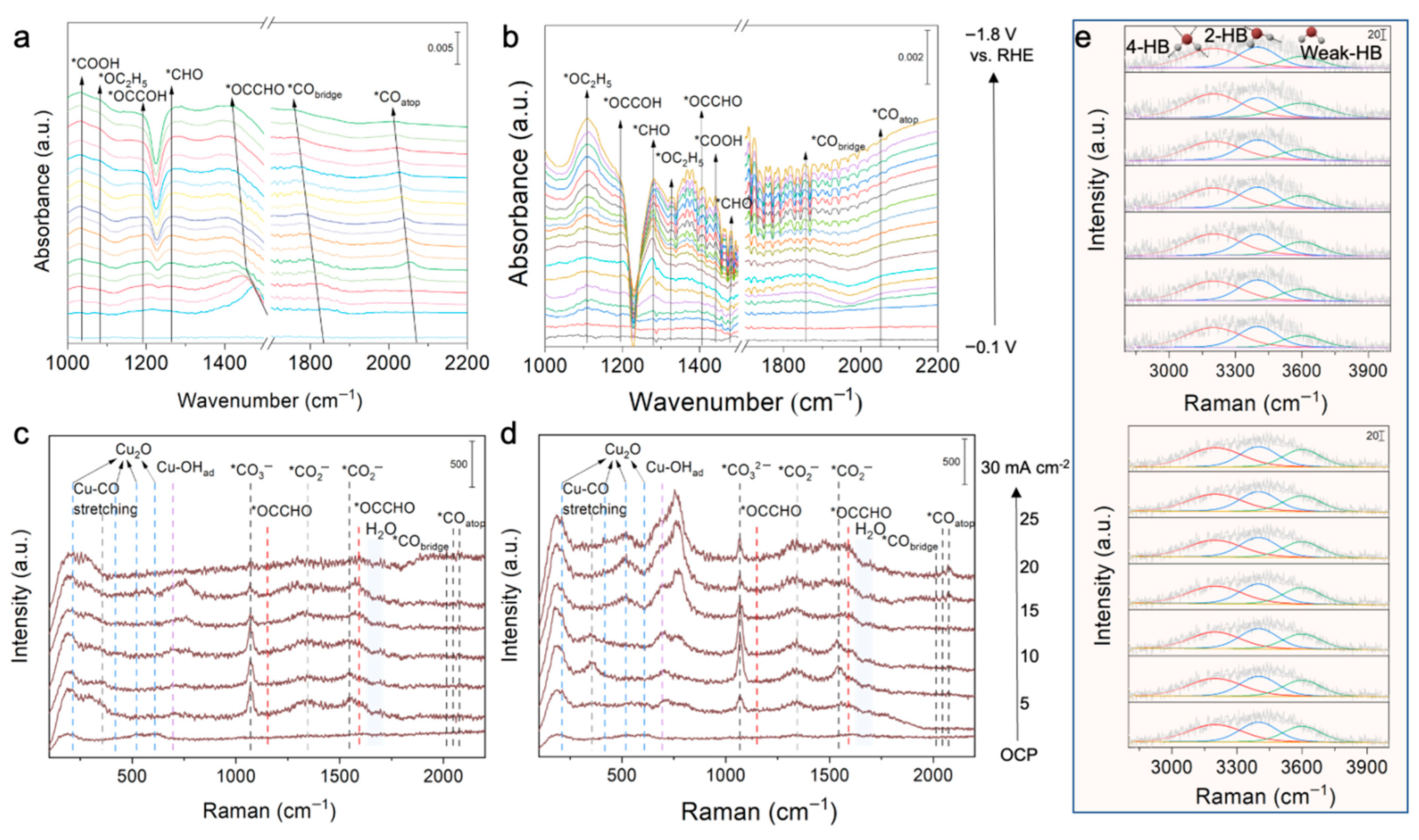
3.3. Investigation of Reaction Mechanism
3.4. Density Functional Theory (DFT) Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, S.; Khan, M.K.; Kim, J. Revolutionary advancements in carbon dioxide valorization via metal-organic framework-based strategies. Carbon Capture Sci. Technol. 2025, 15, 100405. [Google Scholar] [CrossRef]
- Ahmed, S.; Hussain, M.S.; Khan, M.K.; Kim, J. Innovations in catalysis towards efficient electrochemical reduction of CO2 to C1 chemicals. J. Energy Chem. 2025, 107, 622–649. [Google Scholar] [CrossRef]
- Ye, Q.; Zhao, X.; Jin, R.; Dong, F.; Xie, H.; Deng, B. Advances and challenges in membrane electrode assembly electrolyzers for CO2 reduction. J. Mater. Chem. A 2023, 11, 21498–21515. [Google Scholar] [CrossRef]
- Deng, B.; Huang, M.; Zhao, X.; Mou, S.; Dong, F. Interfacial electrolyte effects on electrocatalytic CO2 reduction. ACS Catal. 2021, 12, 331–362. [Google Scholar] [CrossRef]
- Lv, H.; Lv, F.; Qin, H.; Min, X.; Sun, L.; Han, N.; Xu, D.; Li, Y.; Liu, B. Single-crystalline mesoporous palladium and palladium-copper nanocubes for highly efficient electrochemical CO2 reduction. CCS Chem. 2022, 4, 1376–1385. [Google Scholar] [CrossRef]
- Deng, B.; Zhao, X.; Li, Y.; Huang, M.; Zhang, S.; Dong, F. Active site identification and engineering during the dynamic evolution of copper-based catalysts for electrocatalytic CO2 reduction. Sci. China Chem. 2022, 66, 78–95. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Q.; Luo, T.; Herran, M.; Cao, X.; Liao, W.; Zhu, L.; Li, H.; Stefancu, A.; Lu, Y.R.; et al. Potential alignment in tandem catalysts enhances CO2-to-C2H4 conversion efficiencies. J. Am. Chem. Soc. 2024, 146, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Lou, Z.X.; Chen, J.; Liu, Y.; Wu, X.; Zhao, J.Y.; Yuan, H.Y.; Zhu, M.; Dai, S.; Wang, H.F.; et al. Direct OC-CHO coupling towards highly C2+ products selective electroreduction over stable Cu0/Cu2+ interface. Nat. Commun. 2023, 14, 7681. [Google Scholar] [CrossRef]
- Su, X.; Jiang, Z.; Zhou, J.; Liu, H.; Zhou, D.; Shang, H.; Ni, X.; Peng, Z.; Yang, F.; Chen, W.; et al. Complementary operando spectroscopy identification of in-situ generated metastable charge-asymmetry Cu2-CuN3 clusters for CO2 reduction to ethanol. Nat. Commun. 2022, 13, 1322. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Huang, M.; Li, K.; Zhao, X.; Geng, Q.; Chen, S.; Xie, H.; Dong, X.; Wang, H.; Dong, F. The crystal plane is not the key factor for CO2-to-methane electrosynthesis on reconstructed Cu2O microparticles. Angew. Chem. Int. Ed. 2022, 61, e202114080. [Google Scholar] [CrossRef]
- Lian, Z.; Dattila, F.; López, N. Stability and lifetime of diffusion-trapped oxygen in oxide-derived copper CO2 reduction electrocatalysts. Nat. Catal. 2024, 7, 401–411. [Google Scholar] [CrossRef]
- Feng, J.; Wu, L.; Liu, S.; Xu, L.; Song, X.; Zhang, L.; Zhu, Q.; Kang, X.; Sun, X.; Han, B. Improving CO2-to-C2+ product electroreduction efficiency via atomic lanthanide dopant-induced tensile-Strained CuOx catalysts. J. Am. Chem. Soc. 2023, 145, 9857–9866. [Google Scholar] [CrossRef]
- Xu, L.; Feng, J.; Wu, L.; Song, X.; Tan, X.; Zhang, L.; Ma, X.; Jia, S.; Du, J.; Chen, A.; et al. Identifying the optimal oxidation state of Cu for electrocatalytic reduction of CO2 to C2+ products. Green Chem. 2023, 25, 1326–1331. [Google Scholar] [CrossRef]
- Liu, C.; Guo, R.-T.; Zhu, H.-W.; Cui, H.-F.; Liu, M.-Y.; Pan, W.-G. Cu2O-based catalysts applied for electrocatalytic CO2 reduction: A review. J. Mater. Chem. A 2024, 12, 31769–31796. [Google Scholar] [CrossRef]
- Fan, Q.; Zhang, X.; Ge, X.; Bai, L.; He, D.; Qu, Y.; Kong, C.; Bi, J.; Ding, D.; Cao, Y.; et al. Manipulating Cu nanoparticle surface oxidation states tunes catalytic selectivity toward CH4 or C2+ products in CO2 electroreduction. Adv. Energy Mater. 2021, 11, 2101424. [Google Scholar] [CrossRef]
- Jia, B.; Li, L.; Xue, C.; Kang, J.; Liu, L.M.; Guo, T.; Wang, Z.; Huang, Q.; Guo, S. Restraining interfacial Cu2+ by using amorphous SnO2 as sacrificial Protection boosts CO2 electroreduction. Adv. Mater. 2023, 35, e2305587. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, X.; Duan, D.; He, C.; Ma, J.; Zhang, W.; Liu, H.; Long, R.; Li, Z.; Kong, T.; et al. Structural reconstruction of Cu2O superparticles toward electrocatalytic CO2 reduction with high C2+ products selectivity. Adv. Sci. 2022, 9, e2105292. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Rebollar, D.; He, H.; Chong, L.; Liu, Y.; Liu, C.; Sun, C.-J.; Li, T.; Muntean, J.V.; Winans, R.E.; et al. Highly selective electrocatalytic CO2 reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper. Nat. Energy 2020, 5, 623–632. [Google Scholar] [CrossRef]
- Yue, K.; Qin, Y.; Huang, H.; Lv, Z.; Cai, M.; Su, Y.; Huang, F.; Yan, Y. Stabilized Cu0 -Cu1+ dual sites in a cyanamide framework for selective CO2 electroreduction to ethylene. Nat. Commun. 2024, 15, 7820. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, J.; Yang, X.; Xu, Y.; Sun, W.; Zhou, J. MOF-derived Cu@Cu2O heterogeneous electrocatalyst with moderate intermediates adsorption for highly selective reduction of CO2 to methanol. Chem. Eng. J. 2022, 431, 134171. [Google Scholar] [CrossRef]
- Han, M.; Liu, J.; Zheng, K.; Deng, C.; Mu, Y.; Guo, J.; Chu, Y.; Zou, Z.; Yu, F.; Li, W.; et al. Pressure-induced dense and robust Ge architecture for superior volumetric lithium storage. Adv. Energy Mater. 2024, 14, 2401065. [Google Scholar] [CrossRef]
- Ouyang, J.; Feng, C.; Ji, X.; Li, L.; Gutti, H.K.; Kim, N.Y.; Artzi, D.; Xie, A.; Kong, N.; Liu, Y.N.; et al. 2D monoelemental germanene quantum dots: Synthesis as robust photothermal agents for photonic cancer nanomedicine. Angew. Chem. Int. Ed. Engl. 2019, 58, 13405–13410. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Song, H.; Lin, S.; Wu, H.; Shi, Y.; Yao, J. GeO2 encapsulated Ge nanostructure with enhanced lithium-storage properties. Adv. Funct. Mater. 2019, 29, 1807946. [Google Scholar] [CrossRef]
- Min, S.; Xu, X.; He, J.; Sun, M.; Lin, W.; Kang, L. Construction of cobalt porphyrin-modified Cu2O nanowire array as a tandem electrocatalyst for enhanced CO2 reduction to C2 products. Small 2024, 20, e2400592. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Bi, J.; Liu, J.; Zhu, Q.; Chen, C.; Sun, X.; Zhang, J.; Han, B. In situ dual doping for constructing efficient CO2-to-methanol electrocatalysts. Nat. Commun. 2022, 13, 1965. [Google Scholar] [CrossRef]
- Chou, T.C.; Chang, C.C.; Yu, H.L.; Yu, W.Y.; Dong, C.L.; Velasco-Velez, J.J.; Chuang, C.H.; Chen, L.C.; Lee, J.F.; Chen, J.M.; et al. Controlling the oxidation state of the Cu electrode and reaction intermediates for electrochemical CO2 reduction to ethylene. J. Am. Chem. Soc. 2020, 142, 2857–2867. [Google Scholar] [CrossRef]
- Kim, J.; Lee, T.; Jung, H.D.; Kim, M.; Eo, J.; Kang, B.; Jung, H.; Park, J.; Bae, D.; Lee, Y.; et al. Vitamin C-induced CO2 capture enables high-rate ethylene production in CO2 electroreduction. Nat. Commun. 2024, 15, 192. [Google Scholar] [CrossRef]
- Qiao, Y.; Shen, S.; Mao, C.; Xiao, Y.; Lai, W.; Wang, Y.; Zhong, X.; Lu, Y.; Li, J.; Ge, J.; et al. Interfacial oxygen vacancy-copper pair sites on inverse CeO2/Cu catalyst enable efficient CO2 electroreduction to ethanol in acid. Angew. Chem. Int. Ed. Engl. 2025, 64, e202424248. [Google Scholar] [CrossRef]
- Li, S.; Wu, G.; Mao, J.; Chen, A.; Liu, X.; Zeng, J.; Wei, Y.; Wang, J.; Zhu, H.; Xia, J.; et al. Tensile-strained Cu penetration electrode boosts asymmetric C-C coupling for ampere-level CO2-to-C2+ reduction in acid. Angew. Chem. Int. Ed. Engl. 2024, 63, e202407612. [Google Scholar] [CrossRef]
- Wang, S.; Li, F.; Zhao, J.; Zeng, Y.; Li, Y.; Lin, Z.Y.; Lee, T.J.; Liu, S.; Ren, X.; Wang, W.; et al. Manipulating C-C coupling pathway in electrochemical CO2 reduction for selective ethylene and ethanol production over single-atom alloy catalyst. Nat. Commun. 2024, 15, 10247. [Google Scholar] [CrossRef]
- Wang, R.; Dong, L.-Z.; Shi, J.-W.; Zhang, M.; Li, S.-L.; Lan, Y.-Q.; Liu, J. Switching the symmetry of a trinuclear copper cluster catalyst for electroreducing CO2 to an asymmetric C2 product in an acidic electrolyte. ACS Catal. 2024, 14, 741–750. [Google Scholar] [CrossRef]
- de Ruiter, J.; Benning, V.R.M.; Yang, S.; den Hartigh, B.J.; Wang, H.; Prins, P.T.; Dorresteijn, J.M.; Janssens, J.C.L.; Manna, G.; Petukhov, A.V.; et al. Multiscale X-ray scattering elucidates activation and deactivation of oxide-derived copper electrocatalysts for CO2 reduction. Nat. Commun. 2025, 16, 373. [Google Scholar] [CrossRef]
- Ma, X.; Xu, L.; Liu, S.; Zhang, L.; Tan, X.; Wu, L.; Feng, J.; Liu, Z.; Sun, X.; Han, B. Electrochemical C-C coupling between CO2 and formaldehyde into ethanol. Chem Catal. 2022, 2, 3207–3224. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, S. An investigation of active sites for electrochemical CO2 reduction reactions: From in situ characterization to rational design. Adv. Sci. 2021, 8, 2003579. [Google Scholar] [CrossRef]
- Lu, X.; Wang, L.; Zhao, X.; Pan, B.; Li, Z.; Du, X.; Zhang, S.; Dong, F.; Deng, B. Unlocking asymmetric C-C coupling pathways on commercial Cu catalysts via Cu (100) grain boundaries for efficient and durable CO electroreduction. Chinese J. Catal. 2025, 76, 198–209. [Google Scholar] [CrossRef]
- Li, H.; Wei, P.; Gao, D.; Wang, G. In situ Raman spectroscopy studies for electrochemical CO2 reduction over Cu catalysts. Curr. Opin. Green Sust. 2022, 34, 100589. [Google Scholar] [CrossRef]
- Zou, C.J.; Du, Z.Y.; Tang, W.; Liu, Q.; Liu, X.B.; Dong, J.C.; Fang, P.P.; Li, J.F. Interfacial water on Ag/Ag2S nanowires enhancing the ethanol selectivity for CO2 electroreduction. Adv. Mater. 2025, 37, 2503010. [Google Scholar] [CrossRef]
- He, X.; Lin, L.; Li, X.; Zhu, M.; Zhang, Q.; Xie, S.; Mei, B.; Sun, F.; Jiang, Z.; Cheng, J.; et al. Roles of copper(I) in water-promoted CO2 electrolysis to multi-carbon compounds. Nat. Commun. 2024, 15, 9923. [Google Scholar] [CrossRef]
- Sun, L.; Zheng, X.; Li, Y.; Lin, M.; Zeng, X.; Yu, J.; Song, Z.; Zhang, L. Nanoconfinement and tandem catalysis over yolk-shell catalysts towards electrochemical reduction of CO2 to multi-carbon products. J. Colloid Interface Sci. 2025, 687, 733–741. [Google Scholar] [CrossRef]
- Yang, C.; Wang, R.; Yu, C.; Xiao, J.; Huang, Z.; Lv, B.; Zhao, H.; Wu, X.; Jing, G. Engineering stable Cu+-Cu0 sites and oxygen defects in boron-doped copper oxide for electrocatalytic reduction of CO2 to C2+ products. Chem. Eng. J. 2024, 484, 149710. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Kong, Y.; Chen, Q.; Zhao, S. Stabilization of Cu2O catalyst via strong electronic interaction for selective electrocatalytic CO2 reduction to ethanol. Chem. Eng. J. 2024, 499, 156065. [Google Scholar] [CrossRef]
- Jin, P.; Wang, Q.; Gu, X.; Huang, L.; Qin, W.; Chong, Y.; Pruksawan, S.; Zhang, S.; Wang, F.; Lin, X. MOFs derived N doped CuNix@C dual metallic core-shell electrocatalysts for CO2 electrocatalytic reduction. Int. J. Electrochem. Sci. 2024, 19, 100427. [Google Scholar] [CrossRef]
- Da, Y.; Chen, J.; Fan, L.; Jiang, R.; Xiao, Y.; Wang, M.; Chen, G.; Tian, Z.; Zhang, H.; Jin, H.; et al. Selective and energy efficient electrocatalytic CO2-to-ethanol conversion through anion modulation. Angew. Chem. Int. Ed. Engl. 2025, 64, e202506867. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Song, Q.; Huang, H.; He, Y.; Sun, S.; Wang, Z. Direct observation of surface-bound intermediates in CO/CO2 reduction on a polycrystalline Cu electrode using broad-band SFG spectroscopy. J. Phys. Chem. C 2023, 127, 23675–23686. [Google Scholar] [CrossRef]
- Yu, Z.; Gu, M.; Wang, H.; Jiang, L.; Sun, P.; Liu, X.; Pei, Y.; Wang, G.; Zhang, X. Dual sites on graphitic biochar for electrocatalytic CO2 reduction to ethanol conversion. J. Phys. Chem. C 2024, 128, 4171–4179. [Google Scholar] [CrossRef]
- Angerasa, F.T.; Moges, E.A.; Chang, C.-Y.; Lakshmanan, K.; Dessie, T.A.; Huang, W.-H.; Edao, H.G.; Dilebo, W.B.; Guta, C.B.; Chang, C.-C.; et al. Selective electrochemical reduction of CO2 to ethanol on a heteroatom-coordinated dual-atom catalyst of Fe/Cu-NC. Chem. Mater. 2025, 37, 2474–2484. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Wang, L.; Xie, H.; Li, Z.; Du, X.; Deng, B. Ge4+ Stabilizes Cu1+ Active Sites to Synergistically Regulate the Interfacial Microenvironment for Electrocatalytic CO2 Reduction to Ethanol. Appl. Sci. 2025, 15, 11420. https://doi.org/10.3390/app152111420
Lu X, Wang L, Xie H, Li Z, Du X, Deng B. Ge4+ Stabilizes Cu1+ Active Sites to Synergistically Regulate the Interfacial Microenvironment for Electrocatalytic CO2 Reduction to Ethanol. Applied Sciences. 2025; 15(21):11420. https://doi.org/10.3390/app152111420
Chicago/Turabian StyleLu, Xianlong, Lili Wang, Hongtao Xie, Zhendong Li, Xiangfei Du, and Bangwei Deng. 2025. "Ge4+ Stabilizes Cu1+ Active Sites to Synergistically Regulate the Interfacial Microenvironment for Electrocatalytic CO2 Reduction to Ethanol" Applied Sciences 15, no. 21: 11420. https://doi.org/10.3390/app152111420
APA StyleLu, X., Wang, L., Xie, H., Li, Z., Du, X., & Deng, B. (2025). Ge4+ Stabilizes Cu1+ Active Sites to Synergistically Regulate the Interfacial Microenvironment for Electrocatalytic CO2 Reduction to Ethanol. Applied Sciences, 15(21), 11420. https://doi.org/10.3390/app152111420





