Real-Time Monitoring of Occupational Radiation Exposure in Nuclear Medicine Technologists: An Initial Study
Abstract
1. Introduction
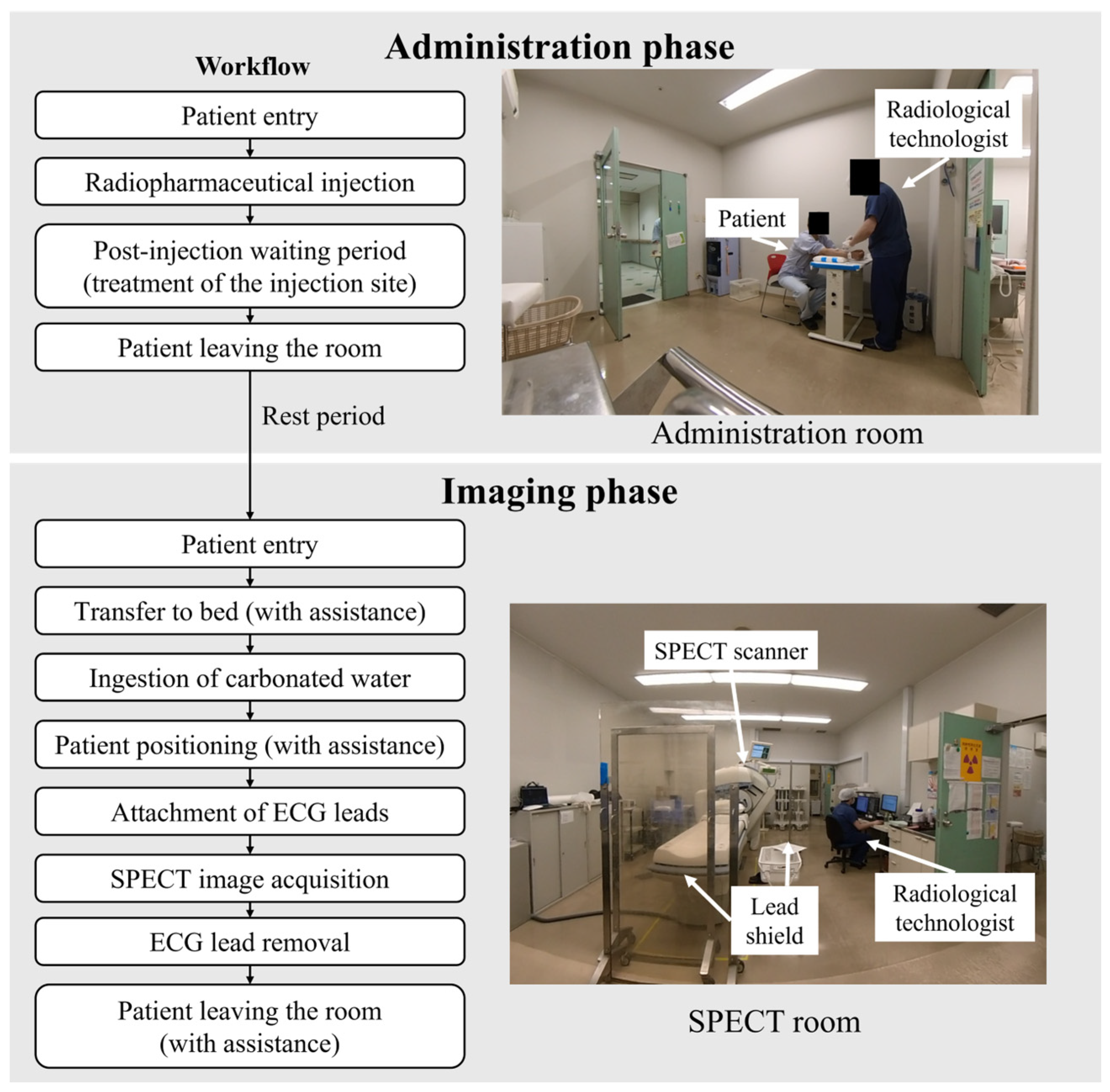
2. Materials and Methods
2.1. Subjects
2.2. Dosimetry
2.3. Data Analysis
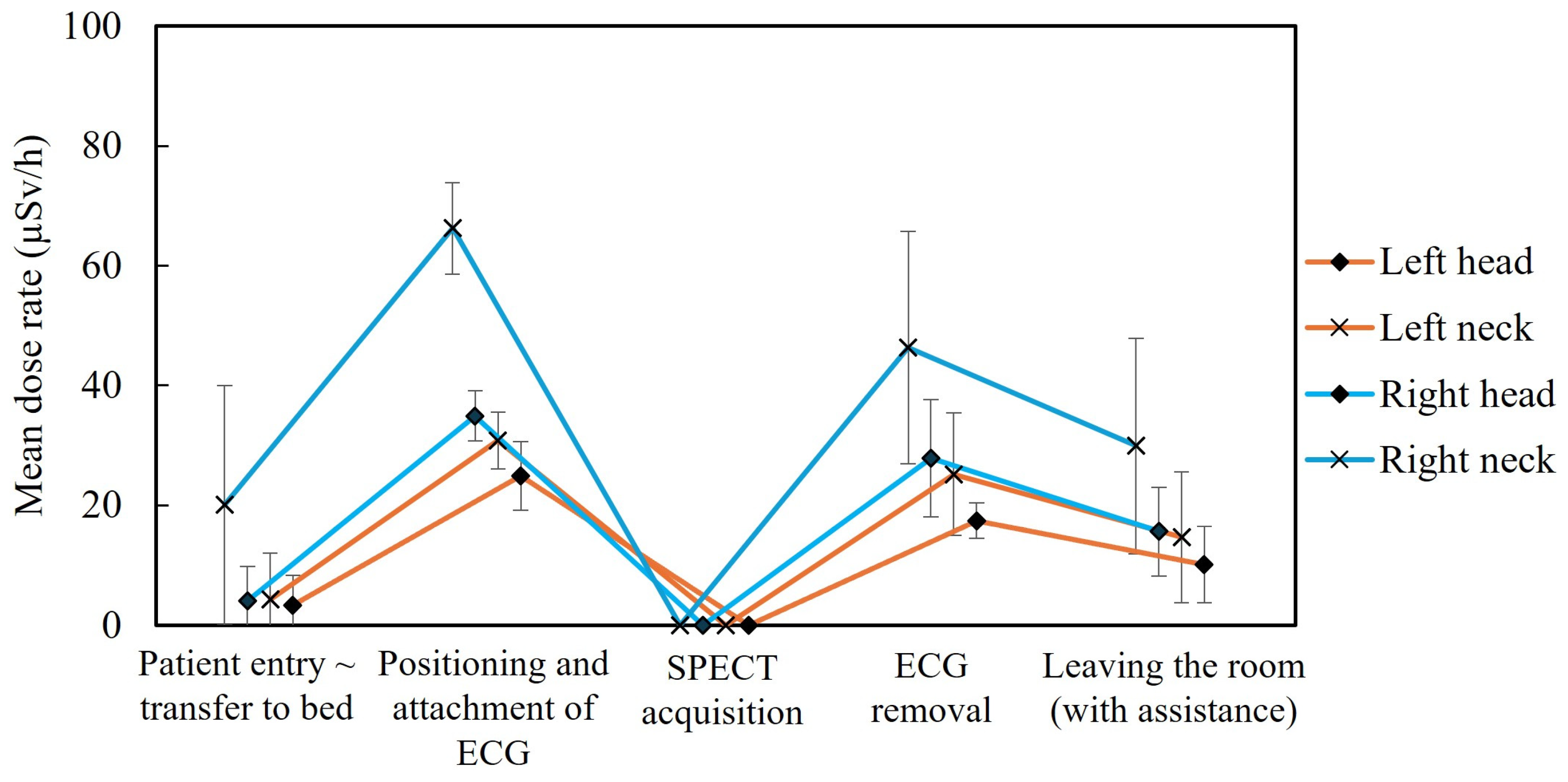
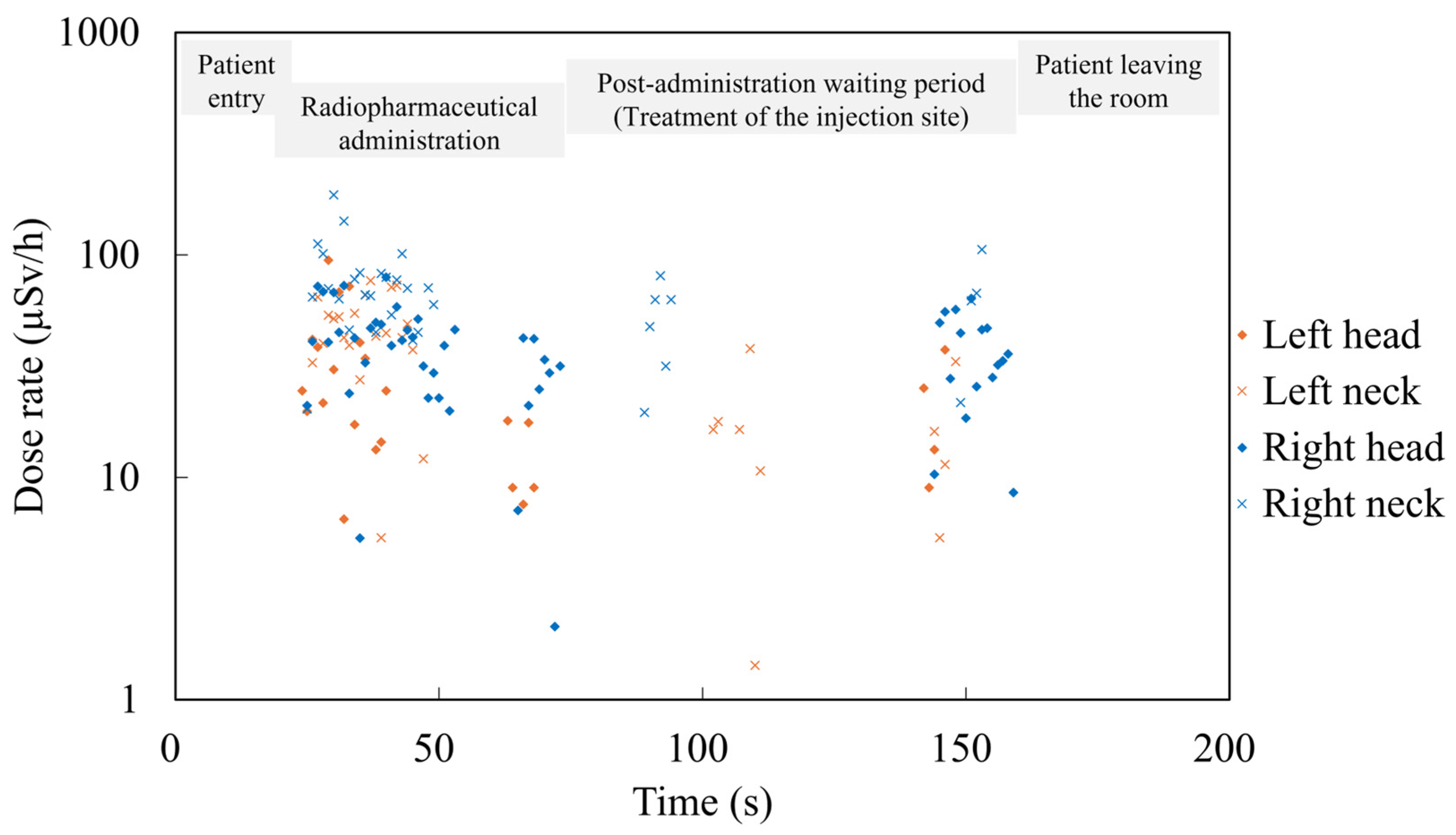
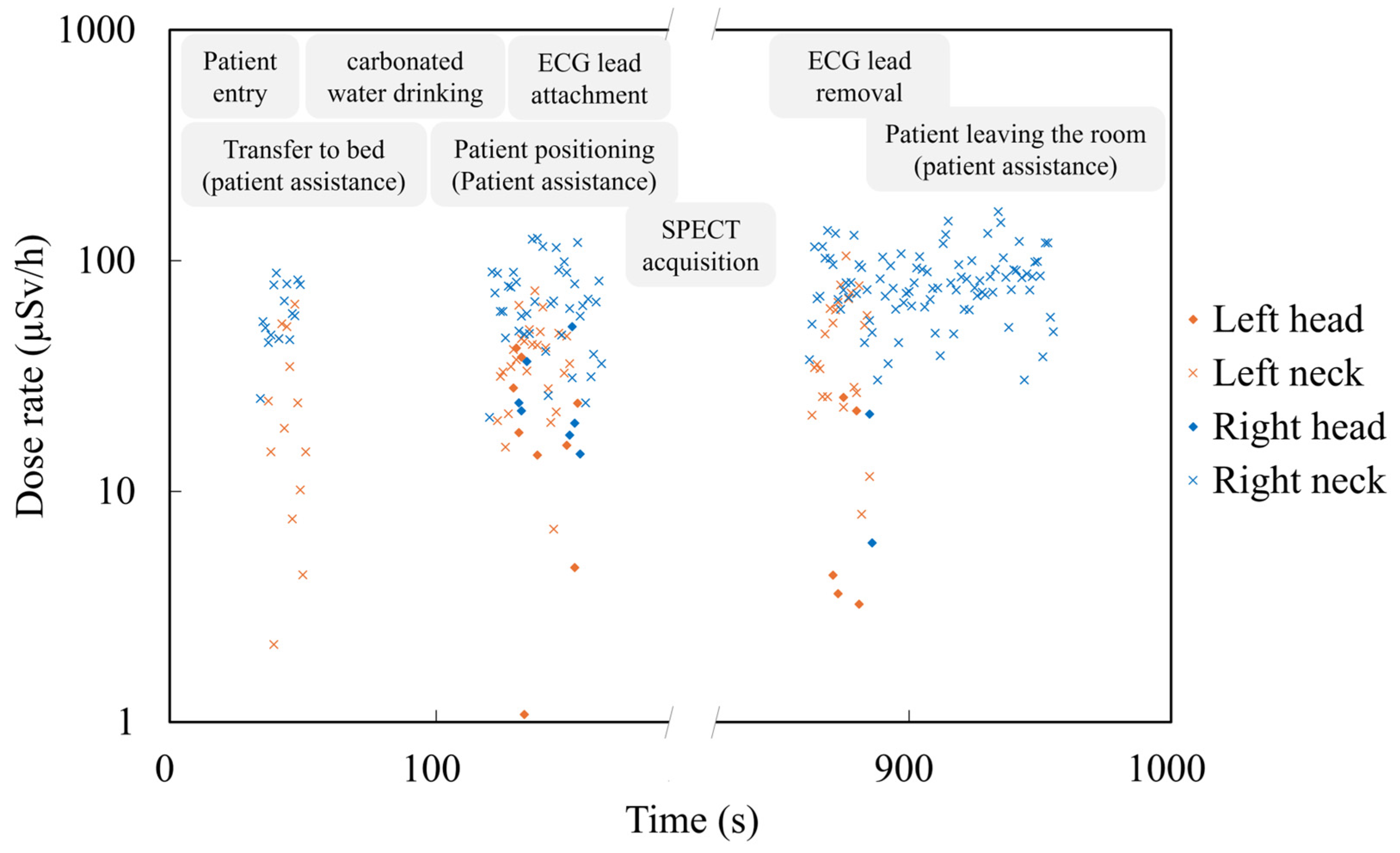
3. Results
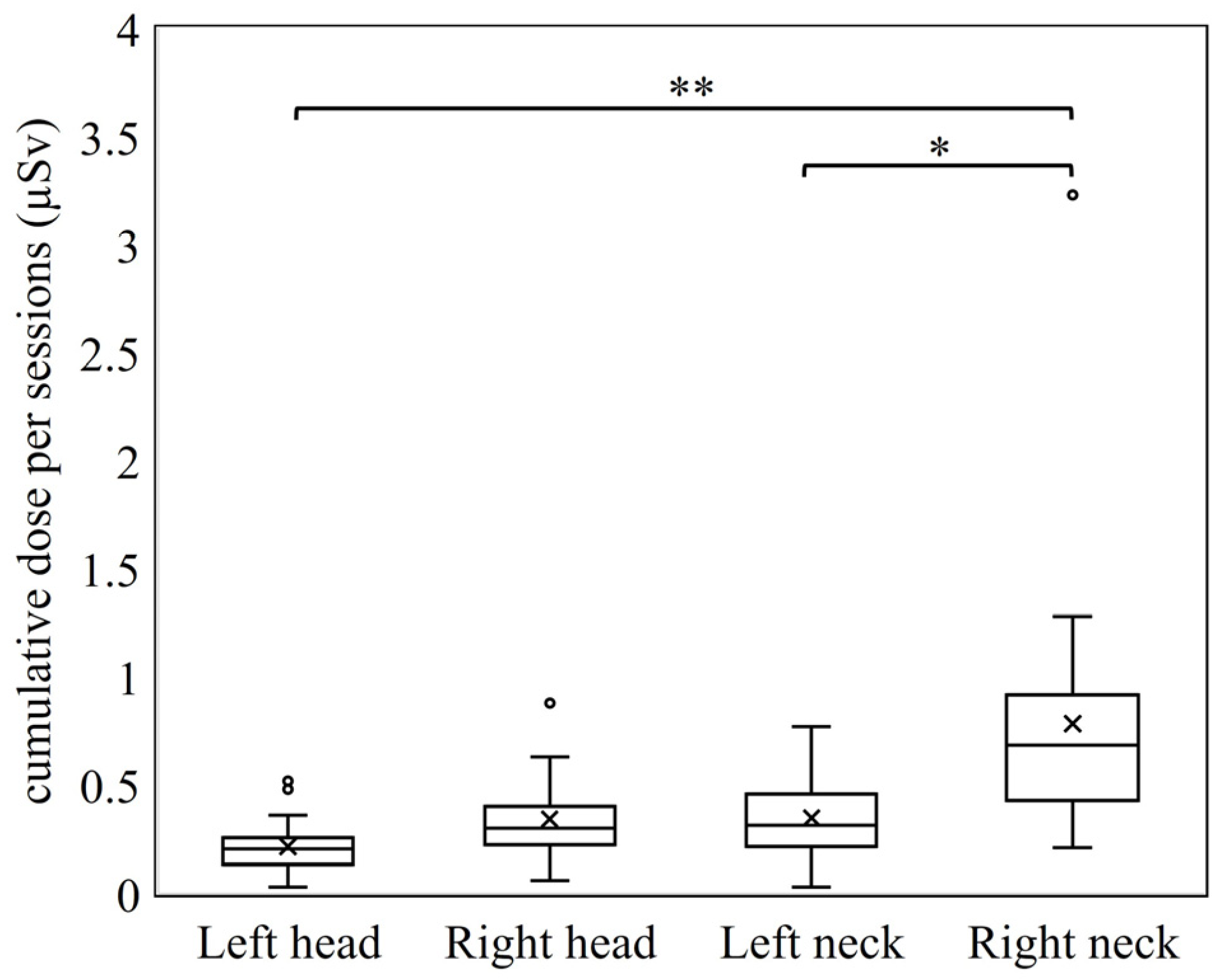
3.1. Differences by Dosimetry Position
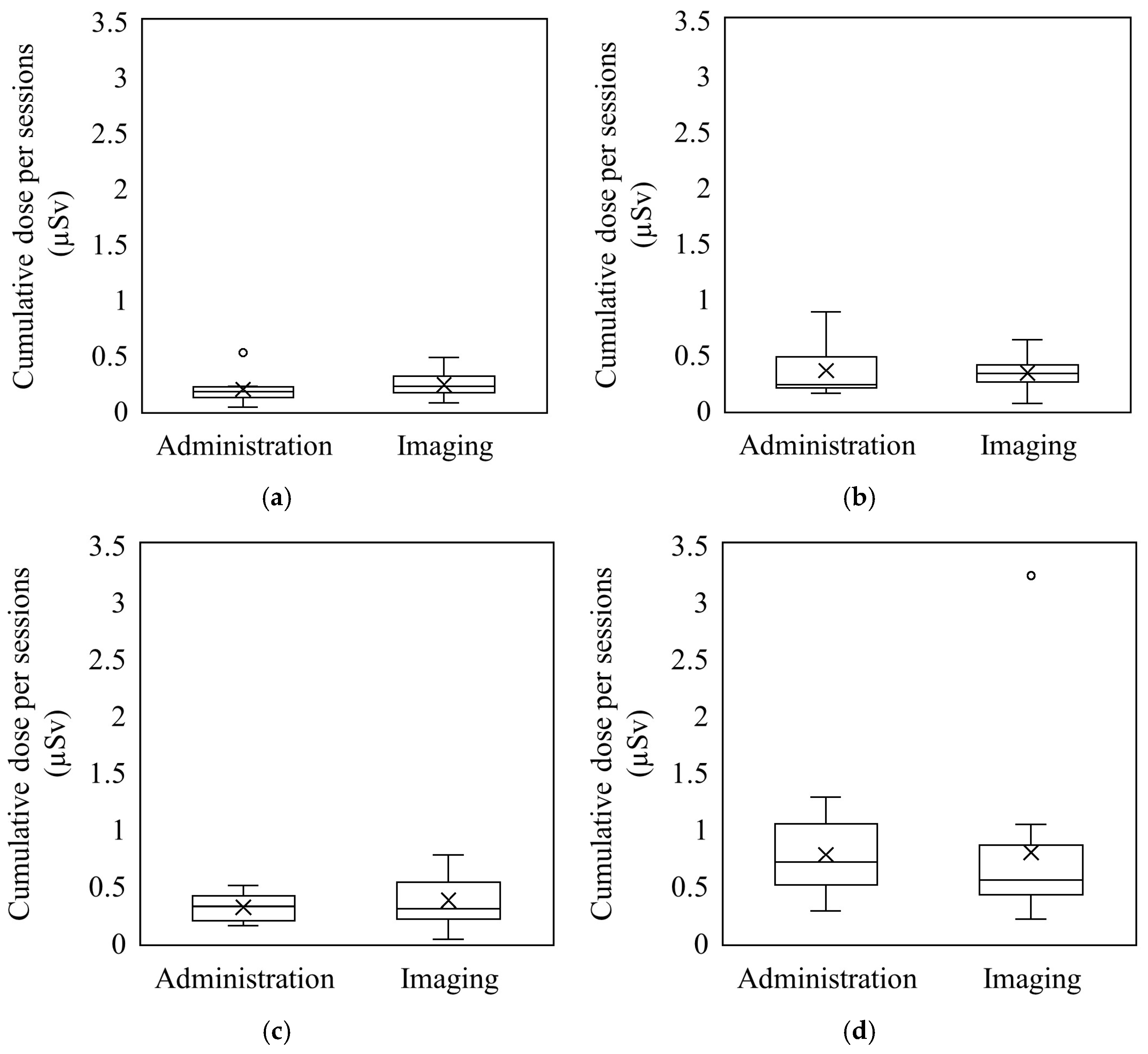
3.2. Comparison of Radiation Exposure Between the Administration and Imaging Phases
3.3. Relationship Between Administered Activity and Exposure
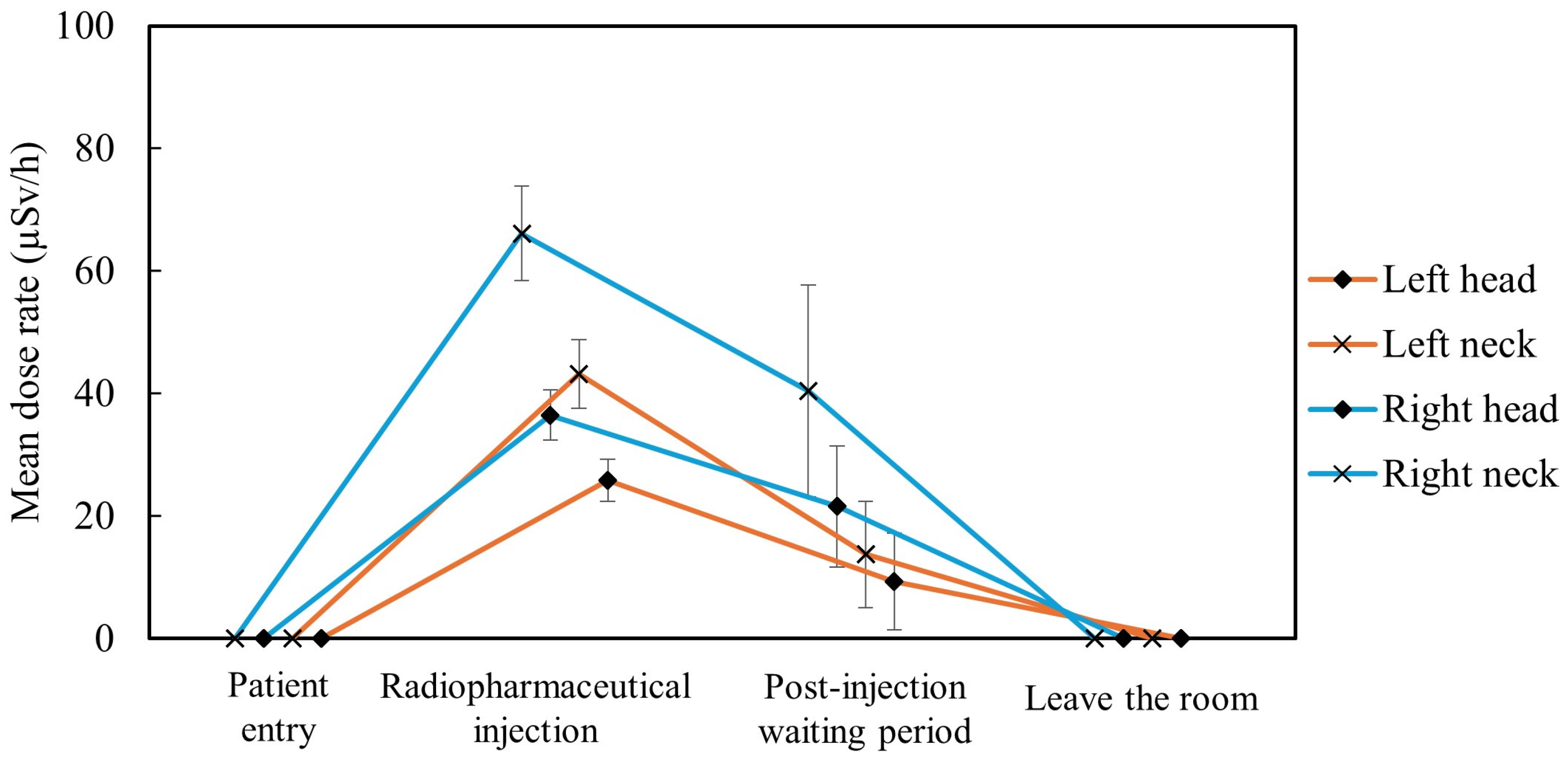
3.4. Identification of Exposure-Causing Actions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECG | Electrocardiogram |
| IAEA | International Atomic Energy Agency |
| ICRP | International Commission on Radiological Protection |
| MIBI | Methoxyisobutylisonitrile |
| SPECT | Single-Photon Emission Computed Tomography |
References
- Rajaraman, P.; Doody, M.M.; Yu, C.L.; Preston, D.L.; Miller, J.S.; Sigurdson, A.J.; Freedman, D.M.; Alexander, B.H.; Little, M.P.; Miller, D.L.; et al. JOURNAL CLUB: Cancer Risks in U.S. Radiologic Technologists Working with Fluoroscopically Guided Interventional Procedures, 1994–2008. Am. J. Roentgenol. 2016, 206, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, Y.; Yoshinaga, S.; Zhu, W.; Tokonami, S.; Zou, J.; Tan, G.; Tsuji, M.; Akiba, S.; Sun, Q. Lens Opacity Prevalence among the Residents in High Natural Background Radiation Area in Yangjiang, China. J. Radiat. Res. 2021, 62, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Little, M.P.; Cahoon, E.K.; Kitahara, C.M.; Simon, S.L.; Hamada, N.; Linet, M.S. Occupational Radiation Exposure and Excess Additive Risk of Cataract Incidence in a Cohort of US Radiologic Technologists. Occup. Environ. Med. 2020, 77, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hijikata, Y.; Yamashita, K.; Hatsusaka, N.; Nagata, T.; Kitamura, H.; Morota, K.; Matsuzaki, S.; Nakagami, K.; Hitomi, G.; Kuriyama, T.; et al. Prevalence of Cataractous Changes in the Eyes and Chronic Inflammatory Changes in the Hands Among Spine Surgeons. J. Bone Jt. Surg. 2025, 107, e25. [Google Scholar] [CrossRef]
- Preston, D.L.; Kitahara, C.M.; Freedman, D.M.; Sigurdson, A.J.; Simon, S.L.; Little, M.P.; Cahoon, E.K.; Rajaraman, P.; Miller, J.S.; Alexander, B.H.; et al. Breast Cancer Risk and Protracted Low-to-Moderate Dose Occupational Radiation Exposure in the US Radiologic Technologists Cohort, 1983–2008. Br. J. Cancer 2016, 115, 1105–1112. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Linet, M.S.; Drozdovitch, V.; Alexander, B.H.; Preston, D.L.; Simon, S.L.; Freedman, D.M.; Brill, A.B.; Miller, J.S.; Little, M.P.; et al. Cancer and Circulatory Disease Risks in US Radiologic Technologists Associated with Performing Procedures Involving Radionuclides. Occup. Environ. Med. 2015, 72, 770–776. [Google Scholar] [CrossRef]
- Berrington de Gonzalez, A.; Daniels, R.D.; Cardis, E.; Cullings, H.M.; Gilbert, E.; Hauptmann, M.; Kendall, G.; Laurier, D.; Linet, M.S.; Little, M.P.; et al. Epidemiological Studies of Low-Dose Ionizing Radiation and Cancer: Rationale and Framework for the Monograph and Overview of Eligible Studies. J. Natl. Cancer Inst. Monogr. 2020, 2020, 97–113. [Google Scholar] [CrossRef]
- International Commission on Radiological Protection (ICRP). Avoidance of Radiation Injuries from Medical Interventional Procedures; ICRP Publication 85; ICRP: Pergamon, Turkey; Oxford, UK, 2000; Volume 30. [Google Scholar]
- International Commission on Radiological Protection (ICRP). Radiation dose to patients from radiopharmaceuticals: A compendium of current information related to frequently used substances. ICRP Publication 128. Ann. ICRP 2015, 47, 7–321. [Google Scholar]
- Forbrig, R.; Trumm, C.G.; Reidler, P.; Kunz, W.G.; Dimitriadis, K.; Kellert, L.; Rückel, J.; Liebig, T.; Stahl, R. Optimizing Radiation Dose and Image Quality in Stroke CT Protocols: Proposed Diagnostic Reference Levels for Multiphase CT Angiography and Perfusion Imaging. Diagnostics 2024, 14, 2866. [Google Scholar] [CrossRef]
- Yoshinaga, S.; Mabuchi, K.; Sigurdson, A.J.; Doody, M.M.; Ron, E. Cancer Risks among Radiologists and Radiologic Technologists: Review of Epidemiologic Studies. Radiology 2004, 233, 313–321. [Google Scholar] [CrossRef]
- Boice, J., Jr.; Dauer, L.T.; Kase, K.R.; Mettler, F.A., Jr.; Vetter, R.J. Evolution of Radiation Protection for Medical Workers. Br. J. Radiol. 2020, 93, 20200282. [Google Scholar] [CrossRef] [PubMed]
- Linet, M.S.; Kim, K.P.; Miller, D.L.; Kleinerman, R.A.; Simon, S.L.; de Gonzalez, A.B. Historical Review of Occupational Exposures and Cancer Risks in Medical Radiation Workers. Radiat. Res. 2010, 174, 793–808. [Google Scholar] [CrossRef]
- Endo, M.; Haga, Y.; Sota, M.; Tanaka, A.; Otomo, K.; Murabayashi, Y.; Abe, M.; Kaga, Y.; Inaba, Y.; Suzuki, M.; et al. Evaluation of Novel X-Ray Protective Eyewear in Reducing the Eye Dose to Interventional Radiology Physicians. J. Radiat. Res. 2021, 62, 414–419. [Google Scholar] [CrossRef]
- International Commission on Radiological Protection (ICRP). Occupational radiological protection in interventional procedures. ICRP Publication 139. Ann. ICRP 2018, 47, 1–118. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). Occupational Radiation Protection: General Safety Guide; IAEA Safety Standards Series No. GSG-7; IAEA: Vienna, Austria, 2018; pp. 1–152. [Google Scholar]
- International Atomic Energy Agency (IAEA). Radiation Protection and Safety of Radiation Sources: International Basic Safety Standards; IAEA Safety Standards Series No. GSR Part 3; IAEA: Vienna, Austria, 2014; pp. 1–436. [Google Scholar]
- Shindo, R.; Ohno, S.; Yamamoto, K.; Konta, S.; Inaba, Y.; Suzuki, M.; Zuguchi, M.; Chida, K. Comparison of Shielding Effects of Over-Glasses-Type and Regular Eyewear in Terms of Occupational Eye Dose Reduction. J. Radiol. Prot. 2024, 44, 023501. [Google Scholar] [CrossRef]
- Kato, M.; Chida, K.; Munehisa, M.; Sato, T.; Inaba, Y.; Suzuki, M.; Zuguchi, M. Non-Lead Protective Aprons for the Protection of Interventional Radiology Physicians from Radiation Exposure in Clinical Settings: An Initial Study. Diagnostics 2021, 11, 1613. [Google Scholar] [CrossRef]
- McVey, S.; Sandison, A.; Sutton, D.G. An Assessment of Lead Eyewear in Interventional Radiology. J. Radiol. Prot. 2013, 33, 647. [Google Scholar] [CrossRef]
- Fujisawa, M.; Haga, Y.; Takahira, S.; Sota, M.; Kato, T.; Abe, M.; Kaga, Y.; Inaba, Y.; Suzuki, M.; Chida, K. Development of a Headgear-Based Eye Protection Device for Physicians Performing Fluoroscopy-Guided Bronchoscopy. J. Radiat. Res. 2025, 66, 372–384. [Google Scholar] [CrossRef]
- Lin, H.-H.; Lai, L.-H.; Tang, K.-T.; Ting, C.-Y.; Lai, C.-S. Radiation Dose Assessment of the Fog Lead Acrylic Shields during Coronary Angiography: A Phantom Study. Appl. Sci. 2021, 11, 10743. [Google Scholar] [CrossRef]
- Lindholm, C.; Pekkarinen, A.; Sipilä, O.; Manninen, A.-L.; Lehtinen, M.; Siiskonen, T. Estimation of Hp(3) among Staff Members in Two Nuclear Medicine Units in Finland. Radiat. Prot. Dosim. 2020, 190, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Guiu-Souto, J.; Sánchez-García, M.; Vázquez-Vázquez, R.; Otero, C.; Luna, V.; Mosquera, J.; Busto, R.L.; Aguiar, P.; Ruibal, Á.; Pardo-Montero, J.; et al. Evaluation and Optimization of Occupational Eye Lens Dosimetry during Positron Emission Tomography (PET) Procedures. J. Radiol. Prot. 2016, 36, 299. [Google Scholar] [CrossRef]
- Krajewska, G.; Krajewski, P. Evaluation of Internal Exposure of Nuclear Medicine Staff Working with Radioiodine in Poland. Int. J. Occup. Med. Environ. Health 2023, 36, 587–595. [Google Scholar] [CrossRef]
- Struelens, L.; Aalbersberg, E.; Beels, L.; Cherbuin, N.; D’Asseler, Y.; De Monte, F.; Lopez Medina, A.; del Carmen Riveira Martin, M.; Schoonjans, W.; Terwinghe, C.; et al. How Much Do 68Ga-, 177Lu- and 131I-Based Radiopharmaceuticals Contribute to the Global Radiation Exposure of Nuclear Medicine Staff? EJNMMI Phys. 2024, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Mettler, F.A.; Guiberteau, M.J. Occupational Exposure in General Radiology and Nuclear Medicine: A Changing Target. Radiology 2021, 300, 613–614. [Google Scholar] [CrossRef]
- Villoing, D.; Yoder, R.C.; Passmore, C.; Bernier, M.-O.; Kitahara, C.M. A U.S. Multicenter Study of Recorded Occupational Radiation Badge Doses in Nuclear Medicine. Radiology 2018, 287, 676–682. [Google Scholar] [CrossRef]
- Villoing, D.; Borrego, D.; Preston, D.L.; Alexander, B.H.; Rose, A.; Salasky, M.; Linet, M.S.; Lee, C.; Kitahara, C.M. Trends in Occupational Radiation Doses for U.S. Radiologic Technologists Performing General Radiologic and Nuclear Medicine Procedures, 1980–2015. Radiology 2021, 300, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.K.; Sirieak, N.; Karnkorn, P.; Keawtong, V.; Hayeeabdunromae, A.; Noomad, N.; Durawee, W.; Cheewakul, J. Nuclear Medicine Radiological Hot Laboratory Simulation: A Mixed-Method Intervention Study on Immersive Virtual Reality for Sustainable Education. Appl. Sci. 2024, 14, 5041. [Google Scholar] [CrossRef]
- Marshall, S.K.; Hayeeabdunromae, A.; Noomad, N.; Durawee, W.; Sirieak, N.; Karnkorn, P.; Keawtong, V. Evaluation of Environmental Radiation Exposure and Algorithms for Determining the Occupational Effective Dose During 99mTc-MDP Bone Scintigraphy: A Comprehensive Analysis. Appl. Sci. 2024, 14, 11211. [Google Scholar] [CrossRef]
- Wrzesień, M.; Królicki, L.; Albiniak, Ł.; Olszewski, J. Is Eye Lens Dosimetry Needed in Nuclear Medicine? J. Radiol. Prot. 2018, 38, 763. [Google Scholar] [CrossRef]
- Wrzesień, M.; Albiniak, Ł. Personal Dose Equivalent Hp(0.07) during 68Ga-DOTA-TATE Production Procedures. Radiat. Environ. Biophys. 2023, 62, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.K.; Prom-on, P.; Sangkue, S.; Thiangsook, W. Assessment of Radiation Exposure in a Nuclear Medicine Department during 99mTc-MDP Bone Scintigraphy. Toxics 2023, 11, 814. [Google Scholar] [CrossRef]
- Mettler, F.A.; Bhargavan, M.; Faulkner, K.; Gilley, D.B.; Gray, J.E.; Ibbott, G.S.; Lipoti, J.A.; Mahesh, M.; McCrohan, J.L.; Stabin, M.G.; et al. Radiologic and Nuclear Medicine Studies in the United States and Worldwide: Frequency, Radiation Dose, and Comparison with Other Radiation Sources—1950–2007. Radiology 2009, 253, 520–531. [Google Scholar] [CrossRef]
- Slomka, P.J.; Pan, T.; Berman, D.S.; Germano, G. Advances in SPECT and PET Hardware. Prog. Cardiovasc. Dis. 2015, 57, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.A.; Akleyev, A.V.; Hauer-Jensen, M.; Hendry, J.H.; Kleiman, N.J.; Macvittie, T.J.; Aleman, B.M.; Edgar, A.B.; Mabuchi, K.; Muirhead, C.R. ICRP Publication 118: ICRP Statement on Tissue Reactions and Early and Late Effects of Radiation in Normal Tissues and Organs–Threshold Doses for Tissue Reactions in a Radiation Protection Context. Ann. ICRP 2012, 41, 1–322. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). Implications for Occupational Radiation Protection of the New Dose Limit for the Lens of the Eye; IAEA: Vienna, Austria, 2013; Volume 1731, pp. 1–34. [Google Scholar]
- Yamada, A.; Haga, Y.; Sota, M.; Abe, M.; Kaga, Y.; Inaba, Y.; Suzuki, M.; Tada, N.; Zuguchi, M.; Chida, K. Eye Lens Radiation Dose to Nurses during Cardiac Interventional Radiology: An Initial Study. Diagnostics 2023, 13, 3003. [Google Scholar] [CrossRef]
- Ohno, S.; Konta, S.; Shindo, R.; Yamamoto, K.; Isobe, R.; Inaba, Y.; Suzuki, M.; Zuguchi, M.; Chida, K. Effect of Backscatter Radiation on the Occupational Eye-Lens Dose. J. Radiat. Res. 2024, 65, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Osanai, M.; Sato, H.; Sato, K.; Kudo, K.; Hosoda, M.; Hosokawa, S.; Kitajima, M.; Tsushima, M.; Fujita, A.; Hosokawa, Y.; et al. Occupational Radiation Dose, Especially for Eye Lens: Hp(3), in Medical Staff Members Involved in Computed Tomography Examinations. Appl. Sci. 2021, 11, 4448. [Google Scholar] [CrossRef]
- Inaba, Y.; Hitachi, S.; Watanuki, M.; Chida, K. Occupational Radiation Dose to Eye Lenses in CT-Guided Interventions Using MDCT-Fluoroscopy. Diagnostics 2021, 11, 646. [Google Scholar] [CrossRef]
- Bernier, M.-O.; Journy, N.; Villoing, D.; Doody, M.M.; Alexander, B.H.; Linet, M.S.; Kitahara, C.M. Cataract Risk in a Cohort of U.S. Radiologic Technologists Performing Nuclear Medicine Procedures. Radiology 2018, 286, 592–601. [Google Scholar] [CrossRef]
- Vañó, E.; Rosenstein, M.; Liniecki, J.; Rehani, M.M.; Martin, C.J.; Vetter, R.J. ICRP Publication 113. Education and Training in Radiological Protection for Diagnostic and Interventional Procedures. Ann. ICRP 2009, 39, 7–68. [Google Scholar] [CrossRef]
- Cousins, C.; Miller, D.L.; Bernardi, G.; Rehani, M.M.; Schofield, P.; Vañó, E.; Einstein, A.J.; Geiger, B.; Heintz, P.; Padovani, R.; et al. ICRP Publication 120: Radiological Protection in Cardiology. Ann. ICRP 2013, 42, 1–125. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, A.; Leichert, H.J.; König, A.M.; Owczarek, A.D.; Mahnken, A.H. Efficiency in Radiation Protection of a Novel Exoskeleton-Based Interventional Radiology Apron and Correlation with Conventional Aprons. Eur. J. Radiol. 2025, 184, 111946. [Google Scholar] [CrossRef]
- Crowhurst, J.A.; Tse, J.; Mirjalili, N.; Savage, M.L.; Raffel, O.C.; Gaikwad, N.; Walters, D.L.; Dautov, R. Trial of a Novel Radiation Shielding Device to Protect Staff in the Cardiac Catheter Laboratory. Am. J. Cardiol. 2023, 203, 429–435. [Google Scholar] [CrossRef]
- Lundvall, L.-L.; Sandborg, M. Does Radiological Protection Training or a Real-Time Staff Dosemeter Display Reduce Staff Doses During X-Ray-Guided Pulmonary Bronchoscopy? Radiat. Prot. Dosim. 2022, 198, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Casazza, R.; Malik, B.; Hashmi, A.; Fogel, J.; Montagna, E.; Gibson, D.; Palacio, A.; Beginyazova, H.; Frankel, R.; Shani, J. The Influence of Height on Occupational Radiation Exposure of Interventional Cardiologists During Selective Coronary Angiography Using a Radial Artery Approach. Am. J. Cardiol. 2025, 251, 1–8. [Google Scholar] [CrossRef]
- Casazza, R.; Malik, B.; Hashmi, A.; Fogel, J.; Montagna, E.; Frankel, R.; Borgen, E.; Ayzenberg, S.; Friedman, M.; Moskovits, N.; et al. Operator Radiation Exposure Comparing the Left Radial Artery Approach and a Uniform Hyper-Adducted Right Radial Artery Approach: The HARRA Study. Circ.-Cardiovasc. Interv. 2025, 18, e014602. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, M.; Haga, Y.; Sota, M.; Abe, M.; Kaga, Y.; Inaba, Y.; Suzuki, M.; Meguro, T.; Hosoi, Y.; Chida, K. Evaluation of Lens Doses among Medical Staff Involved in Nuclear Medicine: Current Eye Radiation Exposure among Nuclear-Medicine Staff. Appl. Sci. 2023, 13, 9182. [Google Scholar] [CrossRef]
- JCS Joint Working Group. Guidelines for Clinical Use of Cardiac Nuclear Medicine (JCS 2010). Circ. J. 2012, 76, 761–767. [Google Scholar] [CrossRef]
- Hattori, K.; Inaba, Y.; Kato, T.; Fujisawa, M.; Yasuno, H.; Yamada, A.; Haga, Y.; Suzuki, M.; Zuguchi, M.; Chida, K. Evaluation of a New Real-Time Dosimeter Sensor for Interventional Radiology Staff. Sensors 2023, 23, 512. [Google Scholar] [CrossRef]
- RaySafe i3—Instructions for Use. 2020. Available online: https://www.raysafe.com/sites/default/files/2020-10/RaySafe%20i3%20Instructions%20for%20Use%20%28multilingual%29_0.pdf (accessed on 8 October 2025).
- International Atomic Energy Agency. Manual for First Responders to a Radiological Emergency; Emergency Preparedness and Response; International Atomic Energy Agency: Vienna, Austria, 2006. [Google Scholar]
- Chida, K. What Are Useful Methods to Reduce Occupational Radiation Exposure among Radiological Medical Workers, Especially for Interventional Radiology Personnel? Radiol. Phys. Technol. 2022, 15, 101–115. [Google Scholar] [CrossRef]
- Inaba, Y.; Hitachi, S.; Watanuki, M.; Chida, K. Radiation Eye Dose for Physicians in CT Fluoroscopy-Guided Biopsy. Tomography 2022, 8, 438–446. [Google Scholar] [CrossRef]
- Konta, S.; Ohno, S.; Shindo, R.; Yamamoto, K.; Haga, Y.; Kato, T.; Sota, M.; Kaga, Y.; Abe, M.; Chida, K. Assessment of Lens Absorbed Dose by Radiological Technologists during Mobile X-Ray Radiography: A Comparison between Computed Radiography and Flat Panel Detector Systems. Radiol. Phys. Technol. 2025, 18, 766–774. [Google Scholar] [CrossRef]
- Ohno, S.; Shindo, R.; Konta, S.; Yamamoto, K.; Inaba, Y.; Chida, K. Radiation Exposure to the Brains of Interventional Radiology Staff: A Phantom Study. Bioengineering 2024, 11, 1083. [Google Scholar] [CrossRef]
- Sagehashi, K.; Haga, Y.; Takahira, S.; Tanabe, M.; Nakamura, M.; Sota, M.; Kaga, Y.; Abe, M.; Tada, N.; Chida, K. Evaluation of Radiation Dose to the Lens in Interventional Cardiology Physicians before and after Dose Limit Regulation Changes. J. Radiol. Prot. 2024, 44, 031512. [Google Scholar] [CrossRef]
- Haga, Y.; Chida, K.; Kaga, Y.; Sota, M.; Meguro, T.; Zuguchi, M. Occupational Eye Dose in Interventional Cardiology Procedures. Sci. Rep. 2017, 7, 569. [Google Scholar] [CrossRef] [PubMed]
- Penfold, S.N.; Marcu, L.; Lawson, J.M.; Asp, J. Evaluation of physician eye lens doses during permanent seed implant brachytherapy for prostate cancer. J. Radiol. Prot. 2012, 32, 339. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Jingu, K.; Fujisawa, M.; Otomo, K.; Ishii, H.; Kato, T.; Murabayashi, Y.; Suzuki, M.; Zuguchi, M.; Chida, K. Evaluation of Radiation Doses Received by Physicians during Permanent 198Au Grain Implant Brachytherapy for Oral Cancer. Appl. Sci. 2024, 14, 6010. [Google Scholar] [CrossRef]
- Higley, B.; Smith, F.W.; Smith, T.; Gemmell, H.G.; Gupta, P.D.; Gvozdanovic, D.V.; Graham, D.; Hinge, D.; Davidson, J.; Lahiri, A. Technetium-99m-1,2-Bis[Bis(2-Ethoxyethyl) Phosphino]Ethane: Human Biodistribution, Dosimetry and Safety of a New Myocardial Perfusion Imaging Agent. J. Nucl. Med. 1993, 34, 30–38. [Google Scholar] [PubMed]
- Günay, O.; Sarıhan, M.; Yarar, O.; Abuqbeitah, M.; Demir, M.; Sönmezoğlu, K.; Abamor, E.; Kara, Ö.E.; İpek Işıkcı, N.; Aközcan, S.; et al. Determination of Radiation Dose from Patients Undergoing Tc-99m Sestamibi Nuclear Cardiac Imaging. Int. J. Environ. Sci. Technol. 2019, 16, 5251–5258. [Google Scholar] [CrossRef]


| Session Number | LH | RH | LN | RN | RT (A/B) | AD (MBq) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD (µSv) | MDR (µSv/h) | CD (µSv) | MDR (µSv/h) | CD (µSv) | MDR (µSv/h) | CD (µSv) | MDR (µSv/h) | |||
| 1 | 0.03 | 41.36 | 0.19 | 55.78 | 0.15 | 108.3 | 0.28 | 104.72 | A | 644.7 |
| 2 | 0.14 | 61.12 | 0.23 | 56.85 | 0.23 | 74.59 | 0.70 | 98.96 | A | 636.3 |
| 3 | 0.12 | 57.83 | 0.39 | 88.12 | 0.31 | 108.3 | 0.94 | 141.07 | A | 726.2 |
| 4 | 0.22 | 70.64 | 0.21 | 87.76 | 0.33 | 130.89 | 0.34 | 107.96 | A | 683.5 |
| 5 | 0.52 | 89.01 | 0.88 | 82.44 | 0.36 | 86.33 | 0.71 | 102.54 | A | 619.3 |
| 6 | 0.17 | 59.10 | 0.35 | 74.52 | 0.16 | 61.93 | 0.88 | 114.05 | A | 615.4 |
| 7 | 0.20 | 94.64 | 0.57 | 79.31 | 0.32 | 76.75 | 0.66 | 186.14 | A | 614.2 |
| 8 | 0.12 | 87.90 | 0.15 | 78.68 | 0.46 | 102.34 | 1.15 | 125.74 | B | 903.9 |
| 9 | 0.21 | 64.26 | 0.23 | 71.85 | 0.50 | 108.00 | 1.28 | 134.34 | B | 862.3 |
| Mean | 0.19 | 0.35 | 0.31 | 0.77 | 700.64 | |||||
| SD | 0.13 | 0.22 | 0.12 | 0.32 | 103.83 | |||||
| Session Number | LH | RH | LN | RN | RT (A/B) | AD (MBq) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD (µSv) | MDR (µSv/h) | CD (µSv) | MDR (µSv/h) | CD (µSv) | MDR (µSv/h) | CD (µSv) | MDR (µSv/h) | |||
| 1 | 0.22 | 56.14 | 0.41 | 79.66 | 0.60 | 114.43 | 0.91 | 133.72 | A | 731.9 |
| 2 | 0.21 | 76.11 | 0.33 | 106.45 | 0.22 | 58.11 | 0.80 | 136.95 | A | 486.7 |
| 3 | 0.17 | 52.86 | 0.19 | 97.66 | 0.20 | 69.53 | 0.46 | 141.85 | A | 944.5 |
| 4 | 0.22 | 54.35 | 0.25 | 78.62 | 0.20 | 64.94 | 0.50 | 137.90 | A | 691.8 |
| 5 | 0.17 | 74.82 | 0.25 | 81.09 | 0.03 | 34.24 | 0.37 | 96.96 | A | 639.3 |
| 6 | 0.36 | 107.65 | 0.27 | 96.62 | 0.44 | 63.30 | 0.55 | 153.80 | A | 644.7 |
| 7 | 0.15 | 100.42 | 0.26 | 85.92 | 0.28 | 77.96 | 0.21 | 134.94 | A | 636.3 |
| 8 | 0.28 | 62.85 | 0.35 | 60.85 | 0.46 | 72.24 | 0.63 | 145.09 | A | 683.5 |
| 9 | 0.48 | 82.05 | 0.63 | 99.94 | 0.28 | 74.25 | 0.44 | 119.58 | A | 619.3 |
| 10 | 0.35 | 107.59 | 0.40 | 83.22 | 0.38 | 112.00 | 0.40 | 92.89 | A | 615.4 |
| 11 | 0.25 | 66.99 | 0.55 | 81.09 | 0.30 | 107.90 | 0.77 | 150.30 | A | 614.2 |
| 12 | 0.07 | 41.71 | 0.06 | 51.80 | 0.69 | 104.51 | 3.23 | 185.17 | B | 903.9 |
| 13 | 0.11 | 56.10 | 0.38 | 80.12 | 0.77 | 88.96 | 1.04 | 135.00 | B | 862.3 |
| Mean | 0.23 | 0.33 | 0.37 | 0.79 | 697.98 | |||||
| SD | 0.10 | 0.14 | 0.20 | 0.71 | 121.40 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujisawa, M.; Sota, M.; Haga, Y.; Tanaka, S.; Kataoka, N.; Kato, T.; Kaga, Y.; Abe, M.; Suzuki, M.; Inaba, Y.; et al. Real-Time Monitoring of Occupational Radiation Exposure in Nuclear Medicine Technologists: An Initial Study. Appl. Sci. 2025, 15, 11008. https://doi.org/10.3390/app152011008
Fujisawa M, Sota M, Haga Y, Tanaka S, Kataoka N, Kato T, Kaga Y, Abe M, Suzuki M, Inaba Y, et al. Real-Time Monitoring of Occupational Radiation Exposure in Nuclear Medicine Technologists: An Initial Study. Applied Sciences. 2025; 15(20):11008. https://doi.org/10.3390/app152011008
Chicago/Turabian StyleFujisawa, Masaki, Masahiro Sota, Yoshihiro Haga, Shigehisa Tanaka, Nozomi Kataoka, Toshiki Kato, Yuji Kaga, Mitsuya Abe, Masatoshi Suzuki, Yohei Inaba, and et al. 2025. "Real-Time Monitoring of Occupational Radiation Exposure in Nuclear Medicine Technologists: An Initial Study" Applied Sciences 15, no. 20: 11008. https://doi.org/10.3390/app152011008
APA StyleFujisawa, M., Sota, M., Haga, Y., Tanaka, S., Kataoka, N., Kato, T., Kaga, Y., Abe, M., Suzuki, M., Inaba, Y., & Chida, K. (2025). Real-Time Monitoring of Occupational Radiation Exposure in Nuclear Medicine Technologists: An Initial Study. Applied Sciences, 15(20), 11008. https://doi.org/10.3390/app152011008






