Comparative Analysis of Different AI Approaches to Stroke Patients’ Gait Analysis
Abstract
1. Introduction
- Limited validation in diverse populations due to any AI models have been developed and validated in controlled settings with small sample sizes, often characterized by a lack of diversity in age, gender, and ethnicity. This limits their generalizability to broader patient populations [30].
- Lack of standardization because a lack of consensus on standardized protocols for data collection, feature extraction, or model evaluation leads to inconsistencies across studies and hinders comparison of results or their integration into clinical practice [31].
- Data privacy concerns because the use of wearable sensors and video recordings poses significant privacy issues, especially for sensitive medical data. Ensuring data security and patient consent is a priority but often insufficiently addressed.
- Interpretability of AI models since many AI systems act as “black boxes,” providing predictions without clear explanations. This lack of interpretability can hinder clinician trust and acceptance, as understanding the rationale behind decisions is crucial to decision-making [32].
- Integration with clinical processes because AI tools often operate in isolation and are not seamlessly integrated with existing clinical processes or electronic health record systems, limiting their utility in real-world settings [33].
- Practical application: most AI models are tested in controlled environments, but their effectiveness in real-world settings, such as patients’ homes or community clinics, remains understudied [34].
- Challenges with longitudinal monitoring because continuous gait monitoring over extended periods is essential to assessing rehabilitation progress, but current AI systems may lack long-term tracking capabilities or be impractical for daily use [35].
- Multimodal data integration: Integrating data from various sources, such as video, wearable sensors, and clinical assessments, poses challenges in data fusion and analysis but is essential for comprehensive gait analysis [36].
- Personalization of interventions because AI models often provide generalized conclusions without tailoring recommendations to individual patient needs, which is crucial for effective rehabilitation [37].
- Regulatory and ethical issues due to implementing AI in clinical settings raises regulatory concerns about safety, effectiveness, and ethical issues that are still being addressed by policymakers and regulators.
- Methods for rapid and easy-to-automate gait analysis;
- New gait parameters, including fuzzy and fractal logic;
- Methods for objectifying the assessment of gait rehabilitation effectiveness.
2. Materials and Methods
2.1. Dataset
- Ethics and consent: all participants are recruited only after informed consent, explaining how gait data, and health information will be collected, stored, and used, and consent forms specify data-sharing conditions;
- De-identification and anonymization: raw clinical identifiers (name, date of birth, medical record numbers) are replaced with randomized study IDs;
- Data storage and transfer security: data are stored in encrypted servers compliant with GDPR standards, Transfer between sensors and servers uses end-to-end encryption (VPN), Role-based access control ensures only authorized clinicians/researchers access sensitive data;
- Minimization and purpose limitation: only the minimum necessary dataset for analysis is collected (e.g., gait parameters instead of raw continuous video whenever possible).
2.2. Statistical Analysis
2.3. Computational Methods
3. Results
4. Discussion
- Overarching study design: technical/bench testing, prospective observational validation, clinical impact trial;
- Participant selection & sample size (practical guidance): broad range of post-stroke patients (from acute to chronic), varying severity (functional levels), assistive device users vs. independent walkers and comorbidities typical for clinic, sample size guidance (algorithm internal validation, external validation);
- Data collection;
- Outcome measures: clinically meaningful outcomes, technical performance metrics;
- Evaluation metrics;
- Extended statistical analysis (within dataset, between model comparisons, agreement and subgroup analysis;
- Blindig, bias and generalizability,
- Safety of patients and clinical workflow testing;
- Regulatory, ethical and data governance issues;
- Practical logistics;
- Reporting and transparency.
4.1. Limitations of Previous Studies and the Current Study
4.2. Technological Implications
4.3. Economical Implications
4.4. Societal Implications
4.5. Ethical and Legal Implications
4.6. Directions for Further Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Arificial intelligence |
| CNN | Convolutional neural network |
| DL | Deep learning |
| ML | Machine learning |
| ReLU | Rectification linear unit |
| RF | Random forests |
| SVM | Support vector machine |
References
- Mohan, D.M.; Khandoker, A.H.; Wasti, S.A.; Ismail Ibrahim Ismail Alali, S.; Jelinek, H.F.; Khalaf, K. Assessment Methods of Post-stroke Gait: A Scoping Review of Technology-Driven Approaches to Gait Characterization and Analysis. Front. Neurol. 2021, 12, 650024. [Google Scholar] [CrossRef]
- Pohl, J.; Ryser, A.; Veerbeek, J.M.; Verheyden, G.; Vogt, J.E.; Luft, A.R.; Easthope, C.A. Accuracy of gait and posture classification using movement sensors in individuals with mobility impairment after stroke. Front. Physiol. 2022, 13, 933987. [Google Scholar] [CrossRef]
- Morizio, C.; Compagnat, M.; Boujut, A.; Labbani-Igbida, O.; Billot, M.; Perrochon, A. Immersive Virtual Reality during Robot-Assisted Gait Training: Validation of a New Device in Stroke Rehabilitation. Medicina 2022, 58, 1805. [Google Scholar] [CrossRef]
- Igarashi, T.; Tani, Y.; Takeda, R.; Asakura, T. Minimal detectable change in inertial measurement unit-based trunk acceleration indices during gait in inpatients with subacute stroke. Sci. Rep. 2023, 13, 19262. [Google Scholar] [CrossRef]
- Andrenelli, E.; Ippoliti, E.; Coccia, M.; Millevolte, M.; Cicconi, B.; Latini, L. Features and predictors of activity limitations and participation restriction 2 years after intensive rehabilitation following first-ever stroke. Eur. J. Phys. Rehabil. Med. 2015, 51, 575–585. [Google Scholar]
- Baker, R.; Esquenazi, A.; Benedetti, M.G.; Desloovere, K. Gait analysis: Clinical facts. Eur. J. Phys. Rehabil. Med. 2016, 52, 560–574. [Google Scholar] [PubMed]
- Calabrò, R.S.; Sorrentino, G.; Cassio, A.; Mazzoli, D.; Andrenelli, E.; Bizzarini, E. Robotic-assisted gait rehabilitation following stroke: A systematic review of current guidelines and practical clinical recommendations. Eur. J. Phys. Rehabil. Med. 2021, 57, 460–471. [Google Scholar] [CrossRef]
- Chen, Z.; Li, M.; Cui, H.; Wu, X.; Chen, F.; Li, W. Effects of kinesio taping therapy on gait and surface electromyography in stroke patients with hemiplegia. Front. Physiol. 2022, 13, 1040278. [Google Scholar] [CrossRef]
- Imoto, D.; Hirano, S.; Mukaino, M.; Saitoh, E.; Otaka, Y. A novel gait analysis system for detecting abnormal hemiparetic gait patterns during robot-assisted gait training: A criterion validity study among healthy adults. Front. Neurorobot. 2022, 16, 1047376. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, I.; Imoto, D.; Hirano, S.; Konosu, H.; Otaka, Y. Gait analysis system for assessing abnormal patterns in individuals with hemiparetic stroke during robot-assisted gait training: A criterion-related validity study in healthy adults. Front Neurorobot. 2025, 19, 1558009. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Kawasaki, T.; Hamada, H.; Sihan Lim, V.; Shimada, R.; Nakazato, R.; Horimoto, Y.; Ikeda, Y. Changes in Gait Metrics and Motor Strategies Post-Neurocognitive Rehabilitation in Subacute Stroke: A Retrospective Mixed-Methods Study. Cureus 2025, 17, e86264. [Google Scholar] [CrossRef]
- Alammari, B.J.; Schoenwether, B.; Ripic, Z.; Kirk-Sanchez, N.; Eltoukhy, M.; Bishop, L. Validity of AI-Driven Markerless Motion Capture for Spatiotemporal Gait Analysis in Stroke Survivors. Sensors 2025, 25, 5315. [Google Scholar] [CrossRef] [PubMed]
- Scataglini, S.; Abts, E.; Van Bocxlaer, C.; Van den Bussche, M.; Meletani, S.; Truijen, S. Accuracy, Validity, and Reliability of Markerless Camera-Based 3D Motion Capture Systems versus Marker-Based 3D Motion Capture Systems in Gait Analysis: A Systematic Review and Meta-Analysis. Sensors 2024, 24, 3686. [Google Scholar] [CrossRef]
- Lam, W.W.T.; Tang, Y.M.; Fong, K.N.K. A systematic review of the applications of markerless motion capture (MMC) technology for clinical measurement in rehabilitation. J. Neuroeng. Rehabil. 2023, 20, 57. [Google Scholar] [CrossRef]
- Desai, R.; Martelli, D.; Alomar, J.A.; Agrawal, S.; Quinn, L.; Bishop, L. Validity and reliability of inertial measurement units for gait assessment within a post stroke population. Top. Stroke Rehabil. 2024, 31, 235–243. [Google Scholar] [CrossRef]
- Yoma, M.; Herrington, L.; Starbuck, C.; Llurda, L.; Jones, R. Between-Day Reliability of Kinematic Variables Using Markerless Motion Capture for Single-Leg Squat and Single-Leg Landing Tasks. Int. J. Sports Phys. Ther. 2025, 20, 1160–1175. [Google Scholar] [CrossRef] [PubMed]
- Louie, D.R.; Simpson, L.A.; Mortenson, W.B.; Field, T.S.; Yao, J.; Eng, J.J. Prevalence of Walking Limitation After Acute Stroke and Its Impact on Discharge to Home. Phys. Ther. 2022, 102, pzab246. [Google Scholar] [CrossRef] [PubMed]
- Heshmatollah, A.; Darweesh, S.K.L.; Dommershuijsen, L.J.; Koudstaal, P.J.; Ikram, M.A.; Ikram, M.K. Quantitative Gait Impairments in Patients with Stroke or Transient Ischemic Attack: A Population-Based Approach. Stroke 2020, 51, 2464–2471. [Google Scholar] [CrossRef]
- Balaban, B.; Tok, F. Gait Disturbances in Patients with Stroke. PM&R 2014, 6, 635–642. [Google Scholar] [CrossRef]
- Perry, J.; Garrett, M.; Gronley, J.K.; Mulroy, S.J. Classification of walking handicap in the stroke population. Stroke 1995, 26, 982–989. [Google Scholar] [CrossRef]
- Min, Y.S.; Jung, T.D.; Lee, Y.S.; Kwon, Y.; Kim, H.J.; Kim, H.C.; Lee, J.C.; Park, E. Biomechanical Gait Analysis Using a Smartphone-Based Motion Capture System (OpenCap) in Patients with Neurological Disorders. Bioengineering 2024, 11, 911. [Google Scholar] [CrossRef]
- Schmid, A. Improvements in speed-based gait classifications are meaningful. Stroke 2007, 38, 2096–2100. [Google Scholar] [CrossRef]
- Grau-Pellicer, M.; Chamarro-Lusar, A.; Medina-Casanovas, J.; Serda Ferrer, B.C. Walking speed as a predictor of community mobility and quality of life after stroke. Top. Stroke Rehabil. 2019, 26, 349–358. [Google Scholar] [CrossRef]
- Pound, P.; Gompertz, P.; Ebrahim, S. A patient-centred study of the consequences of stroke. Clin. Rehabil. 1998, 12, 255–264. [Google Scholar] [CrossRef]
- Bergamini, E. Multi-sensor assessment of dynamic balance during gait in patients with subacute stroke. J. Biomech. 2017, 61, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Olsen, T.S. Recovery of walking function in stroke patients: The Copenhagen stroke study. Arch. Phys. Med. Rehabil. 1995, 76, 27–32. [Google Scholar] [CrossRef]
- Beyaert, C.; Vasa, R.; Frykberg, G.E. Gait post-stroke: Pathophysiology and rehabilitation strategies. Clin. Neurophysiol. 2015, 45, 335–355. [Google Scholar] [CrossRef]
- Price, R.; Choy, N.L. Investigating the Relationship of the Functional Gait Assessment to Spatiotemporal Parameters of Gait and Quality of Life in Individuals with Stroke. J. Geriatr. Phys. Ther. 2019, 42, 256–264. [Google Scholar] [CrossRef]
- Coleman, E.R.; Moudgal, R.; Lang, K.; Hyacinth, H.I.; Awosika, O.O.; Kissela, B.M.; Feng, W. Early Rehabilitation After Stroke: A Narrative Review. Curr. Atheroscler. Rep. 2017, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Martiš, P.; Košutzká, Z.; Kranzl, A. A Step Forward Understanding Directional Limitations in Markerless Smartphone-Based Gait Analysis: A Pilot Study. Sensors 2024, 24, 3091. [Google Scholar] [CrossRef]
- Tiddia, G.; Mainas, F.; Retico, A.; Oliva, P. Explainable AI Highlights the Most Relevant Gait Features for Neurodegenerative Disease Classification. Appl. Sci. 2025, 15, 8078. [Google Scholar] [CrossRef]
- Rojek, I.; Prokopowicz, P.; Dorożyński, J.; Mikołajewski, D. Novel Methods of AI-Based Gait Analysis in Post-Stroke Patients. Appl. Sci. 2023, 13, 6258. [Google Scholar] [CrossRef]
- Wankhede, V.; Verma, P.; Gahane, S. A Review on the Developments in the Field of AI-Based Gait Analysis. In Proceedings of the 2024 2nd DMIHER International Conference on Artificial Intelligence in Healthcare, Education and Industry (IDICAIEI), Wardha, India, 29–30 November 2024; IEEE: New York, NY, USA, 2024; pp. 1–5. [Google Scholar]
- Kim, G.; Kim, H.; Kim, Y.H.; Kim, S.J.; Choi, M.T. Deep Temporal Clustering of Pathological Gait Patterns in Post-Stroke Patients Using Joint Angle Trajectories: A Cross-Sectional Study. Bioengineering 2025, 12, 55. [Google Scholar] [CrossRef]
- Romano, F.; Perpetuini, D.; Cardone, D.; Merla, A. A machine learning framework for gait and EMG analysis for post-stroke motor dysfunctions assessment. In Proceedings of the European Medical and Biological Engineering Conference 2024, Portorož, Slovenia, 9–13 June 2024; SpringerNature: Cham, Switzerland, 2024; pp. 15–22. [Google Scholar]
- Teng, T.H.; Kim, G.; Kim, H.; Choi, M.T. Deep Temporal Clustering for Long-Term Gait Recovery Patterns of Post-Stroke Patients using Joint Kinematic Data. In Proceedings of the 2025 11th International Conference on Computing and Artificial Intelligence (ICCAI), Kyoto, Japan, 28–31 March 2025; IEEE: New York, NY, USA, 2025; pp. 654–658. [Google Scholar]
- Feng, L.; Duan, Q.; Lai, R.; Liu, W.; Song, X.; Lyu, Y. Development of a three-dimensional muscle-driven lower limb model developed using an improved CFD-FE method. Comput. Methods Biomech. Biomed. Eng. 2025, 28, 314–325. [Google Scholar] [CrossRef]
- Al_Hassani, R.T.; Atilla, D.C. Human Activity Detection Using Smart Wearable Sensing Devices with Feed Forward Neural Networks and PSO. Appl. Sci. 2023, 13, 3716. [Google Scholar] [CrossRef]
- Zia Ur Rehman, M.; Khan, M.; Paul, A. AI-Powered Human Activity Recognition with Attention Networks. In Proceedings of the International Conference on Intelligent Information Hiding and Multimedia Signal Processing, Daegu, Republic of Korea, 5–7 December 2023; Springer Nature: Singapore, 2023; pp. 129–136. [Google Scholar]
- Wang, H.; Wang, P.; Wang, C.; Xiao, Z.; Sun, Y. Intelligent Backpack Based on Wireless Mobile Technology. In Proceedings of the International Conference on Artificial Intelligence Security and Privacy, Guangzhou, China, 6–7 December 2024; Springer Nature: Singapore, 2024; pp. 44–58. [Google Scholar]
- Chimamiwa, G.; Alirezaie, M.; Pecora, F.; Loutfi, A. Multi-sensor dataset of human activities in a smart home environment. Data Brief. 2021, 34, 106632. [Google Scholar] [CrossRef]
- Kvist, A.; Tinmark, F.; Bezuidenhout, L.; Reimeringer, M.; Conradsson, D.M.; Franzén, E. Validation of algorithms for calculating spatiotemporal gait parameters during continuous turning using lumbar and foot mounted inertial measurement units. J. Biomech. 2024, 162, 111907. [Google Scholar] [CrossRef]
- Fawden, T.; Roberts, I.V.; Goldin, S.; Sharma, Y.; Dunne, H.; Stone, T.; Bance, M. Detection of Gait Events Using Ear-Worn IMUs During Functional Movement Tasks. Sensors 2025, 25, 3629. [Google Scholar] [CrossRef] [PubMed]
- Cordillet, S.; Drapier, S.; Leh, F.; Dumont, A.; Bidet, F.; Bonan, I.; Jamal, K. Detecting Freezing of Gait in Parkinson Disease Using Multiple Wearable Sensors Sets During Various Walking Tasks Relative to Medication Conditions (DetectFoG): Protocol for a Prospective Cohort Study. JMIR Res. Protoc. 2025, 14, e58612. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.M.; O’Brien, E.S.; Kamrud, K.E.; Roberts, S.M.; Rooney, T.A.; Thibodeau, K.P.; Balakrishnan, S.; Gell, N.; Mohapatra, S. Utilization of wearable technology to assess gait and mobility post-stroke: A systematic review. J. Neuroeng. Rehabil. 2021, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Shin, M.J.; Park, T.S.; Park, J.H. Clinometric Gait Analysis Using Smart Insoles in Patients With Hemiplegia After Stroke: Pilot Study. JMIR Mhealth Uhealth 2020, 8, e22208. [Google Scholar] [CrossRef] [PubMed]
- Romijnders, R.; Warmerdam, E.; Hansen, C.; Schmidt, G.; Maetzler, W. A Deep Learning Approach for Gait Event Detection from a Single Shank-Worn IMU: Validation in Healthy and Neurological Cohorts. Sensors 2022, 22, 3859. [Google Scholar] [CrossRef]
- Su, D.; Hu, Z.; Wu, J.; Shang, P.; Luo, Z. Review of adaptive control for stroke lower limb exoskeleton rehabilitation robot based on motion intention recognition. Front. Neurorobot. 2023, 17, 1186175. [Google Scholar] [CrossRef] [PubMed]
- Liaw, J.C.; Raab, D.; Weber, M.; Siebler, M.; Hefter, H.; Zietz, D.; Jäger, M.; Kecskeméthy, A.; Geu Flores, F. An Intelligible AI-Driven Decision Support System For Poststroke Mobility Assessment. J. Rehabil. Med. Clin. Commun. 2025, 8, 42379. [Google Scholar] [CrossRef]
- Benson, L.C.; Clermont, C.A.; Bošnjak, E.; Ferber, R. The use of wearable devices for walking and running gait analysis outside of the lab: A systematic review. Gait Posture. 2018, 63, 124–138. [Google Scholar] [CrossRef]
- Bedon, C.; Sciomenta, M.; Mazelli, A. Use of Smartphone-Based Experimental Data for the Calibration of Biodynamic Spring-Mass-Damper (SMD) Pedestrian Models. Sensors 2025, 25, 1387. [Google Scholar] [CrossRef]
- Ma, P.; Bian, Q.; Kim, J.M.; Alsayed, K.; Ding, Z. Measuring Lower-Limb Kinematics in Walking: Wearable Sensors Achieve Comparable Reliability to Motion Capture Systems and Smartphone Cameras. Sensors 2025, 25, 2899. [Google Scholar] [CrossRef]
- Dibbern, K.N.; Krzak, M.G.; Olivas, A.; Albert, M.V.; Krzak, J.J.; Kruger, K.M. Scoping Review of Machine Learning Techniques in Marker-Based Clinical Gait Analysis. Bioengineering 2025, 12, 591. [Google Scholar] [CrossRef]
- Luvizutto, G.J.; Silva, G.F.; Nascimento, M.R.; Sousa Santos, K.C.; Appelt, P.A.; de Moura Neto, E.; de Souza, J.T.; Wincker, F.C.; Miranda, L.A.; Hamamoto Filho, P.T.; et al. Use of artificial intelligence as an instrument of evaluation after stroke: A scoping review based on international classification of functioning, disability and health concept. Top. Stroke Rehabil. 2022, 29, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Senadheera, I.; Hettiarachchi, P.; Haslam, B.; Nawaratne, R.; Sheehan, J.; Lockwood, K.J.; Alahakoon, D.; Carey, L.M. AI Applications in Adult Stroke Recovery and Rehabilitation: A Scoping Review Using AI. Sensors 2024, 24, 6585. [Google Scholar] [CrossRef]
- Daly, J.J.; McCabe, J.P.; Gor-García-Fogeda, M.D.; Nethery, J.C. Update on an Observational, Clinically Useful Gait Coordination Measure: The Gait Assessment and Intervention Tool (G.A.I.T.). Brain Sci. 2022, 12, 1104. [Google Scholar] [CrossRef]
- Adams, J.L.; Dinesh, K.; Snyder, C.W.; Xiong, M.; Tarolli, C.G.; Sharma, S.; Dorsey, E.R.; Sharma, G. A real-world study of wearable sensors in Parkinson’s disease. NPJ Park. Dis. 2021, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Nicora, G.; Pe, S.; Santangelo, G.; Billeci, L.; Aprile, I.G.; Germanotta, M.; Bellazzi, R.; Parimbelli, E.; Quaglini, S. Systematic review of AI/ML applications in multi-domain robotic rehabilitation: Trends, gaps, and future directions. J. Neuroeng. Rehabil. 2025, 22, 79. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Kim, T.W.; Beom, J.; Won, S.; Jeon, D. AI Therapist Realizing Expert Verbal Cues for Effective Robot-Assisted Gait Training. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2805–2815. [Google Scholar] [CrossRef] [PubMed]
- Mikołajewska, E. The value of the NDT-Bobath method in post-stroke gait training. Adv. Clin. Exp. Med. 2013, 22, 261–272. [Google Scholar]
- Mikołajewska, E. Associations between results of post-stroke NDT-Bobath rehabilitation in gait parameters, ADL and hand functions. Adv. Clin. Exp. Med. 2013, 22, 731–738. [Google Scholar]
- Wu, Y.C.; Huang, Y.J.; Han, C.C.; Cheng, Y.Y.; Chang, C.S. Development of an IMU-Based Post-Stroke Gait Data Acquisition and Analysis System for the Gait Assessment and Intervention Tool. Sensors 2025, 25, 1994. [Google Scholar] [CrossRef]
- Lanotte, F.; Okita, S.; O’Brien, M.K.; Jayaraman, A. Enhanced gait tracking measures for individuals with stroke using leg-worn inertial sensors. J. Neuroeng. Rehabil. 2024, 21, 219. [Google Scholar] [CrossRef]
- Silva, R.S.D.; Silva, S.T.D.; Cardoso, D.C.R.; Quirino, M.A.F.; Silva, M.H.A.; Gomes, L.A.; Fernandes, J.D.; Oliveira, R.A.N.D.S.; Fernandes, A.B.G.S.; Ribeiro, T.S. Psychometric properties of wearable technologies to assess post-stroke gait parameters: A systematic review. Gait Posture. 2024, 113, 543–552. [Google Scholar] [CrossRef]
- Song, Q.; Qin, Q.; Suen, L.K.P.; Liang, G.; Qin, H.; Zhang, L. Effects of wearable device training on upper limb motor function in patients with stroke: A systematic review and meta-analysis. J. Int. Med. Res. 2024, 52, 3000605241285858. [Google Scholar] [CrossRef]
- Rojek, I.; Mikołajewski, D.; Dostatni, E.; Macko, M. AI-Optimized Technological Aspects of the Material Used in 3D Printing Processes for Selected Medical Applications. Materials 2020, 13, 5437. [Google Scholar] [CrossRef] [PubMed]
- Rojek, I.; Mikołajewski, D.; Kopowski, J.; Kotlarz, P.; Piechowiak, M.; Dostatni, E. Reducing Waste in 3D Printing Using a Neural Network Based on an Own Elbow Exoskeleton. Materials 2021, 14, 5074. [Google Scholar] [CrossRef] [PubMed]
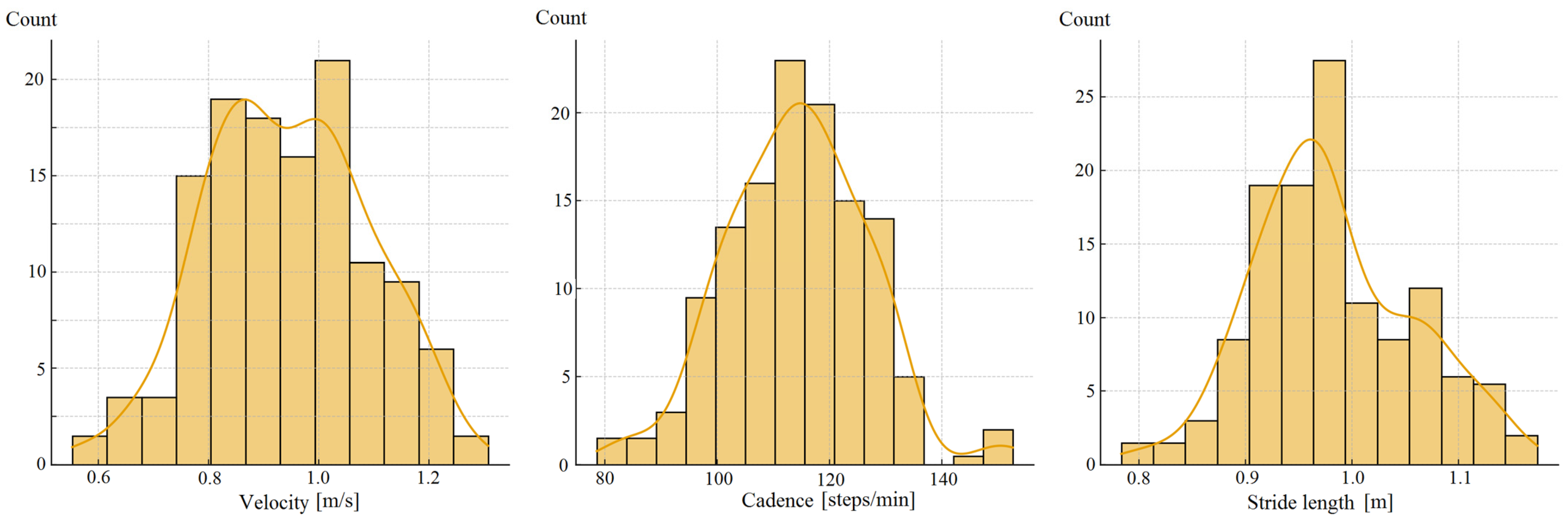
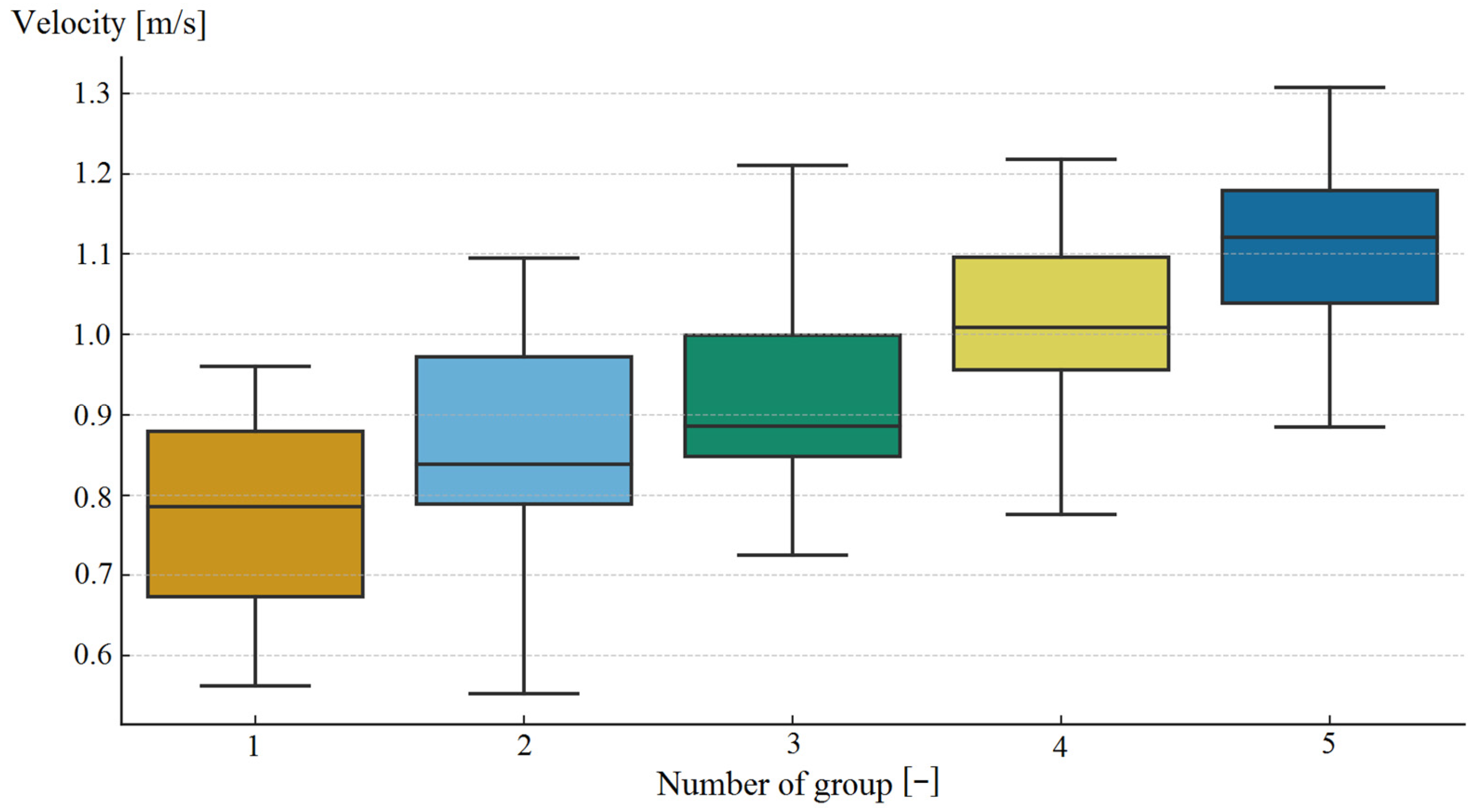
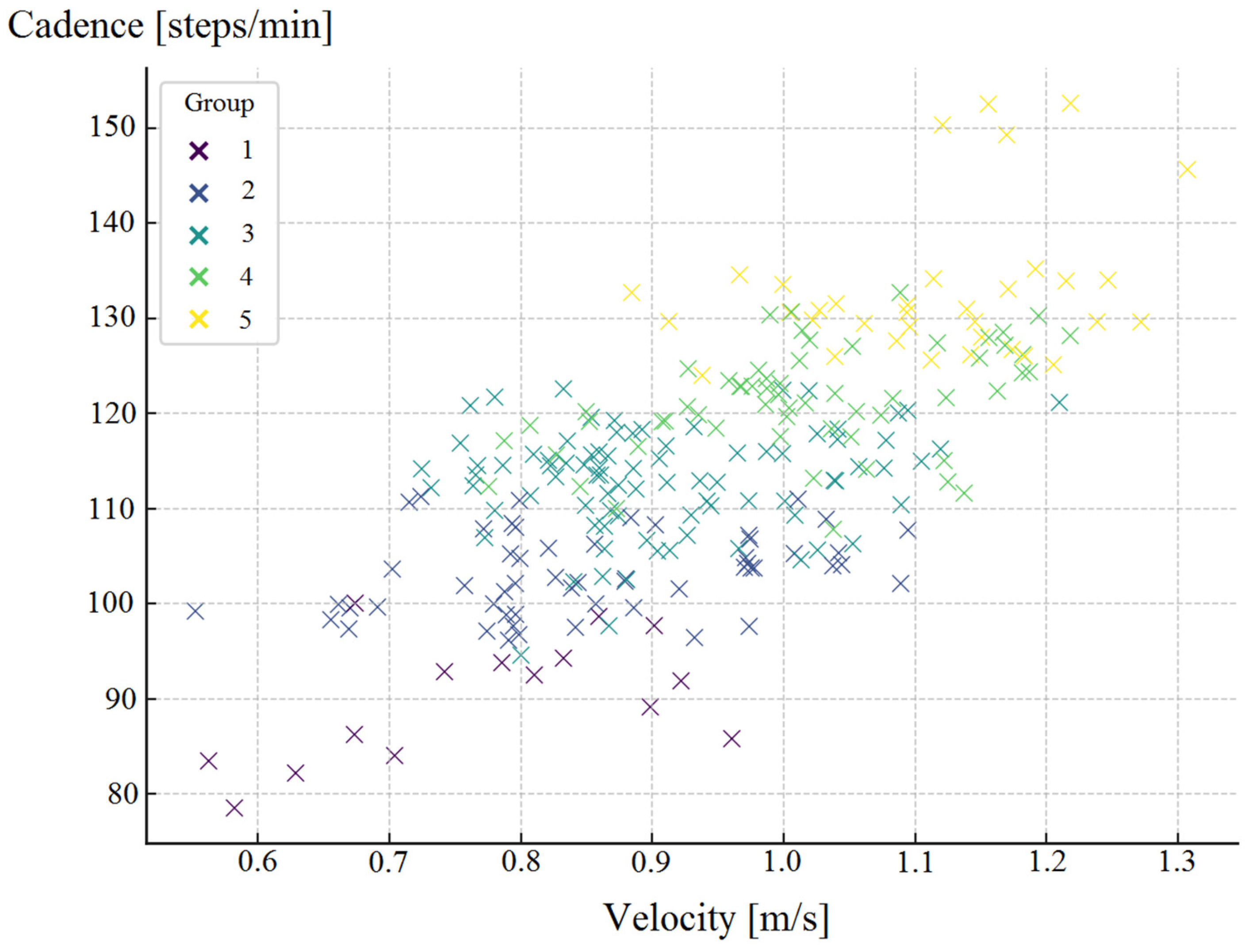
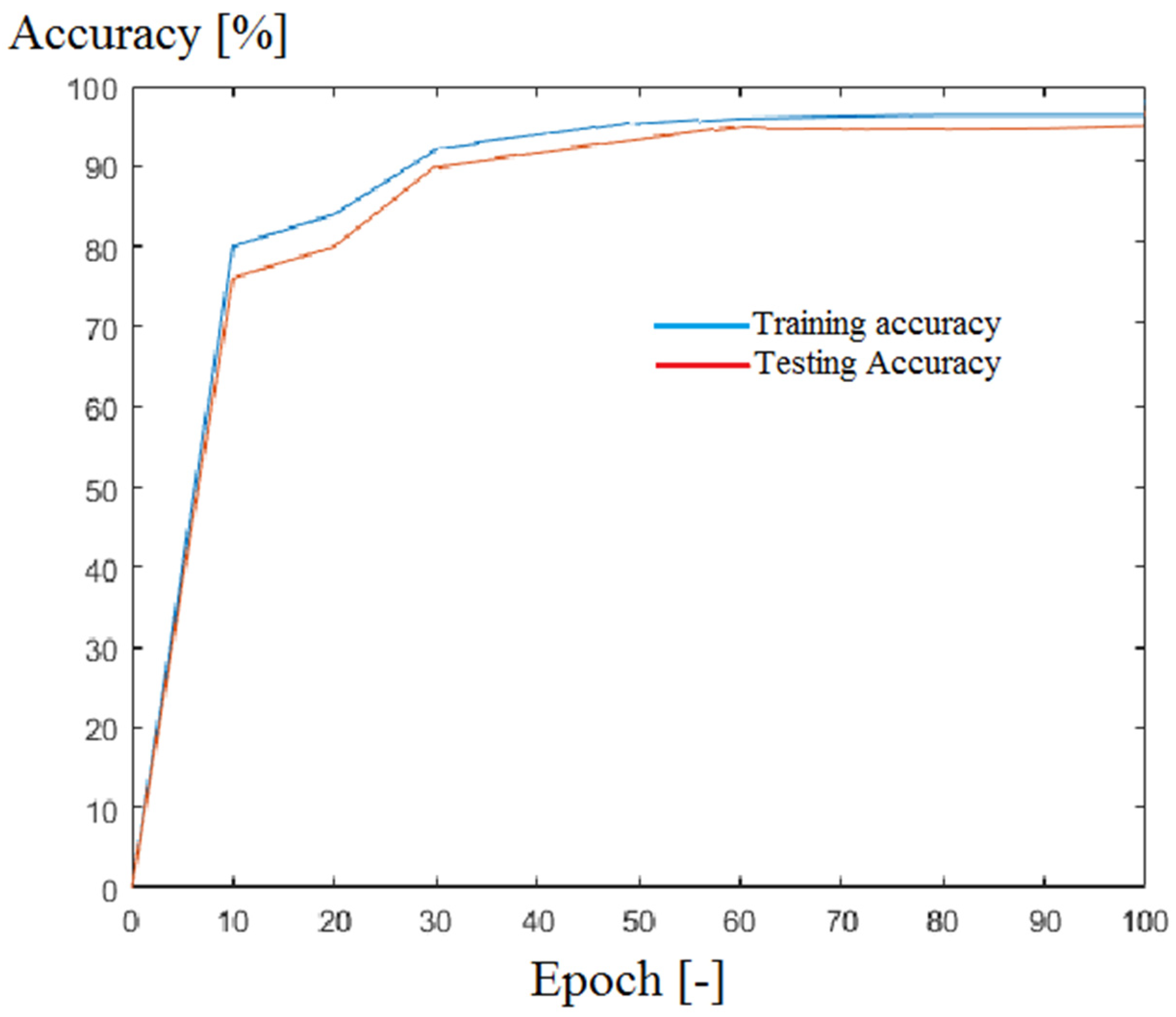
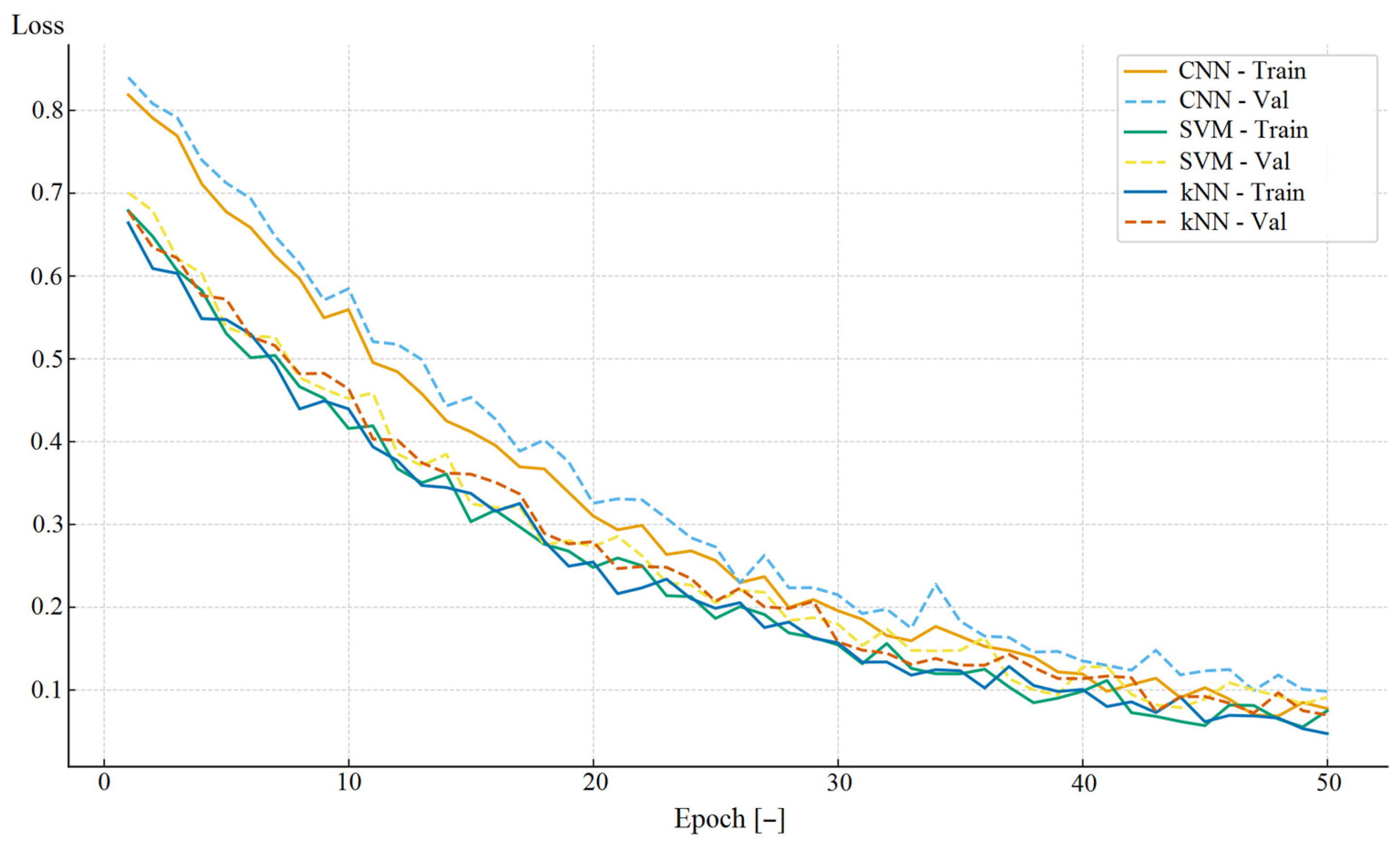
| Parameter | Study Group (n = 120) |
|---|---|
| Age [years] | |
| mean | 62.5 |
| SD | 8.91 |
| min | 40 |
| Q1 | 59 |
| Median | 64 |
| Q3 | 71 |
| Max | 81 |
| Gender | |
| male | 60 (50%) |
| female | 60 (50%) |
| Time after stroke [weeks] | |
| mean | 56.7 |
| SD | 7.98 |
| min | 15 |
| Q1 | 22 |
| Median | 61 |
| Q3 | 102 |
| Max | 143 |
| Side affected | |
| Left | 51 (42.50%) |
| Right | 69 (57.50%) |
| Subset | Number of Patients | Percentage of Total | Class 1 [%] | Class 2 [%] |
|---|---|---|---|---|
| Training | 84 | 70 | 50 | 50 |
| Validation | 12 | 10 | 50 | 50 |
| Testing | 24 | 20 | 50 | 50 |
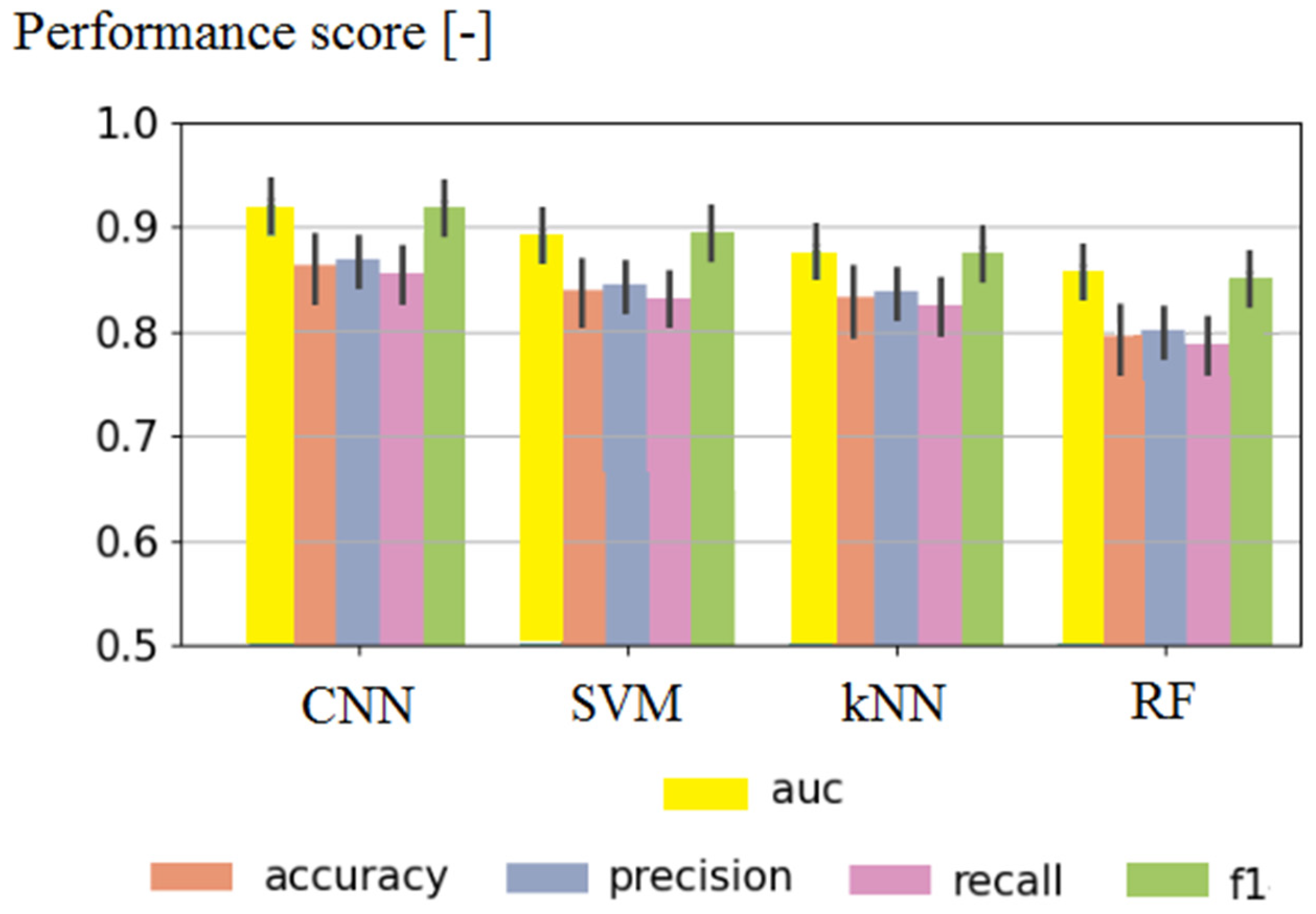
| Algorithm | Accuracy [%] | RMSE [-] |
|---|---|---|
| CNN | 91.88 | 0.001 |
| SVM | 89.91 | 0.01 |
| kNN | 87.52 | 0.02 |
| RF | 85.41 | 0.02 |
| Class | Precision | Recall | F1 | 95% CI (F1) |
|---|---|---|---|---|
| 1 | 91.12 | 91.12 | 91.88 | 0.88–0.94 |
| 2 | 91.02 | 91.02 | 91.56 | 0.88–0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojek, I.; Mikołajewska, E.; Małolepsza, O.; Kozielski, M.; Mikołajewski, D. Comparative Analysis of Different AI Approaches to Stroke Patients’ Gait Analysis. Appl. Sci. 2025, 15, 10896. https://doi.org/10.3390/app152010896
Rojek I, Mikołajewska E, Małolepsza O, Kozielski M, Mikołajewski D. Comparative Analysis of Different AI Approaches to Stroke Patients’ Gait Analysis. Applied Sciences. 2025; 15(20):10896. https://doi.org/10.3390/app152010896
Chicago/Turabian StyleRojek, Izabela, Emilia Mikołajewska, Olga Małolepsza, Mirosław Kozielski, and Dariusz Mikołajewski. 2025. "Comparative Analysis of Different AI Approaches to Stroke Patients’ Gait Analysis" Applied Sciences 15, no. 20: 10896. https://doi.org/10.3390/app152010896
APA StyleRojek, I., Mikołajewska, E., Małolepsza, O., Kozielski, M., & Mikołajewski, D. (2025). Comparative Analysis of Different AI Approaches to Stroke Patients’ Gait Analysis. Applied Sciences, 15(20), 10896. https://doi.org/10.3390/app152010896









