Characterization of Cantal and Salers Protected Designation of Origin Cheeses Based on Sensory Analysis, Physicochemical Characteristics and Volatile Compounds
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Selection
2.2. Physicochemical Analysis
2.3. Color
2.4. Volatile Compound Analysis
2.5. Sensory Analysis
2.5.1. Training Session
2.5.2. Evaluation Session
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics of Cheeses
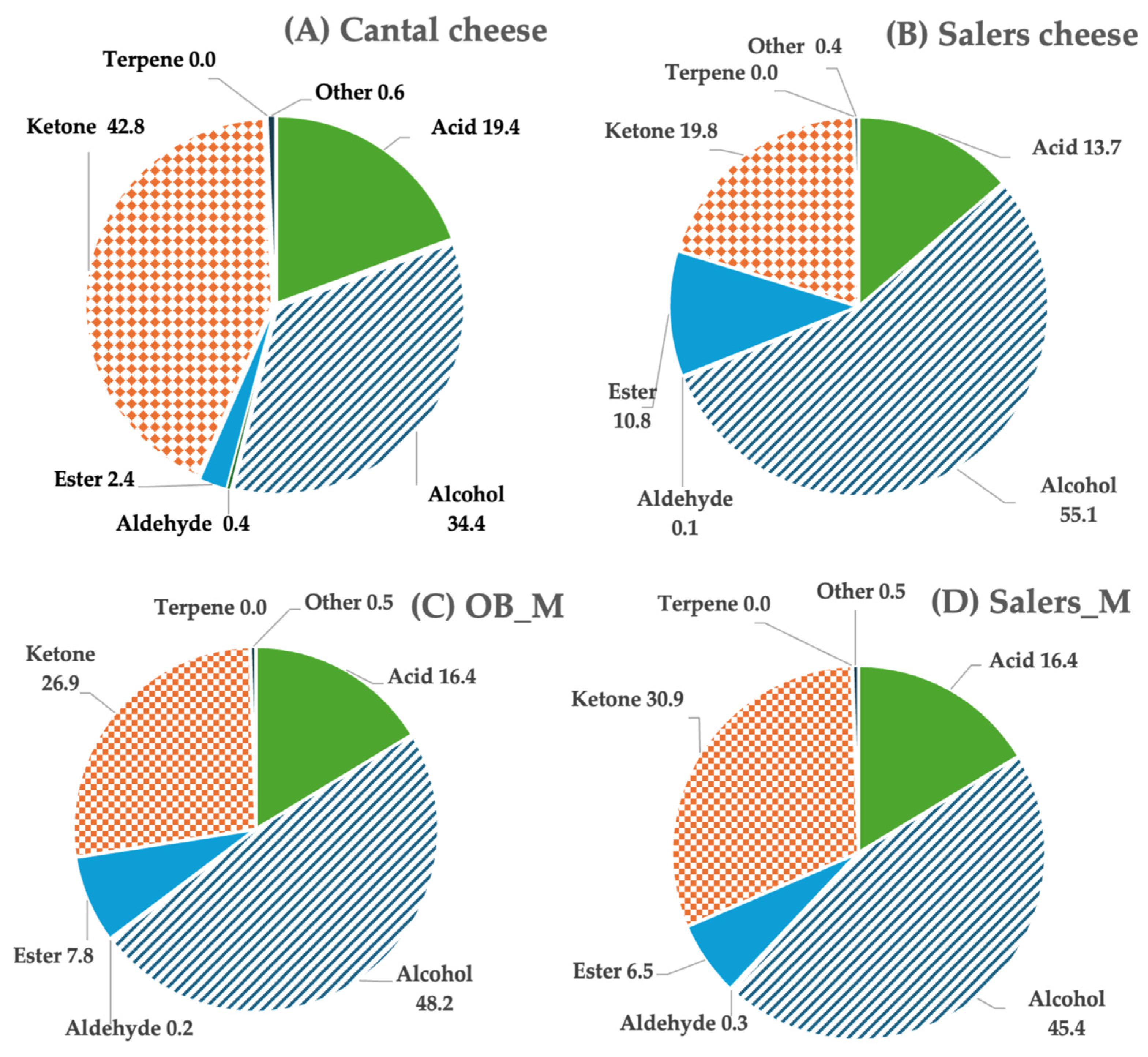
3.2. Volatile Compound Profiles in Cheeses
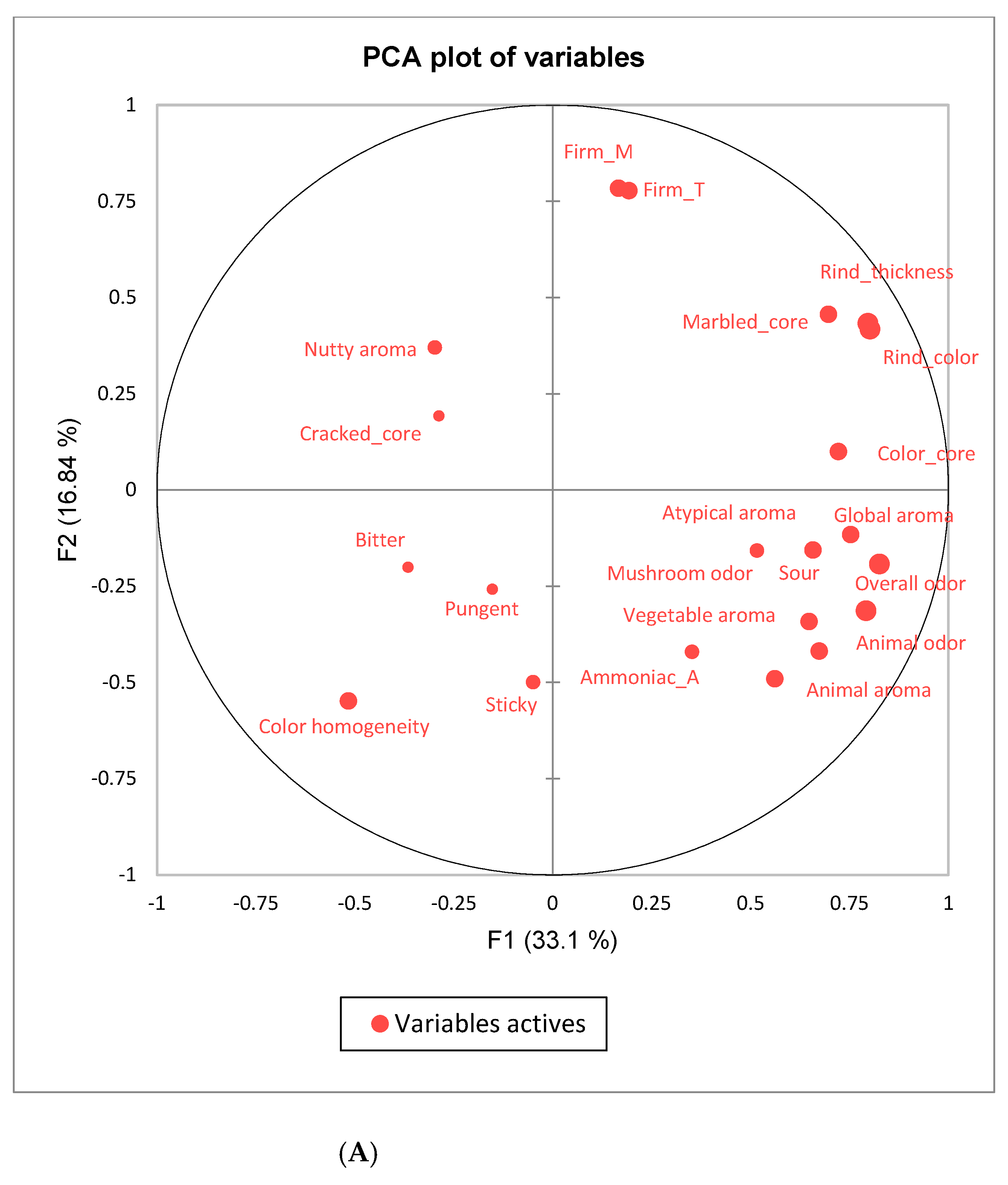
3.3. Sensory Properties of Cheeses
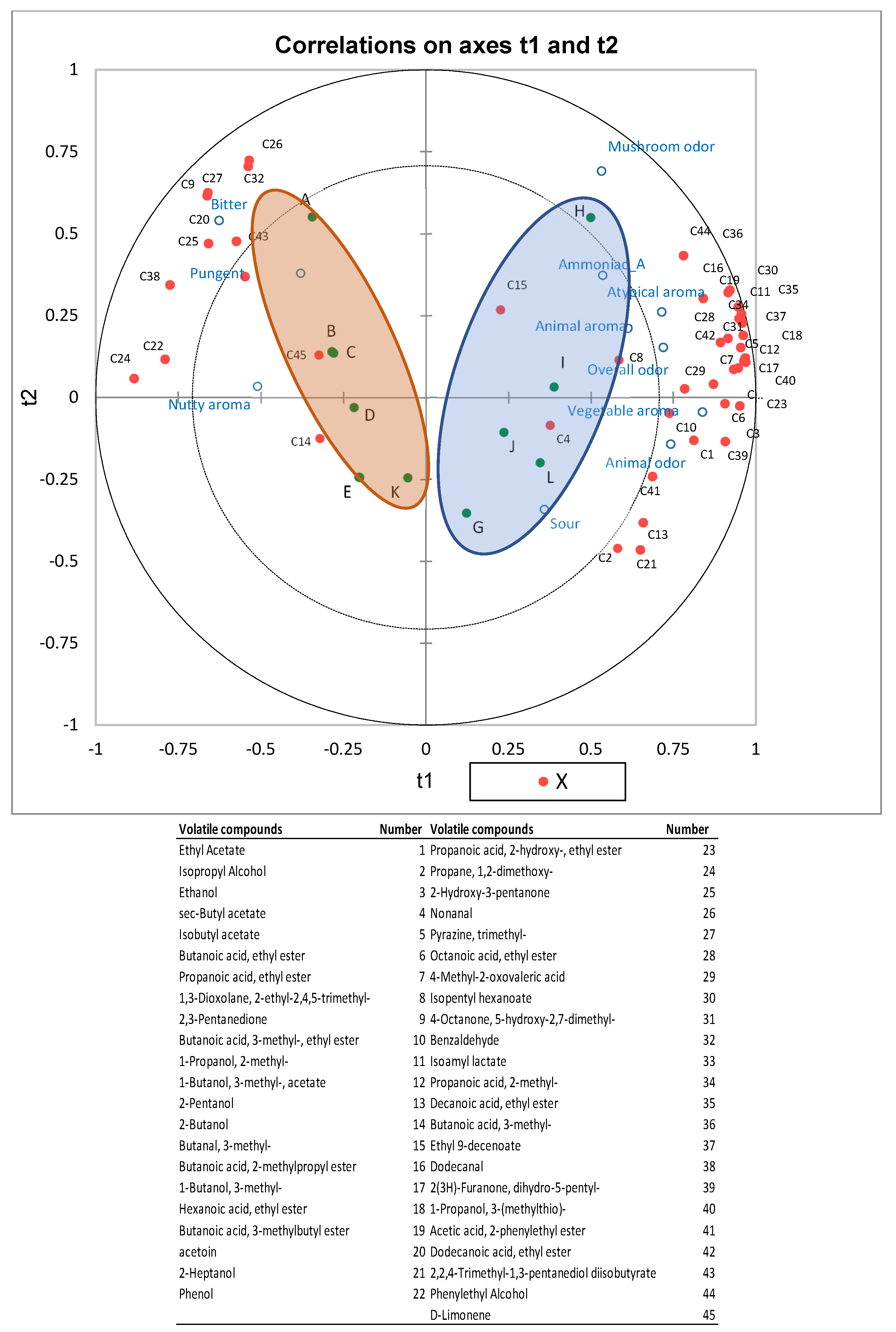
3.4. Correlations Between Volatile Compounds and Flavor Sensory Attributes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, P.; Fark, T.; de Wijk, R.A.; Roudnitzky, N.; Iannilli, E.; Seo, H.-S.; Hummel, T. Modulation of sensory perception of cheese attributes intensity and texture liking via ortho- and retro-nasal odors. Food Qual. Prefer. 2019, 73, 1–7. [Google Scholar] [CrossRef]
- Agabriel, J.; Faure, B.; Lebreton, F.X.; Lherm, M.; Micol, D.; Garcia-Launay, F.; Pradel, P.; Angeon, V.; Martin, B. La race bovine Salers: Un atout pour le développement de son territoire d’origine par son identité forte et des produits qualifiés. Cah. Agric. 2014, 23, 138–147. [Google Scholar] [CrossRef]
- Bérard, L.; Casabianca, F.; Montel, M.-C.; Agabriel, C.; Bouche, R. Salers Protected Designation of Origin cheese, France. The diversity and paradox of local knowledge in geographical indications. Cult. Hist. Digit. J. 2016, 5, e006. [Google Scholar] [CrossRef]
- Curioni PM, G.; Bosset, J.O. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- McSweeney PL, H.; Sousa, M.J. Biochemical pathways for the production of flavour compounds in cheeses during ripening: A review. Le Lait 2000, 80, 293–324. [Google Scholar] [CrossRef]
- Callon, C.; Berdagué, J.L.; Dufour, E.; Montel, M.C. The Effect of Raw Milk Microbial Flora on the Sensory Characteristics of Salers-Type Cheeses. J. Dairy Sci. 2005, 88, 3840–3850. [Google Scholar] [CrossRef] [PubMed]
- Choisy, C.; Demazeaud, M.; Gripon, J.C.; Lamberet, G.; Lenoir, J. The biochemistry of ripening. In Cheese Making: From Science to Quality Assurance, 2nd ed.; Eck, A., Gillis, J.C., Eds.; Lavoisier: Cachan, France, 2000; pp. 82–151. [Google Scholar]
- Coulon, J.-B.; Delacroix-Buchet, A.; Martin, B.; Pirisi, A. Relationships between ruminant management and sensory characteristics of cheeses: A review. Le Lait 2004, 84, 221–241. [Google Scholar] [CrossRef]
- Freitas, I.D.; Pinon, N.; Thierry, A.; Lopez, C.; Maubois, J.-L.; Lortal, S. In depth dynamic characterisation of French PDO Cantal cheese made from raw milk. Lait 2007, 87, 97–117. [Google Scholar] [CrossRef]
- Menci, R.; Martin, B.; Werne, S.; Bord, C.; Ferlay, A.; Lèbre, A.; Leiber, F.; Klaiss, M.; Coppa, M.; Heckendorn, F. Supplementing goats’ diet with sainfoin pellets (versus alfalfa) modifies cheese sensory properties and fatty acid profile. Int. Dairy J. 2022, 132, 105398. [Google Scholar] [CrossRef]
- Frétin, M.; Martin, B.; Buchin, S.; Desserre, B.; Lavigne, R.; Tixier, E.; Cirié, C.; Bord, C.; Montel, M.-C.; Delbès, C.; et al. Milk fat composition modifies the texture and appearance of Cantal-type cheeses but not their flavor. J. Dairy Sci. 2019, 102, 1131–1143. [Google Scholar] [CrossRef]
- Cornu, A.; Kondjoyan, N.; Martin, B.; Verdier-Metz, I.; Pradel, P.; Berdagué, J.-L.; Coulon, J.-B. Terpene profiles in Cantal and Saint-Nectaire-type cheese made from raw or pasteurised milk. J. Sci. Food Agric. 2005, 85, 2040–2046. [Google Scholar] [CrossRef]
- Lebecque, A.; Laguet, A.; Devaux, M.F.; Dufour, E. Delineation of the texture of Salers cheese by sensory analysis and physical methods. Le Lait 2001, 81, 609–624. [Google Scholar] [CrossRef]
- ISO 5534; Cheese and Processed Cheese—Determination of the Total Solid Content (Reference Method). ISO: Geneva, Switzerland, 2004.
- NF V04-287; Fromages—Détermination de la Teneur en Matière Grasse—Méthode Acido-Butyrométrique. AFNOR: St. Denis, France, 2019.
- ISO 8968-1; Milk and Milk Products—Determination of Nitrogen Content—Part 1: Kjeldahl Principle and Crude Protein Calculation. ISO: Geneva, Switzerland, 2014.
- Ferroukhi, I.; Bord, C.; Alvarez, S.; Fayolle, K.; Theil, S.; Lavigne, R.; Chassard, C.; Mardon, J. Functional changes in Bleu d’Auvergne cheese during ripening. Food Chem. 2022, 397, 133850. [Google Scholar] [CrossRef] [PubMed]
- NF ISO 5943/2007; Cheese and Processed Cheese Products—Determination of Chloride Content—Potentiometric Titration Method. ISO: Geneva, Switzerland, 2007.
- Caron, T.; Le Piver, M.; Péron, A.-C.; Lieben, P.; Lavigne, R.; Brunel, S.; Roueyre, D.; Place, M.; Bonnarme, P.; Giraud, T.; et al. Strong effect of Penicillium roqueforti populations on volatile and metabolic compounds responsible for aromas, flavor and texture in blue cheeses. Int. J. Food Microbiol. 2021, 354, 109174. [Google Scholar] [CrossRef]
- Işık, S.; Bozkurt, F.; Guner, S.; Işik, S.; Topalcengiz, Z. Microbiological, physicochemical, textural and volatile characteristics of traditional kashar cheese produced in Muş. Harran Tarım Gıda Bilim. Derg. 2020, 24, 409–419. [Google Scholar] [CrossRef]
- ISO 8586:2023; Sensory Analysis—Selection and Training of Sensory Assessors. ISO: Geneva, Switzerland, 2023.
- ISO 13299:2016; Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. ISO: Geneva, Switzerland, 2016.
- Bord, C.; Lenoir, L.; Schmidt-Filgueras, R.; Benoit, J.; Dechambre, G.; Chassard, C. Discrimination and sensory characterization of Protected Designation of Origin Salers- and Cantal-type cheeses: An approach using descriptive analysis and consumer insights by check-all-that-apply questions. J. Sens. Stud. 2021, 36, e12698. [Google Scholar] [CrossRef]
- Nozière, P.; Graulet, B.; Lucas, A.; Martin, B.; Grolier, P.; Doreau, M. Carotenoids for ruminants: From forages to dairy products. Anim. Feed. Sci. Technol. 2006, 131, 418–450. [Google Scholar] [CrossRef]
- Cozma, A.; Martin, B.; Cirié, C.; Verdier-Metz, I.; Agabriel, J.; Ferlay, A. Influence of the calf presence during milking on dairy performance, milk fatty acid composition, lipolysis and cheese composition in Salers cows during winter and grazing seasons. J. Anim. Physiol. Anim. Nutr. 2017, 101, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Manzocchi, E.; Martin, B.; Bord, C.; Verdier-Metz, I.; Bouchon, M.; De Marchi, M.; Constant, I.; Giller, K.; Kreuzer, M.; Berard, J.; et al. Feeding cows with hay, silage, or fresh herbage on pasture or indoors affects sensory properties and chemical composition of milk and cheese. J. Dairy Sci. 2021, 104, 5285–5302. [Google Scholar] [CrossRef]
- Bergamaschi, M.; Aprea, E.; Betta, E.; Biasioli, F.; Cipolat-Gotet, C.; Cecchinato, A.; Bittante, G.; Gasperi, F. Effects of dairy system, herd within dairy system, and individual cow characteristics on the volatile organic compound profile of ripened model cheeses. J. Dairy Sci. 2015, 98, 2183–2196. [Google Scholar] [CrossRef]
- Nogueira, M.C.L.; Lubachevsky, G.; Rankin, S.A. A study of the volatile composition of Minas cheese. LWT—Food Sci. Technol. 2005, 38, 555–563. [Google Scholar] [CrossRef]
- Meng, H.Y.; Piccand, M.; Fuchsmann, P.; Dubois, S.; Baumeyer, A.; Tena Stern, M.; von Ah, U. Formation of 3-Methylbutanal and 3-Methylbutan-1-ol Recognized as Malty during Fermentation in Swiss Raclette-Type Cheese, Reconstituted Milk, and de Man, Rogosa, and Sharpe Broth. J. Agric. Food Chem. 2021, 69, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Cornu, A.; Rabiau, N.; Kondjoyan, N.; Verdier-Metz, I.; Pradel, P.; Tournayre, P.; Berdagué, J.-L.; Martin, B. Odour-active compound profiles in Cantal-type cheese: Effect of cow diet, milk pasteurization and cheese ripening. Int. Dairy J. 2009, 19, 588–594. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Holland, R.; Crow, V.L. Esters and their biosynthesis in fermented dairy products: A review. Int. Dairy J. 2004, 14, 923–945. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.J.; Wang, Y.D.; Cao, Y.P.; Wang, B.; Liu, Y. The key aroma compounds and sensory characteristics of commercial Cheddar cheeses. J. Dairy Sci. 2021, 104, 7555–7571. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Díaz, R.; González-Córdova, A.F.; del Carmen Estrada-Montoya, M.; Méndez-Romero, J.I.; Mazorra-Manzano, M.A.; Soto-Valdez, H.; Vallejo-Cordoba, B. Volatile and sensory evaluation of Mexican Fresco cheese as affected by specific wild Lactococcus lactis strains. J. Dairy Sci. 2020, 103, 242–253. [Google Scholar] [CrossRef]
- Picon, A.; Gaya, P.; Fernández-García, E.; Rivas-Cañedo, A.; Ávila, M.; Nuñez, M. Proteolysis, lipolysis, volatile compounds, texture, and flavor of Hispánico cheese made using frozen ewe milk curds pressed for different times. J. Dairy Sci. 2010, 93, 2896–2905. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.F.; Singh, T.K.; McSweeney, P.L.H. Biogenesis of Flavour Compounds in Cheese. In Chemistry of Structure-Function Relationships in Cheese; Malin, E.L., Tunick, M.H., Eds.; Springer: New York, NY, USA, 1995; pp. 59–98. [Google Scholar] [CrossRef]
- Dahl, S.; Tavaria, F.K.; Xavier Malcata, F. Relationships between flavour and microbiological profiles in Serra da Estrela cheese throughout ripening. Int. Dairy J. 2000, 10, 255–262. [Google Scholar] [CrossRef]
- Carbonell, M.; Nuñez, M.; Fernández-García, E. Seasonal variation of volatile compounds in ewe raw milk La Serena cheese. Le Lait 2002, 82, 699–711. [Google Scholar] [CrossRef]
- High, R.; Eyres, G.T.; Bremer, P.; Kebede, B. Characterization of blue cheese volatiles using fingerprinting, self-organizing maps, and entropy-based feature selection. Food Chem. 2021, 347, 128955. [Google Scholar] [CrossRef] [PubMed]
- You, L.-Q.; Wang, Y.-R.; Bai, S.; Wang, X.-Y.; Wei, Z.-J. Impact of ripening periods on the key volatile compounds of Cheddar cheese evaluated by sensory evaluation, instrumental analysis and chemometrics method. Appl. Food Res. 2024, 4, 100578. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, J.; Shi, X.; Wang, B.; Zheng, X.; Zheng, X. Characteristic physicochemical indexes and flavor compounds in Xinjiang Kazak cheese during ripening. Food Biosci. 2020, 35, 100586. [Google Scholar] [CrossRef]
- Meinhart, E.; Schreier, P. Study of flavour compounds from Parmigiano Reggiano cheese. Study Flavour Compd. Parmigiano Reggiano Cheese 1986, 41, 689–691. [Google Scholar]
- Ayad, E.H.E.; Verheul, A.; de Jong, C.; Wouters, J.T.M.; Smit, G. Flavour forming abilities and amino acid requirements of Lactococcus lactis strains isolated from artisanal and non-dairy origin. Int. Dairy J. 1999, 9, 725–735. [Google Scholar] [CrossRef]
- Gutiérrez-Méndez, N.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Nevárez-Moorillón, G.V.; Rivera-Chavira, B. Evaluation of Aroma Generation of Lactococcus lactis with an Electronic Nose and Sensory Analysis. J. Dairy Sci. 2008, 91, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Engels, W.J.M. Volatile and Non-Volatile Compounds in Ripened Cheese: Their Formation and Their Contribution to Flavour. Ph.D. Thesis, Agricultural University, Wageningen, The Netherland, 1997. [Google Scholar]
- Lemieux, L.; Simard, R.E. Bitter flavour in dairy products. II. A review of bitter peptides from caseins: Their formation, isolation and identification, structure masking and inhibition. Le Lait 1992, 72, 335–385. [Google Scholar] [CrossRef]
- Sablé, S.; Cottenceau, G. Current Knowledge of Soft Cheeses Flavor and Related Compounds. J. Agric. Food Chem. 1999, 47, 4825–4836. [Google Scholar] [CrossRef] [PubMed]
- Molimard, P.; Spinnler, H.E. Review: Compounds Involved in the Flavor of Surface Mold-Ripened Cheeses: Origins and Properties. J. Dairy Sci. 1996, 79, 169–184. [Google Scholar] [CrossRef]
- Bugaud, C.; Buchin, S.; Hauwuy, A.; Coulon, J.-B. Relationships between flavour and chemical composition of Abondance cheese derived from different types of pastures. Le Lait 2001, 81, 757–773. [Google Scholar] [CrossRef]
- Buchin, S.; Delague, V.; Duboz, G.; Berdague, J.L.; Beuvier, E.; Pochet, S.; Grappin, R. Influence of Pasteurization and Fat Composition of Milk on the Volatile Compounds and Flavor Characteristics of a Semi-hard Cheese. J. Dairy Sci. 1998, 81, 3097–3108. [Google Scholar] [CrossRef]
- Zheng, X.; Shi, X.; Wang, B. A Review on the General Cheese Processing Technology, Flavor Biochemical Pathways and the Influence of Yeasts in Cheese. Front. Microbiol. 2021, 12, 703284. [Google Scholar] [CrossRef] [PubMed]
- Didienne, R.; Defargues, C.; Callon, C.; Meylheuc, T.; Hulin, S.; Montel, M.-C. Characteristics of microbial biofilm on wooden vats (‘gerles’) in PDO Salers cheese. Int. J. Food Microbiol. 2012, 156, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.M.; Sánchez-Palomo, E.; Pérez-Coello, M.S.; Cabezas, L. Volatile composition, olfactometry profile and sensory evaluation of semi-hard Spanish goat cheeses. Dairy Sci. Technol. 2008, 88, 355–367. [Google Scholar] [CrossRef]
- Ianni, A.; Bennato, F.; Martino, C.; Grotta, L.; Martino, G. Volatile Flavor Compounds in Cheese as Affected by Ruminant Diet. Molecules 2020, 25, 461. [Google Scholar] [CrossRef] [PubMed]
- Le Quéré, J.L.; Buchin, S. Cheese flavor. In Encyclopedia of Dairy Sciences; McSweeney, P.L.H., McNamara, J.P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 79–90. [Google Scholar]
- Urbach, G. Contribution of lactic acid bacteria to flavour compound formation in dairy products. Int. Dairy J. 1995, 5, 877–903. [Google Scholar] [CrossRef]
- Bergamini, C.V.; Wolf, I.V.; Perotti, M.C.; Zalazar, C.A. Characterisation of biochemical changes during ripening in Argentinean sheep cheeses. Small Rumin. Res. 2010, 94, 79–89. [Google Scholar] [CrossRef]
- Milesi, M.M.; Wolf, I.V.; Bergamini, C.V.; Hynes, E.R. Two strains of nonstarter lactobacilli increased the production of flavor compounds in soft cheeses. J. Dairy Sci. 2010, 93, 5020–5031. [Google Scholar] [CrossRef] [PubMed]
- Ismail, B.; Nielsen, S.S. Invited review: Plasmin protease in milk: Current knowledge and relevance to dairy industry. J. Dairy Sci. 2010, 93, 4999–5009. [Google Scholar] [CrossRef]
- Guiadeur, M.; Verdier-Metz, I.; Monsallier, F.; Agabriel, J.; Cirie, C.; Montel, M.-C.M.-C.; Martin, B. Traditional Milking of Salers Cows: Influence of Removing Calf on Cheese Making Ability of Milk in Comparison to Holstein Cows. 10. International Meeting on Mountain Cheese. 2011. Available online: https://hal.inrae.fr/hal-02748960 (accessed on 12 February 2024).
- Duthoit, F.; Callon, C.; Tessier, L.; Montel, M.-C. Relationships between sensorial characteristics and microbial dynamics in “Registered Designation of Origin” Salers cheese. Int. J. Food Microbiol. 2005, 103, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Garde, S.; Ávila, M.; Medina, M.; Nuñez, M. Influence of a bacteriocin-producing lactic culture on the volatile compounds, odour and aroma of Hispánico cheese. Int. Dairy J. 2005, 15, 1034–1043. [Google Scholar] [CrossRef]
- Qian, M.; Reineccius, G.A. Quantification of Aroma Compounds in Parmigiano Reggiano Cheese by a Dynamic Headspace Gas Chromatography-Mass Spectrometry Technique and Calculation of Odor Activity Value. J. Dairy Sci. 2003, 86, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Castada, H.; Hanas, K.; Barringer, S. Swiss Cheese Flavor Variability Based on Correlations of Volatile Flavor Compounds, Descriptive Sensory Attributes, and Consumer Preference. Foods 2019, 8, 78. [Google Scholar] [CrossRef] [PubMed]


| Cheese | Cow Breed | Producer/Dairy | Cheese Sample (Code) |
|---|---|---|---|
| Cantal | Salers 1 | Dairy 1 | A |
| Cantal | Dairy 1 | C | |
| Cantal | Other cow breeds 2 | Dairy 1 | D |
| Cantal | Dairy 1 | B | |
| Cantal | Producer 1 | E | |
| Cantal | Producer 2 | F | |
| Salers | Salers 1 | Producer 3 | G |
| Salers | Producer 4 | H | |
| Salers | Producer 5 | I | |
| Salers | Other cow breeds 2 | Producer 6 | J |
| Salers | Producer 7 | K | |
| Salers | Producer 8 | L |
| Parameters | Cheese Category | Cow Breeds’ Milk | Category × Breed | ||||
|---|---|---|---|---|---|---|---|
| Cantal | Salers | Significance | OB_M | Salers_M | Significance | Significance | |
| Dry matter-DM (%) | 62.11 ± 0.5 | 62.38 ± 0.6 | NS | 62.49 ± 0.4 | 61.91 ± 0.4 | NS | NS |
| Fat (%) | 29.33 ± 1.29 | 29.25 ± 1.04 | NS | 30.40 a ± 1.12 | 27.73 b ± 1.15 | *** | NS |
| Fat in DM (%) | 47.27 ± 2.47 | 46.83 ± 3.44 | NS | 48.67 a ± 1.83 | 44.78 b ± 2.56 | *** | NS |
| pH | 5.50 b ± 0.11 | 5.59 a ± 0.12 | * | 5.54 ± 0.12 | 5.56 ± 0.09 | NS | NS |
| Aw | 0.91b ± 0.02 | 0.94 a ± 0.01 | *** | 0.92 ± 0.03 | 0.93 ± 0.02 | NS | NS |
| Salt (%) | 2.34 ± 0.15 | 2.29 ± 0.24 | NS | 2.27 ± 0.14 | 2.37 ± 0.26 | NS | NS |
| Salt in DM (%) | 3.77 ± 0.28 | 3.67 ± 0.50 | NS | 3.63 ± 0.32 | 3.84 ± 0.49 | NS | NS |
| TN | 3.97 ± 0.21 | 4.12 ± 0.27 | NS | 3.97 b ± 0.24 | 4.15 a ± 0.19 | * | NS |
| WSN | 1.21 ± 0.11 | 1.23 ± 0.10 | NS | 1.23 ± 0.10 | 1.22 ± 0.12 | NS | NS |
| WSN/TN (%) | 30.62 ± 2.86 | 30.00 ± 3.02 | NS | 31.03 ± 2.92 | 29.29 ± 2.68 | NS | NS |
| L* (Lightness) | 78.60 a ± 4.13 | 73.20 b ± 3.00 | *** | 76.27 ± 5.14 | 75.37 ± 4.20 | NS | NS |
| a* (redness) | −2.75 b ± 0.53 | −2.37 a ± 0.50 | * | −2.56 ± 0.57 | −2.56 ± 0.51 | NS | NS |
| b* (yellowness) | 25.81 b ± 1.50 | 27.83 a ± 2.35 | *** | 27.35 a ± 2.28 | 26.07 b ± 1.94 | ** | *** |
| Chemical Family | Volatile Compounds | Cheese Category | Cow Breeds’ Milk | Category × Breed | ||||
|---|---|---|---|---|---|---|---|---|
| Cantal | Salers | Significance | OB_M | Salers_M | Significance | Significance | ||
| Acid | 4-Methyl-2-oxovaleric acid | 0.0 ± 0.0 | 3.4 ± 3.3 | ** | 1.9 ± 3.0 | 1.2 ± 2.6 | NS | NS |
| Acetic acid | 773.5 ± 474.5 | 894.9 ± 390.1 | NS | 889.9 ± 403.3 | 827.7 ± 478.8 | NS | * | |
| Butanoic acid | 321.3 ± 222.5 | 261.7 ± 132.6 | NS | 308.7 ± 180.0 | 298.4 ± 204.2 | * | NS | |
| Butanoic acid, 3-methyl- | 3.0 ± 1.8 | 13.0 ± 8.0 | *** | 8.6 ± 7.7 | 7.2 ± 8.6 | NS | NS | |
| Hexanoic acid | 59.6 ± 48.8 | 86.3 ± 47.9 | NS | 78.6 ± 47.5 | 67.1 ± 53.5 | NS | NS | |
| n-Decanoic acid | 2.7 ± 3.4 | 4.4 ± 2.7 | * | 3.9 ± 3.0 | 2.9 ± 3.1 | ** | NS | |
| Octanoic acid | 9.0 ± 7.4 | 14.4 ± 7.9 | * | 12.8 ± 7.4 | 10.3 ± 8.3 | NS | NS | |
| Pentanoic acid | 1.6 ± 0.7 | 1.5 ± 0.8 | NS | 1.6 ± 0.8 | 1.6 ± 0.8 | NS | NS | |
| Propanoic acid | 30.3 ± 32.1 | 59.0 ± 66.1 | NS | 46.6 ± 55.0 | 27.6 ± 27.6 | * | NS | |
| Propanoic acid, 2-methyl- | 2.8 ± 1.8 | 9.8 ± 4.5 | *** | 6.7 ± 4.9 | 5.4 ± 5.1 | NS | NS | |
| Alcohol | 1-Butanol | 118.2 ± 81.0 | 209.5 ± 293.7 | NS | 176.1 ± 221.6 | 110.1 ± 69.8 | NS | NS |
| 1-Butanol, 3-methyl- | 78.4 ± 77.5 | 2499.7 ± 904.4 | *** | 1403.1 ± 1393.9 | 1094.6 ± 1551.7 | ** | ** | |
| 1-Butanol, 3-methyl-, acetate | 12.2 ± 11.4 | 362.7 ± 165.9 | *** | 204.2 ± 214.5 | 156.8 ± 222.3 | NS | NS | |
| 1-Hexanol | 8.3 ± 3.9 | 23.2 ± 30.2 | NS | 16.6 ± 23.3 | 9.6 ± 4.1 | NS | NS | |
| 1-Octanol | 1.3 ± 0.6 | 1.7 ± 1.6 | NS | 1.6 ± 1.3 | 1.3 ± 0.5 | NS | NS | |
| 1-Penten-3-ol | 2.4 ± 2.8 | 0.8 ± 1.1 | * | 1.0 ± 0.9 | 2.0 ± 2.4 | * | NS | |
| 1-Propanol | 205.3 ± 122.3 | 195.8 ± 53.7 | NS | 207.2 ± 93.7 | 196.1 ± 100.4 | NS | NS | |
| 1-Propanol, 2-methyl- | 7.4 ± 1.5 | 533.2 ± 301.3 | *** | 294.3 ± 345.4 | 239.7 ± 378.9 | * | * | |
| 1-Propanol, 3-(methylthio)- | 0.0 ± 0.0 | 2.4 ± 1.3 | ** | 1.3 ± 1.5 | 0.9 ± 1.5 | NS | NS | |
| 2-Butanol | 1613.2 ± 450.3 | 1297.5 ± 572.1 | NS | 1503.1 ± 526.6 | 1513.7 ± 428.6 | NS | * | |
| 2-Heptanol | 8.1 ± 4.4 | 27.3 ± 16.0 | ** | 19.2 ± 14.9 | 13.2 ± 9.7 | NS | NS | |
| 2-Nonanol | 0.8 ± 0.9 | 1.6 ± 1.6 | NS | 1.2 ± 1.4 | 0.8 ± 1.1 | NS | NS | |
| 2-Pentanol | 65.9 ± 31.6 | 190.2 ± 83.2 | ** | 138.0 ± 85.6 | 93.2 ± 52.2 | NS | NS | |
| Ethanol | 7.3 ± 3.5 | 34.0 ± 13.9 | ** | 22.0 ± 17.1 | 18.1 ± 16.2 | NS | NS | |
| Ethanol, 2-(dodecyloxy)- | 1.3 ± 1.9 | 0.3 ± 0.6 | NS | 0.5 ± 1.3 | 0.9 ± 1.7 | NS | NS | |
| Isopropyl Alcohol | 0.6 ± 0.3 | 1.3 ± 0.7 | ** | 1.0 ± 0.6 | 0.8 ± 0.4 | NS | NS | |
| Phenol | 3.3 ± 1.3 | 0.0 ± 0.0 | *** | 1.5 ± 1.9 | 2.2 ± 1.9 | NS | NS | |
| Phenylethyl Alcohol | 0.3 ± 0.7 | 26.9 ± 25.1 | *** | 14.8 ± 22.7 | 13.0 ± 25.6 | NS | NS | |
| Aldehyde | Benzaldehyde | 4.4 ± 2.7 | 1.9 ± 0.6 | *** | 2.5 ± 1.0 | 3.6 ± 2.5 | * | NS |
| Butanal, 3-methyl- | 9.2 ± 2.6 | 10.2 ± 3.7 | NS | 9.6 ± 3.3 | 10.4 ± 3.1 | * | NS | |
| Dodecanal | 4.4 ± 2.1 | 0.0 ± 0.0 | *** | 2.0 ± 2.7 | 2.9 ± 2.7 | NS | NS | |
| Hexanal | 2.2 ± 4.1 | 0.0 ± 0.0 | ** | 0.2 ± 0.7 | 1.4 ± 3.4 | * | * | |
| Nonanal | 2.0 ± 0.7 | 1.3 ± 0.3 | *** | 1.5 ± 0.4 | 1.8 ± 0.6 | * | NS | |
| Ester | Ethyl 9-decenoate | 0.0 ± 0.0 | 2.5 ± 1.6 | *** | 1.3 ± 1.7 | 1.1 ± 1.7 | NS | NS |
| Ethyl Acetate | 14.9 ± 15.4 | 280.8 ± 172.5 | *** | 161.0 ± 183.6 | 117.4 ± 170.9 | NS | NS | |
| Acetic acid ethenyl ester | 39.0 ± 19.6 | 29.9 ± 22.4 | * | 31.6 ± 19.7 | 33.1 ± 18.9 | NS | *** | |
| Acetic acid, 2-phenylethyl ester | 0.0 ± 0.0 | 8.3 ± 7.7 | ** | 4.6 ± 7.0 | 2.7 ± 4.7 | NS | NS | |
| Butanoic acid, 1-methylpropyl ester | 17.0 ± 13.1 | 25.5 ± 16.6 | NS | 23.1 ± 14.6 | 20.8 ± 16.5 | NS | NS | |
| Butanoic acid, 2-methylpropyl ester | 0.0 ± 0.0 | 3.0 ± 2.3 | *** | 1.7 ± 2.2 | 1.2 ± 2.4 | NS | NS | |
| Butanoic acid, 3-methyl-, ethyl ester | 0.0 ± 0.0 | 2.2 ± 1.7 | *** | 1.2 ± 1.7 | 1.1 ± 1.7 | * | * | |
| Butanoic acid, 3-methylbutyl ester | 0.0 ± 0.0 | 25.1 ± 14.8 | *** | 13.7 ± 16.7 | 10.5 ± 18.1 | NS | NS | |
| Butanoic acid, ethyl ester | 20.6 ± 10.3 | 139.6 ± 72.4 | *** | 86.2 ± 79.9 | 61.3 ± 61.3 | NS | NS | |
| Decanoic acid, ethyl ester | 1.4 ± 0.6 | 15.8 ± 9.8 | *** | 9.3 ± 10.2 | 7.2 ± 10.1 | NS | NS | |
| Dodecanoic acid, ethyl ester | 0.0 ± 0.0 | 2.3 ± 1.4 | *** | 1.3 ± 1.5 | 0.9 ± 1.5 | NS | NS | |
| Hexanoic acid, 2-methylpropyl ester | 5.8 ± 4.2 | 11.9 ± 11.1 | NS | 9.5 ± 8.8 | 8.7 ± 9.3 | NS | NS | |
| Hexanoic acid, ethyl ester | 32.8 ± 22.2 | 306.5 ± 156.0 | *** | 183.7 ± 178.8 | 141.2 ± 172.0 | NS | NS | |
| Hexanoic acid, propyl ester | 2.3 ± 2.7 | 3.1 ± 1.2 | NS | 2.9 ± 2.1 | 2.5 ± 2.4 | NS | NS | |
| Isoamyl lactate | 0.0 ± 0.0 | 0.7 ± 0.4 | *** | 0.4 ± 0.5 | 0.3 ± 0.5 | NS | NS | |
| Isobutyl acetate | 0.0 ± 0.0 | 14.3 ± 7.4 | *** | 7.8 ± 9.0 | 6.5 ± 9.6 | * | ** | |
| Isopentyl hexanoate | 0.0 ± 0.0 | 4.7 ± 3.1 | *** | 2.5 ± 3.3 | 2.0 ± 3.6 | NS | NS | |
| Octanoic acid, 2-butyl ester | 1.4 ± 0.9 | 2.1 ± 1.7 | NS | 1.9 ± 1.4 | 1.7 ± 1.5 | NS | NS | |
| Octanoic acid, ethyl ester | 4.8 ± 3.5 | 69.0 ± 43.4 | *** | 40.1 ± 45.2 | 31.6 ± 45.8 | NS | NS | |
| Propanoic acid, 2-hydroxy-, ethyl ester | 0.0 ± 0.0 | 13.2 ± 8.2 | *** | 7.2 ± 9.0 | 5.5 ± 9.0 | NS | NS | |
| Propanoic acid, ethyl ester | 2.0 ± 1.9 | 35.9 ± 20.6 | *** | 20.5 ± 22.9 | 13.0 ± 17.6 | NS | NS | |
| sec-Butyl acetate | 6.9 ± 9.2 | 60.0 ± 51.9 | ** | 36.4 ± 46.3 | 24.4 ± 28.9 | NS | NS | |
| Ketone | 2,3-Pentanedione | 2.0 ± 1.8 | 0.0 ± 0.0 | *** | 0.6 ± 1.1 | 1.3 ± 1.7 | ** | ** |
| 2-Butanone | 1930.9 ± 161.7 | 1604.0 ± 847.9 | NS | 1766.4 ± 648.7 | 1792.1 ± 535.8 | NS | NS | |
| 2-Heptanone | 17.9 ± 13.3 | 18.8 ± 12.4 | NS | 19.0 ± 13.0 | 17.8 ± 13.4 | NS | NS | |
| 2-Hexanone | 0.8 ± 1.3 | 2.1 ± 1.7 | NS | 1.6 ± 1.6 | 1.0 ± 1.5 | NS | NS | |
| 2-Hydroxy-3-pentanone | 3.8 ± 1.8 | 1.1 ± 1.2 | *** | 2.2 ± 2.0 | 2.9 ± 2.2 | ** | *** | |
| 2-Nonanone | 9.9 ± 8.5 | 6.8 ± 2.2 | NS | 8.5 ± 6.6 | 9.1 ± 7.1 | NS | NS | |
| 2-Pentanone | 22.4 ± 16.7 | 31.3 ± 20.7 | NS | 27.6 ± 19.6 | 23.2 ± 19.6 | NS | NS | |
| 2-Propanone, 1-hydroxy- | 4.0 ± 1.5 | 2.9 ± 2.1 | NS | 3.3 ± 1.9 | 3.4 ± 1.6 | NS | * | |
| 2-Undecanone | 0.9 ± 0.5 | 1.0 ± 0.5 | NS | 1.0 ± 0.5 | 1.0 ± 0.6 | NS | NS | |
| 4-Octanone, 5-hydroxy-2,7-dimethyl- | 0.0 ± 0.0 | 4.5 ± 3.6 | *** | 2.5 ± 3.5 | 1.7 ± 3.3 | NS | NS | |
| 8-Nonen-2-one | 1.0 ± 1.0 | 1.0 ± 0.4 | NS | 1.1 ± 0.8 | 1.1 ± 0.9 | NS | NS | |
| Acetoin | 643.7 ± 470.1 | 254.4 ± 128.1 | *** | 389.5 ± 350.8 | 488.5 ± 441.8 | *** | *** | |
| Acetone | 14.3 ± 6.9 | 15.4 ± 8.1 | NS | 14.4 ± 7.5 | 13.8 ± 5.9 | NS | * | |
| Terpene | D-Limonene | 6.0 ± 11.2 | 0.4 ± 0.2 | NS | 3.4 ± 8.6 | 4.1 ± 9.4 | NS | NS |
| Other | 2(3H)-Furanone, dihydro-5-pentyl- | 0.0 ± 0.0 | 2.7 ± 0.7 | *** | 1.5 ± 1.5 | 0.9 ± 1.4 | NS | NS |
| 3-Methyl-2-(2-methyl-2-butenyl)-furan | 1.6 ± 1.9 | 2.4 ± 2.0 | NS | 2.2 ± 2.0 | 1.9 ± 2.3 | NS | NS | |
| 1,3-Dioxolane, 2-ethyl-2,4,5-trimethyl- | 0.0 ± 0.0 | 4.1 ± 6.0 | * | 2.3 ± 4.8 | 2.2 ± 5.2 | NS | NS | |
| 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 1.5 ± 0.3 | 1.3 ± 0.2 | ** | 1.3 ± 0.2 | 1.4 ± 0.3 | NS | NS | |
| 2-Butene | 24.1 ± 18.4 | 26.5 ± 12.1 | NS | 26.0 ± 15.7 | 25.3 ± 15.2 | NS | NS | |
| Dimethyl sulfone | 3.9 ± 1.4 | 4.0 ± 2.4 | NS | 4.0 ± 2.0 | 4.3 ± 2.0 | NS | * | |
| Propane, 1,2-dimethoxy- | 7.8 ± 2.2 | 0.9 ± 2.1 | *** | 4.1 ± 4.2 | 5.2 ± 4.2 | NS | * | |
| Pyrazine, trimethyl- | 0.5 ± 0.4 | 0.0 ± 0.0 | *** | 0.1 ± 0.2 | 0.3 ± 0.4 | *** | *** | |
| Sensory Attributes | Cheese Category | Cow Breeds’ Milk | Category × Breed | |||||
|---|---|---|---|---|---|---|---|---|
| Cantal | Salers | Significance | OB_M | Salers_M | Significance | Significance | ||
| APPEARANCE | Rind color | 5.3 b ± 2.2 | 7.2 a ± 1.6 | *** | 6.6 a ± 2.1 | 5.7 b ± 2.1 | *** | *** |
| Rind thickness | 4.9 b ± 2.2 | 6.9 a ± 1.8 | *** | 6.1 a ± 2.3 | 5.6 b ± 2.2 | *** | ** | |
| Color_core | 5.6 b ± 1.5 | 6.7 a ± 1.5 | *** | 6.1 ± 1.7 | 6.2 ± 1.5 | NS | *** | |
| Color homogeneity | 6.3 a ± 1.7 | 6.1 b ± 2.0 | ** | 6.1b ± 1.9 | 6.4 a ± 1.8 | ** | *** | |
| Marbled_core | 4.6 b ± 2.1 | 5.1 a ± 2.3 | *** | 5.1a ± 2.2 | 4.5 b ± 2.2 | *** | NS | |
| Cracked_core | 5.0 a ± 2.1 | 3.4 b ± 2.5 | *** | 4.5a ± 2.5 | 3.8 b ± 2.2 | ** | NS | |
| Firm_ in Touch | 6.8 a ± 1.6 | 6.4 b ± 1.9 | * | 7.0a ± 1.7 | 6.1 b ± 1.7 | *** | NS | |
| FLAVOR | Overall odor | 5.8 b ± 1.4 | 6.3 a ± 1.3 | *** | 6.0 ± 1.4 | 6.0 ± 1.3 | NS | NS |
| Animal odor | 3.0 b ± 2.1 | 3.8 a ± 2.1 | *** | 3.4 ± 2.2 | 3.4 ± 2.1 | NS | NS | |
| Mushroom odor | 1.6 b ± 1.8 | 1.8 a ± 1.9 | * | 1.6 ± 1.8 | 1.8 ± 1.8 | NS | NS | |
| Lactic_odor | 3.9 ± 2.3 | 3.7 ± 2.2 | NS | 3.8 ± 2.3 | 3.8 ± 2.2 | NS | NS | |
| Global aroma | 6.1 ± 1.4 | 6.3 ± 1.4 | NS | 6.2 ± 1.4 | 6.3 ± 1.4 | NS | NS | |
| Mushroom aroma | 1.8 ± 1.8 | 2.0 ± 2.0 | NS | 1.8 ± 1.8 | 2.0 ± 1.9 | NS | NS | |
| Vegetable aroma | 2.9 b ± 1.9 | 3.4 a ± 2.1 | *** | 3.1 ± 2.0 | 3.4 ± 2.1 | NS | NS | |
| Nutty aroma | 2.3 a ± 2.0 | 1.9 b ± 2.0 | *** | 2.1 ± 2.0 | 2.0 ± 2.2 | NS | * | |
| Animal aroma | 2.2 b ± 1.9 | 2.8 a ± 1.9 | *** | 2.3 b ± 1.9 | 2.8 a ± 1.9 | * | NS | |
| Ammoniac aroma | 1.3 ± 1.7 | 1.5 ± 2.1 | NS | 1.3 b ± 1.8 | 1.6 a ± 2.2 | * | NS | |
| Atypical aroma | 1.7 b ± 2.2 | 2.4 a ± 2.5 | *** | 1.9 ± 2.3 | 2.2 ± 2.4 | NS | NS | |
| Lactic aroma | 3.3 ± 2.2 | 3.2 ± 2.3 | NS | 3.3 ± 2.2 | 3.2 ± 2.3 | NS | NS | |
| Persistance | 5.6 ± 1.8 | 5.8 ± 2.0 | NS | 5.7 ± 1.9 | 5.8 ± 2.0 | NS | NS | |
| Salty | 6.5 ± 1.3 | 6.6 ± 1.3 | NS | 6.5 ± 1.3 | 6.7 ± 1.3 | NS | NS | |
| Sour | 3.3 b ± 2.1 | 3.8 a ± 2.4 | *** | 3.4 ± 2.2 | 3.7 ± 2.3 | NS | NS | |
| Bitter | 3.3 a ± 2.1 | 2.7 b ± 2.2 | *** | 3.0 ± 2.1 | 3.1 ± 2.2 | NS | ** | |
| Pungent | 3.5 a ± 2.6 | 3.1 b ± 2.6 | *** | 3.3 ± 2.6 | 3.4 ± 2.7 | NS | ** | |
| TEXTURE IN MOUTH | Firm_Mouth | 5.8 a ± 1.8 | 5.5 b ± 2.1 | * | 5.8 a ± 1.9 | 5.4 b ± 2.0 | ** | NS |
| Melty | 3.7 ± 2.0 | 3.9 ± 2.2 | NS | 3.8 ± 2.1 | 3.9 ± 2.1 | NS | NS | |
| Crumbly | 3.6 ± 2.1 | 3.6 ± 2.3 | NS | 3.7 ± 2.2 | 3.4 ± 2.1 | NS | NS | |
| Sticky | 3.8 ± 2.0 | 3.6 ± 1.8 | NS | 3.8 a ± 1.9 | 3.5 b ± 1.9 | * | NS | |
| Grainy | 3.8 ± 2.0 | 3.5 ± 2.1 | NS | 3.7 ± 2.1 | 3.6 ± 2.1 | NS | NS | |
| Fatty | 4.7 ± 1.8 | 4.9 ± 1.9 | NS | 4.8 ± 1.8 | 4.7 ± 1.9 | NS | NS | |
| Residue | 4.2 ± 2.3 | 4.1 ± 2.4 | NS | 4.2 ± 2.3 | 4.1 ± 2.4 | NS | NS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bord, C.; Lenoir, L.; Benoit, J.; Guérinon, D.; Dechambre, G.; Chassard, C.; Coelho, C. Characterization of Cantal and Salers Protected Designation of Origin Cheeses Based on Sensory Analysis, Physicochemical Characteristics and Volatile Compounds. Appl. Sci. 2025, 15, 961. https://doi.org/10.3390/app15020961
Bord C, Lenoir L, Benoit J, Guérinon D, Dechambre G, Chassard C, Coelho C. Characterization of Cantal and Salers Protected Designation of Origin Cheeses Based on Sensory Analysis, Physicochemical Characteristics and Volatile Compounds. Applied Sciences. 2025; 15(2):961. https://doi.org/10.3390/app15020961
Chicago/Turabian StyleBord, Cécile, Louis Lenoir, Julie Benoit, Delphine Guérinon, Gilles Dechambre, Christophe Chassard, and Christian Coelho. 2025. "Characterization of Cantal and Salers Protected Designation of Origin Cheeses Based on Sensory Analysis, Physicochemical Characteristics and Volatile Compounds" Applied Sciences 15, no. 2: 961. https://doi.org/10.3390/app15020961
APA StyleBord, C., Lenoir, L., Benoit, J., Guérinon, D., Dechambre, G., Chassard, C., & Coelho, C. (2025). Characterization of Cantal and Salers Protected Designation of Origin Cheeses Based on Sensory Analysis, Physicochemical Characteristics and Volatile Compounds. Applied Sciences, 15(2), 961. https://doi.org/10.3390/app15020961






