1. Introduction
In vitro analyses of behavioral, physiological and morphological cell responses are often a preliminary to in vivo studies. The objective of the present study was the combinatorial use of new methods to characterize cell modifications in response to a given treatment. To this purpose, we have studied the effects of cell exposure to reduced glutathione (GSH) on an olfactory neuroblast cell line (13s24).
Glutathione holds a special place among antioxidant molecules: it is the most abundant small molecular weight thiol compound of living cells [
1]. Although it is a small molecule composed of only three amino acids, it cannot enter directly inside cells. It is broken down in the extracellular space by the enzyme ectoprotein γ-glutamyl transpeptidase to produce dipeptides and free amino acids that are then absorbed by cells to intracellularly resynthesize GSH [
2,
3]. Reduced glutathione (GSH) is a well-known direct antioxidant and a substrate of several antioxidant enzymes [
4]. Glutathione participates to numerous cellular regulations: antioxidant protection, thiol–disulfide exchange of peptides and proteins, cell signaling, gene expression, detoxification of toxic compounds, etc. [
5]. Studies have shown that it is excreted by cells such as liver cells [
6], lymphoid cells [
7], gallbladder [
8], and cancer cells [
9], supporting the hypothesis of local extracellular activities. Specific transport of GSH through the blood–brain barrier has been described [
10]. Glutathione is abundant in the brain (in the range of millimolar) and exerts a protective effect against oxidative stress in neurons [
11,
12]. The endogenous production of oxidative stress is considered the main endogenous factor of aging, and dysregulation of glutathione homeostasis has been linked to loss of neurons in age-related diseases such as Parkinson’s, Alzheimer’s, and Huntington’s [
13]. In a recent study, we demonstrated that both adhesion strength and cell volume are directly regulated by the extracellular GSH concentrations [
14]. Both cell adhesion and reactive oxygen species (ROS) play a crucial role in neurogenesis through regulation of proliferation and differentiation [
15,
16]. Because GSH plays a crucial role as an antioxidant and causes marked cellular changes, cell exposure to GSH is a suitable situation for testing new technological approaches.
In the present study, experiments were carried out on a non-cancerous immortalized cell line representing sensory olfactory neuron precursors (13s24) [
17]. This study of olfactory neuroblasts is of particular interest since (1) the olfactory epithelium is the only location where the brain is in direct contact with the environment and thus exposed to partial oxygen pressure and potential oxidative stress higher than other brain cells, and (2) olfactory sensory neurons are continuously renewed during live from olfactory precursors (neuroblasts) [
18].
For this purpose, we performed a combinatorial study of cell physiological and morphological properties using highly sensitive methods to measure in real-time experiments, adhesion strength (xCELLigence), physical parameters (cell surface occupancy, size, and cell shape), cellular behavior such as motility (HoloMonitor) and changes in refractive index within the cells (Nanolive). The effects on size and shape were validated, after optimized time of exposure with GSH, using fluorescent markers by flow cytometry. Results show that GSH induced both a reduction in adhesion strength and an increase in cell volume. These findings reveal an unexpected role of GSH in the regulation of cell volume and adhesion strength, suggesting that each cell is able to control its own volume. Although the methods studied measure different parameters, their main interest lies in the fact that they are in real-time and do not require marking.
2. Materials and Methods
2.1. Cell Cultures
Rat 13s24 olfactory neuroblasts were grown in Dulbecco’s Modified Eagle Medium with low glucose (1 g/L) containing 10% fetal calf serum and streptomycin plus penicillin (100 units/mL; Sigma Aldrich, St Quentin-Fallavier, France) at 37 °C with 5% CO
2 in a cell culture incubator, SL ShelLab (Sheldon Manufacturing, Inc., Cornelius, NC, USA). Before experiments, cells were dissociated by trypsin (0.25%, Sigma Aldrich) and cell concentration titrations were performed using a Sceptor sensor with 60 µm pipet tips (Millipore, Burlington, VT, USA). Cells were seeded at 10 000 cells/cm
2 in either E-96 plates (ACEA, Agilent Technology, Santa Clara, CA, USA), 96-well plates (Corning, Thermo Fisher Scientific, Illkirch, France), or Ibidi
® glass bottom dishes with inserts (Clinisciences, Nanterre, France). Cells were grown during 48 h before treatments because in previous studies, as we pointed out, using nanometric nanoparticles, that plasma membrane integrity was not fully restored 24 h after dissociation by trypsin [
19]. Freshly prepared stock solutions of reduced glutathione (GSH, Sigma Aldrich) with pH adjusted at 7.4 were filtered at 0.2 μm and serial dilutions were performed in cell culture media.
2.2. Real-Time Cell Analysis of Impedance
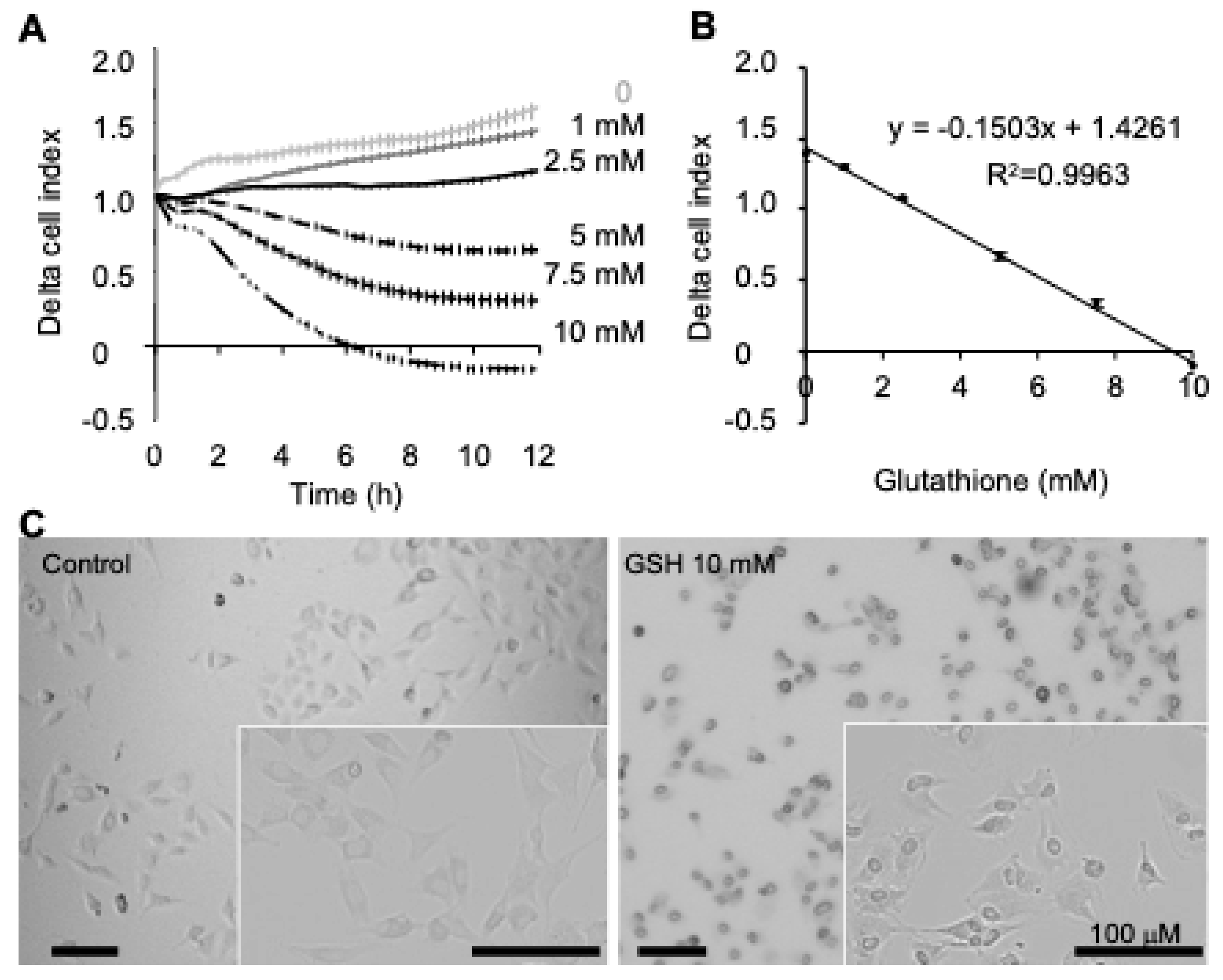
The real-time cell analysis xCELLigence single-plate biosensor (RTCA, ACEA, Agilent) measures cell surface occupancy (i.e., by impedance measurement), expressed as cell index. Cell index depends on cell number, cell size, and cell adhesion force. Cells were grown until the cell index reached around 1 (at least 48 h), i.e., they were in a linear proliferative phase. Then, cells were exposed to fresh culture medium containing different concentrations of GSH (100 µL/well). The impedance was recorded for each well every 15 min and data were retrieved using RTCA 2.01 (ACEA). Results are represented by mean delta cell indexes (DCIs) ± SEM, (n = 6 to 8 replicates), i.e., the cell index at a given time point plus a delta value. The delta value is a constant value for each well and is the difference between the DCI value at time of treatment (t0) and the cell index at the delta time point from t0, as follows: DCItime = CItime + (DCI t0 − CIDelta time). Data were retrieved on Microsoft Excel for analyses and graphical presentation of results.
2.3. Quantitative Phase Imaging
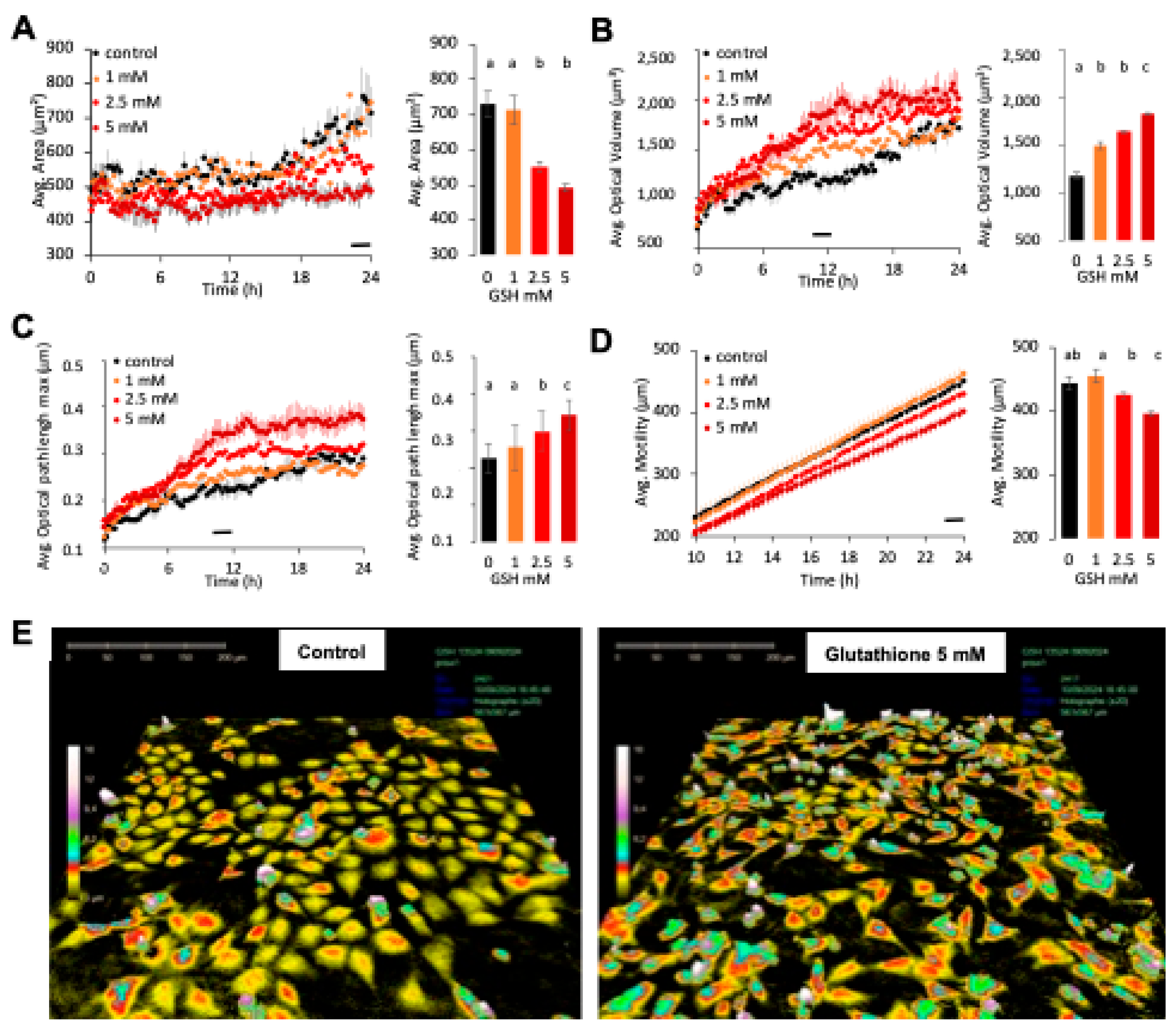
Quantitative phase imaging experiments were performed using the HoloMonitor M4 digital holographic cytometer (Phase Holographic Imaging, PHI, Lund, Sweden). The microscope was housed in cell culture incubator Heracell (Heraeus, Thermo Fischer Scientific) at 37 °C with 5% CO2. Cells were grown during 48 h in 96-well plates and treated with reduced glutathione in 150 µL/well. HoloLids were used to insure an optimum optic signal. More than 30 morphological cell parameters were measured in real time every fifteen minutes for twenty-four hours after treatment with GSH (0 to 5 mM) using the software program HoloStudio M4 2.6.2 and retrieved on Microsoft Excel for analyses and graphical presentation of results. Volume is the optical cell volume. It is based on area and optical thickness.
2.4. Holotomographic Microscopy Time-Lapse Acquisition
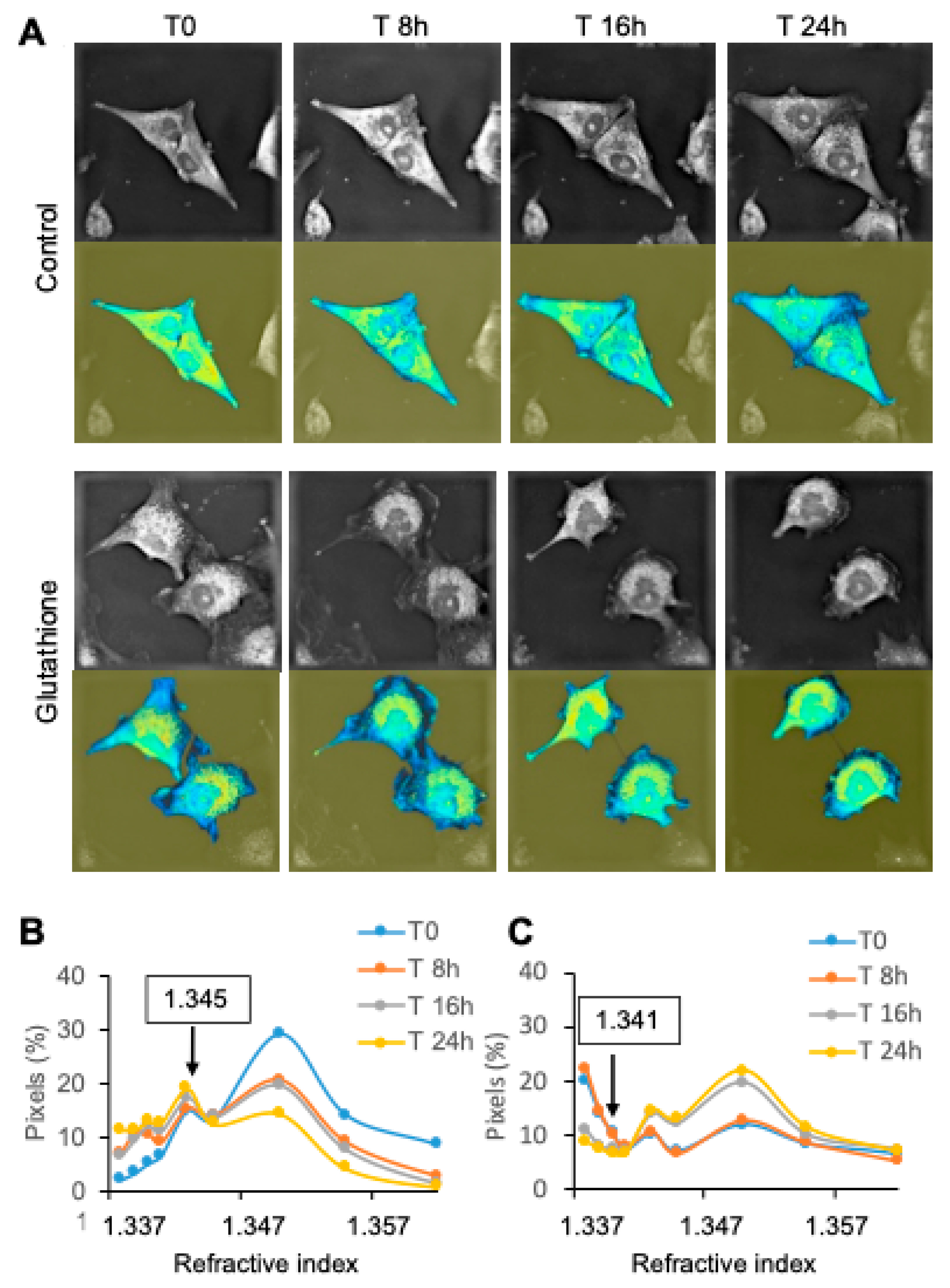
Label-free images were acquired using a Nanolive 3D Cell Explorer microscope (Nanolive SA, Lausanne, Switzerland). A green light laser diode (520 nm) was split into sample and reference beams. Cells grown on IBIDI
® dishes (35 mm diameter) were placed in a heated lid and heated plate (Ibidi), between a high numerical aperture air objective (60× magnification) beneath the sample and a low-power laser (0.2 mW/mm
2) reflected by a mirror on a rotational arm above. The samples were illuminated with the laser beam inclined at 45° rotating 360° around the samples. Each rotation of the laser beam around the sample allows for the capture of one hundred holograms, recorded via a digital camera (CMOS) by combining the beam that had passed through the sample with the reference beam. High-resolution images (∆xy = 200 nm; ∆z = 400 nm) of each sample layer were produced using a synthetic aperture and multiple-viewpoint holographic methods. The software displays a comprehensible 96 z-stack cell image in grayscale for a depth of field of 30 µm. Images were captured using STEVE
® software (Nanolive) to obtain a refractive index (RI)-based z-stack. The microscope is equipped for long-term live cell imaging: temperature, humidity, and gas composition (Ibidi). Samples were kept at 37 °C under a closed heated glass lid to prevent condensation, which was connected to a gas mixer to maintain 5% of CO
2. Raw data were transferred into FIJI, an open-source platform dedicated to biological image analysis [
20]. Additionally, cells were colored according to refractive index to quantitative phase imaging in order to underline differences [
21].
2.5. Fluorescence Imaging and Cell Sorting by Flow Cytometry
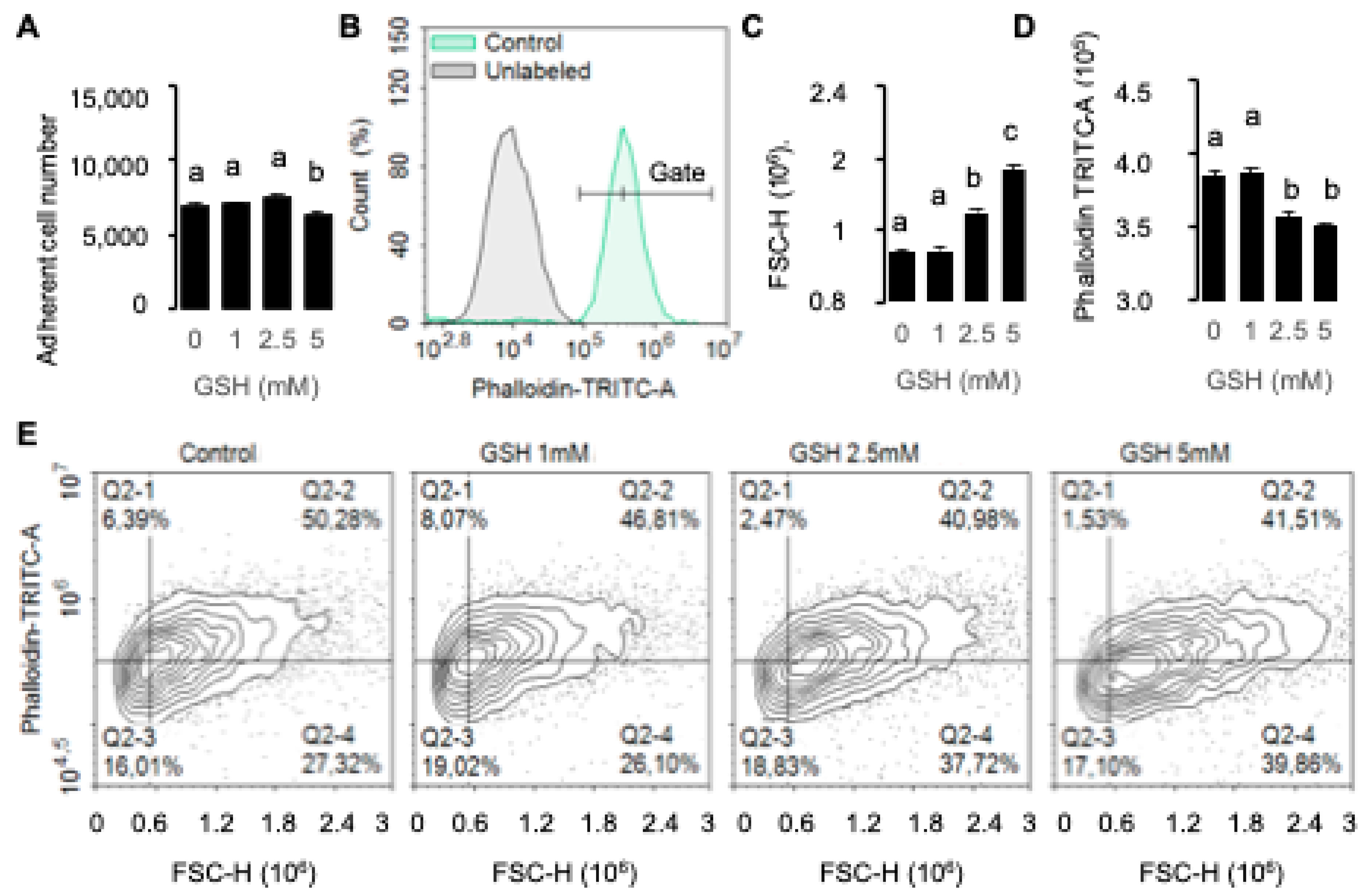
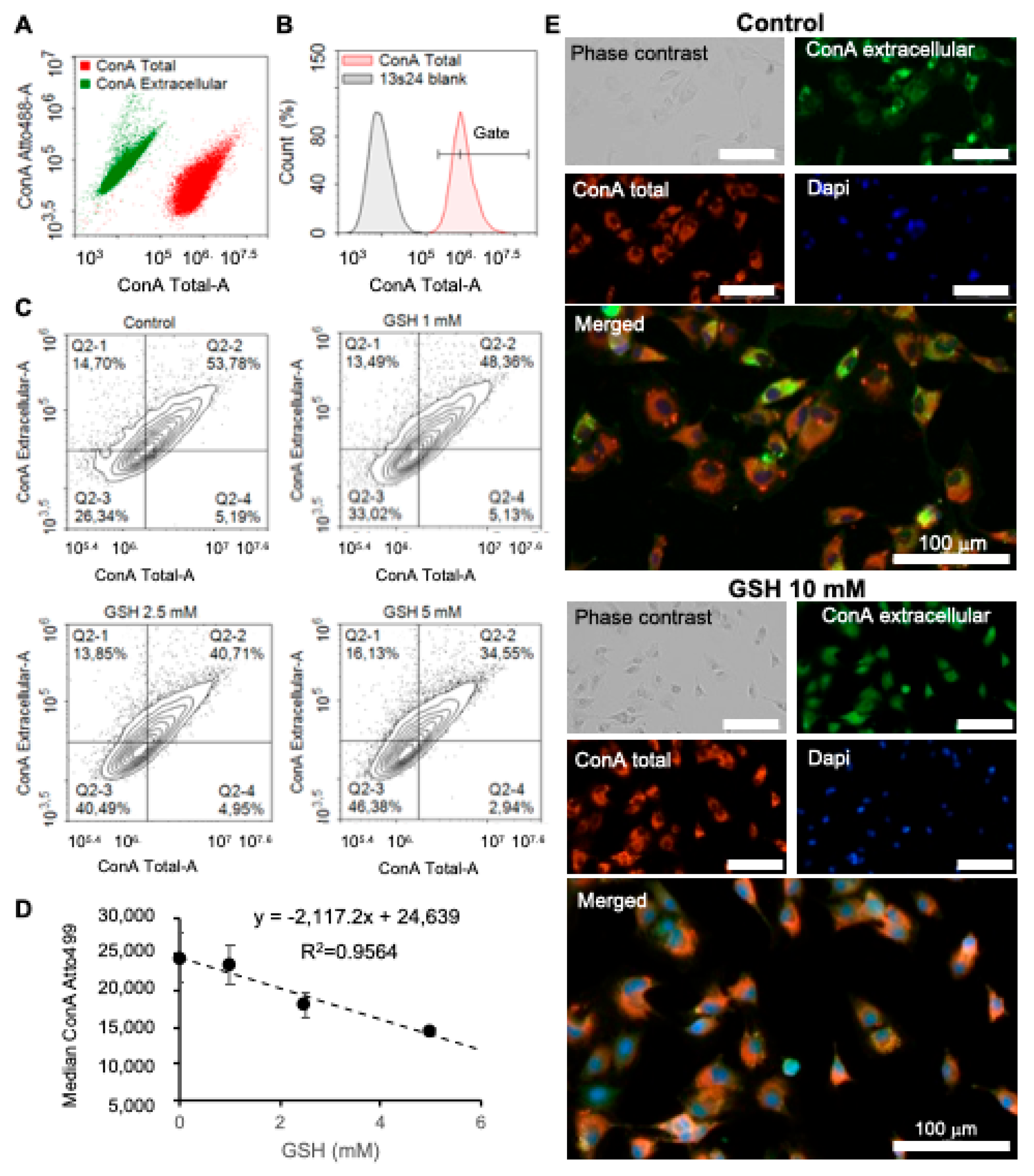
Cell morphological changes and counts induced after GSH treatments were assessed using fluorescent markers for both cell imaging and cell sorting. Images were obtained on a Cytation 3 reader (Biotek Instrument, Agilent) at objectives ×4 or ×20. Cell sorting analyses were obtained with a Novocyte cytometer (Acea Biosciences, San Diego, CA, USA) and data were retrieved using NovoExpress Software1.2.5. Cytoskeleton modifications were analyzed by quantification of polymerized F-actin using fluorescent phalloidin incorporation. At the end of the experiment, cells were dissociated using trypsin, fixed with PBS/formalin 3% (Sigma Aldrich), and then incubated for at least 15 min with PBS, Triton 0.1%, and phalloidin–TRITC (Sigma Aldrich) 10 nM and washed in PBS before analysis. Cell count and fluorescence analyses were performed in at least 4 replicates. Effects on the extracellular matrix were analyzed using glycoprotein-interacting lectin concanavalin A from Canavalia ensiformis conjugated with either Atto488 (FITC filters, ConA Extracellular) or Alexa Fluor 594 (TRITC filters, ConA Total) at 50 μg/mL for cell sorting and 500 μg/mL for cell imaging. After treatments, 13s24 cells were fixed with 5% paraformaldehyde (PFA) (Biotium, Ozyme, Saint-Cyr-l’École, France). A first labeling step without permeabilization was performed in order to quantify extracellular glycosylated sites with ConA-Atto488 in Hank’s Balanced Salt Solution (HBBS; Gibco, Sigma Aldrich) for at least 30 min, then washed twice in HBBS before incubation with ConA AlexaFluor 594 and 0.1%Triton to quantify full glycoproteins in permeabilized cells. Cells were counterstained with Dapi 10 µg/mL for cell imaging (Sigma Aldrich). After two washes in HBSS, ConA fixation was performed using 4% PFA. Cell imaging data were obtained on the Cytation 3 platform using the ×20 objective with identical acquisition parameters (Gen5.03 software).
2.6. Statistical Analyses
The experiments were performed in 6 to 8 biological replicates for the RTCA and HoloMonitor 96-well plate and presented as mean values ± standard error of the mean (SEM). For cytometry analyses, data are presented as the result of mean values ± standard error (4 replicates) or SEM (5 to 6 replicates). Statistical analyses were obtained with StatView 4.5 software (Abacus Corporation, Baltimore, MD, USA) for Windows. The data were analyzed using one-way ANOVA followed by Fisher’s protected least significant difference [PLSD] post-hoc test. Statistical significance was defined as p-values < 0.05. In the figures, different letters indicate significant differences. Significance of correlation was established from the correlation coefficient (r) according to Pearson table, for p-values < 0.05.
4. Discussion
We used different methods to characterize the effects of GSH on 13s24 neuroblasts: cell adhesion (real-time monitoring of impedance on the xCELLigence sensor and flow cytometry analysis with specific markers), cell behavior (time-lapse cytometry on the HoloMonitor microscope), and cell morphology (time-lapse 3D Nanolive refractive index imaging). Of course, the higher the number of analytical methods used, the better the description of GSH effects on cells. However, preliminary distinctions can be made, especially regarding the number of cells analyzed (
Table 1).
The microscopy techniques were performed on a small number of cells (a few cells for Nanolive; a few dozen cells for HoloMonitor), a few thousand cells for xCELLigence, and several tens of thousands for cytometry. Another important difference is the time required to complete the analysis of results. Microscopy is time-consuming (HoloMonitor, Nanolive), while, even including the time needed for labeling or preparing cells, other methods are much faster (xCELLigence, cytometry). Another important distinction is the skill required to carry out measurements, although most device software is user-friendly. For example, xCELLigence is very sensitive in the measurement of cell index; however, precise cell counting and accurate pipetting of volumes are required during E-plate filling; otherwise, the results will be scattered. Cytometry allows for the analysis of a large number of cells and the multi-parametric analysis of a single cell using complex labeling strategies that are highly time-consuming, especially for the characterization of the combinatorial assemblages regulating cell adhesion.
The molecular and cellular mechanisms involved in cell adhesion have been extensively studied [
23]; however, they have been poorly studied in real-time experiments. In the present study, we used three recently developed time-lapse instruments, allowing for complementary contributions to decipher the mechanisms involved in cell response to GSH. The xCELLigence sensor allows for label-free, noninvasive determination of the surface area occupied by the cells on microelectrodes by measuring their impedance, i.e., their resistance to the passage of a low-intensity current. The adhesion strength of cells on the electrodes can markedly trigger the cell index. While this tool was first dedicated to monitoring cell numbers related to proliferation versus death, it is also possible to study the adhesion strength of cells (
Figure 1). In a previous study, we validated this application by measuring lipid uptake by immature adipose cells; indeed, the accumulation of triglycerides in small lipid droplets induces a round cell shape, which is related to a loss of adhesion strength [
24]. The regulation of the cell index by the adhesion force was also validated in a study of the molecular mechanisms involved in cancer cell adhesion [
25]. xCELLigence stations are designed to use 96-well plates, allowing to obtain results from a large number of cells (10
3 to 10
5 cells per well) with a high reproducibility rate. The most recent version of xCELLigence e-Sight is coupled with microscopic observation; there is no doubt that it is a very effective tool for quantifying cellular responses, allowing for primary identification of dose-dependent effects (determination of IC50). However, complementary analyses are then required in order to determine which effects are involved, among the regulation of cell number, cell adhesion force, and/or cell surface occupancy (size, differentiation). Another method for monitoring RTCAs is the optimization of complementary analyses according to time-dependent events to identify short-term (adhesion, toxicity, etc.) and long-term events (proliferation, differentiation, etc.) involving different signaling pathways [
26]. Finally, RTCA data can be monitored and analyzed during experiments, allowing for instant result processing and adjustments to the experiment’s duration. The phase holographic cell imaging system HoloMonitor is amazing; the software brings the possibility to follow single cells in time-lapse experiments to quantify their physical (area, perimeter, volume, irregularity, etc.) and behavioral (motility, migration, speed, etc.) characteristics. It is possible to select few tens of cells. It is also possible to select the place to be analyzed inside the wells, which is very convenient in light of cell cultures often being heterogeneous. Such a process takes time before starting acquisition; it may be problematic to study very fast responses, but it is essential to insure efficient measurements. Analysis of the results is time-consuming; therefore, it is necessary to check selected cells from one frame to the following one in order to insure reliable results (
Table 1). The third time-lapse method, using the 3D Nanolive microscope, allows for an impressive resolution. It is very user-friendly, and time-lapses bring researchers in the close vicinity of cells. Cell analysis based on refractive index is an original and fascinating approach. Further research is required to increase the potential of the microscope, in particular by defining the refractive index of organelles. The main limitation of the microscopic analysis of cells, apart from the low number of cells analyzed, is that the recent history of cells is unknown, i.e., whether they have divided recently, or whether they will divide soon. Such variations in the metabolic status of cells may introduce confounding results. A limitation applicable to both HoloMonitor and Nanolive is that the time required for processing and sample setup makes it difficult to acquire data quickly enough to measure very early responses (less than 30 min).
The different methods show some discrepancies in terms of glutathione’s dose-dependent effect. For example, GSH-induced effects were detected from the lower concentration 1 mM in RTCA experiments with xCELLigence (cell index,
Figure 1A) and HoloMonitor (
Figure 2A), although the effects were detected from 2.5 mM with flow cytometry (
Figure 4 and
Figure 5). Since flow cytometry measurements require cell dissociation and fixation, decreased sensitivity could result from partial cell and extracellular epitope loss, leading to lower sensitivity than the RTCA and HoloMonitor time-lapse monitoring methods. An important experimental parameter rarely indicated, as seen in
Section 2, is the culture media volume in which cells are incubated at the time of measurements. It is usual to refer to the concentrations of molecules tested; however, in the case of molecules having a specific attraction for certain chemical bonds, such as GSH for disulfide bridges, incubating cells in 1 mL represents twice the GSH molecules than in 500 μL. This can lead to significant differences in sensitivities measured by different techniques, especially in 96-well plates (xCELLigence and HoloMonitor) versus Ibidi dishes (Nanolive). In the same order, cell number at the time of measurement may be critically important. Indeed, in the present case, it will change the number of disulfide bridges exposed to GSH. It is not always easy to obtain the same cell density when the cell supports are different: although there is not significant difference between E-96 and 96 well plates, well size and bottom glass of Ibidi dishes have significant impact on cell proliferation.
Besides the comparison of the different methods, it is of interest to comment the effects of GSH on 13s24 cells. The most significant result of this work is the dose-dependent alteration of adhesion strength and increase of olfactory neuron cell size. The dose-response of cell index (xCELLigence) was strongly correlated from control conditions to 10 mM GSH (r
2 = 0.9963,
Figure 1) without significantly affecting cell proliferation and survival (
Figure 4A). Such high GSH concentration has been reported in liver cells [
3]. The decrease in cell index without affecting cell number suggests an effect on cell adhesion strength. GSH-induced decrease in cell motility (4D), reduction in F-actin content (
Figure 4C), and extracellular glycosylation sites (
Figure 5) suggest that GSH reduces cell force adhesion to the extracellular matrix (ECM). Indeed, the regulation of cell adhesion, required for the control of cell growth, migration, as well as differentiation, involves a fine regulation of the cytoskeleton by controlling actin polymerization (F-actin) by integrins, which are highly glycosylated proteins [
27]. Moreover, GSH was found to increase neuroblast cell size in a dose-dependent manner (
Figure 3B and
Figure 4B). GSH is produced in the cytosol of most cells [
3], and several studies have shown that they are able to export GSH. Two types of carriers were identified: protein multidrug resistance protein (MRP) [
28] and organic anion transporting protein (Oatp1) [
29]. Cells produce GSH in their cytosol; that GSH can be exported to the extracellular space and can react with extracellular components. Cell adhesion is regulated by complex assemblages of integrins, which are highly glycosylated proteins containing disulfide bonds in the extracellular domains. The binding capacity of integrins can be modulated by various stimuli; the disulfide bonds maintain the integrin in a less active form. The addition of reducing agents results in increased ligand binding [
30]. Conformational regulation of integrin affinity (α4β1) by reducing agents has been demonstrated in many studies [
31]. GSH regulates integrin α2β3-mediated cell adhesion [
32]. Similarly, the disruption of disulfide restriction at integrin knees induces activation and ligand-independent signaling of α4β7 [
33]. All these observations strengthen our hypothesis that GSH produced in cell cytosol can be exported to interact with integrins, giving the cells an opportunity to control their volume.
One may argue that the GSH concentrations tested in the present study were not physiological. Admittedly, the plasma GSH concentration is low (in the order of μM), but intracellular concentrations are in the millimolar range and can even reach 10 mM in liver cells; much of it is exported across the plasma membrane into extracellular spaces [
34]. It must be noticed that GSH is a highly reactive compound. It is then not a surprise to detect low concentrations of free GSH in the interstitial liquid. Ultimately, what matters is the turnover rate of GSH. GSH turnover rates in most cells are rather fast, with half-lives between 2 and 6 h [
35,
36], indicating high rates of both GSH synthesis and export [
3]. In conclusion, despite rather low concentrations of GSH, high turnover rate and high cell sensitivity are compatible with the role of GSH in the regulation of cell volume. Further, our results already show an increased cell volume at 1 mM GSH, which, according to the literature, seems common in cells [
1,
14]. In a previous study, we observed a similar effect of GSH on human lung epithelial cell volume suggesting that the effect of GSH on cell volume is a common feature. We also showed that the effect of extracellular GSH is counterbalanced by hydrogen peroxide [
14]. Our results support the hypothesis of a major role of GSH exported by cells to interact with the oxidative stress-sensitive integrins. This represents a causal link between the redox state of the cell and the necessary changes in cell volume and motility required for both cell migration and differentiation.
The results obtained on an olfactory neuroblast cell line is of particular interest in the context of the control of neurogenesis, which is a specific feature of the olfactory system in adult humans [
37]. In the olfactory epithelium, continuous neurogenesis occurs to replace bipolar neurons involved in odorant molecule detection by specific apical receptors and signaling through axonal projection to the olfactory bulb [
38]. In vitro studies using primary non-neuronal cell cultures have shown that both biochemical and mechanical stresses are sufficient to induce stem cell differentiation into bipolar sensory neurons [
18]. The regulation of cell morphology by the cytoskeleton contributes to neuronal identity by inducing axonal specification in relation to other neurites that become dendrites [
39], assuming, respectively, the transfer of signal at the nasal level by projection to the CNS and specific odorant response signaling by direct exposition to airway flux. In non-neuronal olfactory 13s24 cells, we found that GSH can transiently affect cell adhesion force and F-actin polymerization. Therefore, local production of GSH could participate in the finely tuned regulation of neuronal differentiation versus proliferation of neuronal stem cell progenitors. Loss of brain GSH is associated with development of neurogenerative diseases [
40]. The antioxidative properties of intracellular GSH are largely described. Further exploration of the mechanisms involved in its extracellular activity on cell adhesion and regulation of size will improve its promising potential in new therapeutic strategies to treat neurodegenerative diseases. Although cell parameters such as cell volume, surface, and motility have not been directly measured, some results from the literature support our conclusions.