Ammonia Gas Adsorption in Fixed Bed and Fluidized Bed Using Bentonite Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents for Pillaring Process
2.2. Preparation Method of Aluminum Pillared Bentonite
2.3. Preparation of Adsorbent Particles
2.4. Equipment for Adsorbent Characterization
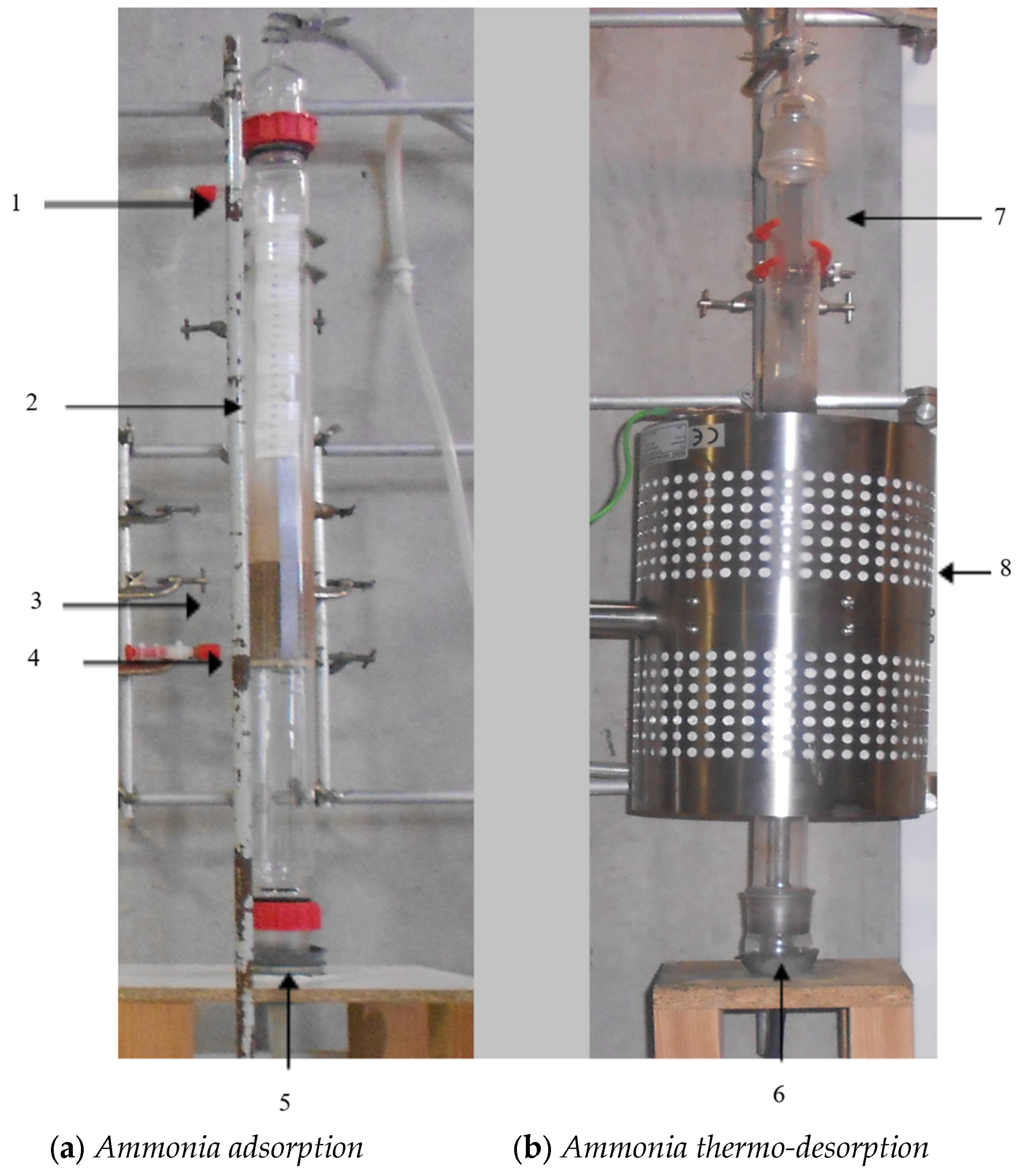
2.5. Ammonia Adsorption and Desorption Procedure
3. Results and Discussion
3.1. Sodium Bentonite Characterization
3.1.1. Granulometric Distribution
3.1.2. Measurement of Surface Acidity and Surface Basicity
3.2. Aluminum Pillared Bentonite Characterization
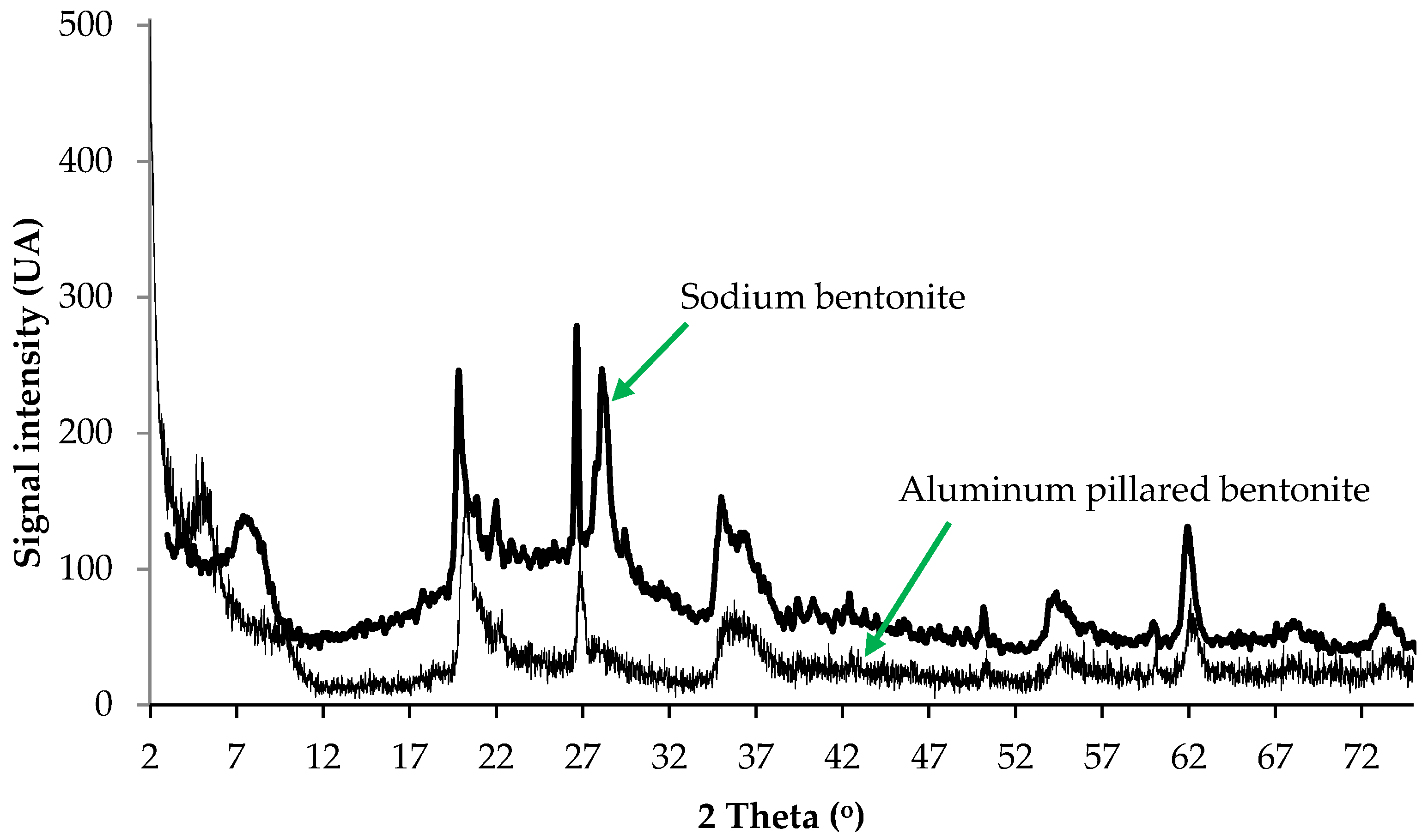
3.2.1. X-Ray Diffraction (XRD)
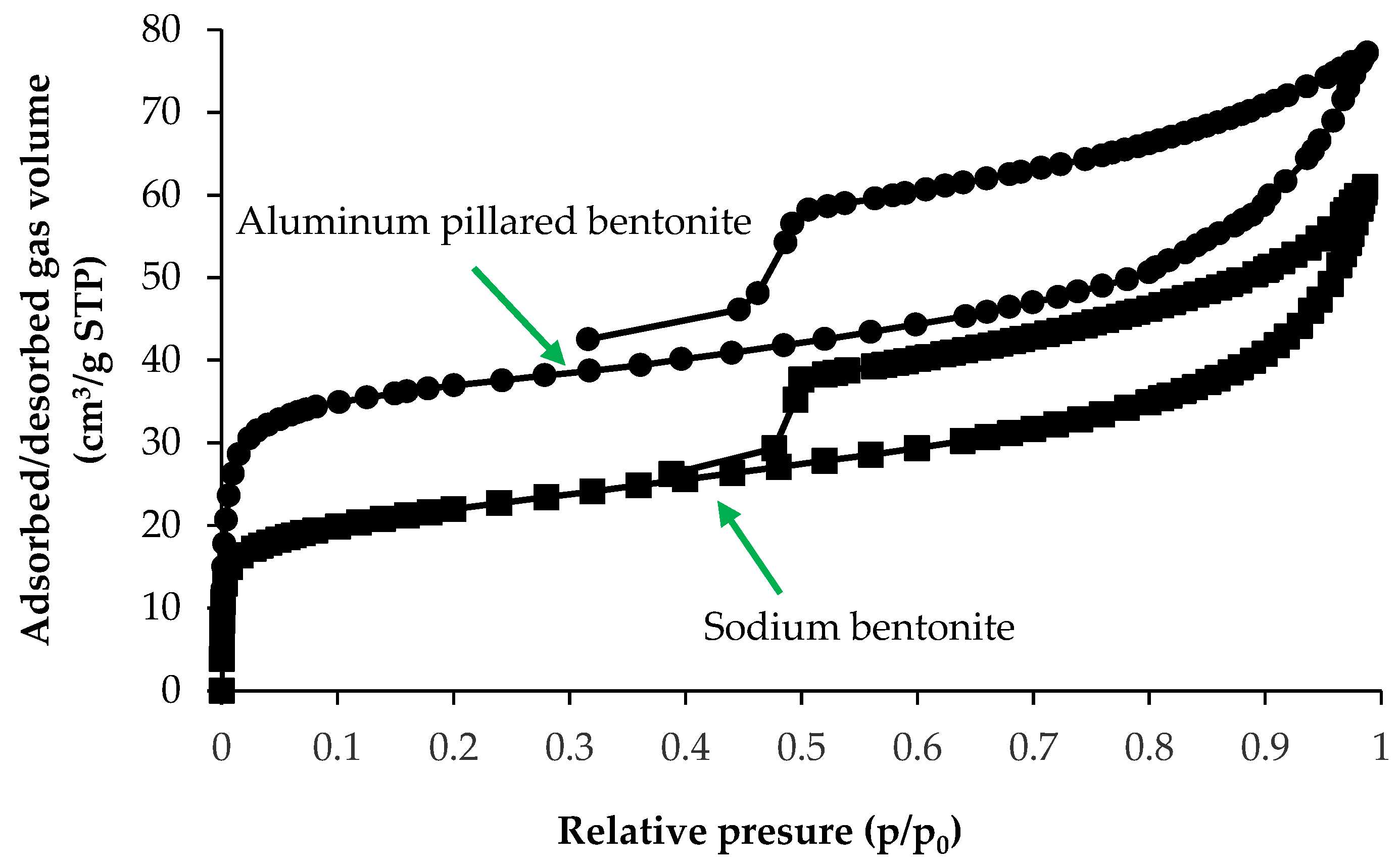
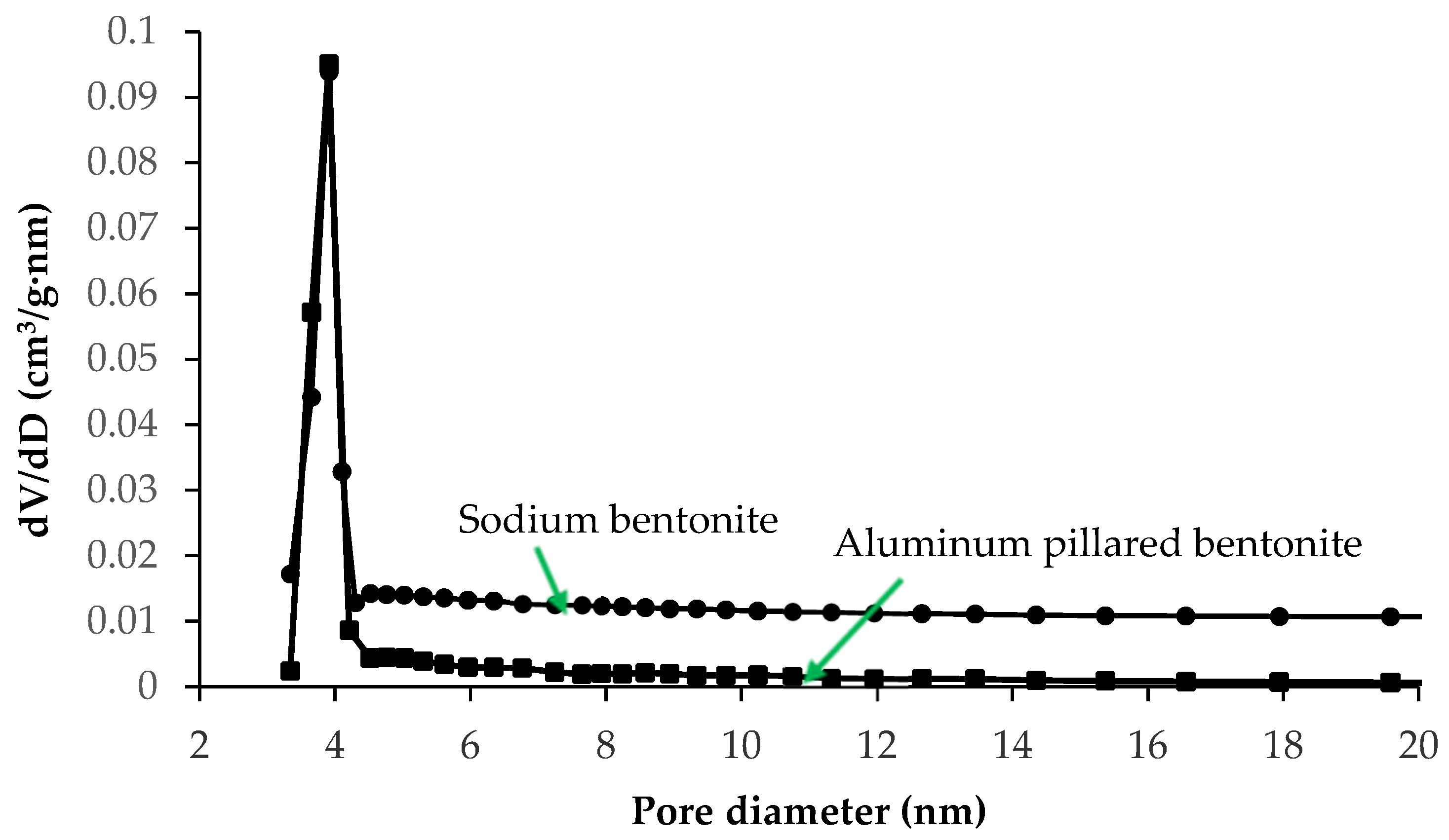
3.2.2. Nitrogen Adsorption–Desorption
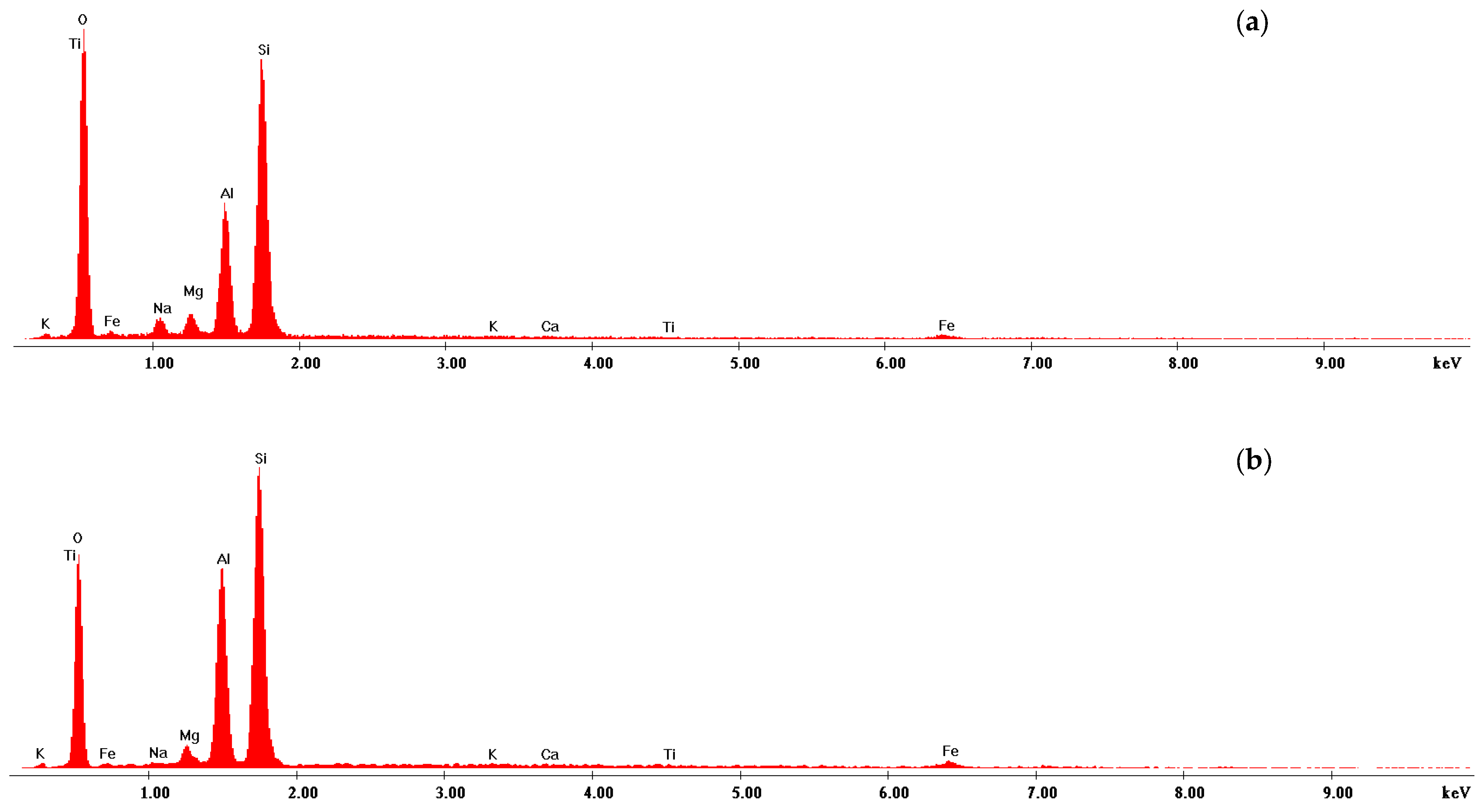
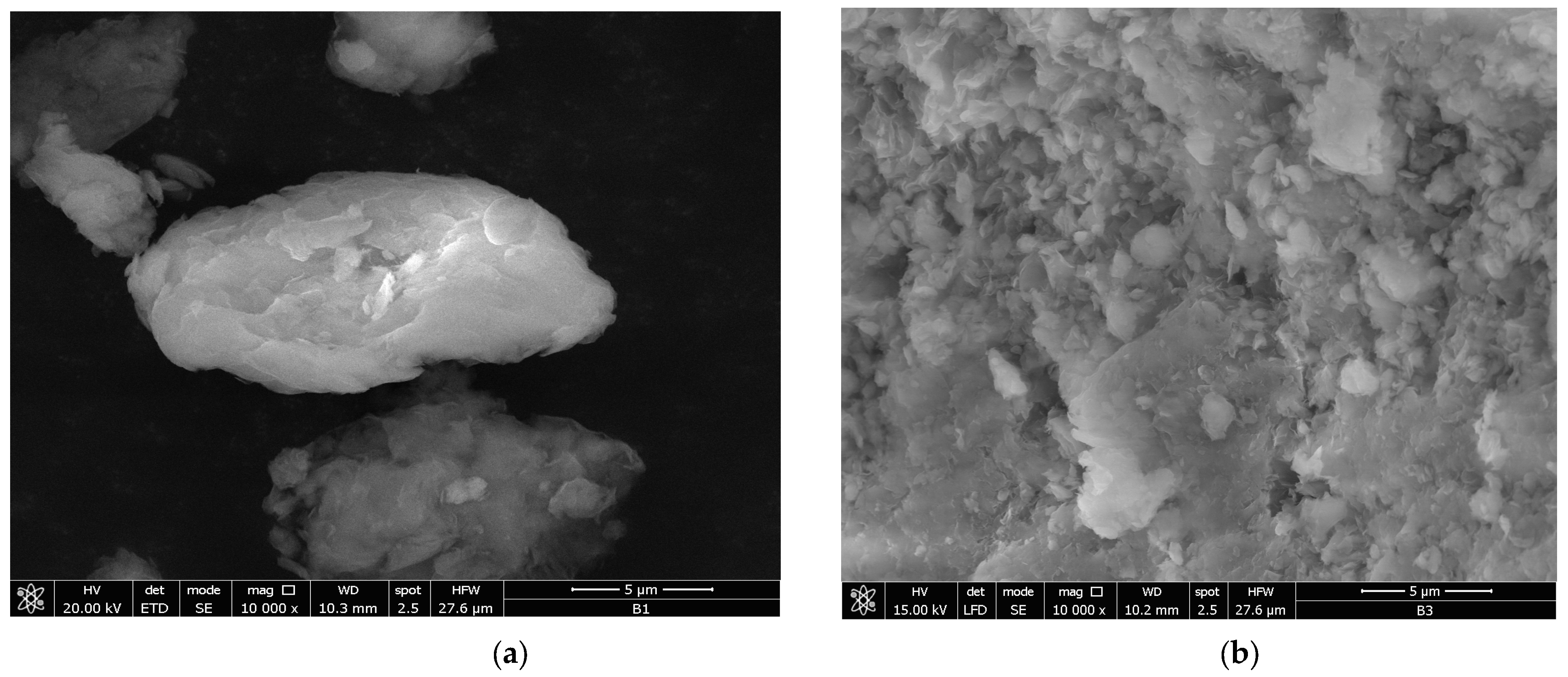
3.2.3. Energy Dispersive X-Ray (EDX) Coupled with Scanning Electron Microscopy
3.3. Ammonia Adsorption
3.3.1. Experimental Matrix
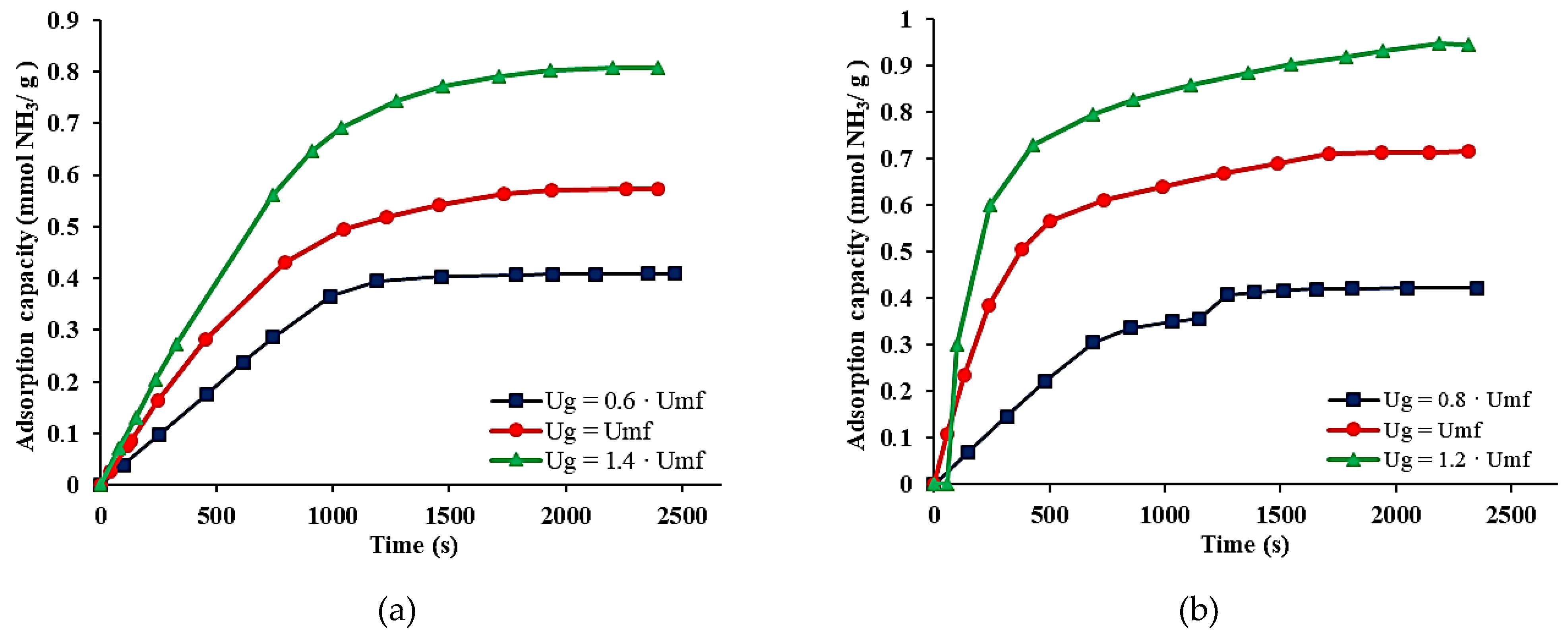
3.3.2. Adsorption Kinetics
3.4. Desorption and Regeneration Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venaruzzo, J.L.; Volzone, C.; Rueda, M.L.; Ortiga, J. Modified bentonitic clay minerals as adsorbents of CO, CO2 and SO2 gases. Microporous Mesoporous Mater. 2002, 56, 73–80. [Google Scholar] [CrossRef]
- Vo, H.T.; Kim, J.; Kim, N.Y.; Lee, J.-K.; Joo, J.B. Effect of pore texture property of mesoporous alumina on adsorption performance of ammonia gas. J. Ind. Eng. Chem. 2020, 91, 129–138. [Google Scholar] [CrossRef]
- Brodny, J.; Tutak, M. The analysis of similarities between the European Union countries in terms of the level and structure of the emissions of selected gases and air pollutants into the atmosphere. J. Clean. Prod. 2021, 279, 123641. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Clay, S.A.; Clay, D.E.; Harper, S.S. Ammonia fertilizer influences atrazine adsorption-desorption characteristics. J. Agric. Food Chem. 1995, 43, 815–819. [Google Scholar] [CrossRef]
- Guo, J.; Yang, H.; Yuan, Y.; Yin, P.; Zhang, N.; Lin, Z.; Ma, Q.; Yang, Q.; Liu, X.; Wang, H.; et al. Blending of Slow-Release N Fertilizer and Urea Improve Rainfed Maize Yield and Nitrogen Use Efficiency While Reducing Apparent N Losses. Agronomy 2025, 15, 11. [Google Scholar] [CrossRef]
- Wyer, K.E.; Kelleghan, D.B.; Blanes-Vidal, V.; Schauberger, G.; Curran, T.P. Ammonia emissions from agriculture and their contribution to fine particulate matter: A review of implications for human health. J. Environ. Manag. 2022, 323, 116285. [Google Scholar] [CrossRef]
- Busca, G.; Pistarino, C. Abatement of ammonia and amines from waste gases: A summary. J. Loss Prevent. Process Ind. 2003, 16, 157–163. [Google Scholar] [CrossRef]
- Behera, S.N.; Sharma, M.; Aneja, V.P.; Balasubramanian, R. Ammonia in the atmosphere: A review on emission sources, atmospheric chemistry and deposition on terrestrial bodies. Environ. Sci. Pollut. Res. 2013, 20, 8092–8131. [Google Scholar] [CrossRef]
- Mofidi, A.; Asilian, H.; Jafari, A.J. Adsorption of volatile organic compounds on fluidized activated carbon bed. Health Scope 2013, 2, 84–89. [Google Scholar] [CrossRef]
- Han, B.; Butterly, C.; Zhang, W.; He, J.-Z.; Chen, D. Adsorbent materials for ammonium and ammonia removal: A review. J. Clean. Prod. 2021, 283, 124611. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, Q.; Zhou, Q.; Xi, Y.; Zhu, J.; He, H. Adsorbents based on montmorillonite for contaminant removal from water: A review. Appl. Clay Sci. 2016, 123, 239–258. [Google Scholar] [CrossRef]
- Adeyemo, A.A.; Adeoye, I.O.; Bello, S.O. Adsorption of dyes using different types of clay: A review. App. Water Sci. 2017, 7, 543–568. [Google Scholar] [CrossRef]
- Volzone, C. Retention of pollutant gases: Comparison between clay minerals and their modified products. Appl. Clay Sci. 2007, 36, 191–196. [Google Scholar] [CrossRef]
- Erdoğan, B.; Ergürha, O.; Ante, A. Influence of acid activation on the NH3-adsorption properties of a Turkish bentonite. Clay Miner. 2021, 56, 178–184. [Google Scholar] [CrossRef]
- Konta, J. Clay and Man: Clay Raw Materials in the Service of Man. Appl. Clay Sci. 1995, 10, 275–335. [Google Scholar] [CrossRef]
- Carriazo, J.G.; Saavedra, M.J.; Molina, M.F. XRD study on the intercalation-pillaring of a 2:1 clay mineral with aluminum polyoxocationic species. Rev. Mex. Ing. Quim. 2009, 8, 299–305. [Google Scholar]
- Worasith, N.; Goodman, B.A. Clay mineral products for improving environmental quality. Appl. Clay Sci. 2023, 242, 106980. [Google Scholar] [CrossRef]
- Ursu, A.V.; Gros, F.; Nistor, D.I.; Djelveh, G. Characterization and utilization of a commercial clay for ammonia adsorption: Influence of operating parameters on gas retaining. Rev. Chim. 2008, 59, 1067–1072. [Google Scholar] [CrossRef]
- Platon, N.; Siminiceanu, I.; Nistor, I.D.; Miron, N.D.; Muntianu, G.; Mares, A.M. Fe—Pillared clay as an efficient Fenton-like heterogeneous catalyst for phenol degradation. Rev. Chim. 2011, 62, 676–679. [Google Scholar]
- Ding, Z.; Kloprogge, J.T.; Frost, R.L. Porous clays and pillared clays-based catalysts. Part 2: A review of the catalytic and molecular sieve applications. J. Porous Mater. 2001, 8, 273–293. [Google Scholar] [CrossRef]
- Hortolomeu, A.; Mirila, D.C.; Roșu, A.-M.; Nedeff, F.M.; Scutaru, I.; Ureche, D.; Sturza, R.; Fînaru, A.-L.; Nistor, I.D. Chemically Modified Clay Adsorbents Used in the Retention of Protein and Polyphenolic Compounds from Sauvignon Blanc White Wine. Nanomaterials 2024, 14, 588. [Google Scholar] [CrossRef] [PubMed]
- Maes, N.; Heylen, I.; Cool, P.; Vansant, E.F. The relation between the synthesis of pillared clays and their resulting porosity. Appl. Clay Sci. 1997, 12, 43–60. [Google Scholar] [CrossRef]
- Muthu, M.; Sadowski, Ł. Evaluation of the Performance of Pervious Concrete Inspired by CO2-Curing Technology. Appl. Sci. 2024, 14, 4202. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Su, W.; Cai, F.; Lan, T.; Dai, Z. Adsorption of Coxsackievirus in Sediments: Influencing Factors, Kinetics, and Isotherm Modeling. Appl. Sci. 2024, 14, 1480. [Google Scholar] [CrossRef]
- Ouakouak, A.; Abdelhamid, M.; Thouraya, B.; Chahinez, H.-O.; Hocine, G.; Hamdi, N.; Syafiuddin, A.; Boopathy, R. Development of a Novel Adsorbent Prepared from Dredging Sediment for Effective Removal of Dye in Aqueous Solutions. Appl. Sci. 2021, 11, 10722. [Google Scholar] [CrossRef]
- Kurian, M.; Sugunan, S. Characterisation of the acid-base properties of pillared montmorillonites. Microporous Mesoporous Mater. 2005, 83, 25–34. [Google Scholar] [CrossRef]
- Shin, Y.S.; Geun, S.; Ha, B.H. Pore structures and acidities of Al-pillared montmorillonite. Korean J. Chem. Eng. 2003, 20, 77–82. [Google Scholar] [CrossRef]
- Rodríguez-González, L.; Rodríguez-Castellón, E.; Jiménez-López, A.; Simon, U. Correlation of TPD and impedance measurements on the desorption of NH3 from zeolite H-ZSM-5. Solid State Ion. 2008, 179, 1968–1973. [Google Scholar] [CrossRef]
- Sakr, A.K.; Cheira, M.F.; Hassanin, M.A.; Mira, H.I.; Mohamed, S.A.; Khandaker, M.U.; Osman, H.; Eed, E.M.; Sayyed, M.I.; Hanfi, M.Y. Adsorption of Yttrium Ions on 3-Amino-5-Hydroxypyrazole Impregnated Bleaching Clay, a Novel Sorbent Material. Appl. Sci. 2021, 11, 10320. [Google Scholar] [CrossRef]
- Stanly, S.; Jelmy, E.J.; Nair, C.P.R.; John, H. Carbon dioxide adsorption studies on modified montmorillonite clay/reduced graphene oxide hybrids at low pressure. J. Environ. Chem. Eng. 2019, 7, 103344. [Google Scholar] [CrossRef]
- Azzouz, A.; Nistor, D.; Miron, D.; Ursu, A.V.; Sajin, T.; Monette, F.; Niquette, P.; Hausler, R. Assessment of acid-base strength distribution of ion-exchanged montmorillonites through NH3 and CO2-TPD measurements. Thermochim. Acta 2006, 449, 27–34. [Google Scholar] [CrossRef]
- Ayari, F.; Srasra, E.; Trabelsi-Ayadi, M. Characterization of bentonitic clays and their use as adsorbent. Desalination 2005, 185, 391–397. [Google Scholar] [CrossRef]
- De Stefanis, A.; Tomlinson, A.A.G. Towards designing pillared clays for catalysis. Catal. Today 2006, 114, 126–141. [Google Scholar] [CrossRef]
- Altunlu, M.; Yapar, S. Effect of OH-/Al3+ and Al3+/clay ratios on the adsorption properties of Al-pillared bentonites. Colloids Surf. A Physicochem. Eng. Asp. 2007, 306, 88–94. [Google Scholar] [CrossRef]
- Cool, P.; Vansant, E.F. Pillared Clays: Preparation, Characterization and Applications. In Synthesis; Part of the Molecular Sieves Book Series (SIEVES, volume 1); Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Salerno, P.; Mendioroz, S. Preparation of Al-pillared montmorillonite from concentrated dispersions. Appl. Clay Sci. 2002, 22, 115–123. [Google Scholar] [CrossRef]
- Georgescu, A.M.; Platon, N.; Arus, V.A.; Jinescu, C.; Nardou, F.; Nistor, I.D. Study on the preparation and characterization of aluminum-pillared clays using montmorillonite K10. Sci. Study Res.-Chem. Chem. Eng. Biotechnol. Food Ind. 2023, 24, 145–153. [Google Scholar]
- Khanh, T.S.T.; Trung, T.Q.; Giang, L.T.T.; Nguyen, T.Q.; Lam, N.D.; Dinh, N.N. Ammonia Gas Sensing Characteristic of P3HT-rGO-MWCNT Composite Films. Appl. Sci. 2021, 11, 6675. [Google Scholar] [CrossRef]
- Muntianu, G.; Georgescu, A.M.; Nistor, I.D.; Jinescu, G. Ammonia adsorption kinetics on clay particles in fluidized bed. Bull. Rom. Chem. Eng. Soc. 2016, 3, 2–11. [Google Scholar]
- Al-Ghurabi, E.H.; Ajbar, A.; Asif, M. Improving Fluidization Hydrodynamics of Group C Particles by Mixing with Group B Particles. Appl. Sci. 2018, 8, 1469. [Google Scholar] [CrossRef]
- Usuda, H.; Mishima, Y.; Kawamoto, T.; Minami, K. Desorption of Ammonia Adsorbed on Prussian Blue Analogs by Washing with Saturated Ammonium Hydrogen Carbonate Solution. Molecules 2022, 27, 8840. [Google Scholar] [CrossRef]
- Ganachari, S.V.; Shilar, F.A.; Patil, V.B.; Khan, T.M.Y.; Saleel, C.A.; Ali, M.A. Optimizing Ammonia Detection with a Polyaniline−Magnesia Nano Composite. Polymers 2024, 16, 2892. [Google Scholar] [CrossRef] [PubMed]
- Apostolov, A.; Panyovska, S.; Stefanov, S.; Dzhonova-Atanasova, D.; Razkazova-Velkova, E.; Michev, S. Integrated Absorption–Adsorption Process for Waste-Free Decontamination of Gases from Sulfur Dioxide, Part 2: CFD Modeling and Experimental Investigation of a Bubble-Cap Tray. Sustainability 2024, 16, 2472. [Google Scholar] [CrossRef]
- Murtaza, M.; Ahmad, H.M.; Kamal, M.S.; Hussain, S.M.S.; Mahmoud, M.; Patil, S. Evaluation of Clay Hydration and Swelling Inhibition Using Quaternary Ammonium Dicationic Surfactant with Phenyl Linker. Molecules 2020, 25, 4333. [Google Scholar] [CrossRef]
- Muntianu, G.; Platon, N.; Mardaru, A.; Nistor, I.D.; Miron, N.D.; Jinescu, G. Use of modified clays obtained by pillaring in gas purification. Univ. Politeh. Buchar. Sci. Bull. Ser. B—Chem. Mater. Sci. 2015, 77, 151–164. [Google Scholar]
- Mugge, J.; Bosch, H.; Reith, T. Measuring and modelling gas adsorption kinetics in single porous particles. Chem. Eng. Sci. 2001, 56, 5351–5360. [Google Scholar] [CrossRef]
- Castillo-Araiza, C.O.; Che-Galicia, G.; Dutta, A.; González, G.G.; Martínez-Vera, C.; Ruíz-Martínez, R.S. Effect of diffusion on the conceptual design of a fixed-bed adsorber. Fuel 2015, 149, 100–108. [Google Scholar] [CrossRef]
- Manohar, D.M.; Noeline, B.F.; Anirudhan, T.S. Adsorption performance of Al-pillared bentonite clay for the removal of cobalt(II) from aqueous phase. App. Clay Sci. 2006, 31, 194–206. [Google Scholar] [CrossRef]
- Ursu, A.V.; Jinescu, C.; Nistor, D.I.; Arus, V.A.; Isopencu, G.; Mares, A.M. Estimation of the Dynamic Parameters of Mono and Bicomponent Granular Particles Beds Fluidization. Rev. Chim. 2010, 61, 1226–1230. [Google Scholar]
- Mirila, D.C.; Pirva, M.S.; Rosu, A.M.; Zichil, V.; Nistor, I.D. Activated adsorption on clay of micropollutants from paper printing industry. Sci. Stud. Res. Chem. 2018, 19, 63–72. [Google Scholar]
- Rodrigues, C.C.; de Moraes, D., Jr.; da Nóbrega, S.W.; Barboza, M.G. Ammonia adsorption in a fixed bed of activated carbon. Bioresour. Technol. 2007, 98, 886–891. [Google Scholar] [CrossRef]
- Muntianu, G.; Simion, A.-I.; Grigoraș, C.-G.; Platon, N.; Nistor, I.-D.; Jinescu, G. Aluminum pillared bentonite—Characterization and synthesis optimization by response surface methodology. Stud. UBB Chem. LXVI 2021, 1, 73–88. [Google Scholar] [CrossRef]
- El Miz, M.; Salhi, S.; Chraibi, I.; El Bachiri, A.; Fauconnier, M.-L.; Tahani, A. Characterization and Adsorption Study of Thymol on Pillared Bentonite. Open J. Phys. Chem. 2014, 4, 98–116. [Google Scholar] [CrossRef]
- Muntianu, G.; Platon, N.; Arus VA Rosu, A.M.; Nistor, D.I.; Jinescu, G. Hydrodynamic study of clay particles in fluidized bed. J. Eng. Stud. Res. 2013, 19, 70–75. [Google Scholar]
- Muntianu, G.; Ursu, A.-V.; Djelveh, G.; Isopencu, G.; Mareş, A.-M.; Nistor, I.D.; Jinescu, C.V. Dynamic parameters for mixtures of pillared clay-magnetic particles in fluidized bed in coaxial magnetic field. Rev. Chim. 2014, 65, 1077–1085. [Google Scholar]



| Mean Diameter by: | Size (μm) | STD (μm) | Conf. (%) | Dimension | D10 (μm) | D50 (μm) | D90 (μm) | Mode (μm) |
|---|---|---|---|---|---|---|---|---|
| Length, D[1,0] | 11.74 | 1.40 | 98.11 | Number | 10.30 | 11.49 | 13.87 | 10.30 |
| Length weighted, D[2,1] | 11.91 | 1.47 | 98.10 | Length | 10.30 | 11.49 | 13.87 | 10.30 |
| Surface, D[2,0] | 11.83 | - | - | - | - | - | - | - |
| Surface weighted, D[3,2] | 12.09 | 1.52 | 47.43 | Surface | 10.30 | 12.08 | 13.87 | 10.30 |
| Volume, D[3,0] | 11.91 | - | - | - | - | - | - | - |
| Volume weighted, D[4,3] | 12.28 | 1.57 | 47.41 | Volume | 10.30 | 12.08 | 14.46 | 10.30 |
| Chemical Element | Sodium Bentonite, (wt. %) | Aluminum Pillared Bentonite, (wt. %) |
|---|---|---|
| O | 57.68 | 54.53 |
| Na | 2.45 | 0.18 |
| Mg | 1.92 | 1.75 |
| Al | 11.01 | 15.05 |
| Si | 26.22 | 27.69 |
| K | 0.08 | 0.07 |
| Ca | 0.08 | 0.07 |
| Ti | 0.05 | 0.10 |
| Fe | 0.52 | 0.54 |
| Total | 100.00 | 100.00 |
| No. Test | Adsorbent Particles | Average Particle Size , μm) | Gas Velocity, (Ug, m/s) | Total Flow Rate, (QT, L/s) |
|---|---|---|---|---|
| 1. | Sodium bentonite | 750 | 0.6 × Umf | 0.3341 |
| 2. | Umf | 0.5436 | ||
| 3. | 1.4 × Umf | 0.7525 | ||
| 4. | 1500 | 0.8 × Umf | 0.3341 | |
| 5. | Umf | 1.34 | ||
| 6. | 1.2 × Umf | 2.3433 | ||
| 7. | Aluminum pillared bentonite | 750 | 0.6 × Umf | 0.3341 |
| 8. | Umf | 0.5436 | ||
| 9. | 1.4 × Umf | 0.7525 | ||
| 10. | 1500 | 0.8 × Umf | 0.3341 | |
| 11. | Umf | 1.34 | ||
| 12. | 1.2 × Umf | 2.3433 |
| No. of Cycles | Sodium Bentonite Particles | Aluminum Pillared Bentonite Particles | ||
|---|---|---|---|---|
| mmol NH3 Desorbed/g Bentonite | % | mmol NH3 Desorbed/g Bentonite | % | |
| 0. | 0.357 | 100 | 0.527 | 100 |
| 1. | 0.245 | 68.62 | 0.464 | 95.44 |
| 2. | 0.158 | 44.25 | 0.345 | 88.04 |
| 3. | 0.062 | 17.36 | 0.268 | 50.85 |
| 4. | - | 0.00 | 0.088 | 16.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muntianu, G.; Georgescu, A.-M.; Rosu, A.-M.; Platon, N.; Arus, V.A.; Jinescu, C.V.; Nistor, I.D. Ammonia Gas Adsorption in Fixed Bed and Fluidized Bed Using Bentonite Particles. Appl. Sci. 2025, 15, 832. https://doi.org/10.3390/app15020832
Muntianu G, Georgescu A-M, Rosu A-M, Platon N, Arus VA, Jinescu CV, Nistor ID. Ammonia Gas Adsorption in Fixed Bed and Fluidized Bed Using Bentonite Particles. Applied Sciences. 2025; 15(2):832. https://doi.org/10.3390/app15020832
Chicago/Turabian StyleMuntianu, Gabriela, Ana-Maria Georgescu, Ana-Maria Rosu, Nicoleta Platon, Vasilica Alisa Arus, Cosmin Valeriu Jinescu, and Ileana Denisa Nistor. 2025. "Ammonia Gas Adsorption in Fixed Bed and Fluidized Bed Using Bentonite Particles" Applied Sciences 15, no. 2: 832. https://doi.org/10.3390/app15020832
APA StyleMuntianu, G., Georgescu, A.-M., Rosu, A.-M., Platon, N., Arus, V. A., Jinescu, C. V., & Nistor, I. D. (2025). Ammonia Gas Adsorption in Fixed Bed and Fluidized Bed Using Bentonite Particles. Applied Sciences, 15(2), 832. https://doi.org/10.3390/app15020832







