Physico-Chemical Characterization of Sesame/Rapeseed Oil Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oil Mixtures Creations
2.3. Fatty Acid Composition
2.4. Determination of Peroxide Value
2.5. Determination of Iodine Value
2.6. Differential Scanning Calorimetry
2.7. Refractive Index Measurement
2.8. UV Spectra
2.9. Color Parameters Measurement
2.10. Statistics
3. Results
3.1. Chemical Characterization of the SO/RSO Mixtures
3.2. Crystallisation and Melting Phenomena in Sesame Oil/Rapeseed Oil Mixtures
3.3. Refractive Index
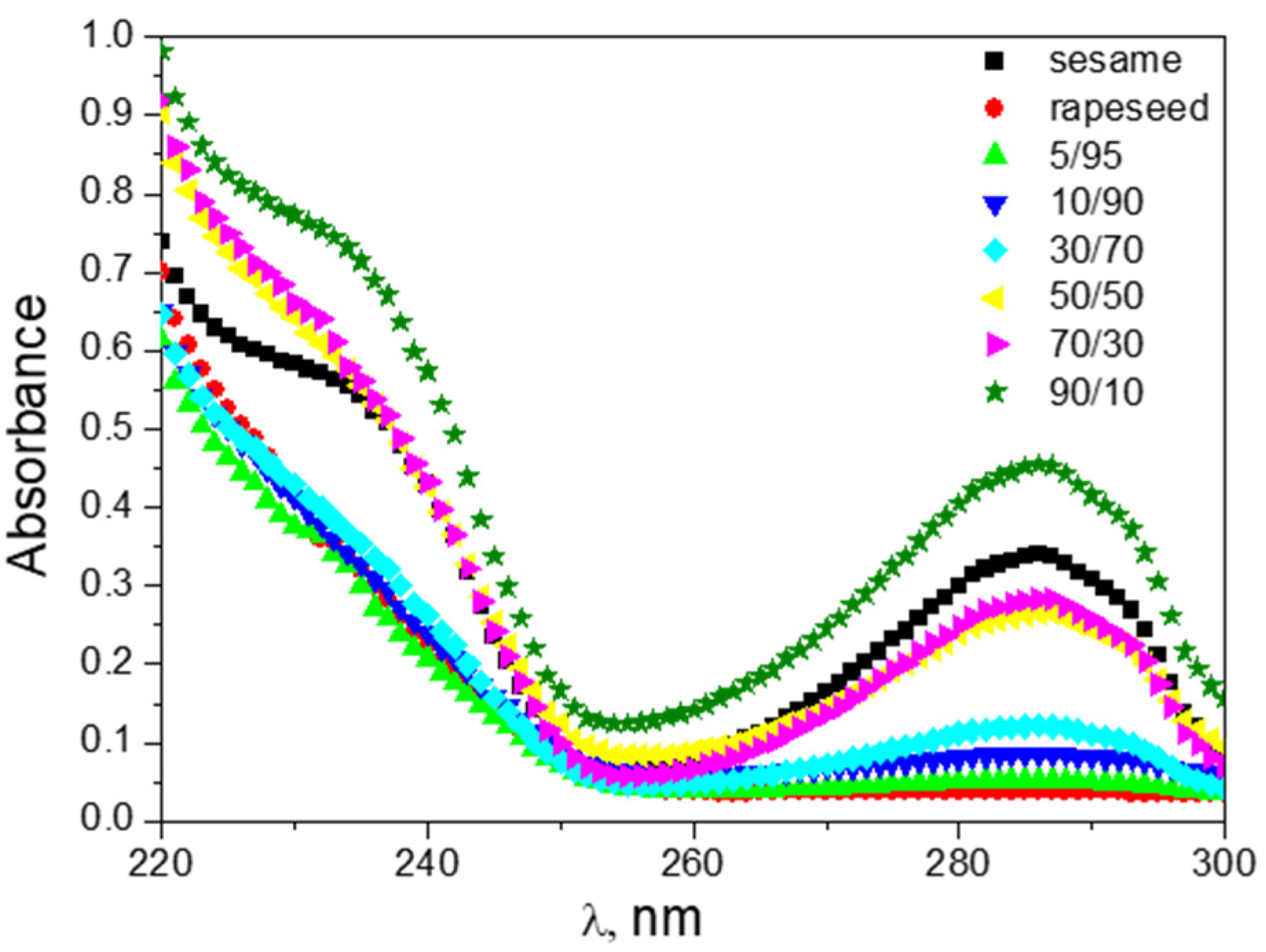
3.4. UV Spectral Analysis
3.5. SO/RSO Mixtures Color Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wen, C.; Shen, M.; Liu, G.; Liu, X.; Liang, L.; Li, Y.; Zhang, J.; Xu, X. Edible vegetable oils from oil crops: Preparation, refining, authenticity identification and application. Process Biochem. 2023, 124, 168–179. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Kalogianni, E.P. The In Vitro, Ex Vivo, and In Vivo Effect of Edible Oils: A Review on Cell Interactions. Pharmaceutics 2023, 15, 869. [Google Scholar] [CrossRef]
- Martysiak-Żurowska, D.; Orzołek, M. The correlation between nutritional and health potential and antioxidant properties of raw edible oils from cultivated and wild plants. Int. J. Food Sci. Technol. 2023, 58, 676–685. [Google Scholar] [CrossRef]
- Szabó, É.; Csölle, I.; Felső, R.; Kuellenberg de Gaudry, D.; Nyakundi, P.N.; Ibrahim, K.; Metzendorf, M.-I.; Ferenci, T.; Lohner, S. Benefits and Harms of Edible Vegetable Oils and Fats Fortified with Vitamins A and D as a Public Health Intervention in the General Population: A Systematic Review of Interventions. Nutrients 2023, 15, 5135. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, S.; Soltani, S.; Ramezani-Jolfaie, N.; Mohammadi, M.; Sherafatmanesh, S.; Lorzadeh, E.; Salehi-Abargouei, A. The effect of different edible oils on body weight: A systematic review and network meta-analysis of randomized controlled trials. BMC Nutr. 2024, 10, 107. [Google Scholar] [CrossRef]
- Voon, P.T.; Ng, C.M.; Ng, Y.T.; Wong, Y.J.; Yap, S.Y.; Leong, S.L.; Yong, X.S.; Lee, S.W.H. Health effects of various edible vegetable oil: An umbrella review. Adv. Nutr. 2024, 15, 100276. [Google Scholar] [CrossRef] [PubMed]
- Sadatasl, S.L.; Delavar, A.; Pakbaz, N.; Yavari, G. Evaluation of climate changes and its effects on the performance and well-being of producers and consumers of oilseeds (sesame and rapeseed). J. Clim. Res. 2024, 1402, 85–93. [Google Scholar]
- Bedigian, D.; Harlan, J.R. Evidence for cultivation of sesame in the ancient world. Econ. Bot. 1986, 40, 137–154. [Google Scholar] [CrossRef]
- Hwang, L.S. Sesame oil. Bailey’s Ind. Oil Fat Prod. 2005, 2, 547–552. [Google Scholar]
- Moazzami, A.; Kamal-Eldin, A. Sesame Seed Oil. In Gourmet and Health-Promoting Specialty Oils; Moreau, R.A., Kamal-Eldin, A., Eds.; AOCS Press: Urbana, IL, USA, 2009; pp. 267–282. [Google Scholar]
- Narasimhulu, C.A.; Selvarajan, K.; Litvinov, D.; Parthasarathy, S. Anti-atherosclerotic and anti-inflammatory actions of sesame oil. J. Med. Food 2015, 18, 11–20. [Google Scholar] [CrossRef]
- Saleem, T.M. Anti-microbial activity of sesame oil. Int. J. Phytochem. Pharmacol. 2011, 1, 21–23. [Google Scholar]
- Monteiro, É.M.H.; Chibli, L.A.; Yamamoto, C.H.; Pereira, M.C.S.; Vilela, F.M.P.; Rodarte, M.P.; De Oliveira Pinto, M.A.; Da Penha Henriques do Amaral, M.; Silvério, M.S.; De Matos Araújo, A.L.S.; et al. Antinociceptive and anti-inflammatory activities of the sesame oil and sesamin. Nutrients 2014, 6, 1931–1944. [Google Scholar] [CrossRef] [PubMed]
- Gauthaman, K.; Saleem, T.M. Nutraceutical value of sesame oil. Pharmacogn. Rev. 2009, 3, 264. [Google Scholar]
- Elkhaleefa, A.; Shigidi, I. Optimization of Sesame Oil Extraction Process Conditions. Adv. Chem. Eng. Sci. 2015, 5, 305–310. [Google Scholar] [CrossRef]
- Raymer, P.L. Canola: An emerging oilseed crop. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002; Volume 1, pp. 122–126. [Google Scholar]
- Przybylski, R.; Mag, T. Canola/rapeseed oil. In Vegetable Oils in Food Technology: Composition, Properties and Uses; Frank, D., Ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 107–136. [Google Scholar]
- Dupont, J.; White, P.J.; Johnston, K.M.; Heggtveit, H.A.; McDonald, B.E.; Grundy, S.M.; Bonanome, A. Food safety and health effects of canola oil. J. Am. Coll. Nutr. 1989, 8, 360–375. [Google Scholar] [CrossRef]
- Lin, L.; Allemekinders, H.; Dansby, A.; Campbell, L.; Durance-Tod, S.; Berger, A.; Jones, P.J. Evidence of health benefits of canola oil. Nutr. Rev. 2013, 71, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Banaś, K.; Piwowar, A.; Harasym, J. The potential of rapeseed (canola) oil nutritional benefits wide spreading via oleogelation. Food Biosci. 2023, 56, 103162. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Chemical, Rheological and Nutritional Characteristics of Sesame and Olive Oils Blended with Linseed Oil. Adv. Pharm. Bull. 2018, 8, 107–113. [Google Scholar] [CrossRef]
- Oparanti, S.O.; Yapi, K.M.; Fofana, I.; Rao, U.M. Preliminary studies on Improving the Properties of Canola Oil by Addition of Methyl Ester from a Saturated Vegetable Oil. In Proceedings of the 2023 IEEE Electrical Insulation Conference (EIC), Quebec, QC, Canada, 18–21 June 2023. [Google Scholar]
- Ramroudi, F.; Yasini Ardakani, S.A.; Dehghani-tafti, A.; Khalili Sadrabad, E. Investigation of the Physicochemical Properties of Vegetable Oils Blended with Sesame Oil and Their Oxidative Stability during Frying. Int. J. Food Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Upadhyay, R.; Mahato, D.K.; Mishra, H.N. Thermal and oxidative stability assessment of synergistic blends of sunflower and sesame oils tailored for nutritionally stable composition of omega fatty acids. J. Therm. Anal. Calorim. 2019, 135, 2389–2398. [Google Scholar] [CrossRef]
- Moghtaderi, F.; Amiri, M.; Raeisi-Dehkordi, H.; Zimorovat, A.; Mohyadini, M.; Salehi-Abargouei, A. The effect of sesame, canola, and sesame-canola oils on cardiometabolic risk factors in overweight adults: A three-way randomized triple-blind crossover clinical trial. Phytother. Res. 2022, 36, 1043–1057. [Google Scholar] [CrossRef]
- Amiri, M.; Ghaneian, M.T.; Zare-Sakhvidi, M.J.; Rahmanian, M.; Nadjarzadeh, A.; Moghtaderi, F.; Raeisi-Dehkordi, H.; Zimorovat, A.; Jafari, F.; Zavar-Reza, J.; et al. The effect of canola oil compared with sesame and sesame-canola oil on cardio-metabolic biomarkers in patients with type 2 diabetes: Design and research protocol of a randomized, triple-blind, three-way, crossover clinical trial. ARYA Atheroscler. 2019, 15, 168–178. [Google Scholar] [PubMed]
- Chung, J.; Lee, J.; Choe, E. Oxidative Stability of Soybean and Sesame Oil Mixture during Frying of Flour Dough. J. Food Sci. 2004, 69, 574–578. [Google Scholar] [CrossRef]
- Heshmati, M.K.; Jafarzadeh-Moghaddam, M.; Pezeshki, A.; Shaddel, R. The oxidative and thermal stability of optimal synergistic mixture of sesame and grapeseed oils as affected by frying process. Food Sci. Nutr. 2022, 10, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Farhoosh, R.; Esmaeilzadeh Kenari, R. Anti-Rancidity Effects of Sesame and Rice Bran Oils on Canola Oil During Deep Frying. J. Am. Oil Chem. Soc. 2009, 86, 539–544. [Google Scholar] [CrossRef]
- ISO 12966-1:2014; Animal and vEgetable Fats and Oils. Gas Chromatography of Fatty acid Methyl Esters—Part 1: Guidelines on Modern Gas Chromatography of Fatty Acid Methyl Esters. ISO: Geneva, Switzerland, 2014.
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils. Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. ISO: Geneva, Switzerland, 2017.
- ISO 3960:2017; Animal and Vegetable Fats and Oils. Determination of Peroxide value—Iodometric (Visual) Endpoint Determination. ISO: Geneva, Switzerland, 2017.
- AOCS (American Oil Chemists Society). Official Methods and Recommended Practices of the American Oil Chemists Society, Calculated Iodine Value, 5th ed.; AOCS Press: Champaign, IL, USA, 1999; p. Cd 1c-8. [Google Scholar]
- Gerlei, M.; Pierson, H.; Ponçot, M.; Kahn, C.J.; Linder, M. Chemical Composition and Crystallization Behavior of Oil and Fat Blends for Spreadable Fat Applications. Foods 2024, 13, 3305. [Google Scholar] [CrossRef] [PubMed]
- Codex-Stan 210-1999; Codex Standard for Named Vegetable Oils. FAO/WHO: Rome, Italy, 2011.
- Mohammadi, T.; Hatami, M.; Mirzaee Sisaabad, Y.; Hooshiari, A.; Nejatian, M. Formulation of Blend Oil Containing Canola and Ses-ame Oils without Synthetic Antioxidants. Iranian J. Nutr. Sci. Food Technol. 2014, 9, 83–92. [Google Scholar]
- Tan, C.P.; Man, Y.B.C. Differential scanning calorimetric analysis of edible oils: Comparison of thermal properties and chemical composition. J. Am. Oil Chem. Soc. 2000, 77, 143–155. [Google Scholar] [CrossRef]
- Minar, M.; Hassanien, M.; Adel, G. Abdel-Razek, Improving the Stability of Edible Oils by Blending With Roasted Sesame Seed Oil as a Source of Natural Antioxidants. J. Asian Sci. Res. 2012, 8, 4074–4083. [Google Scholar]
- Aly, A.; Ali, H.; Abdeldaiem, M. Improvement of the sunflower oil stability by blending with moringa or sesame oils. Potravin. Slovak J. Food Sci. 2021, 15, 111–120. [Google Scholar] [CrossRef]
- El Khier, M.K.; Ishag, K.E.; Yagoub, A.E. Chemical composition and characteristics of sesame seed cultivars grown in Sudan. Res. J. Agric. Biol. Sci. 2009, 4, 761–766. [Google Scholar]
- Alhibshi, E.A.; Ibraheim, J.A.; Hadad, A.S. Effect of Heat Processing and Storage on Characteristic and Stability of Some Edible Oils. In Proceedings of the 6th Int’l Conference on Agriculture, Environment and Biological Sciences, Kuala Lumpur, Malaysia, 21–22 December 2016; pp. 105–109. [Google Scholar]
- Malavi, D.; Nikkhah, A.; Raes, K.; Van Haute, S. Hyperspectral Imaging and Chemometrics for Authentication of Extra Virgin Olive Oil: A Comparative Approach with FTIR, UV-VIS, Raman, and GC-MS. Foods 2023, 12, 429. [Google Scholar] [CrossRef]
- Hashem, H.; Almoselhy, R.I.; El-Waseif, M.; Magdy, A. Rapid Authentication of Extra Virgin Olive Oil using UV and FTIR Spectroscopy. Middle East J. Appl. Sci. 2020, 10, 263–271. [Google Scholar]
- Souza, F.H.; Almeida, L.R.; Batista, F.; Rios, M.A. UV-Visible Spectroscopy Study of Oxidative Degradation of Sunflower Biodiesel. Energy Sci. Technol. 2011, 2, 56–61. [Google Scholar]
- Kılıç, K.; Onal-Ulusoy, B.; Boyacı, İ. A novel method for color determination of edible oils in L* a* b* format. Eur. J. Lipid Sci. Technol. 2007, 109, 157–164. [Google Scholar] [CrossRef]
- Giacomelli, L.M.; Mattea, M.; Ceballos, C.D. Analysis and characterization of edible oils by chemometric methods. J. Am. Oil Chem. Soc. 2006, 83, 303–308. [Google Scholar] [CrossRef]


| Title 1 | Sesame Oil | Rapeseed Oil | |
|---|---|---|---|
| C8:0 | Caprylic | 0.1 ± 0.0 | n.d. * |
| C16:0 | Palmitic | 10.4 ± 0.2 a | 4.4 ± 0.1 b |
| C16:1 | Palmitoleic | 0.2 ± 0.0 a | 0.2 ± 0.0 a |
| C17:0 | Margaric | 0.1 ± 0.0 | n.d. |
| C17:1 | Heptadecenoic | 0.1 ± 0.0 a | 0.2 ± 0.0 b |
| C18:0 | Stearic | 2.7 ± 0.1 a | 1.4 ± 0.1 b |
| C18:1 | Oleic | 36.7 ± 0.4 a | 64.7 ± 0.3 b |
| C18:2 | Linoleic | 48.3 ± 0.2 a | 18.8 ± 0.2 b |
| C18:3(n−3) | α-Linolenic | 0.8 ± 0.1 a | 8.5 ± 0.2 b |
| C20:0 | Arachidic | 0.4 ± 0.1 a | 0.5 ± 0.1 a |
| C20:1 | Eicosenoic | 0.1 ± 0.0 a | 1.1 ± 0.2 b |
| C20:2 | Eicosadienoic | n.d. | 0.1 ± 0.0 |
| C22:0 | Behenic | 0.1 ± 0.0 a | 0.1 ± 0.0 a |
| Saturated fatty acids, % | 13.8 | 6.4 | |
| Unsaturated fatty acids, % | 86.2 | 93.6 | |
| Monounsaturated fatty acids, % | 37.1 | 66.2 | |
| Polyunsaturated fatty acids, % | 49.1 | 27.4 | |
| Iodine value, gJ2/100 g | 122.6 ± 1.0 a | 115.5 ± 1.2 b | |
| Vegetable Oil | Peroxide Value, meqO2/kg |
|---|---|
| Sesame | 1.1 ± 0.2 |
| Rapeseed | 4.3 ± 0.3 |
| Fatty Acid Composition, % | 5/95 | 10/90 | 30/70 | 50/50 | 70/30 | 90/10 | |
|---|---|---|---|---|---|---|---|
| C8:0 | Caprylic | 0.3 ± 0.1 a | 0.3 ± 0.1 a | 0.3 ± 0.1 a | 0.2 ± 0.1 a | 0.3 ± 0.1 a | 0.2 ± 0.1 a |
| C10:0 | Capric | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | n.d. * |
| C14:0 | Myristic | 0.1 ± 0.0 a | 0.1 ± 0.0 a | n.d. | n.d. | n.d. | n.d. |
| C15:1 | Pentadecenic | 0.4 ± 0.1 a | 0.3 ± 0.1 a | 0.3 ± 0.1 a | 0.2 ± 0.0 b | 0.3 ± 0.1 a | 0.2 ± 0.0 b |
| C16:0 | Palmitic | 4.9 ± 0.2 a | 5.1 ± 0.3 a | 6.2 ± 0.2 b | 7.6 ± 0.3 c | 8.3 ± 0.3 c | 9.6 ± 0.2 d |
| C16:1 | Palmitoleic | 0.2 ± 0.0 a | 0.2 ± 0.1 a | 0.2 ± 0.0 a | 0.3 ± 0.1 a | 0.2 ± 0.1 a | 0.2 ± 0.0 a |
| C17:0 | Margaric | 0.5 ± 0.1 a | 0.2 ± 0.1 b | 0.2 ± 0.1 b | 0.2 ± 0.1 b | 0.2 ± 0.1 b | 0.2 ± 0.1 b |
| C17:1 | Heptadecenoic | 0.5 ± 0.1 a | 0.4 ± 0.1 a b | 0.4 ± 0.1 a b | 0.3 ± 0.1 b | 0.4 ± 0.2 a b | 0.3 ± 0.1 b |
| C18:0 | Stearic | 1.3 ± 0.1 a | 1.7 ± 0.2 b | 2.3 ± 0.1 c | 2.7 ± 0.2 d | 3.3 ± 0.3 e | 3.1 ± 0.1 e |
| C18:1 | Oleic | 61.7 ± 0.4 a | 60.3 ± 0.3 b | 55.4 ± 0.4 c | 49.6 ± 0.5 d | 45.2 ± 0.2 e | 38.6 ± 0.3 f |
| C18:2 | Linoleic | 20.0 ± 0.2 a | 21.5 ± 0.3 b | 26.6 ± 0.2 c | 32.9 ± 0.3 d | 36.8 ± 0.2 e | 45.1 ± 0.2 f |
| C18:3(n−3) | α-Linolenic | 8.3 ± 0.1 a | 8.0 ± 0.2 b | 6.3 ± 0.1 c | 4.9 ± 0.3 d | 3.5 ± 0.2 e | 1.6 ± 0.1 f |
| C20:0 | Arachidic | 0.5 ± 0.1 a | 0.5 ± 0.1 a | 0.5 ± 0.0 a | 0.4 ± 0.1 a | 0.5 ± 0.1 a | 0.4 ± 0.1 a |
| C20:1 | Eicosenoic | 1.0 ± 0.1 a | 1.0 ± 0.1 a | 0.9 ± 0.2 a | 0.5 ± 0.2 b | 0.5 ± 0.1 b | 0.3 ± 0.1 b |
| C20:2 | Eicosadienoic | n.d. | 0.1 ± 0.0 a | 0.1 ± 0.0 a | n.d. | 0.1 ± 0.0 a | n.d. |
| C22:0 | Behenic | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.2 ± 0.1 a | 0.1 ± 0.0 a |
| C22:1 | Erucic | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | n.d. | 0.1 ± 0.0 a | 0.1 ± 0.0 a |
| Saturated fatty acids, % | 7.8 | 8.1 | 9.7 | 11.3 | 12.9 | 13.6 | |
| Unsaturated fatty acids, % | 92.2 | 91.9 | 90.3 | 88.7 | 87.1 | 86.4 | |
| Monounsaturated fatty acids, % | 63.9 | 62.3 | 57.3 | 50.9 | 46.7 | 39.7 | |
| Polyunsaturated fatty acids, % | 28.3 | 29.6 | 33.0 | 37.8 | 40.4 | 46.7 | |
| Iodine value, gJ2/100 g | 114.5 ± 1.0 a | 115.1 ± 1.4 a | 115.3 ± 1.0 a | 117.6 ± 1.8 ab | 116.9 ± 1.1 a | 119.1 ± 0.8 b | |
| SO/RSO | Peroxide Value, meqO2/kg |
|---|---|
| 5/95 | 4.5 ± 0.2 d |
| 10/90 | 5.0 ± 0.2 e |
| 30/70 | 2.7 ± 0.1 c |
| 50/50 | 2.2 ± 0.1 b |
| 70/30 | 2.5 ± 0.1 c |
| 90/10 | 1.6 ± 0.2 a |
| Crystallization | Melting | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SO/RSO | T1, °C | H1, J/g | T2, °C | H2, J/g | H3, J/g | T3, °C | T1, °C | T2, °C | H, J/g |
| 100/0 | −9.3 | −5.575 | - | - | −27.85 | −53.2 | −28.8 | −15.0 | 65.48 |
| 90/10 | −9.7 | −5.625 | - | - | −29.24 | −52.3 | −27.9 | −14.9 | 64.06 |
| 70/30 | −11.3 | −4.101 | - | - | −35.29 | −50.4 | −26.6 | −14.9 | 66.91 |
| 50/50 | −11.8 | −2.184 | - | - | −37.02 | −47.8 | −26.0 | −14.4 | 63.99 |
| 30/70 | −14.9 | −1.444 | - | - | −46.6 | −46.1 | - | −13.6 | 69.63 |
| 10/90 | −16.9 | −1.267 | −35.0 | −1.140 | −49.86 | −44.7 | - | −12.5 | 69.38 |
| 5/95 | −17.5 | −0.652 | −35.0 | −2.059 | −53.6 | −41.8 | - | −11.8 | 71.35 |
| 0/100 | −18.0 | −0.308 | −35.1 | −2.412 | −58.38 | −40.9 | - | −12.1 | 74.21 |
| R2 | 0.9838 | 0.9459 | 0.9741 | 0.9655 | 0.9123 | 0.7463 | |||
| Refractive Indices | SO | 5/95 | 10/90 | 30/70 | 50/50 | 70/30 | 90/10 | RSO |
| 1.4729 ± 0.0001 d | 1.4711 ± 0.0002 a | 1.4711 ± 0.0002 a | 1.4711 ± 0.0003 a | 1.4713 ± 0.0002 a | 1.4720 ± 0.0001 bc | 1.4724 ± 0.0001 c | 1.4718 ± 0.0002 b |
| Sesame Oil/Rapeseed Oil | L | a* | b* |
|---|---|---|---|
| SO | 97.3 ± 0.7 a | 1.1 ± 0.4 g | 14.7 ± 1.3 i |
| 90/10 | 96.9 ± 0.2 b | 1.5 ± 0.1 f | 23.7 ± 2.1 g |
| 70/30 | 96.3 ± 1.2 c | 5.3 ± 0.2 a | 33.1 ± 1.6 f |
| 50/50 | 95.9 ± 1.1 d | 2.9 ± 0.1 e | 55.5 ± 1.1 e |
| 30/70 | 95.5 ± 0.7 e | 3.6 ± 0.5 d | 68.1 ± 0.9 d |
| 10/90 | 95.2 ± 0.5 e | 4.2 ± 0.4 c | 78.5 ± 0.7 c |
| 5/95 | 95.3 ± 0.1 e | 1.1 ± 0.3 g | 82.8 ± 1.1 b |
| RSO | 95.2 ± 0.3 e | 4.4 ± 0.5 b | 87.8 ± 0.8 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marudova, M.; Viraneva, A.; Antova, G.; Nikolova, K.; Petkova, Z.; Teneva, O. Physico-Chemical Characterization of Sesame/Rapeseed Oil Mixtures. Appl. Sci. 2025, 15, 704. https://doi.org/10.3390/app15020704
Marudova M, Viraneva A, Antova G, Nikolova K, Petkova Z, Teneva O. Physico-Chemical Characterization of Sesame/Rapeseed Oil Mixtures. Applied Sciences. 2025; 15(2):704. https://doi.org/10.3390/app15020704
Chicago/Turabian StyleMarudova, Maria, Asya Viraneva, Ginka Antova, Krastena Nikolova, Zhana Petkova, and Olga Teneva. 2025. "Physico-Chemical Characterization of Sesame/Rapeseed Oil Mixtures" Applied Sciences 15, no. 2: 704. https://doi.org/10.3390/app15020704
APA StyleMarudova, M., Viraneva, A., Antova, G., Nikolova, K., Petkova, Z., & Teneva, O. (2025). Physico-Chemical Characterization of Sesame/Rapeseed Oil Mixtures. Applied Sciences, 15(2), 704. https://doi.org/10.3390/app15020704







