Abstract
Background/Objectives: Computer-assisted mandibular reconstruction requires sophisticated technical expertise alongside surgical knowledge. This study aims to establish and validate an efficient collaborative protocol between oral and maxillofacial surgeons and bioengineers for virtual surgical planning in mandibular reconstruction. Methods: We developed a structured protocol with four sequential phases: (1) generation of 3D models from CT data, (2) virtual resection planning, (3) reconstruction design, and (4) surgical guide fabrication. Protocol efficiency was assessed through seven simulation trials measuring planning duration and required revisions. Clinical validation was performed in four mandibular reconstruction cases. Accuracy was evaluated by comparing virtual surgical plans to postoperative outcomes using 3-matic 13.0 software analysis. Results: Protocol implementation showed consistent efficiency across simulations with a mean planning duration of 2.86 working days (SD = 1.35). Only two of seven simulations required design revisions. Clinical application in four cases (three ameloblastomas, one odontogenic myxoma) demonstrated high precision with a mean virtual-to-actual discrepancy of 0.90 mm (SD = 0.34). Successful reconstructions were achieved across varying defect spans (29–53 mm) using both bicortical deep circumflex iliac artery (DCIA) flaps and monocortical iliac block bone grafts. The collaborative workflow resulted in optimized surgical guide design, reduced planning iterations, and improved surgical precision. Conclusions: The established surgeon–bioengineer collaborative protocol enhances the efficiency and accuracy of computer-assisted mandibular reconstruction while making advanced surgical planning techniques more accessible. While initial results are promising, future studies with larger patient cohorts and extended follow-up periods are needed to fully validate the protocol’s long-term benefits and broader applicability.
1. Introduction
Mandibular reconstruction after tumor resection is one of the most challenging procedures in oral and maxillofacial surgery [1]. The complex 3D anatomy of the mandible and its critical role in esthetics, mastication, and phonation require high-precision reconstruction to restore form and function [2]. Several methods have been developed for mandibular reconstruction, each with its own advantages and limitations. Autologous bone grafts include non-vascularized grafts from the iliac crest, rib, or calvarium. While they are easy to harvest, they are limited in size and may have high resorption rates for larger defects. Vascularized free flaps have become the gold standard for large mandibular defects. Common donor sites include the fibula, iliac crest, scapula, and radial forearm. Each donor site has specific advantages in terms of bone stock, soft tissue availability, and donor site morbidity [3,4,5].
Traditionally, surgeons have relied on their experience and intraoperative judgment to harvest and position bone grafts. However, this approach can result in unfavorable esthetic and functional outcomes. More recently, the advent of virtual surgical planning and 3D printing technologies has revolutionized the approach to mandibular reconstruction [6,7,8]. These technologies offer the potential for increased accuracy, shorter operative times, and improved patient outcomes. CAD/CAM technology allows for detailed preoperative planning based on high-resolution computed tomography (CT) images. This allows surgeons to accurately plan resection margins, design customized bone grafts, and digitally simulate mandibular reconstruction before surgery [8].
The virtual surgical planning process involves a number of sophisticated steps [9]. First, high-resolution CT scans of the patient’s mandible and potential donor site, such as the iliac crest [10], are transformed into intricate three-dimensional renderings [11]. Using state-of-the-art software, surgeons meticulously perform a virtual tumor resection [12], create an optimal reconstructive design, and delineate the exact dimensions and contours of the required bone graft [13]. This advanced level of preoperative planning not only maximizes donor bone utilization but also ensures superior congruency of the reconstructed mandible segment.
In addition, the ability to create patient-specific cutting and positioning guides through additive manufacturing represents a paradigm shift in the translation of virtual surgical plans to the surgical field. These customized guides serve as exquisite templates for mandibular resection and bone graft procurement, ensuring a high degree of fidelity between the virtual plan and the actual surgical procedure. The use of these meticulously crafted guides has demonstrably shortened intraoperative decision-making processes and significantly improved the precision of mandibular reconstruction.
Recent studies have demonstrated the benefits of computer-assisted mandibular reconstruction. These benefits include increased precision of osteotomies, decreased operative time, and improved esthetic outcomes. Empirical studies have shown that computer-guided techniques can achieve reconstructions with an accuracy of 1–2 mm relative to the virtual plan, a level of precision that remains elusive with conventional methods. Furthermore, the implementation of virtual planning and three-dimensional printed surgical guides has been associated with reduced ischemia time for free flaps, potentially leading to improved flap survival rates [14,15].
Despite these benefits, computer-aided reconstruction is not without its challenges. Implementation of this approach requires specialized software, expertise in virtual surgical planning, and access to additive manufacturing technology. In addition, there is a significant learning curve associated with integrating these advanced technologies into established surgical practices. The additional cost and time required for preoperative planning must also be weighed against the potential benefits of these techniques [16,17].
The complexity and time-intensive nature of computer-aided mandibular reconstruction, coupled with the steep learning curve for surgeons, underscores the critical need for collaboration between oral and maxillofacial surgeons and bioengineers. While surgeons have the clinical expertise and understanding of patient-specific needs, bioengineers bring specialized knowledge in 3D modeling, computer-aided design, and additive manufacturing processes. This interdisciplinary collaboration can significantly reduce the burden on surgeons, allowing them to focus on critical clinical decisions while utilizing the technical expertise of bioengineers [18].
Bioengineers can play a critical role in various stages of the virtual surgical planning process, from the initial segmentation of CT scans and creation of 3D models to the design and optimization of patient-specific cutting and positioning guides. Their expertise can ensure the technical accuracy and manufacturability of these guides, potentially reducing errors and improving the overall efficiency of the surgical procedure. In addition, bioengineers can contribute to the continuous improvement of the virtual planning process by incorporating the latest advances in software and manufacturing technologies. The synergy between surgical expertise and bioengineering skills has the potential to overcome many of the challenges associated with computer-assisted mandibular reconstruction. By establishing a structured collaborative protocol, surgical teams can more effectively integrate these advanced technologies into their practice, potentially leading to improved patient outcomes, reduced operative times, and more predictable esthetic and functional results.
This paper aims to address the need for such collaboration by proposing an efficient working protocol between surgeons and bioengineers in the process of virtual surgical planning for mandibular reconstruction. By detailing the workflow, leveraging cloud-based collaboration software for efficient collaboration, and highlighting key decision points where interdisciplinary expertise is most beneficial, this study aims to provide a blueprint for effective collaboration that improves the quality of patient care and makes advanced reconstructive techniques more accessible to the broader surgical teams.
2. Virtual Surgical Planning Protocol Between Surgeons and Bioengineers
2.1. Generation of 3D Models
Accurate segmentation of the mandible and lesions from cone-beam computed tomography (CBCT) data to create stereolithography (STL) files is critical for both precise surgical guide fabrication and accurate surgical planning. To take full advantage of virtual surgical planning, which provides a three-dimensional understanding of anatomical structures and lesions, it is necessary to segment not only the external shape of the mandible and lesions, but also anatomical structures such as tooth roots and inferior alveolar nerve bundles. However, these processes are time-consuming.
Hounsfield unit slices are used to segment the mandible and surrounding anatomical structures for virtual surgical planning. The resulting 3D mandibular model, while too large for computer-aided design due to the inclusion of internal bone structures, is advantageous for virtual surgical planning because it allows simultaneous visualization of anatomical structures, such as tooth roots and the inferior alveolar nerve bundle. For computer-aided planning, a 3D model of the mandible focused on the cortical bone area without inappropriate holes is preferred, and this task is best performed by a bioengineer.
Lesion segmentation is the most critical aspect of virtual surgical planning materials. Accurate lesion identification and appropriate safety margin setting are essential to reduce the risk of recurrence and minimize deviations from the plan during actual surgery. Therefore, lesion segmentation should be performed by the surgeon. This allows identification of ambiguous areas in the lesion and can be supplemented with other medical imaging data, such as magnetic resonance imaging (MRI) or positron emission tomography–computed tomography (PET-CT). It also assists in setting resection margins during virtual surgery planning.
Surgeon’s role: Provide high-quality CT scans, identify critical anatomical structures, and segment lesions.
Bioengineer’s role: Segment CT data to create accurate 3D models of the mandible.
2.2. Setting the Resection Margin
The surgeon sets the resection margin based on the Hounsfield unit (HU) threshold-based mandible model and the lesion model. Adding a safety margin offset to the 3D lesion model can assist in setting the resection margin. The HU threshold-based mandibular model, including internal bone structures, is critical in determining whether to preserve or sacrifice adjacent teeth and the inferior alveolar nerve. It also allows the surgical approach (intraoral or submandibular) to be determined and instrument accessibility to be considered. This three-dimensional approach to structures and lesions allows planning of a safe yet conservative resection margin. Saving the resection margin as a plane is beneficial for subsequent reconstruction planning and collaboration between the surgeon and the bioengineer.
Surgeon’s role: Define resection margins based on clinical judgment and 3D visualization.
Bioengineer’s role: Implement resection margins in the 3D model, highlight potential problems.
2.3. Reconstruction Planning
Based on the established resection margin, a plan is developed to reconstruct the defect area. This process requires close collaboration between the surgeon and bioengineer, led by the surgeon. The surgeon can formulate a surgical plan considering the length, width, height, and shape of the defect area, as well as potential donor sites such as fibular or iliac crest free flaps. A partial osteotomy of the bone flap may be necessary given the curvature of the defect area, and the characteristics of the soft tissue flap and the position of the donor site’s supply vessels should also be considered.
The surgeon provides the overall direction for reconstruction based on clinical experience and anatomical knowledge, while the bioengineer implements and fine-tunes this in 3D software. The final reconstruction plan is determined through several rounds of feedback and modifications. After the surgeon approves the final reconstruction plan, the template model is printed, and the bioengineer begins designing the guide.
Surgeon’s role: Select donor site and determine overall direction of reconstruction and give final approval.
Bioengineer’s role: Fine-tune and optimize the reconstruction plan.
2.4. Fabrication of Surgical Guides
Surgical guide fabrication is the final stage of virtual surgical planning and is critical to the accurate execution of the plan during the actual surgery. Surgical guides can be broadly categorized into mandibular resection guides and reconstruction guides. This process can be performed by the bioengineer under the guidance of the surgeon.
For the mandibular resection guide, the fitting area is determined considering the lesion characteristics and approach method. An extraoral approach can take advantage of the mandibular inferior border curvature, while an intraoral approach can benefit from alveolar bone adaptation. The Resection Guides can also be used to position the mandible by connecting the proximal and distal portions, and screw holes can be preset for accurate fitting of custom plates.
Reconstruction guides are fabricated with donor site characteristics in mind. Osteotomy lines for partial bone cuts and screw holes for accurate custom plate fitting are pre-set to ensure plate positioning according to the surgical plan.
Surgeon’s Role: Define the surgical approach and determine the overall shape of the surgical guide.
Bioengineer’s role: Design and 3D print patient-specific surgical guides.
2.5. Collaboration Software
The efficiency of surgeon–bioengineer collaboration was enhanced through a specialized software ecosystem. The surgeon primarily used Mimics 18.0 software (Materialise, Leuven, Belgium) for initial segmentation and surgical planning, while bioengineers utilized Reconeasy Collabo software (SEEANN solution, Seoul, Republic of Korea) for design optimization. Data exchange occurred through standardized STL files, ensuring compatibility across platforms.
A key innovation in the workflow was the implementation of Reconeasy Collabo software, a cloud-based collaboration platform. This software enabled the following:
- Real-time 3D visualization of surgical plans on both computers and mobile devices;
- Asynchronous communication through embedded commenting systems;
- Version control of design iterations;
- Remote accessibility for all team members.
3. Simulation of Surgeon–Bioengineer Collaboration
To validate the efficiency of our protocol, simulations mirroring actual patient surgery schedules were conducted. When the actual surgical schedule of a patient requiring mandibular reconstruction surgery among the patients visiting Chosun University Dental Hospital was set, a simulation was performed based on the patient’s medical image data and surgical plan. Seven simulations were performed, tracking the duration and number of redesign iterations required. All collaborations took place between the same surgeon team and bioengineer team. (Table 1) Redesign iterations were caused by improper guide placement, guide size that did not match the actual surgical approach, and improper donor site location. All guides and template models were 3D printed using resin. The total printing time was 8 to 10 h depending on the number and size of guides per case.

Table 1.
Simulation result of surgeon–bioengineer collaboration.
4. Materials and Methods
During the virtual surgical planning (VSP) simulation process, actual surgery was applied in a total of four cases. EO gas sterilization was performed on the 3D-printed surgical guide and template model, and the surgery was performed by applying it during the actual surgery. A postoperative CT was taken two weeks after the surgery, and a 3D model of the mandible was created from the postoperative CT. Accuracy analysis was performed on the created postoperative mandible model and the preoperative VSP model. For the accuracy analysis, the preoperative and postoperative models were overlapped based on the condyle of the mandible, and the point-to-point differences of the two overlapped models were analyzed to measure the average error and standard deviation.
5. Results
Four mandibular reconstruction surgeries were performed, including the cases described above. The details of each case are summarized in Table 2. The preoperative planning and postoperative results were evaluated in the same manner as described above and summarized in Table 3.

Table 2.
Details of patients.

Table 3.
Comparison of preoperative plan and postoperative results.
6. Case Presentation
A 25-year-old female was referred to the Department of Oral and Maxillofacial Surgery from a local dental clinic with a primary concern of an intraosseous lesion located below the root apex of the right mandibular first molar. The patient did not report any clinical symptoms. Panorama revealed a multilocular radiolucent lesion with relatively well-defined borders and sclerotic margins in the region of the right mandibular molar. In addition, root resorption was observed in the right mandibular second premolar and first molar (Figure 1). Cone-beam computed tomography (CBCT) revealed thinning of the lingual cortical bone in the areas surrounding the right mandibular second premolar and first molar, as well as inferior displacement of the right mandibular canal (Figure 2). Both the right mandibular second premolar and first molar exhibited slight mobility and percussion sensitivity, while electric pulp testing was positive. Incisional biopsy was the ameloblastoma. To treat the lesion and minimize the risk of recurrence, resection and reconstruction were planned, and virtual surgical planning was performed in collaboration with the SEEANN solution (SEEANN solution, Seoul, Korea). To ensure accurate translation of the VSP to the actual surgery, template models were fabricated for preoperative simulation and intraoperative reference, and surgical guides were designed with multiple registration points for optimal positioning verification during the procedure.
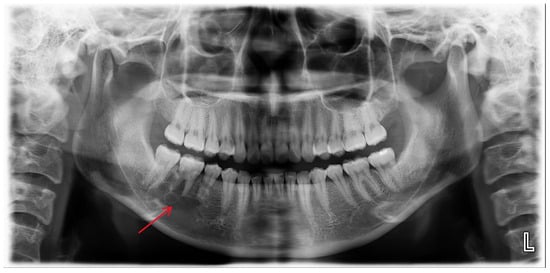
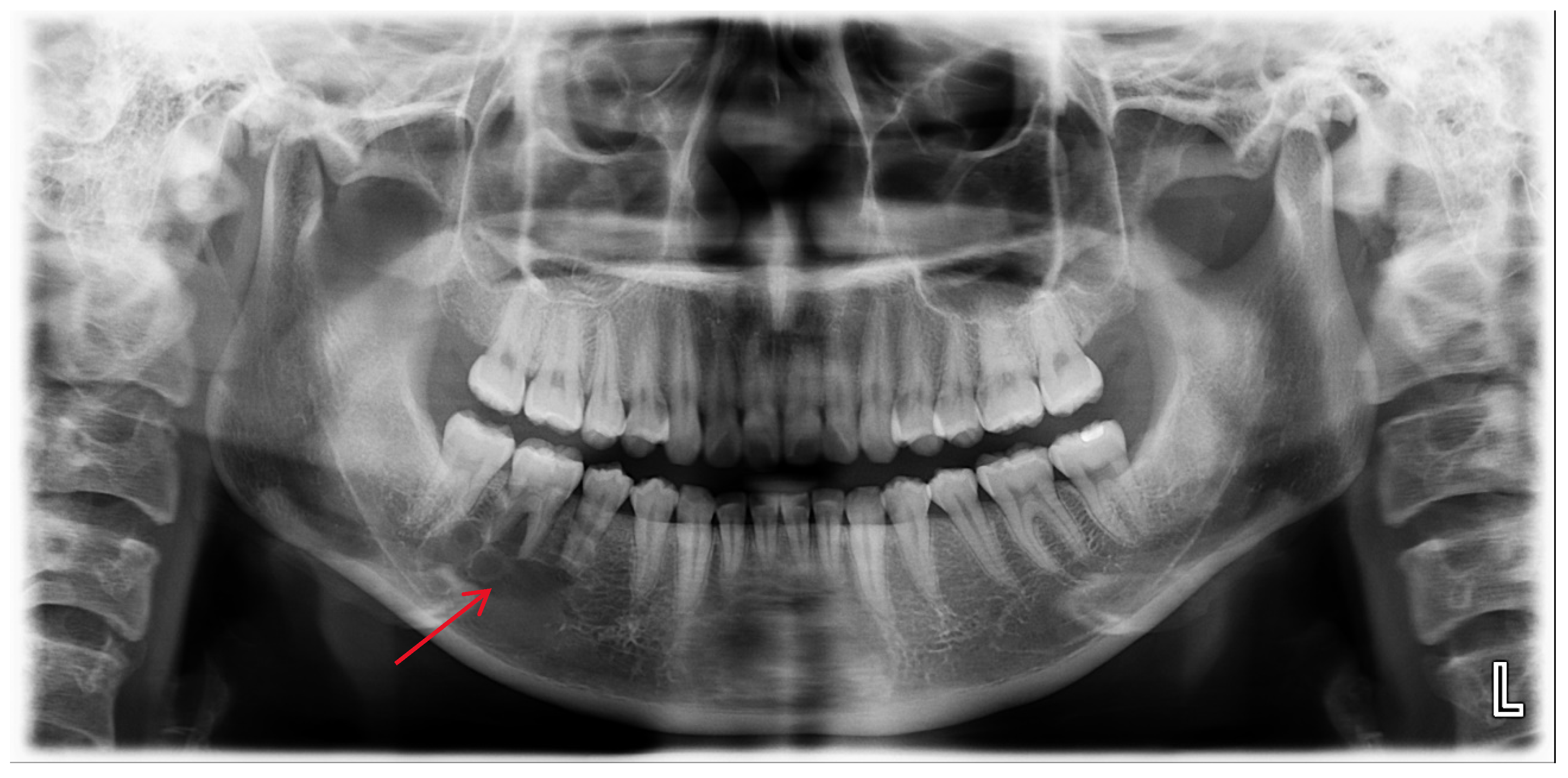
Figure 1.
A multilocular radiolucent lesion with relatively well-defined borders and sclerotic margins in the right mandibular molar region, along with root resorption of the right mandibular second premolar and first molar (red arrow).
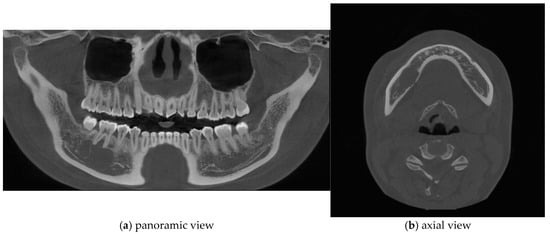
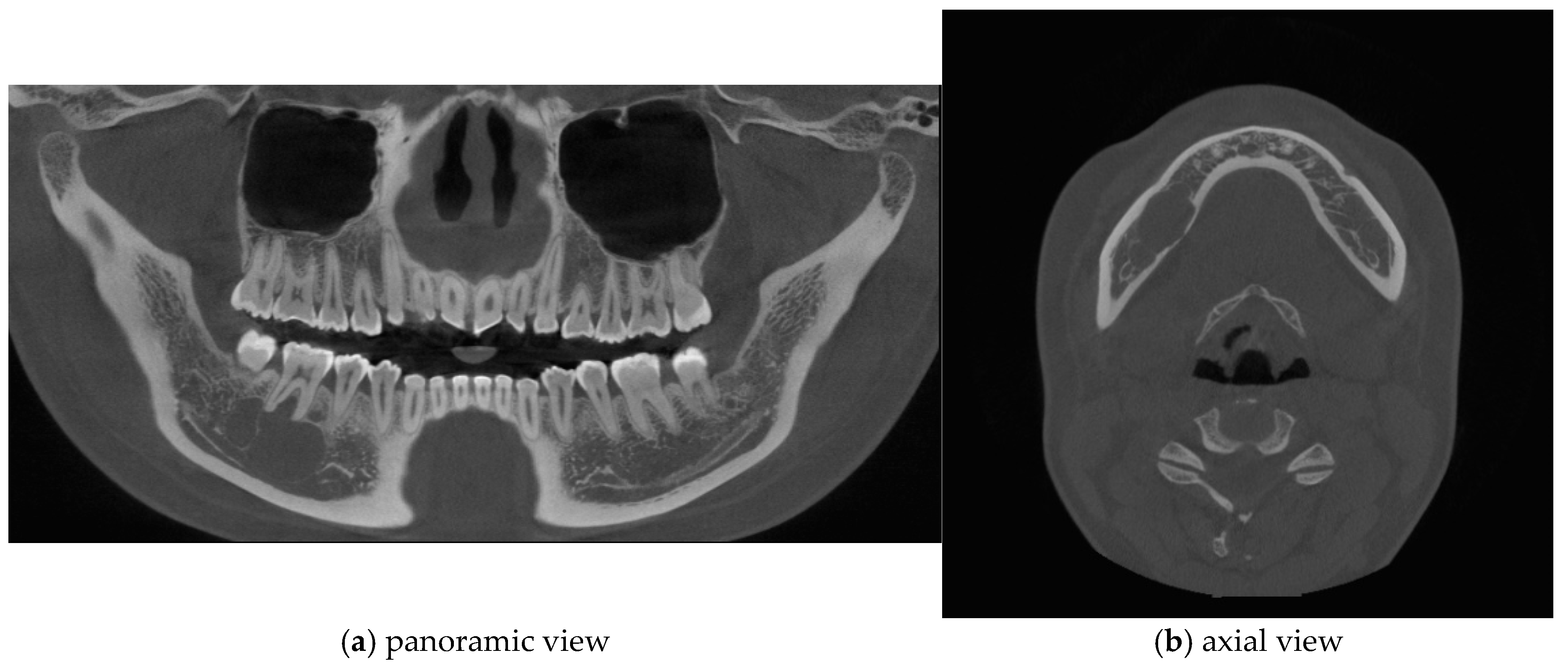
Figure 2.
Cone-beam computed tomography (CBCT) imaging revealed thinning of the lingual cortical bone in the region of the right mandibular second premolar and first molar, as well as inferior displacement of the right mandibular canal.
6.1. Generation of 3D Models
Based on CBCT DICOM data, the surgeon created a mandible 3D model based on HU threshold using mimics 18.0 software (Materialise, Leuven, Belgium) and segmented the lesion to create a 3D model (Figure 3). SEEANN solution created a mandible 3D model.
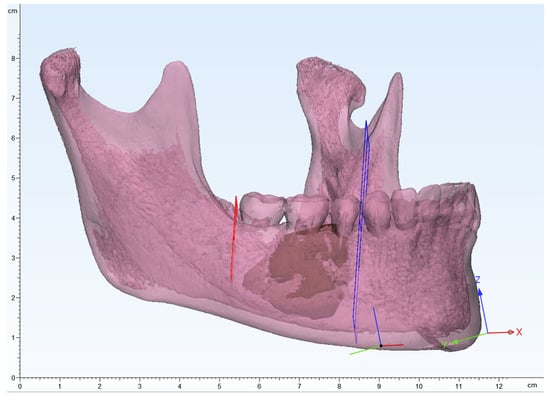
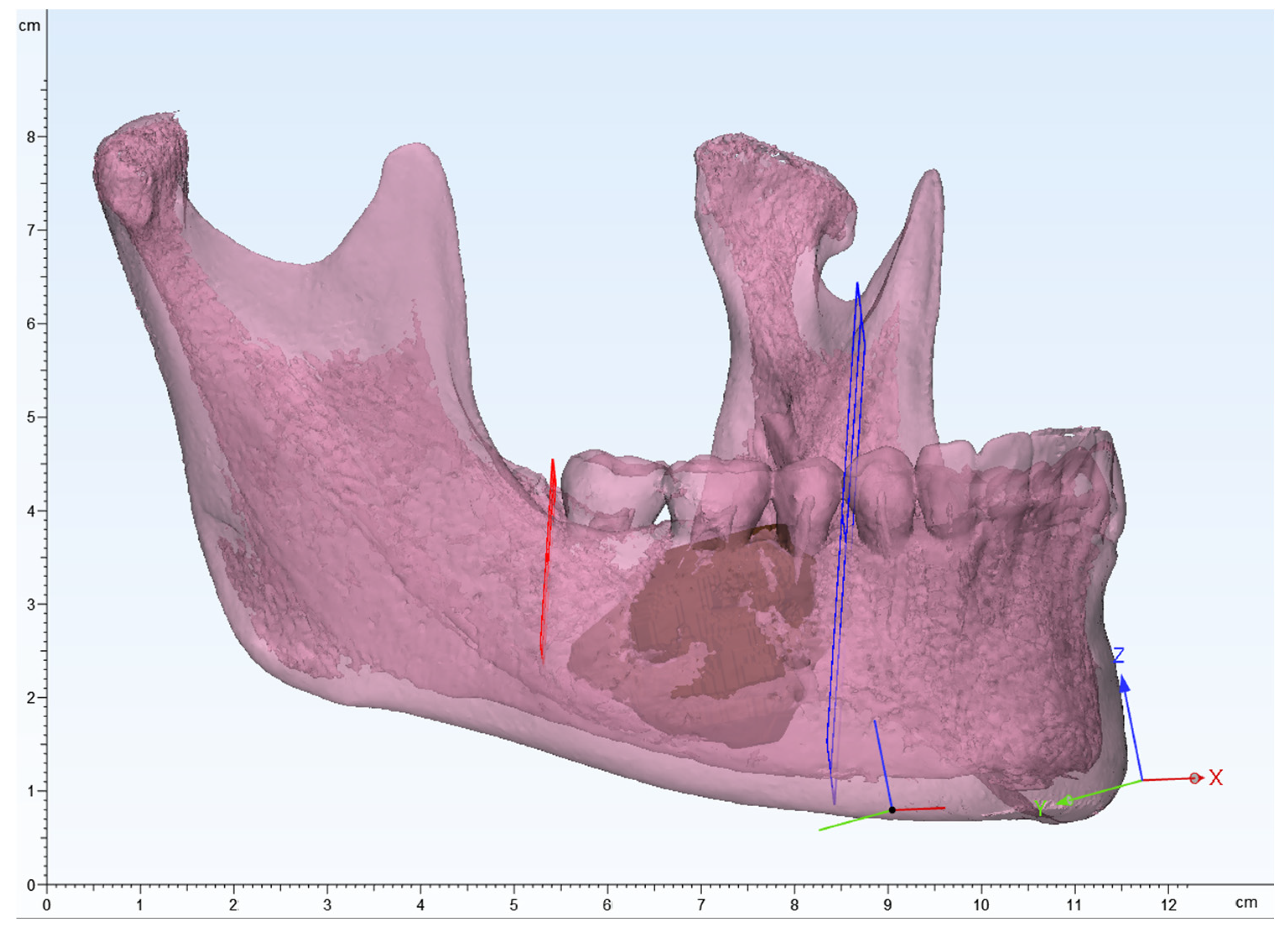
Figure 3.
3D modeling image of the mandible, segmented lesion, and determining the resection margin. Red and blue planes indicate the resection margin.
6.2. Setting the Resection Margin
The surgeon planned an osteotomy based on the size and aggressiveness of the lesion and determined the resection margin (Figure 3). The relevant data were transferred to the bioengineer.
6.3. Reconstruction Planning
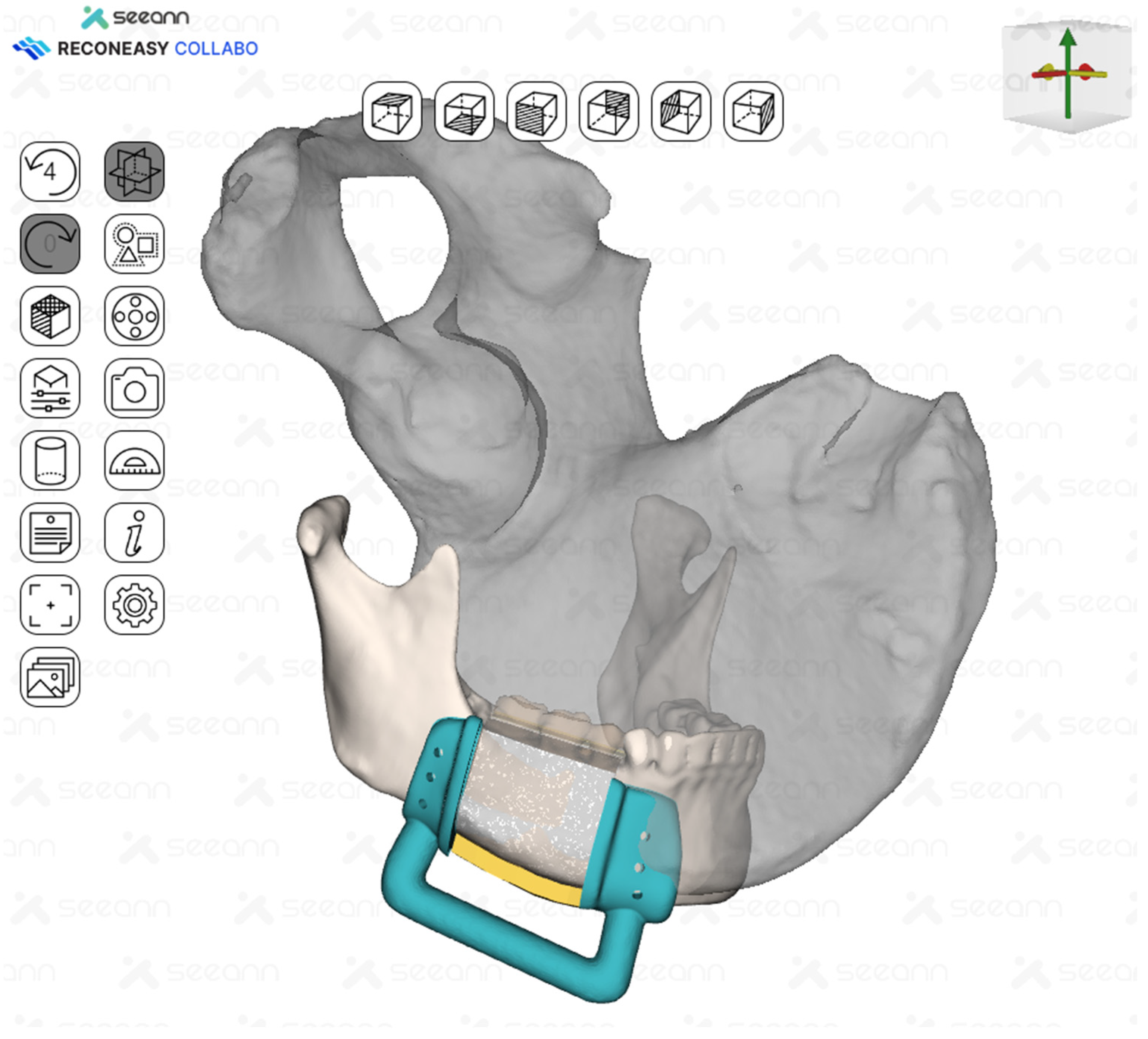
Given the length of the resected mandible, reconstruction with a deep circumflex iliac artery (DCIA) flap was planned. In collaboration with a bioengineer, the area that could reproduce the natural curvature of the mandible while minimizing donor site morbidity was planned (Figure 4 and Figure 5).
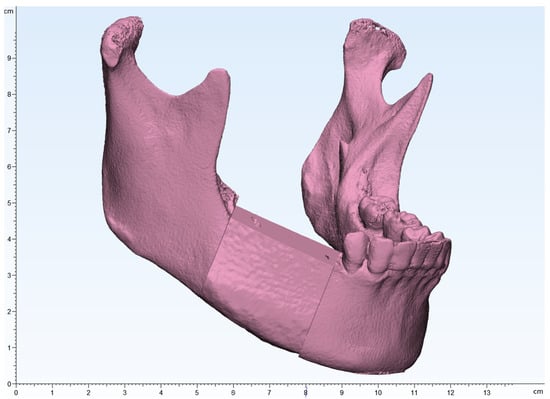
Figure 4.
The mandibular and iliac bone images were merged to design an iliac flap corresponding to the tumor resection margins.
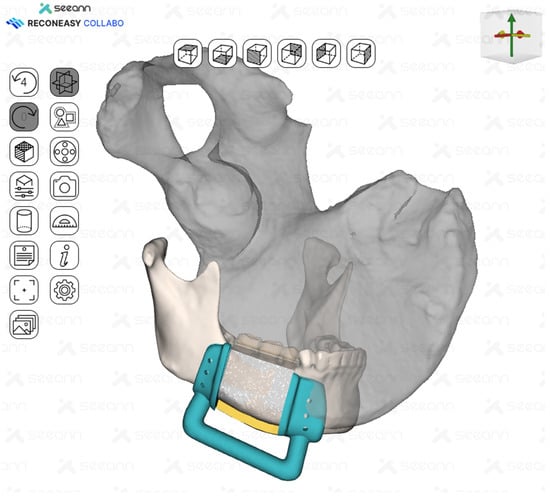
Figure 5.
Surgical guides for tumor resection and iliac flap harvesting were fabricated.
6.4. Fabrication of Surgical Guides
A surgical guide was fabricated by making a full cover of the inferior border of the mandible. In addition, the mesial resection guide and the distal resection guide were connected to each other for fabrication so that they could be used as positioning guides for the mandible. The design and control of the guide was performed in close collaboration between the surgeon and the bioengineer using Reconeasy Collabo software (Figure 5). The patient-specific plate was also fabricated.
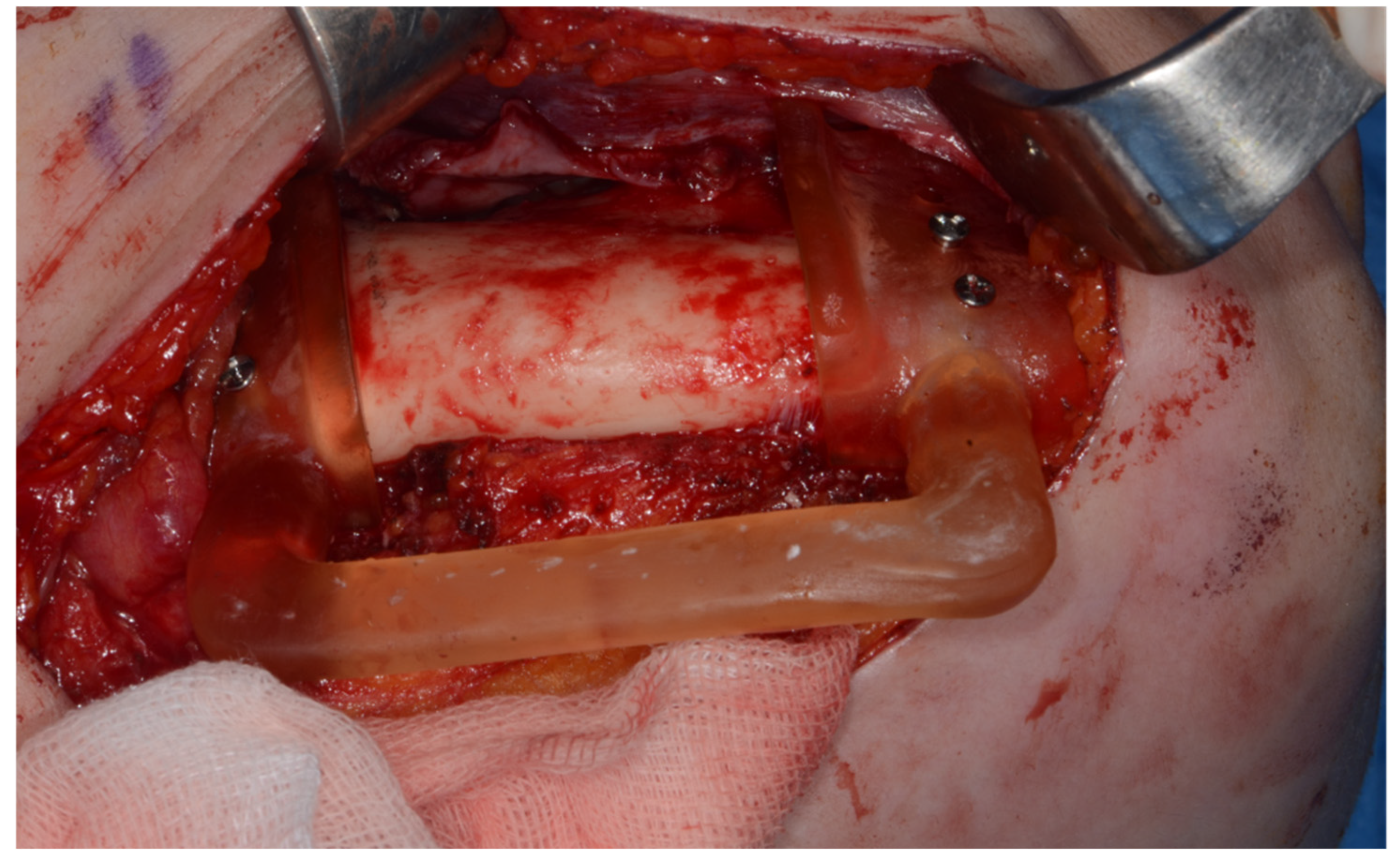
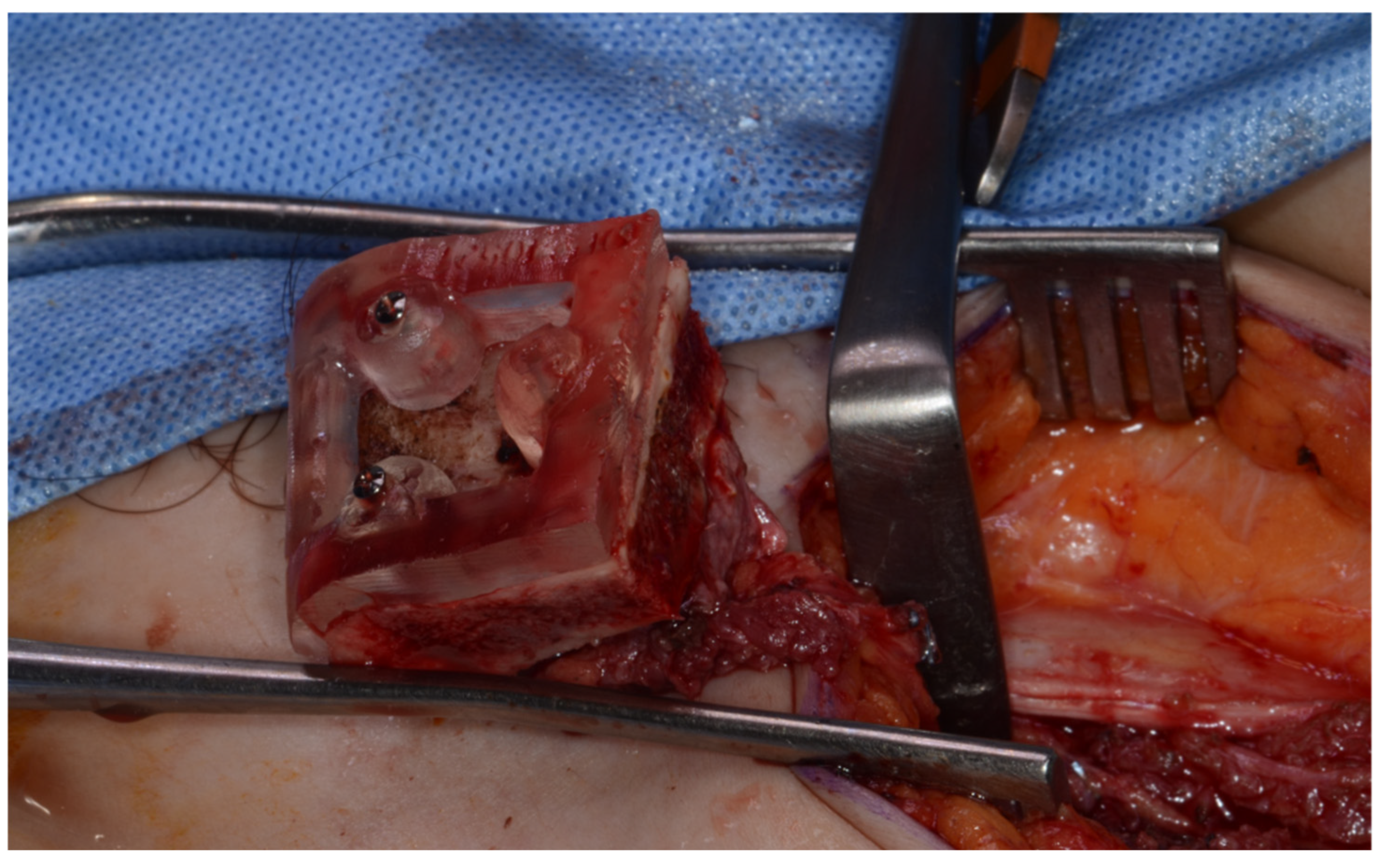
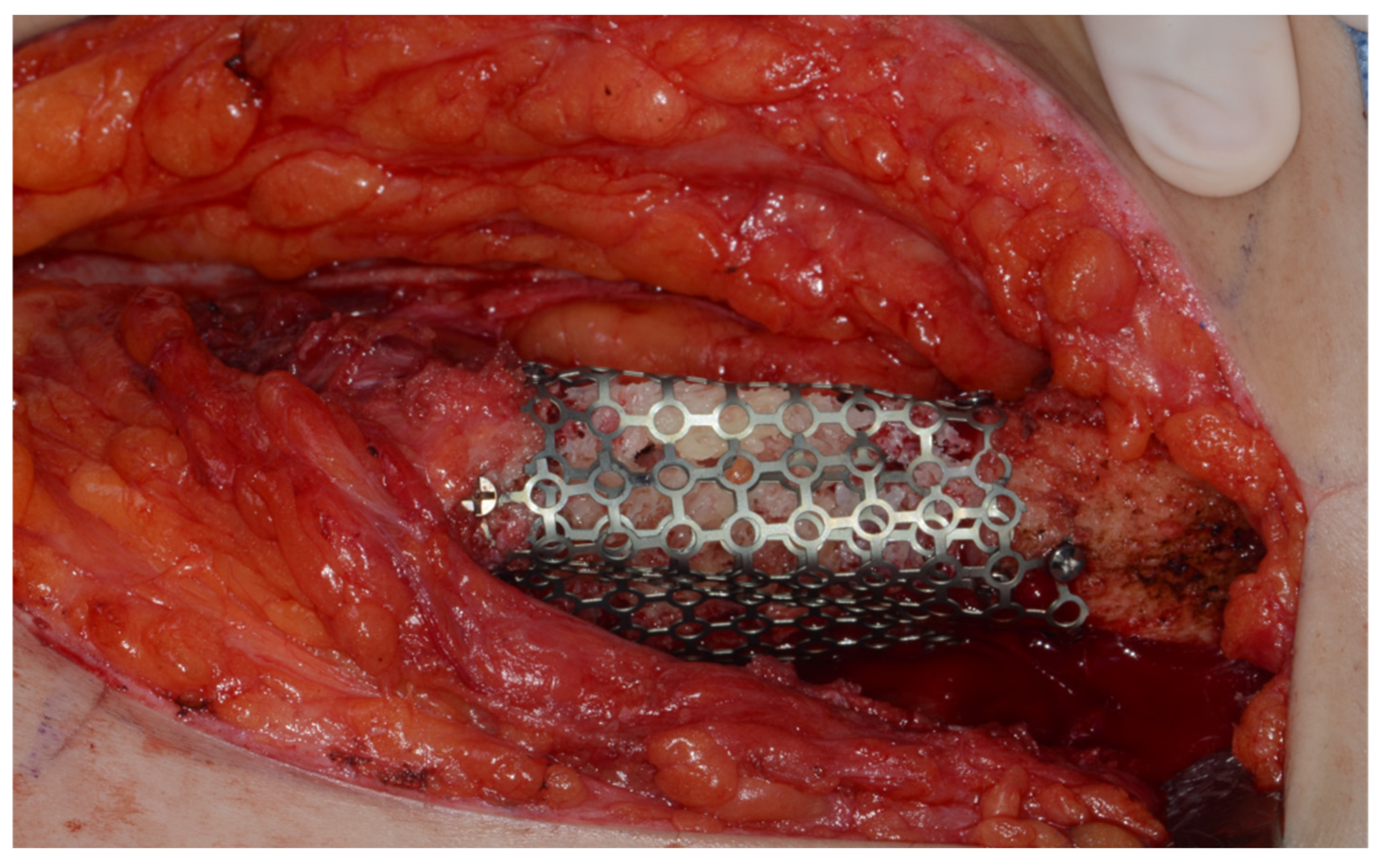
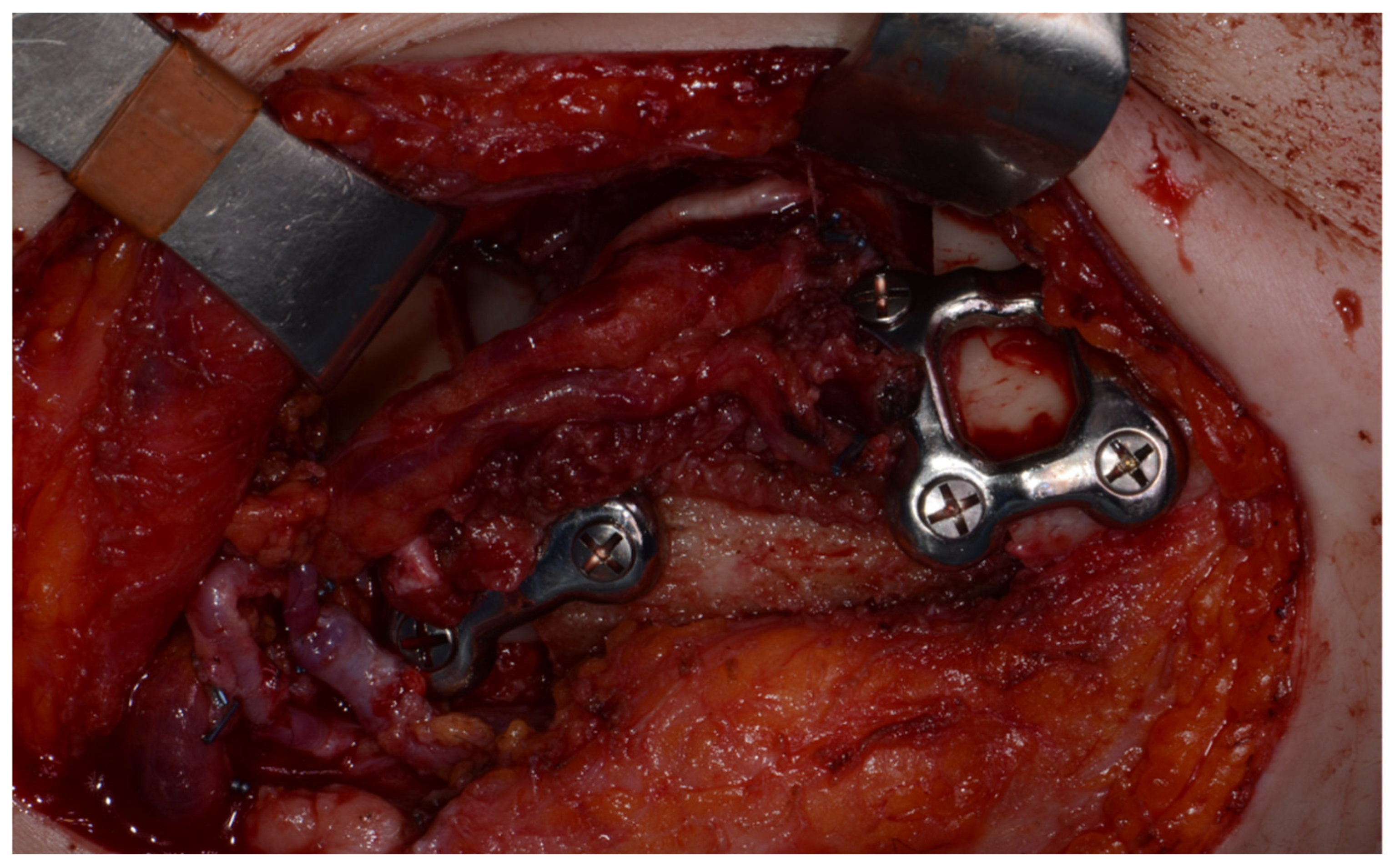
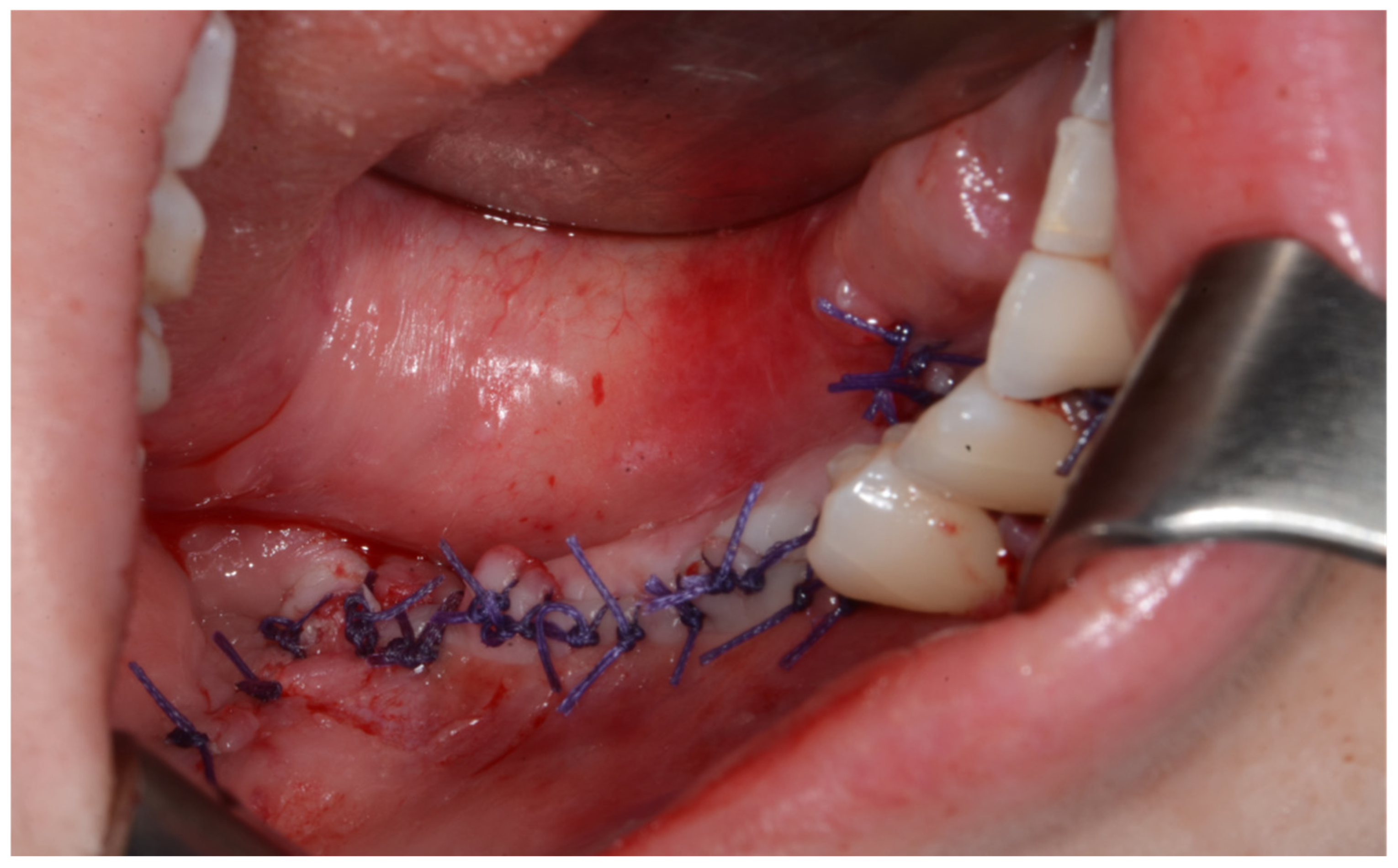
Surgery was performed under general anesthesia. #45, 46, and 47 were extracted. Segmental mandibulectomy was performed using a surgical guide through a submandibular approach. (Figure 6). The left iliac crestal DCIA flap was harvested according to the surgical guide. (Figure 7) To minimize the morbidity of the donor site, the donor site was reconstructed using titanium mesh and allobone chip graft. (Figure 8) Microvascular anastomosis was performed using the facial artery and facial vein. Fixation of the resected mandible and the bicortical iliac bone flap was achieved using a customized reconstruction plate (Cusmedi ©, Sungnam, Republic of Korea) designed to have a thickness of 2 to 3 mm according to the patient’s mandibular morphology and the periphery of the iliac bone flap and 3D printed by the selective laser melting (SLM) method and eight 2.3 mm reconstruction screws. (Figure 9). A silastic drain was inserted into the submandibular region and sutured. (Figure 10) The final diagnosis was ameloblastoma.
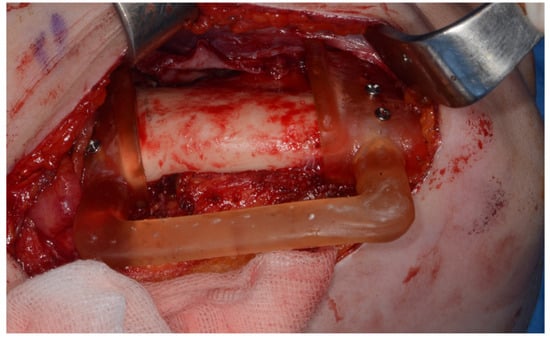
Figure 6.
Surgical guide for segmental mandibulectomy.
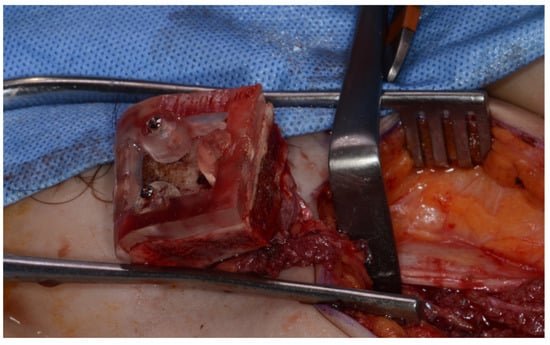
Figure 7.
Harvested DCIA iliac crestal bone and internal oblique muscle flap with an iliac guide.
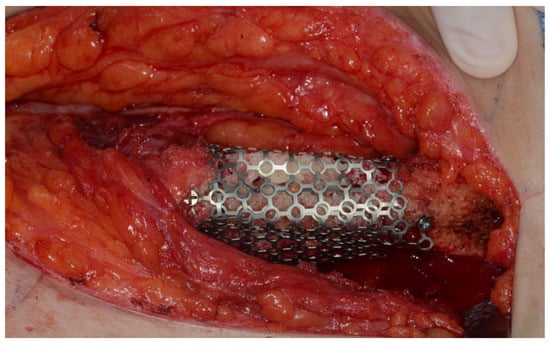
Figure 8.
Donor site reconstruction with titanium mesh and allograft.
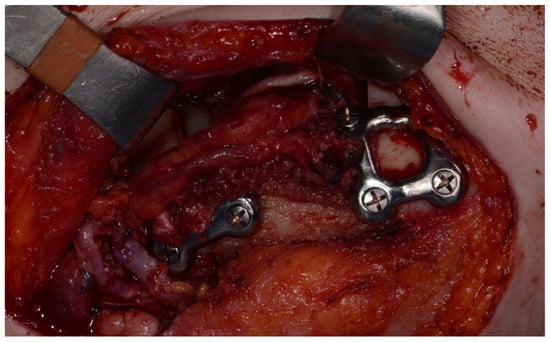
Figure 9.
Mandible fixation with customized reconstruction plate.
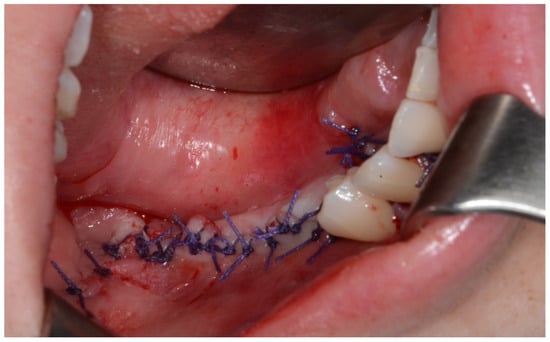
Figure 10.
After primary closure.
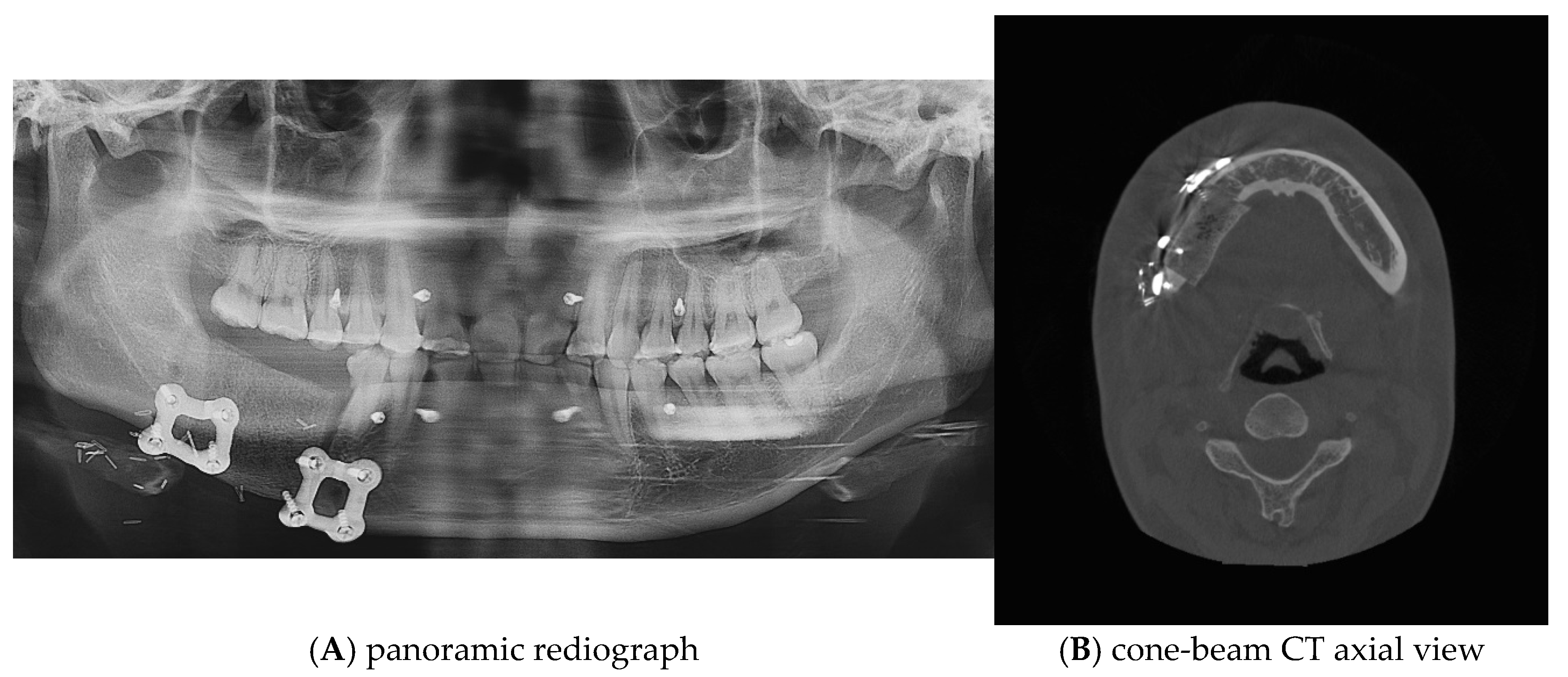
Postoperative panoramic radiographs and CBCT imaging were obtained to evaluate the error compared to the preoperative plan. (Figure 11) The evaluation was performed using Materialize 3-matic software. After aligning the preoperative plan STL model and the postoperative STL model, the average value was obtained through analysis of the difference between the two models. the average discrepancy was 0.79 mm. During the 6-month follow-up period after surgery, occlusion and mouth opening were stable.
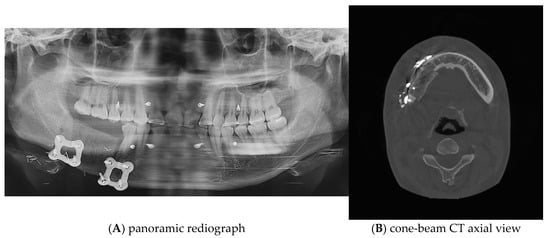
Figure 11.
Postoperative panoramic radiograph and cone-beam CT. A high level of precision was achieved in the reconstruction.
7. Discussion
Analysis of the simulation data showed that the mean time for the virtual surgery planning process was 2.86 working days (standard deviation 1.35 days). This suggests that in most cases the planning can be completed within 3 days. Of note, only 2 of the 7 simulations required redesign iterations, which is an indicator of the accuracy and efficiency of the initial plan. In addition, a trend of increasing efficiency was observed over time. Later simulations generally required fewer working days, which seems to reflect the learning curve of the team and the gradual improvement of the protocol. This trend demonstrates the potential for the surgeon–bioengineer collaboration protocol to become more efficient over time. The results of this analysis suggest that the proposed collaboration protocol is efficient and can be operated within a time frame applicable to real-world clinical settings. It also demonstrates the potential for the process to become more efficient as the team gains experience.
This case report demonstrates the successful implementation of a proposed collaborative protocol between surgeons and bioengineers in virtual surgical planning for mandibular reconstruction. The results highlight several key benefits of this interdisciplinary approach while also identifying areas for potential improvement and further research.
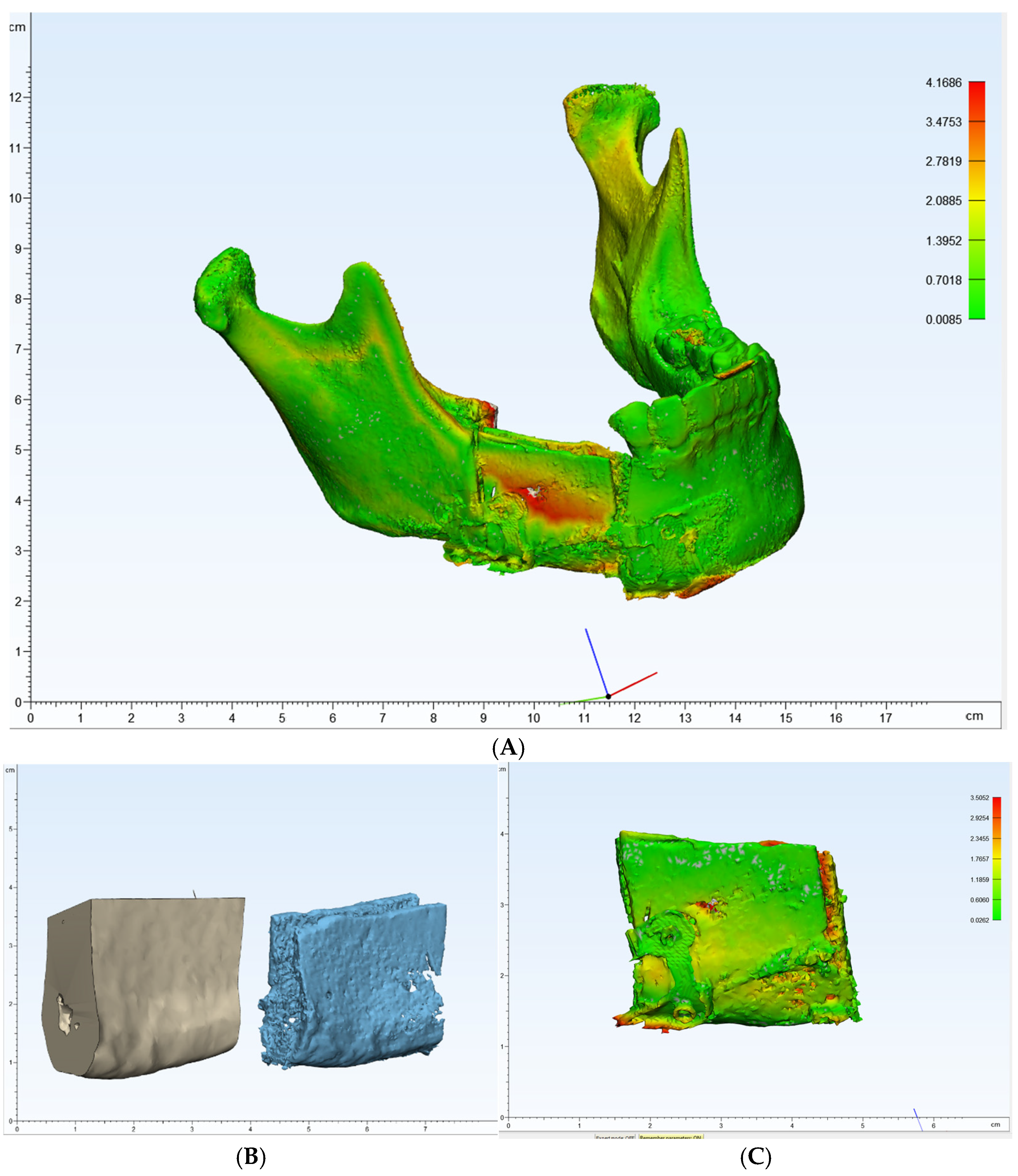
One of the primary benefits observed in this case was the high level of precision achieved in the reconstruction. Postoperative panoramic radiographs and cone-beam CT scans showed that the actual reconstruction closely matched the virtual surgical plan (Figure 12A,C). When comparing the overall preoperative and postoperative results, the average discrepancy was 0.90 mm. This level of accuracy is consistent with findings in the literature, where studies have reported average differences between virtual plans and postoperative results ranging from 0.9 mm to 3.0 mm [9,19,20,21,22,23,24,25,26,27].
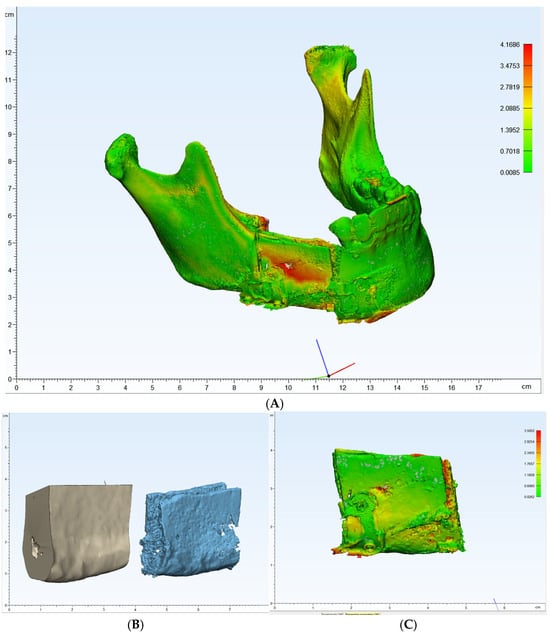
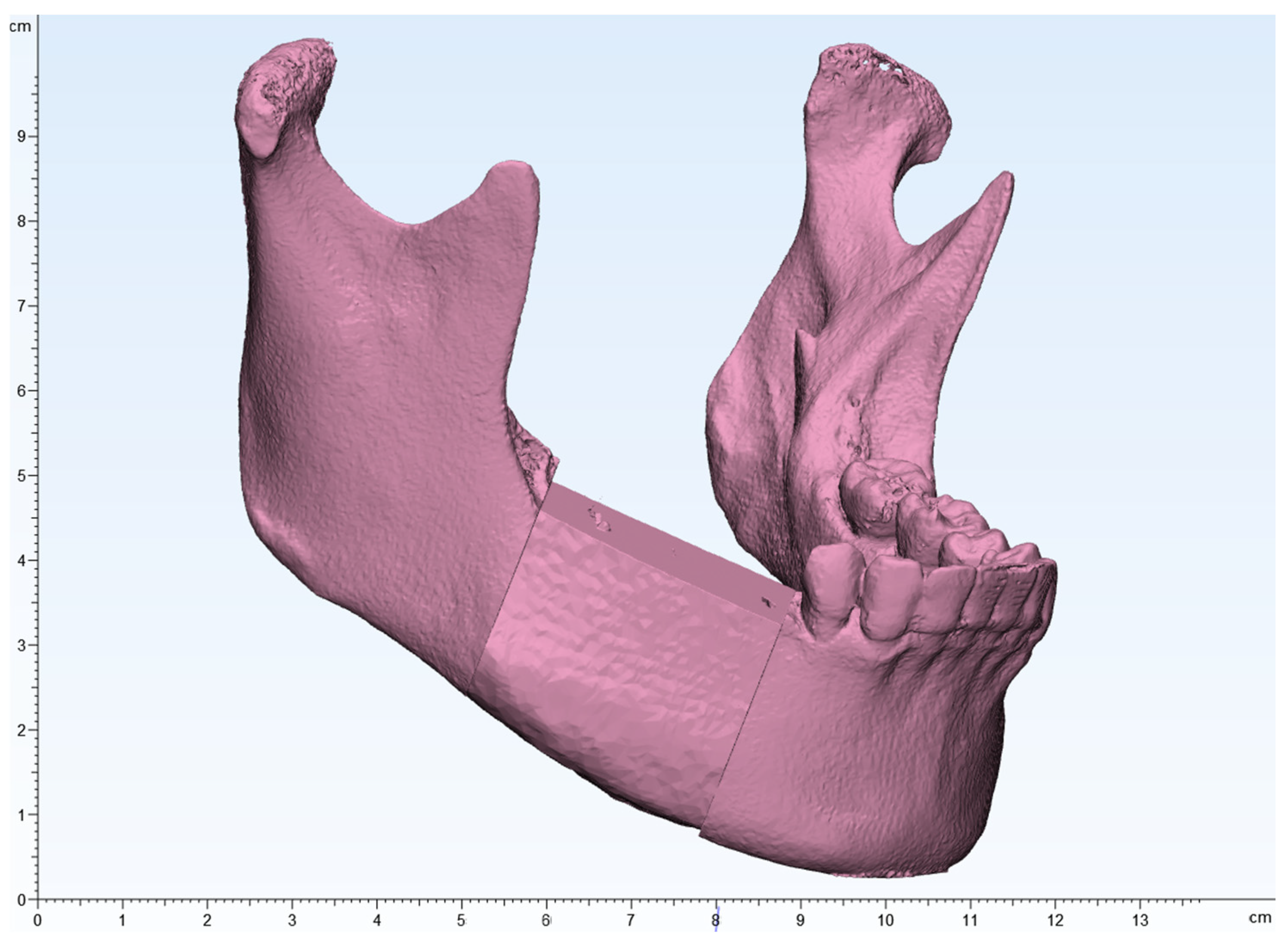
Figure 12.
(A–C): Comparison of preoperative and postoperative flap designs. The rulers on the left and bottom indicate length (cm).
While virtual surgical planning and guided surgery improve accuracy and reduce operative time, especially flap ischemic time [13,28], they require greater expertise and preoperative planning. The proposed collaborative protocol demonstrated significant time savings in preoperative planning. The clear division of tasks between surgeons and bioengineers allowed parallel workflows, optimizing the overall process. Surgeons focused on critical clinical decisions, while bioengineers handled the technical aspects of 3D modeling and guide fabrication. This efficient use of expertise not only reduced operative time but also potentially reduced the risk of surgical complications associated with prolonged procedures.
The synergy between surgical expertise and bioengineering skills was particularly evident in the flap design phase. The surgeon’s understanding of the anatomical and functional requirements guided the bioengineer’s optimization of the virtual flap design. This collaboration resulted in a flap that minimized the need for intraoperative adjustments by maximizing the natural contour of the iliac crest.
The implementation of this collaborative protocol demonstrated the importance of clear communication and mutual understanding between surgeons and bioengineers. While there was an initial learning curve in adopting these techniques, team efficiency improved over time as each discipline became more familiar with the capabilities and limitations of the other. This underscores the need for ongoing interdisciplinary training and education to maximize the benefits of computer-assisted surgery.
The advantage of the protocol presented in this article is that it can be performed without a large adaptation period even when working with other bioengineers. In fact, we worked with a bioengineering team rather than with a single bioengineer, and we were able to complete the work without a large change in time. This is thought to be because the surgeon directly performs important decisions in the VSP process.
The protocol aimed to optimize resource allocation by leveraging the unique skills of both surgeons and bioengineers. Although there are additional costs associated with virtual surgical planning and 3D printing, the improved outcomes and potential time savings justify the investment [29]. However, a comprehensive cost-effectiveness analysis with a larger case series would be beneficial.
Despite the success of this case, several limitations of the current collaborative approach have been identified. The accuracy of the reconstruction still relies heavily on the precise placement of the cutting guides, which remains the responsibility of the surgeon. Future iterations of the protocol could explore ways for bioengineers to provide real-time assistance during guide placement, possibly through augmented reality technologies.
In addition, while the current protocol is excellent at addressing bony reconstruction, it does not fully address soft tissue considerations. Future collaborations could involve bioengineers in soft tissue analysis and planning, potentially improving overall esthetic outcomes.
Another limitation of this study is the absence of a control group. Therefore, the advantages of surgery through VSP, such as shortened surgical time, shortened ischemic time, and improved accuracy, could not be compared and proven. However, since the current standard treatment for mandibular reconstruction is surgery through VSP, it is difficult to set a control group from an ethical perspective. In order to prove the advantages of surgery through VSP in the future, it is thought that a study that retrospectively analyzes and compares the medical records of patients before the introduction of VSP is necessary.
While immediate postoperative results are promising, long-term follow-up is essential to assess the stability of the reconstruction and evaluate functional outcomes. This follow-up should include both surgical and bioengineering perspectives to continually refine the collaborative protocol. Future studies with larger patient cohorts and longer follow-up periods will be essential to fully understand the long-term benefits of this interdisciplinary approach to computer-assisted mandibular reconstruction.
8. Conclusions
This study reports the successful implementation of a collaborative protocol between surgeons and bioengineers for virtual surgical planning in mandibular reconstruction. The protocol resulted in high precision, with an average discrepancy of 0.90 mm between the virtual plan and the postoperative results. It enabled an efficient workflow, optimizing both preoperative planning and intraoperative procedures. The synergy between surgical and bioengineering expertise particularly benefited the flap design phase, minimizing the need for intraoperative adjustments. Although promising, this approach requires further validation through larger cohort studies and long-term follow-up to assess its full potential for improving surgical outcomes and cost-effectiveness in mandibular reconstruction.
Author Contributions
Conceptualization, S.-Y.M. and H.-J.K.; methodology, D.-H.S.; software, D.-H.S. and H.-J.K.; validation, D.-H.S., J.-S.O. and H.-J.K.; formal analysis, D.-H.S. and H.-J.K.; investigation, D.-H.S. and H.-J.K.; resources, S.-Y.M.; data curation, D.-H.S. and H.-J.K.; writing—original draft preparation, D.-H.S.; writing—review and editing, S.-Y.M., J.-S.O. and H.-J.K.; visualization, D.-H.S.; supervision, S.-Y.M. and J.-S.O.; project administration, S.-Y.M.; funding acquisition, S.-Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Industry–University–Research Institute platform cooperation R&D funded by the Korean Ministry of SMEs and Startups in 2022 (grant number RS-2022-TI023679).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Chosun University Dental Hospital (CUDHIRB 2301 002 and 25 May 2023).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lonie, S.; Herle, P.; Paddle, A.; Pradhan, N.; Birch, T.; Shayan, R. Mandibular reconstruction: Meta-analysis of iliac- versus fibula-free flaps. ANZ J. Surg. 2016, 86, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.; Jacobson, A.S.; Buchbinder, D.; Urken, M.L. Contemporary reconstruction of the mandible. Oral. Oncol. 2010, 46, 71–76. [Google Scholar] [CrossRef]
- Kumar, B.P.; Venkatesh, V.; Kumar, K.J.; Yadav, B.Y.; Mohan, S.R. Mandibular reconstruction: Overview. J. Maxillofac. Oral. Surg. 2016, 15, 425–441. [Google Scholar] [CrossRef]
- Maurer, P.; Eckert, A.W.; Kriwalsky, M.S.; Schubert, J. Scope and limitations of methods of mandibular reconstruction: A long-term follow-up. Br. J. Oral. Maxillofac. Surg. 2010, 48, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.F.; Wushou, A.; Zheng, J.; Li, G. An alternative approach for mandible reconstruction. J. Craniofacial Surg. 2013, 24, e195–e198. [Google Scholar] [CrossRef] [PubMed]
- Dupret-Bories, A.; Vergez, S.; Meresse, T.; Brouillet, F.; Bertrand, G. Contribution of 3D printing to mandibular reconstruction after cancer. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2018, 135, 133–136. [Google Scholar] [CrossRef]
- Hirsch, D.L.; Garfein, E.S.; Christensen, A.M.; Weimer, K.A.; Saddeh, P.B.; Levine, J.P. Use of computer-aided design and computer-aided manufacturing to produce orthognathically ideal surgical outcomes: A paradigm shift in head and neck reconstruction. J. Oral. Maxillofac. Surg. 2009, 67, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Wilde, F.; Cornelius, C.P.; Schramm, A. Computer-Assisted Mandibular Reconstruction using a Patient-Specific Reconstruction Plate Fabricated with Computer-Aided Design and Manufacturing Techniques. Craniomaxillofac Trauma. Reconstr. 2014, 7, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Berrone, M.; Crosetti, E.; Tos, P.L.; Pentenero, M.; Succo, G. Fibular osteofasciocutaneous flap in computer-assisted mandibular reconstruction: Technical aspects in oral malignancies. Acta Otorhinolaryngol. Ital. 2016, 36, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Modabber, A.; Mohlhenrich, S.C.; Ayoub, N.; Hajji, M.; Raith, S.; Reich, S.; Steiner, T.; Ghassemi, A.; Holzle, F. Computer-Aided Mandibular Reconstruction With Vascularized Iliac Crest Bone Flap and Simultaneous Implant Surgery. J. Oral. Implant. 2015, 41, e189–e194. [Google Scholar] [CrossRef]
- Tarsitano, A.; Del Corso, G.; Ciocca, L.; Scotti, R.; Marchetti, C. Mandibular reconstructions using computer-aided design/computer-aided manufacturing: A systematic review of a defect-based reconstructive algorithm. J. Craniomaxillofac Surg. 2015, 43, 1785–1791. [Google Scholar] [CrossRef]
- Deek, N.; Wei, F.C. Computer-Assisted Surgery for Segmental Mandibular Reconstruction with the Osteoseptocutaneous Fibula Flap: Can We Instigate Ideological and Technological Reforms? Plast. Reconstr. Surg. 2016, 137, 963–970. [Google Scholar] [CrossRef]
- Kirke, D.N.; Owen, R.P.; Carrao, V.; Miles, B.A.; Kass, J.I. Using 3D computer planning for complex reconstruction of mandibular defects. Cancers Head Neck 2016, 1, 17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, L.; Liu, Z.; Li, B.; Yu, H.; Shen, S.G.; Wang, X. Evaluation of computer-assisted mandibular reconstruction with vascularized fibular flap compared to conventional surgery. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2016, 121, 139–148. [Google Scholar] [CrossRef] [PubMed]
- De Maesschalck, T.; Courvoisier, D.S.; Scolozzi, P. Computer-assisted versus traditional freehand technique in fibular free flap mandibular reconstruction: A morphological comparative study. Eur. Arch. Otorhinolaryngol. 2017, 274, 517–526. [Google Scholar] [CrossRef]
- Han, H.H.; Kim, H.Y.; Lee, J.Y. The Pros and Cons of Computer-Aided Surgery for Segmental Mandibular Reconstruction after Oncological Surgery. Arch. Craniofac Surg. 2017, 18, 149–154. [Google Scholar] [CrossRef]
- Tarsitano, A.; Battaglia, S.; Crimi, S.; Ciocca, L.; Scotti, R.; Marchetti, C. Is a computer-assisted design and computer-assisted manufacturing method for mandibular reconstruction economically viable? J. Craniomaxillofac Surg. 2016, 44, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Probst, F.A.; Liokatis, P.; Mast, G.; Ehrenfeld, M. Virtual planning for mandible resection and reconstruction. Innov. Surg. Sci. 2023, 8, 137–148. [Google Scholar] [CrossRef]
- Mascha, F.; Winter, K.; Pietzka, S.; Heufelder, M.; Schramm, A.; Wilde, F. Accuracy of computer-assisted mandibular reconstructions using patient-specific implants in combination with CAD/CAM fabricated transfer keys. J. Craniomaxillofac Surg. 2017, 45, 1884–1897. [Google Scholar] [CrossRef]
- Ritschl, L.M.; Mucke, T.; Fichter, A.; Gull, F.D.; Schmid, C.; Duc, J.M.P.; Kesting, M.R.; Wolff, K.D.; Loeffelbein, D.J. Functional Outcome of CAD/CAM-Assisted versus Conventional Microvascular, Fibular Free Flap Reconstruction of the Mandible: A Retrospective Study of 30 Cases. J. Reconstr. Microsurg. 2017, 33, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Xi, Q.; Zhang, L.; Liu, H.; Shi, Y.; Zhang, M. Computer-Aided Design and Three-Dimensional-Printed Surgical Templates for Second-Stage Mandibular Reconstruction. J. Craniofac Surg. 2018, 29, 2101–2105. [Google Scholar] [CrossRef] [PubMed]
- Tarsitano, A.; Battaglia, S.; Ricotta, F.; Bortolani, B.; Cercenelli, L.; Marcelli, E.; Cipriani, R.; Marchetti, C. Accuracy of CAD/CAM mandibular reconstruction: A three-dimensional, fully virtual outcome evaluation method. J. Craniomaxillofac Surg. 2018, 46, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- van Baar, G.J.C.; Forouzanfar, T.; Liberton, N.; Winters, H.A.H.; Leusink, F.K.J. Accuracy of computer-assisted surgery in mandibular reconstruction: A systematic review. Oral. Oncol. 2018, 84, 52–60. [Google Scholar] [CrossRef]
- Yang, C.; Shen, S.; Wu, J.; Zhang, S. A New Modified Method for Accurate Mandibular Reconstruction. J. Oral. Maxillofac. Surg. 2018, 76, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Geusens, J.; Sun, Y.; Luebbers, H.T.; Bila, M.; Darche, V.; Politis, C. Accuracy of Computer-Aided Design/Computer-Aided Manufacturing-Assisted Mandibular Reconstruction with a Fibula Free Flap. J. Craniofac. Surg. 2019, 30, 2319–2323. [Google Scholar] [CrossRef] [PubMed]
- Xingzhou, Q.; Wang, Z.; Ong, H.S.; Chenping, Z.; Abdelrehem, A. Accuracy of Computer-Aided Design/Computer-Aided Manufacturing Surgical Template for Guidance of Dental Implant Distraction in Mandibular Reconstruction with Free Fibula Flaps. J. Craniofac Surg. 2020, 31, 355–359. [Google Scholar] [CrossRef]
- Crosetti, E.; Tos, P.; Berrone, M.; Battiston, B.; Arrigoni, G.; Succo, G. Long-Term Follow-Up of Computer-Assisted Microvascular Mandibular Reconstruction: A Retrospective Study. J. Clin. Med. 2024, 13, 3899. [Google Scholar] [CrossRef]
- Sieira Gil, R.; Roig, A.M.; Obispo, C.A.; Morla, A.; Pages, C.M.; Perez, J.L. Surgical planning and microvascular reconstruction of the mandible with a fibular flap using computer-aided design, rapid prototype modelling, and precontoured titanium reconstruction plates: A prospective study. Br. J. Oral. Maxillofac. Surg. 2015, 53, 49–53. [Google Scholar] [CrossRef]
- Nyirjesy, S.C.; Heller, M.; von Windheim, N.; Gingras, A.; Kang, S.Y.; Ozer, E.; Agrawal, A.; Old, M.O.; Seim, N.B.; Carrau, R.L.; et al. The role of computer aided design/computer assisted manufacturing (CAD/CAM) and 3- dimensional printing in head and neck oncologic surgery: A review and future directions. Oral. Oncol. 2022, 132, 105976. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).