Abstract
Background: The management of pain and inflammation after third molar extraction is essential for patient comfort and recovery. While conventional ibuprofen is widely used for pain relief, ibuprofen arginate, a formulation aimed at faster absorption, has shown potential for faster onset and enhanced efficacy. The aim of the present scoping review was to assess current evidence on the effectiveness of ibuprofen arginate in reducing pain and inflammation following third molar extraction compared to conventional ibuprofen. Methods: A comprehensive literature search was conducted in the MEDLINE database for studies published between 2002 and 2024, focusing on ibuprofen arginate’s impact on postoperative sequelae after third molar extractions. The studies included randomized controlled trials, cohort studies, and case–control studies in English. Results: Four studies, with a combined sample of 1245 patients, met the inclusion criteria. The findings suggest that ibuprofen arginate (200/400 mg) offers a faster onset of pain relief, with significant effects noticeable within 1–2 h. At six hours post administration, pain control was found to be similar between ibuprofen arginate and conventional ibuprofen. Additionally, a study found that ibuprofen arginate (600 mg) reduced postoperative swelling more effectively, although its impact on trismus was limited. Conclusions: Ibuprofen arginate seems to offer quicker pain relief and better control of swelling after third molar extractions compared to regular ibuprofen, making it a promising option for faster recovery. However, more studies are needed to fully understand its benefits and potential uses.
1. Introduction
Third molar extractions can be considered one of the most common oral surgery procedures and may lead to severe postoperative pain, significantly affecting patients’ quality of life [1,2]. To avoid postoperative pain, pharmacological painkillers are divided into two major categories: opioid analgesics, which work exclusively on the central nervous system, at opioid receptor sites, and non-opioid analgesics, which include non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen [3,4].
Ibuprofen is a propionic acid derivative with non-steroidal anti-inflammatory analgesic and antipyretic properties [5]. The primary analgesic mechanism of ibuprofen, like other NSAIDs, involves reducing the hyperalgesia associated with tissue damage. This effect is achieved by competing with arachidonic acid for cyclooxygenase (COX) enzymes, thereby inhibiting the production of inflammatory and hyperalgesic prostaglandins in peripheral tissues [4,6,7,8]. Ibuprofen is a racemic mixture that contains both R(−) and S(+) enantiomers. The inhibition of cyclooxygenase due to the S(+) enantiomer leads to anti-inflammatory, analgesic, and anti-platelet effects [9,10,11]. On the other hand, the R(−) enantiomer is less effective at inhibiting prostaglandin (PG) synthesis, but it still possesses certain pharmacological properties that contribute to the overall anti-inflammatory effect [12]. However, after oral absorption, in the intestinal tract and liver, 50–60% of the R(−) enantiomer of ibuprofen is metabolically converted into the S(+) form [13,14].
Regarding the oral formulations of ibuprofen, they are highly effective within a dose range of 200–800 mg, with a maximum total daily dose of 3200 mg. The 800 mg dose provides maximum analgesic effects, but clinicians should only consider it when the benefits of treating severe pain outweigh the increased risks of adverse effects. In fact, under most circumstances, a dosage of 400 or 600 mg taken every 6 h is quite effective against moderate inflammatory pain [15]. In fact, there have been conflicting results concerning the optimal dosing regimen and timing of administration of oral ibuprofen [16].
Ibuprofen is rapidly absorbed by the upper gastrointestinal tract, with a time to reach the maximum concentration (Cmax) (Tmax) of less than 0.25 h for granules and approximately 2 h for tablets. However, absorption can be delayed when ibuprofen is taken with food [17]. The plasma S/R ratio is influenced by the drug’s release characteristics, with sustained-release formulations exhibiting higher ratios than immediate-release formulations [14]. Analgesic effects typically begin within 1 h of administration or less, and the duration of action for the drug is at least 6 to 8 h [5,18,19,20].
The bioavailability of ibuprofen, which is a weak acid, is enhanced through salification with agents such as lysine, sodium, and arginine. Several products currently available on the market contain salified forms of ibuprofen, with a reduced Tmax and an increased Cmax compared to the free-acid form [21,22,23,24].
Ibuprofen arginate is produced by bonding racemic ibuprofen to the amino acid L-arginine [25,26], and this could subsequently impact the level of inversion, the availability of the active S-(+) enantiomer in the body, and the overall therapeutic effectiveness of ibuprofen [26].
Salifying ibuprofen with arginine appears to enhance its pain relief effectiveness and speed up its action, thanks to its improved solubility and quicker absorption through the stomach and intestinal tract [27,28].
In this regard, it could be interesting to understand whether ibuprofen arginate could offer any advantages in patient recovery after third molar surgery.
The aim of this scoping review was to assess the current state of knowledge regarding the use of ibuprofen arginate for managing postoperative inflammation after third molar extraction, in comparison to conventional ibuprofen.
2. Materials and Methods
2.1. Eligibility Criteria
According to the PCC (population (or participants)/ concept/context) framework, the following research question was formulated (Table 1): “What is known from the literature about the use of ibuprofen arginate compared to conventional ibuprofen to manage postoperative inflammatory sequalae in patients undergoing third molar extraction?”

Table 1.
Population/concept/context (PCC) from The Joanna Briggs Institute. Joanna Briggs Institute Reviewers’ Manual: 2015 edition/supplement, Adelaide, The Joanna Briggs Institute, 2015 [29].
The following inclusion criteria were used: randomized controlled trials (RCTs), prospective cohort studies, non-randomized controlled trials, retrospective cohort studies, and case–control studies investigating the use of ibuprofen arginate compared to conventional ibuprofen for managing surgical outcomes after third molar extraction. Only studies published in English until July 2024 were included. Studies not published in English, as well as reviews, case reports, consensus statements, study protocols, and non-human studies, were excluded from this review.
2.2. Search Strategy
A comprehensive search of the literature was conducted using the MEDLINE, Embase, and Cochrane Library databases. The search query aimed to identify clinical trials that studied the effects of ibuprofen arginate, either alone or in comparison with conventional ibuprofen, both administered orally to manage postoperative pain and edema in patients undergoing third molar extraction. The search terms used were ‘ibuprofen arginate OR ibuprofen arginine AND third molar surgery OR postoperative dental pain’. The search terms are presented in Table 2. The search included all studies available in English.

Table 2.
Search strategy.
2.3. Selection of Sources of Evidence
Screening was performed independently by two reviewers (C.L. and I.C.) who firstly examined the titles and, in case of eligibility, the abstracts. The articles selected for full-text reading were reviewed by two reviewers (C.L. and I.C.) and tabulated, while freeform data were extracted based on the main questions of the review. Inclusion and discussion of relevant data were conducted through consensus. Systematic reviews and meta-analyses were separately identified through electronic and manual searches and read in full text by all authors for potential data related to the aims of the review. Additionally, a manual search was conducted on the reference lists of the systematic reviews and meta-analyses.
2.4. Data Charting Process
In the first stage, all the titles and abstracts resulting from the literature search were reviewed by two independent authors (C.L. and I.C.). Duplicate studies, studies not published in English, and those without abstracts were removed. Suddenly, the eligibility criteria were verified by the reviewers. In case of disagreement, a third author (P.C.) was consulted until a consensus was obtained. The full text of the eligible articles was retrieved, and the reference lists of the full text articles were manually checked to ensure that no relevant eligible studies were overlooked.
2.5. Data Items
The following key information was extracted and summarized: first author, date of publication, population included, medications (with dosage) taken in the intervention and control groups, and timing of administration.
3. Results
A total of 3993 studies were identified through the database search (Figure 1). No additional studies were found through forward and backward citation searching.
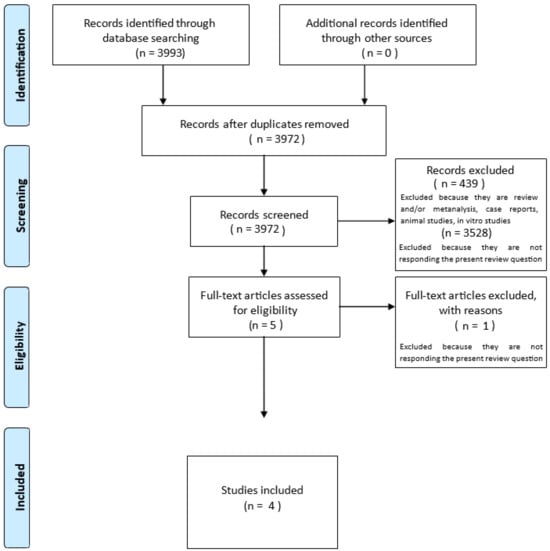
Figure 1.
PRISMA-ScR flow diagram for the scoping review process. Modified: The Joanna Briggs Institute reviewers’ manual [29].
After removing the duplicates, 3972 studies remained and underwent title and abstract screening. At this stage, 3528 studies were deemed irrelevant, and 439 were excluded because they were reviews, case reports, or non-human studies. The full texts of five studies were collected and further examined. Four studies met the inclusion criteria and were included into the qualitative synthesis. Data from 1245 patients were included in the scoping review. The study characteristics are summarized in Table 3.

Table 3.
Characteristics of the studies. * VAS (visual analog scale): a 10 cm line, with two end points representing 0 = no pain to 10 = worst imaginable pain. ** PID (pain intensity difference).
Black et al. (2002), Mehlisch et al. (2002), and Desjardins et al. (2002) conducted randomized controlled trials comparing the efficacy of pain relief between ibuprofen arginate (200 or 400 mg), conventional ibuprofen (200 or 400 mg), and a placebo. These three randomized clinical trials concluded that the onset of analgesia was significantly faster with ibuprofen arginate (both 200 and 400 mg) compared to conventional ibuprofen (200 and 400 mg) [28,31,32].
The most recent RCT regarding the use of ibuprofen arginate to manage inflammatory sequalae after third molar extraction is the one of Ramos et al. (2022) [30]. The studies previously discussed evaluated the pain intensity difference (PID), calculated by comparing the patient’s self-reported pain intensity at different time points after treatment with their baseline pain intensity. In the study of Ramos et al., pain was evaluated using the visual analog scale (VAS), an 11-point scale (BS-11) with two end points representing 0 = no pain to 10 = worst imaginable pain. For the ibuprofen arginate group, the pain level was significantly lower (2.6 ± 1) than for the conventional ibuprofen group (5.0 ± 1) [30].
Desjardins et coll. (2002)’s results were as follow: ibuprofen arginate 200 mg, 0.6 ± 0.8; ibuprofen arginate 200 mg × 2, 0.8 ± 0.9; ibuprofen 200 mg, 0.7 ± 0.9; and ibuprofen 200 mg × 2, 1.0 ± 1.0. Ibuprofen arginate (both the 200 mg and 400 mg doses) provided significantly better pain relief compared to ibuprofen (both the 200 mg and 400 mg doses) in the early phase post administration, particularly within the first 15 to 60 min [32]. In the study of Black et al. (2002), at the 6 h mark (360 min), the pain intensity differences (PIDs) were the following: ibuprofen arginate (200 mg), −0.5 ± 0.8 − 0.5; ibuprofen arginate (2 × 200 mg), −0.3 ± 0.9 − 0.3; ibuprofen (200 mg), −0.4 ± 0.8 − 0.4; and ibuprofen (2 × 200 mg), −0.3 ± 1.0 − 0.3. According to the results presented in this study, ibuprofen arginate was significantly better than ibuprofen in terms of pain relief within the first 15 to 120 min after administration for both the 200 mg and 2 × 200 mg doses [28]. According to Mehlisch et al. (2002), at the 6 h mark (360 min), the mean pain intensity differences (PIDs) were as follows: ibuprofen arginate (200 mg), −0.5 ± 0.8 − 0.5; ibuprofen arginate (2 × 200 mg), −0.3 ± 0.9 − 0.3; ibuprofen (200 mg), −0.4 ± 0.8 − 0.4; and ibuprofen (2 × 200 mg), −0.3 ± 1.0 − 0.3. Ibuprofen arginate (both the 200 mg and 2 × 200 mg doses) consistently showed an advantage over ibuprofen in terms of pain reduction from 10 min up to 120 min after administration. This advantage diminished after 2 h, as the pain relief levels between ibuprofen arginate and ibuprofen became more comparable at the later time points [31].
4. Discussion
The present scoping review highlights findings regarding the use of ibuprofen arginate for managing postoperative inflammatory sequelae following third molar extraction. It may be concluded that ibuprofen arginate could lead to a faster onset of analgesia compared to conventional ibuprofen, which makes it a valuable option for acute pain management after surgery. This rapid onset is attributed to the enhanced bioavailability of ibuprofen in its arginate salt form, which allows for quicker absorption and higher peak plasma concentrations over a shorter time frame. As a matter of fact, the results of Desjardins et al. (2002), Black et al. (2002), and Mehlisch et al. (2002) show that ibuprofen arginate gave significantly better pain relief than conventional ibuprofen, respectively, within the first 15 to 60 min, 15 to 120 min, and 10 min up to 120 min.
These results suggest that ibuprofen arginate has a faster onset of action and provides more pain relief during this initial period, without leading to a shorter overall duration of pain relief compared to conventional ibuprofen. Moreover, it must be pointed out that, after 6 h, ibuprofen arginate does not significantly differ in terms of pain reduction compared to ibuprofen at equivalent doses.
Desjardins et coll. (2002) observed that the time of re-medication was the same (4 h) for both ibuprofen arginate 400 mg and ibuprofen 400 mg, and both were significantly longer than the time recorded for ibuprofen 200 mg (2.6 h). Additionally, the re-medication time for ibuprofen arginate (3 h) was longer compared to ibuprofen 200 mg [31]. In the study of Black et al. (2002), the median times to re-medication were 4.0 h for ibuprofen arginate 200 mg and 4.5 h for 400 mg, while, for ibuprofen 200 mg and 400 mg, the times were 4.2 h and 5.2 h, respectively [28]. According to Mehlisch et al. (2002), the re-medication time was significantly longer for both ibuprofen arginate groups compared to patients treated with conventional ibuprofen 200 mg (4.5 h for ibuprofen arginate 200 mg and 4.4 h for ibuprofen arginate 400 mg vs. 3.8 h for conventional ibuprofen 200 mg and 4.2 h for conventional ibuprofen 400 mg [31].
All three studies evaluated the effect of ibuprofen arginate and conventional ibuprofen when local anesthesia dissipated, and the patients’ pain was of moderate or severe intensity.
Meanwhile, Lau et al. (2009) assessed the effectiveness of 400 mg ibuprofen arginate when used as a preemptive or postoperative analgesic. Their study found no differences between preemptive and postoperative administration in terms of time to first rescue medication, total amount of rescue medication consumed, number of pain-free patients, pain scores, and global assessment scores [33].
The most recent randomized controlled trial by Ramos et al. (2022) further supports the benefits of ibuprofen arginate. The authors evaluated the pain intensity using the visual analog scale (VAS), and the ibuprofen arginate group showed pain level significantly lower (2.6 ± 1) compared to the conventional ibuprofen group (5.0 ± 1). In comparison to the ones mentioned above, this study went beyond pain management, also exploring the drug’s effects on controlling postoperative edema and trismus following third molar extractions. The trial demonstrated that ibuprofen arginate was superior to conventional ibuprofen in reducing edema, though its effect on trismus was less pronounced [30].
Salification with arginine may enhance the anti-inflammatory effect of ibuprofen because L-arginine serves as a substrate for nitric oxide (NO)-synthesizing enzymes (NOSs). Nitric oxide regulates the activity of immune-competent cells and may help reduce inflammation [34,35].
When administered at nanomolar physiological concentrations, nitric oxide exhibits protective antiapoptotic effects [36,37,38,39,40], including on epithelial cells of the gastrointestinal tract [41,42], increases mucin formation, promotes the synthesis of prostaglandins E2 and gastrin, and enhances the effects of prostacyclin on gastric epithelial cells [43,44,45,46,47]. Furthermore, nitric oxide may boost blood flow in the gastric mucosa and promote ulcer healing due to its vasodilatory and angiogenic properties [48,49,50]. These biological effects may counteract the gastrointestinal damage caused by ibuprofen [35].
Other authors have investigated substances that, when combined with ibuprofen, provide faster pain relief.
The review of Moore et al. (2014) shows that maximum ibuprofen concentrations with fast-acting formulations occurred before the 50 min mark (20–40 min after dosing for arginine, lysine, and sodium salts, and 30–50 min after dosing for liquid-filled soft capsules). Conventional ibuprofen took about 90–120 min. Overall, fast-acting formulations were found to be slightly more effective than standard ibuprofen, especially in terms of achieving 50% of the maximum total pain relief (P50% maxTOTPAR). This was particularly noticeable with the 200 mg dose of ibuprofen arginate, where fewer patients needed to take additional medication compared to those on standard formulations. The 200 mg dose of ibuprofen arginate showed the greatest improvement in pain relief compared to the same dose of standard ibuprofen, highlighting the benefit of the fast-acting formulation [51].
Another studied combination involves ibuprofen and caffeine, which has been shown to enhance analgesic efficacy and reduce the time to the onset of pain relief.
In the study of Forbes et al. (1991), ibuprofen 200 mg with caffeine consistently demonstrated higher PID scores than ibuprofen 200 mg alone at each hour mark, suggesting that caffeine enhanced the analgesic effect of ibuprofen, which was particularly notable at the 2 and 3 h mark. The results of this study were that ibuprofen 200 mg had a PID of 1.00 and ibuprofen 200 mg plus caffeine had a PID of 1.25 after 2 h; meanwhile, ibuprofen 200 mg had a PID of 0.98 and ibuprofen 200 mg plus caffeine had a PID of 1.36 after 3 h. A 200 mg ibuprofen dose was considered in the above study for both cases. Mehlisch et al. (2002) showed PIDs at 2 h of 2.8 ± 1.3 and 2.6 ± 1.4 at 3 h [31], while Black et al. (2002) presented PIDs of 1.3 ± 0.9 and 1.1 ± 1.1, respectively, after 2 and 3 h [28], and Desjardins et al. (2002) showed PIDs of 0.4 ± 1 at 2 h and –0.1 ± 1 at 3 h [32]. Ibuprofen arginate, based on the studies of Mehlisch et al. (2002) [31] and Black et al. (2002) [28], shows higher PID scores, suggesting superior pain relief properties compared to ibuprofen with caffeine. Only Desjardins et al. (2002) [32] reported lower scores for ibuprofen arginate compared to ibuprofen plus caffeine. However, it must be pointed out that, in recent years, some new possible medications have been proposed to limit postoperative pain and also prevent complications after this type of extractions [52,53].
Limitations
The main limitation of this scoping review is intrinsic to its study design, lacking a risk of bias assessment and a meta-analysis, together with the limited overall number of studies on this topic. Only four studies met the inclusion criteria for this review, with the most recent study dating back to 2022. The paucity of research limits the generalizability of the findings and suggests the need for further large-scale randomized clinical trials to confirm the enhanced anti-inflammatory activity of ibuprofen arginate.
5. Conclusions
The rapid onset of the analgesic effect of ibuprofen arginate after third molar extraction may help minimize post-extraction discomfort, reducing the anxiety and stress related to postoperative recovery. Moreover, faster pain relief may decrease the need for additional medications or emergency interventions, streamlining postoperative management and improving the overall recovery time.
Within the limitations of this study, ibuprofen arginate seems to have a faster onset of action compared to conventional ibuprofen for managing postoperative pain following third molar extraction, without reducing the overall duration of pain relief. Additionally, ibuprofen arginate may offer enhanced anti-inflammatory effects compared to conventional ibuprofen. Further investigations with the same clinical protocol and pain scale values are needed to better understand the anti-inflammatory potential and proprieties of ibuprofen arginate.
Author Contributions
C.L., I.C., V.M., P.C., C.A. and A.M.P. equally contributed to conceptualization, methodology validation, investigation and writing. C.L. and I.C. performed the articles screening. All authors have read and agreed to the published version of the manuscript.
Funding
The present research did not receive any external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon reasonable request from the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Degirmenci, A.; Yalcin, E. The effect of pregabalin and ibuprofen combination for pain after third molar surgery. Niger. J. Clin. Pract. 2019, 22, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-C.; Choi, S.-S.; Wang, S.-J.; Kim, S.-G. Minor complications after mandibular third molar surgery: Type, incidence, and possible prevention. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 102, e4–e11. [Google Scholar] [CrossRef] [PubMed]
- Mehlisch, D.R. Review of the comparative analgesic efficacy of salicylates, acetaminophen, and pyrazolones. Am. J. Med. 1983, 75, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Mehlisch, D.R.; Sollecito, W.A.; Heffrick, J.F.; Leibold, D.G.; Markowitz, R.; Schow, C.E.; Shultz, R.; Waite, D.E. Multicenter clinical trial of ibuprofen and acetaminophen in the treatment of postoperative dental pain. J. Am. Dent. Assoc. 1990, 121, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.A.; Moore, E.M.; Allen, H.W.; Beaver, W.T. Evaluation of an Ibuprofen Controlled-Release Tablet and Placebo in Postoperative Oral Surgery Pain. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1991, 11, 242–248. [Google Scholar] [CrossRef]
- Hersh, E.V.; Desjardin, P.J.; Trummel, C.L. Nonopioid analgesics, nonsteroidal anti-inflammatory drugs. In Pharmacology and Therapeutics for Dentistry, 6th ed.; Mosby Elsevier: St. Louis, MO, USA, 2011; pp. 346–348. [Google Scholar]
- Vane, J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 1971, 231, 232–235. [Google Scholar] [CrossRef]
- Moore, P.A.; Hersh, E.V. Combining ibuprofen and acetaminophen for acute pain management after third-molar extractions: Translating clinical research to dental practice. J. Am. Dent. Assoc. 2013, 144, 898–908. [Google Scholar] [CrossRef]
- Adams, S.S.; Bresloff, P.; Mason, C.G. Pharmacological differences between the optical isomers of ibuprofen: Evidence for metabolic inversion of the (-)-isomer. J. Pharm. Pharmacol. 1976, 28, 256–257. [Google Scholar] [CrossRef]
- Gaut, Z.N.; Baruth, H.; Randall, L.O.; Ashley, C.; Paulsrud, J.R. Stereoisomeric relationships among anti-inflammatory activity, inhibition of platelet aggregation, and inhibition of prostaglandin synthetase. Prostaglandins 1975, 10, 59–66. [Google Scholar] [CrossRef]
- Evans, A.M.; Nation, R.L.; Sansom, L.N.; Bochner, F.; Somogyi, A.A. Effect of racemic ibuprofen dose on the magnitude and duration of platelet cyclo-oxygenase inhibition: Relationship between inhibition of thromboxane production and the plasma unbound concentration of S(+)-ibuprofen. Br. J. Clin. Pharmacol. 1991, 31, 131–138. [Google Scholar] [CrossRef]
- Rainsford, K.D. Discovery, mechanisms of action and safety of ibuprofen. Int. J. Clin. Pract. Suppl. 2003, 3–8. [Google Scholar] [PubMed]
- Jamali, F.; Mehvar, R.; Russell, A.S.; Sattari, S.; Yakimets, W.W.; Koo, J. Human pharmacokinetics of ibuprofen enantiomers following different doses and formulations: Intestinal chiral inversion. J. Pharm. Sci. 1992, 81, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Carosi, P.; Gallucci, G.O.; Nagy, K.; Nardi, A.; Arcuri, L. Accuracy of complete-arch digital implant impression with intraoral optical scanning and stereophotogrammetry: An in vivo prospective comparative study. Clin. Oral Implant. Res. 2023, 34, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.; Abbott, P.V. Drugs for pain management in dentistry. Aust. Dent. J. 2005, 50 (Suppl. 2), S14–S22. [Google Scholar] [CrossRef]
- La Monaca, G.; Pranno, N.; Annibali, S.; Polimeni, A.; Pompa, G.; Cristalli, M.P. Effects of ibuprofen administration timing on oral surgery pain: A randomized clinical trial. Oral Dis. 2022, 28, 796–804. [Google Scholar] [CrossRef]
- Davies, N.M. Clinical pharmacokinetics of ibuprofen. The first 30 years. Clin. Pharmacokinet. 1998, 34, 101–154. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.A. The relative efficacy of ibuprofen in dental pain. Compend. Contin. Educ. Dent. 1986, 7, 578. [Google Scholar]
- Forbes, J.A.; Barkaszi, B.A.; Ragland, R.N.; Hankle, J.J. Analgesic effect of fendosal, ibuprofen and aspirin in postoperative oral surgery pain. Pharmacotherapy 1984, 4, 385–391. [Google Scholar] [CrossRef]
- Cooper, S.A. Five studies on ibuprofen for postsurgical dental pain. Am. J. Med. 1984, 77, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Sádaba, B.; Campanero, M.A.; Muñoz-Juarez, M.J.; Gil-Aldea, I.; García-Quetglas, E.; Esteras, A.; Azanza, J.R. A comparative study of the pharmacokinetics of ibuprofen arginate versus dexibuprofen in healthy volunteers. Eur. J. Clin. Pharmacol. 2006, 62, 849–854. [Google Scholar] [CrossRef]
- Fini, A.; Fazio, G.; Feroci, G. Solubility and solubilization properties of non-steroidal anti-inflammatory drugs. Int. J. Pharm. 1995, 126, 95–102. [Google Scholar] [CrossRef]
- Carosi, P.; Lorenzi, C.; Di Gianfilippo, R.; Papi, P.; Laureti, A.; Wang, H.L.; Arcuri, C. Immediate vs. Delayed Placement of Immediately Provisionalized Self-Tapping Implants: A Non-Randomized Controlled Clinical Trial with 1 Year of Follow-Up. J. Clin. Med. 2023, 12, 489. [Google Scholar] [CrossRef]
- De Palma, C.; Di Paola, R.; Perrotta, C.; Mazzon, E.; Cattaneo, D.; Trabucchi, E.; Cuzzocrea, S.; Clementi, E. Ibuprofen–arginine generates nitric oxide and has enhanced anti-inflammatory effects. Pharmacol. Res. 2009, 60, 221–228. [Google Scholar] [CrossRef]
- Cattaneo, D.; Clementi, E. Clinical pharmacokinetics of ibuprofen arginine. Curr. Clin. Pharmacol. 2010, 5, 239–245. [Google Scholar] [CrossRef]
- Cajaraville, J.P. Ibuprofen arginate for rapid-onset pain relief in daily practice: A review of its use in different pain conditions. J. Pain Res. 2021, 14, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, P.; Black, P.; Papageorge, M.; Norwood, T.; Shen, D.D.; Norris, L.; Ardia, A. Ibuprofen arginate provides effective relief from postoperative dental pain with a more rapid onset of action than ibuprofen. Eur. J. Clin. Pharmacol. 2002, 58, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Black, P.; Max, M.B.; Desjardins, P.; Norwood, T.; Ardia, A.; Pallotta, T. A randomized, double-blind, placebo-controlled comparison of the analgesic efficacy, onset of action, and tolerability of ibuprofen arginate and ibuprofen in postoperative dental pain. Clin. Ther. 2002, 24, 1072–1089. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.; Godfrey, C.M.; McInerney, P.; Soares, C.B.; Khalil, H.; Parker, D. The Joanna Briggs Institute Reviewers’ Manual 2015: Methodology for JBI Scoping Reviews; The Joanna Briggs Institute: Adelaide, SA, Australia, 2015. [Google Scholar]
- Ramos, E.U.; Benetti, L.P.; Oliveira, J.C.S.; Bassi, A.P.F. Single-Dose Ibuprofen-Arginine as a Preventive for Pain, Edema, and Trismus After Impacted Lower Third Molar Surgery: A Randomized Split-Mouth Clinical Trial. Eur. J. Dent. 2022, 16, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Mehlisch, D.R.; Ardia, A.; Pallotta, T. A controlled comparative study of ibuprofen arginate versus conventional ibuprofen in the treatment of postoperative dental pain. J. Clin. Pharmacol. 2002, 42, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, C.; Lio, F.; Papi, P.; Mazzetti, V.; Laureti, A.; Arcuri, C. Clinical Reliability of Complete-Arch Fixed Prostheses Supported by Narrow-Diameter Implants to Support Complete-Arch Restorations. Appl. Sci. 2023, 13, 538. [Google Scholar] [CrossRef]
- Lau, S.L.; Chow, R.L.K.; Yeung, R.W.K.; Samman, N. Pre-emptive ibuprofen arginate in third molar surgery: A double-blind randomized controlled crossover clinical trial. Aust. Dent. J. 2009, 54, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Vong, L. NSAID-induced gastrointestinal damage and the design of GI-sparing NSAIDs. Curr. Opin. Investig. Drugs 2008, 9, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- De Nadai, C.; Sestili, P.; Cantoni, O.; Lièvremont, J.-P.; Sciorati, C.; Barsacchi, R.; Moncada, S.; Meldolesi, J.; Clementi, E. Nitric oxide inhibits tumor necrosis factor-alpha-induced apoptosis by reducing the generation of ceramide. Proc. Natl. Acad. Sci. USA 2000, 97, 5480–5485. [Google Scholar] [CrossRef]
- Bulotta, S.; Barsacchi, R.; Rotiroti, D.; Borgese, N.; Clementi, E. Activation of the endothelial nitric-oxide synthase by tumor necrosis factor-alpha. A novel feedback mechanism regulating cell death. J. Biol. Chem. 2001, 276, 6529–6536. [Google Scholar] [CrossRef]
- Lièvremont, J.-P.; Sciorati, C.; Morandi, E.; Paolucci, C.; Bunone, G.; Della Valle, G.; Meldolesi, J.; Clementi, E. The p75(NTR)-induced apoptotic program develops through a ceramide-caspase pathway negatively regulated by nitric oxide. J. Biol. Chem. 1999, 274, 15466–15472. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Carosi, P.; Laureti, A.; Mattheos, N.; Pimkhaokham, A.; Chow, J.; Arcuri, L. Accuracy of navigation guided implant surgery for immediate loading complete arch restorations: Prospective clinical trial. Clin. Implant. Dent. Relat. Res. 2024, 26, 954–971. [Google Scholar] [CrossRef]
- De Palma, C.; Falcone, S.; Panzeri, C.; Radice, S.; Bassi, M.T.; Clementi, E. Endothelial nitric oxide synthase overexpression by neuronal cells in neurodegeneration: A link between inflammation and neuroprotection. J. Neurochem. 2008, 106, 193–204. [Google Scholar] [CrossRef]
- Lorenzi, C.; Leggeri, A.; Cammarota, I.; Carosi, P.; Mazzetti, V.; Arcuri, C. Hyaluronic Acid in Bone Regeneration: Systematic Review and Meta-Analysis. Dent. J. 2024, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Leggeri, A.; Carosi, P.; Mazzetti, V.; Arcuri, C.; Lorenzi, C. Techniques to Improve the Accuracy of Intraoral Digital Impression in Complete Edentulous Arches: A Narrative Review. Appl. Sci. 2023, 13, 7068. [Google Scholar] [CrossRef]
- Franco, L.; Doria, D. Nitric oxide enhances prostaglandin production in ethanol-induced gastric mucosal injury in rats. Eur. J. Pharmacol. 1998, 348, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Konturek, J.W.; Fischer, H.; Gromotka, P.M.; Konturek, S.J.; Domschke, W. Endogenous nitric oxide in the regulation of gastric secretory and motor activity in humans. Aliment. Pharmacol. Ther. 1999, 13, 1683–1691. [Google Scholar] [CrossRef]
- Carosi, P.; Ottria, L.; Lio, F.; Laureti, A.; Papi, P. The health of soft tissues around four dental implants loaded immediately supporting a 4-year-old fixed screw-retained prosthesis. J. Biol. Regul. Homeost. Agents 2021, 35 (Suppl. 1), 57–66. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Muscoli, C.; Masini, E.; Cuzzocrea, S.; Salvemini, D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol. Rev. 2005, 57, 217–252. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, C.; Lio, F.; Mazzetti, V.; Carosi, P.; Lamelza, S.; Pistoia, E.S.; Pica, F.; Gaziano, R. Synergistic Effect of Metronidazole and Chlorhexidine against Porphyromonas gingivalis Growth: An In Vitro Study. Dent. J. 2024, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L. Prostaglandins, NSAIDs, and gastric mucosal protection: Why doesn’t the stomach digest itself? Physiol. Rev. 2008, 88, 1547–1565. [Google Scholar] [CrossRef] [PubMed]
- Gyires, K. Gastric mucosal protection: From prostaglandins to gene-therapy. Curr. Med. Chem. 2005, 12, 203–215. [Google Scholar] [CrossRef]
- Duda, D.G.; Fukumura, D.; Jain, R.K. Role of eNOS in neovascularization: NO for endothelial progenitor cells. Trends Mol. Med. 2004, 10, 143–145. [Google Scholar] [CrossRef]
- Moore, A.R.; Derry, S.; Straube, S.; Ireson-Paine, J.; Wiffen, P.J. Faster, higher, stronger? Evidence for formulation and efficacy for ibuprofen in acute pain. Pain 2014, 155, 14–21. [Google Scholar] [CrossRef]
- Uzeda, M.J.; Silva, A.M.; Costa, L.N.; Brito, F.S.; Fernandes, G.V.; Resende, R.F. Evaluating the effectiveness of low-level laser therapy in patients undergoing lower third molar extraction: A double-blinded randomized controlled trial. Med. Oral Patol. Oral. Cir. Bucal. 2025, 30, e129–e134. [Google Scholar] [CrossRef]
- Monteiro, R.J.S.V.; Moura-Netto, C.; Veiga, N.J.; Amaral, S.A.; Fernandes, G.V.D.O. Periodontal regeneration after third molar extraction causing attachment loss in distal and furcation sites of the second molar: A case report with 12 months follow-up. J. Clin. Rev. Case Rep. 2022, 7, 128–132. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).