Saline–CO2 Solution Effects on the Mechanical Properties of Sandstones: An Experimental Study
Abstract
1. Introduction
2. Experimental Designs
2.1. Sample Preparation
2.2. Test Equipment
2.3. Test Methods
3. Test Results and Analysis
3.1. Sandstone Mechanical Experimental Results
3.2. The Results of the NMR Experiment
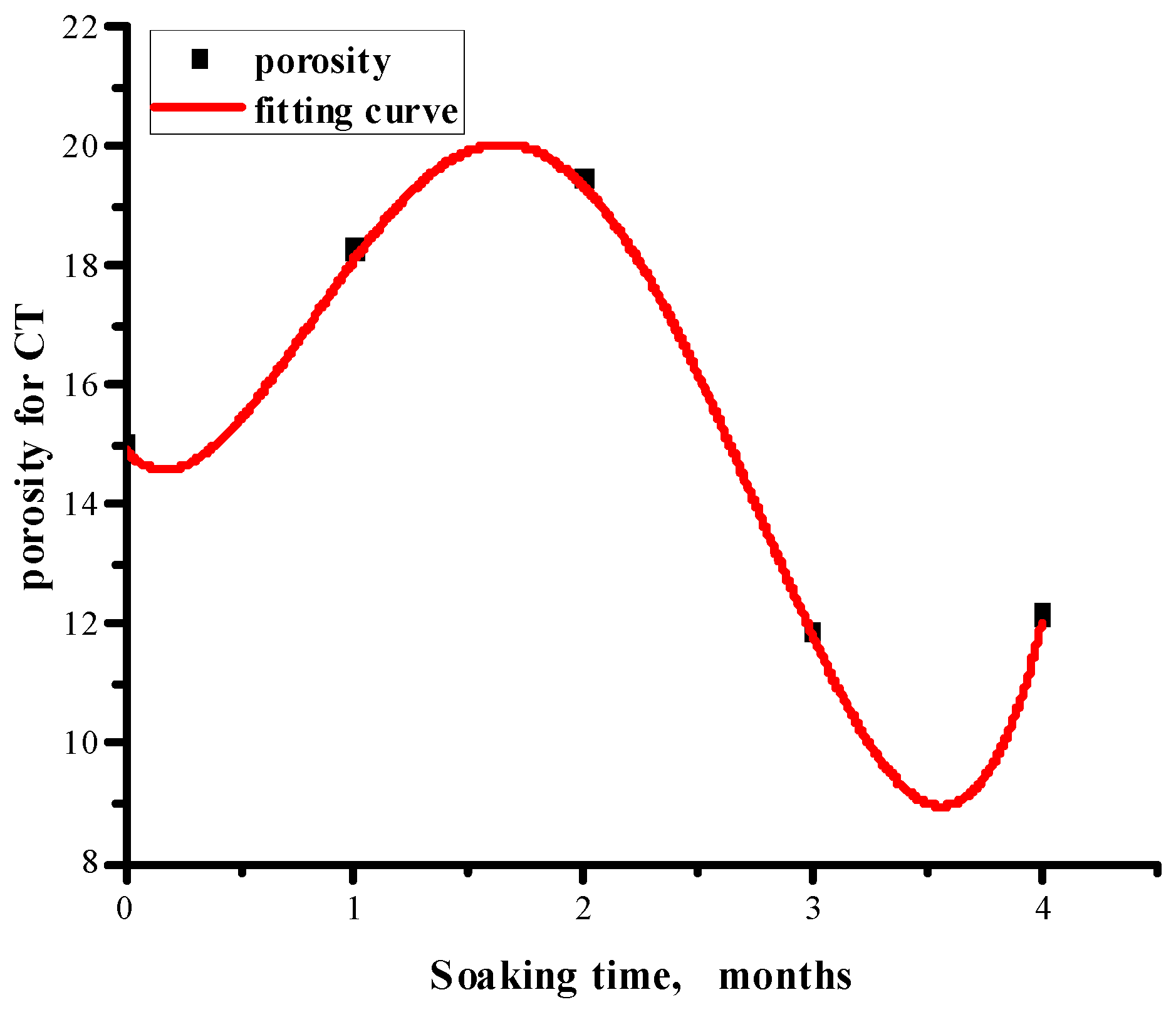
3.3. Results of the CT
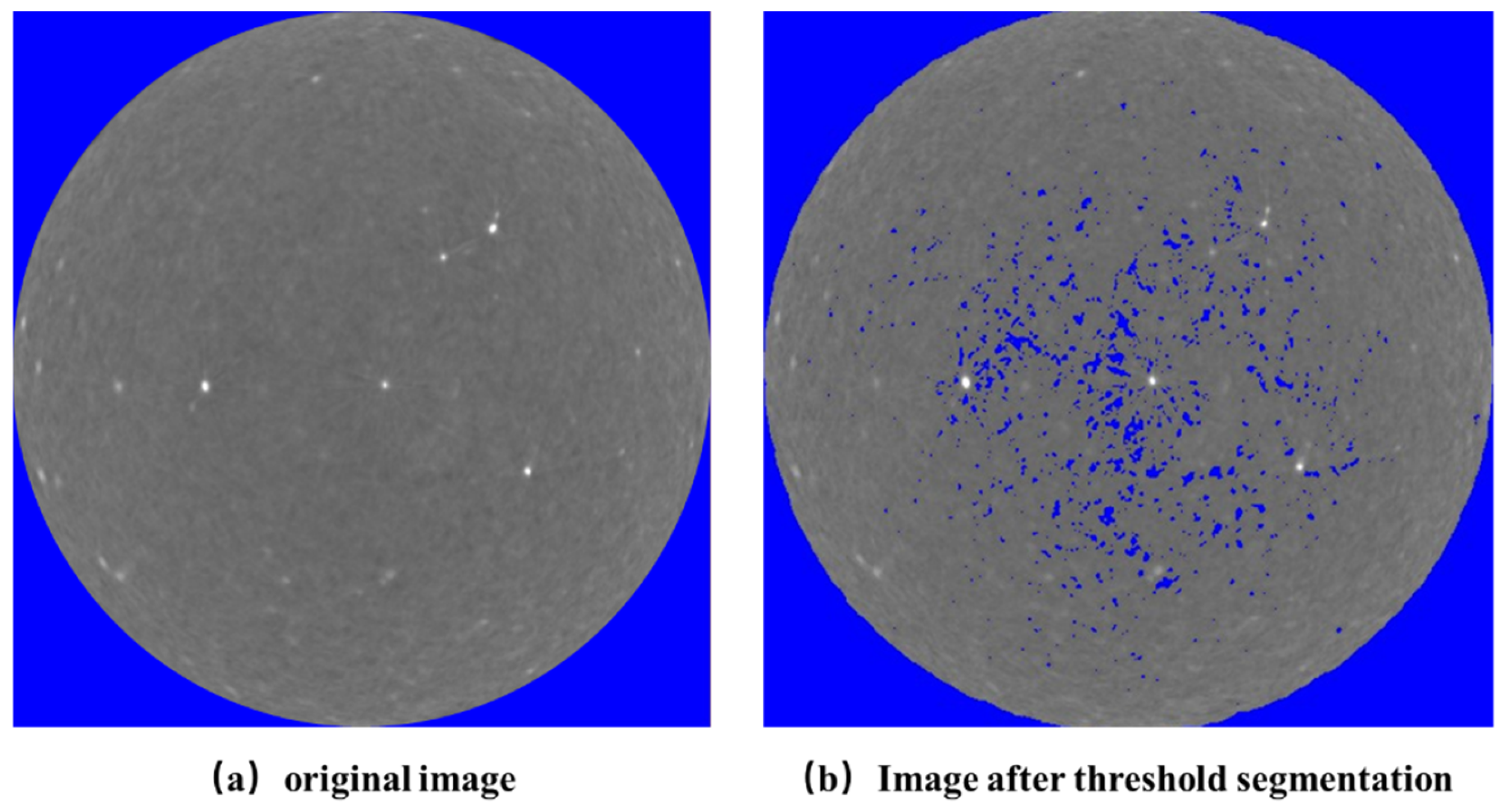
3.3.1. Processing of CT Images
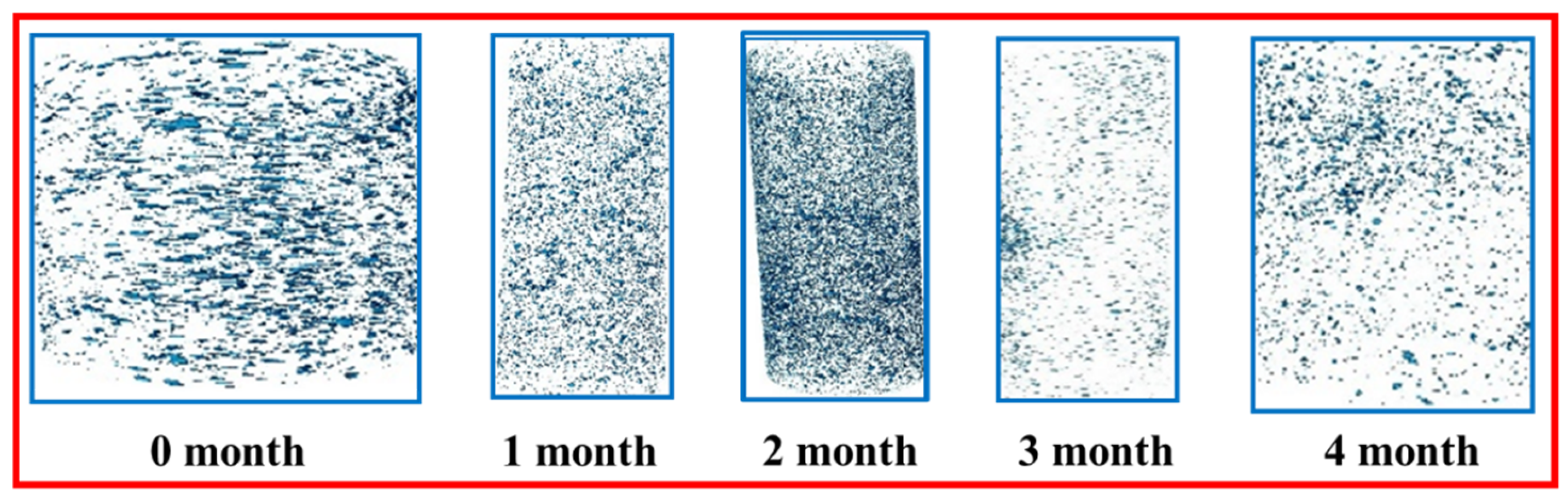
3.3.2. Visualization of Pore Space Under CT Scanning
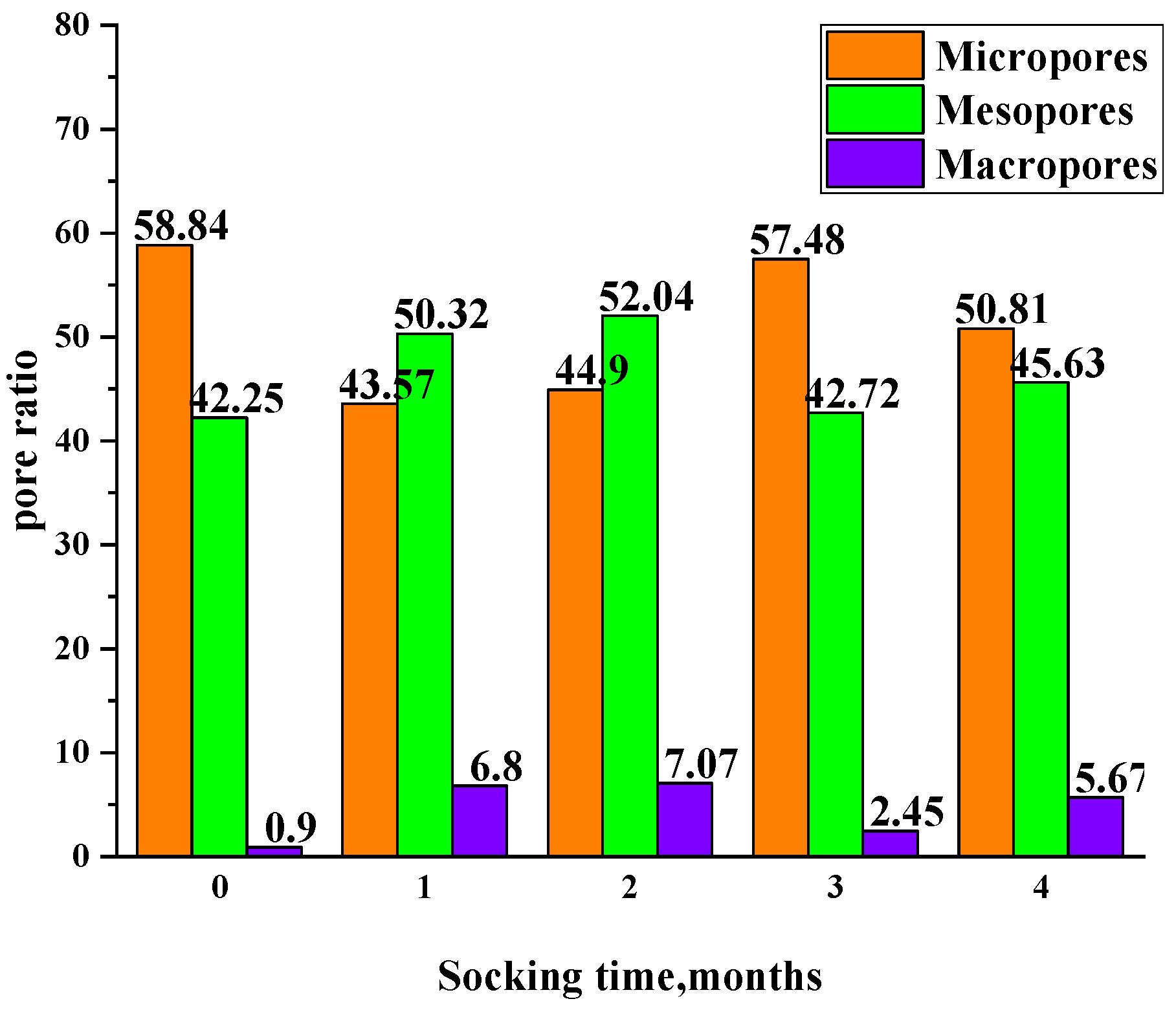
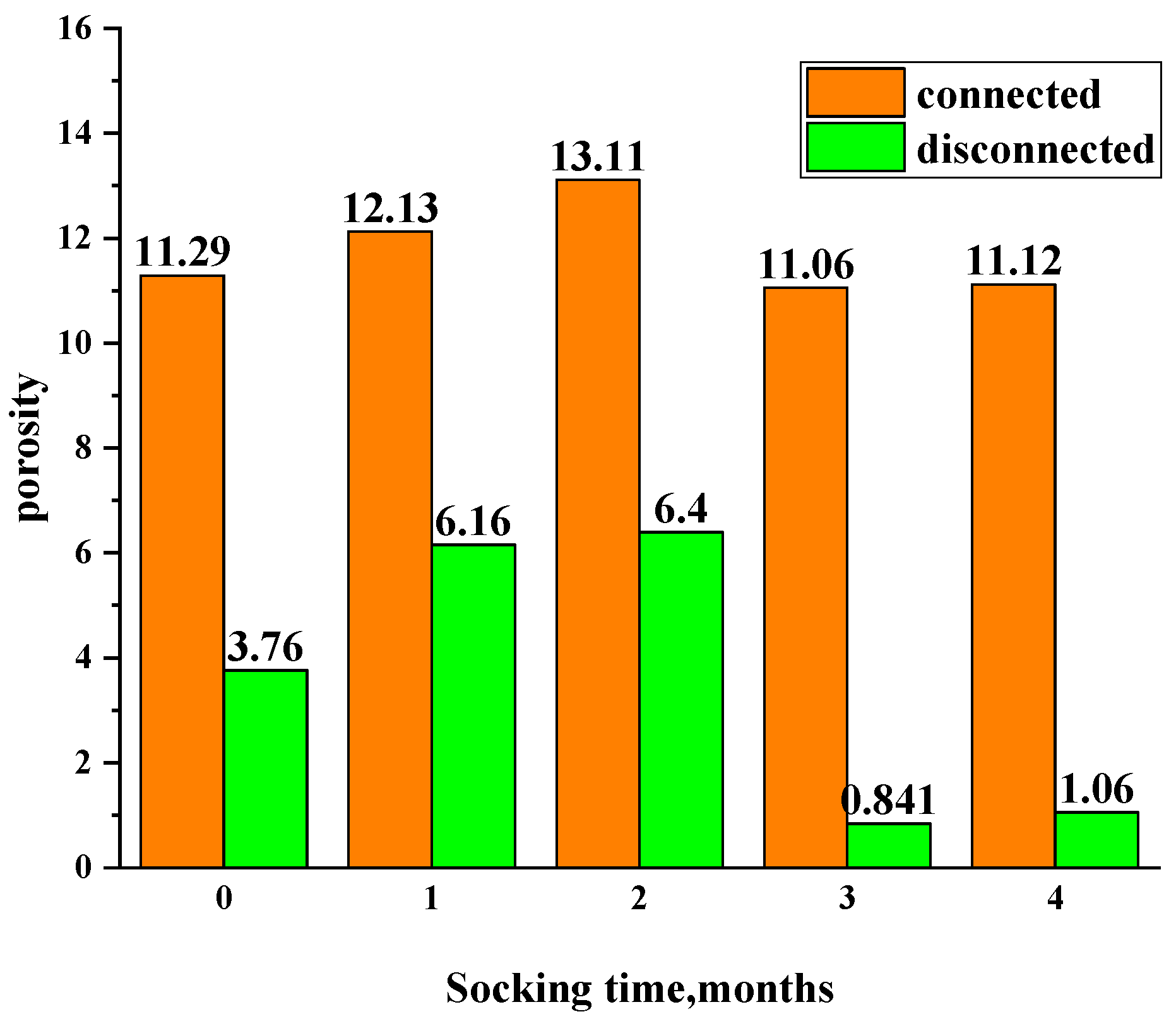
3.3.3. Changes in Non-Connected and Connected Pores at Different Soaking Times
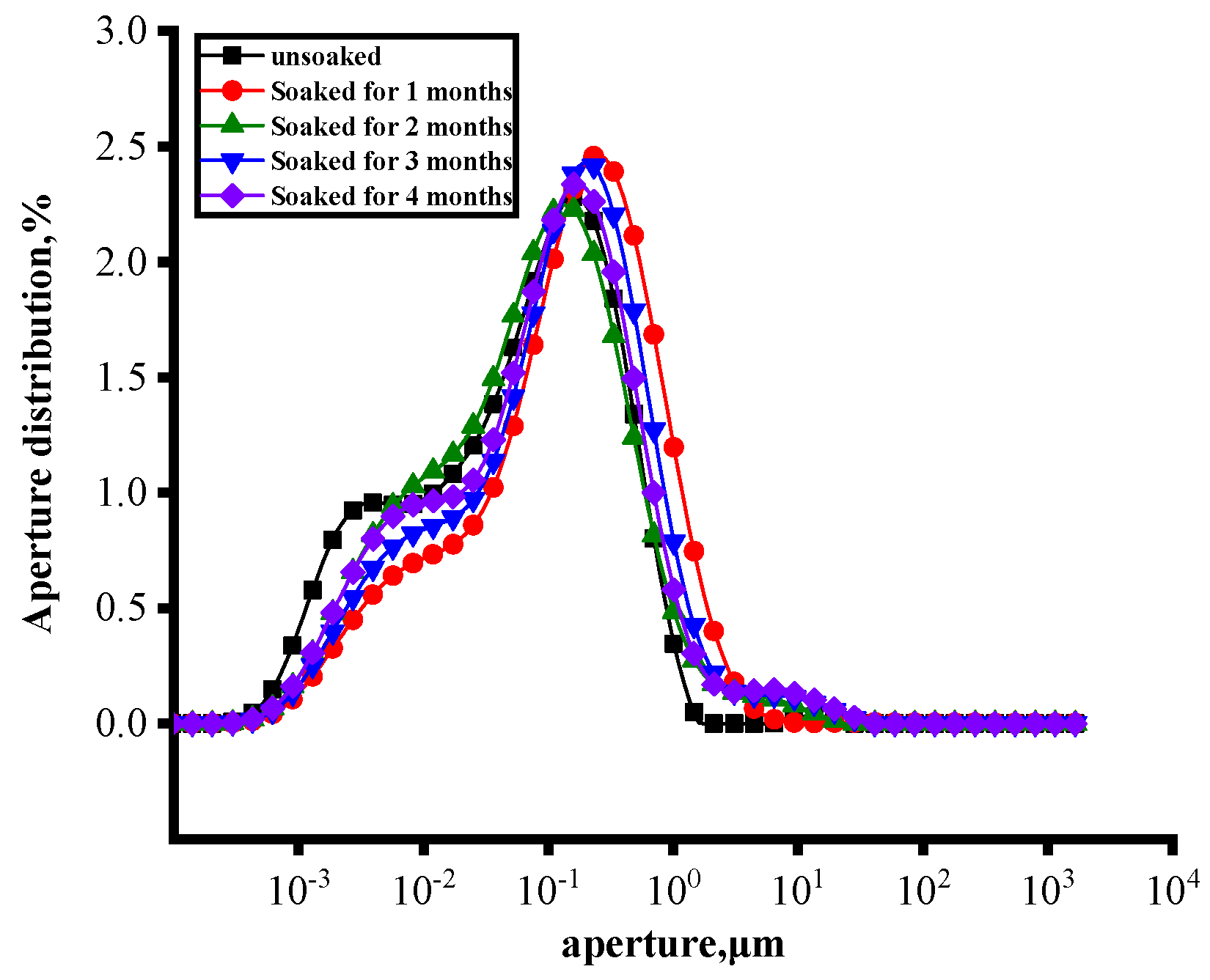
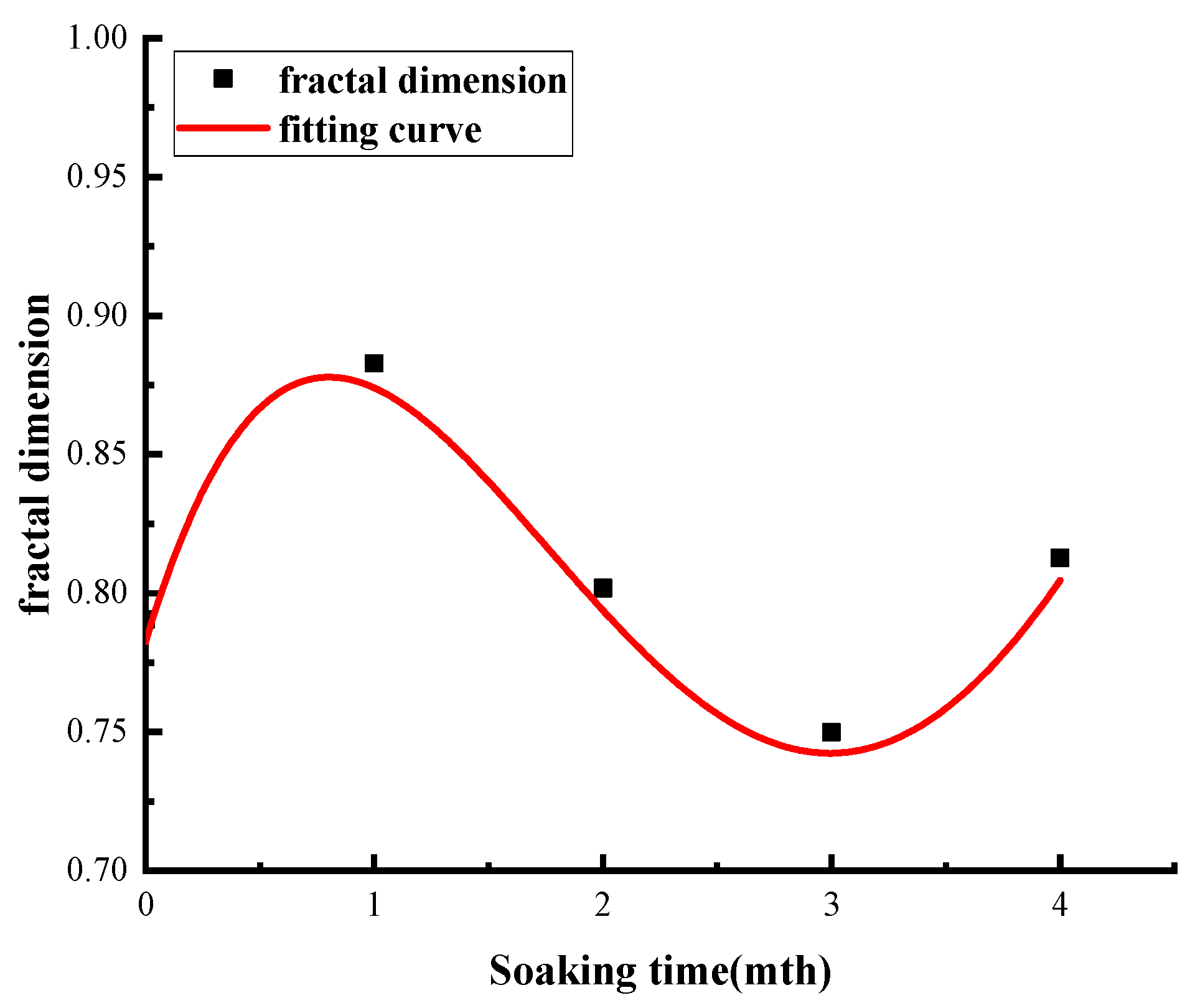
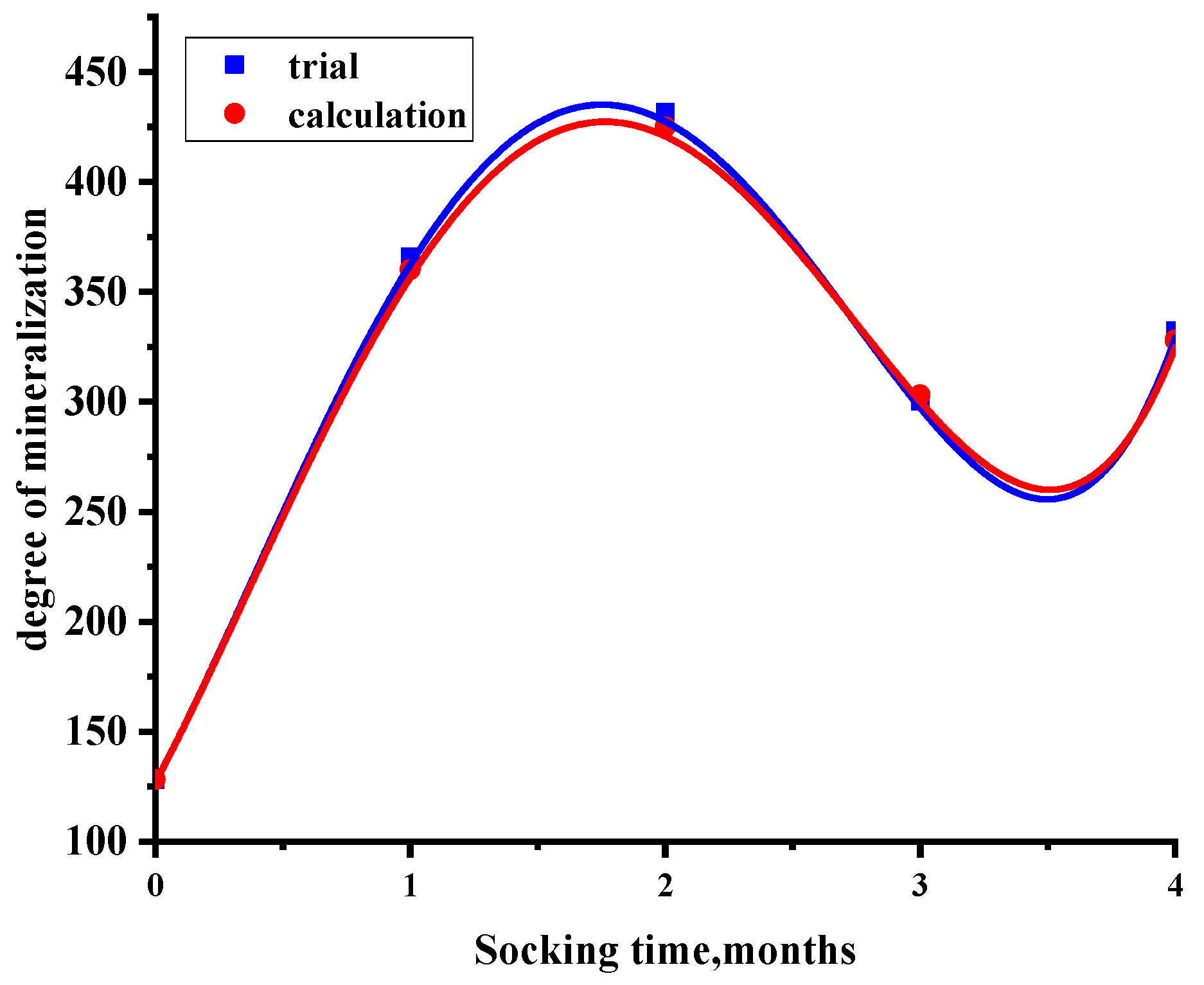
3.3.4. Changes in Pore Fractal Characteristics at Different Soaking Times
4. Discussion
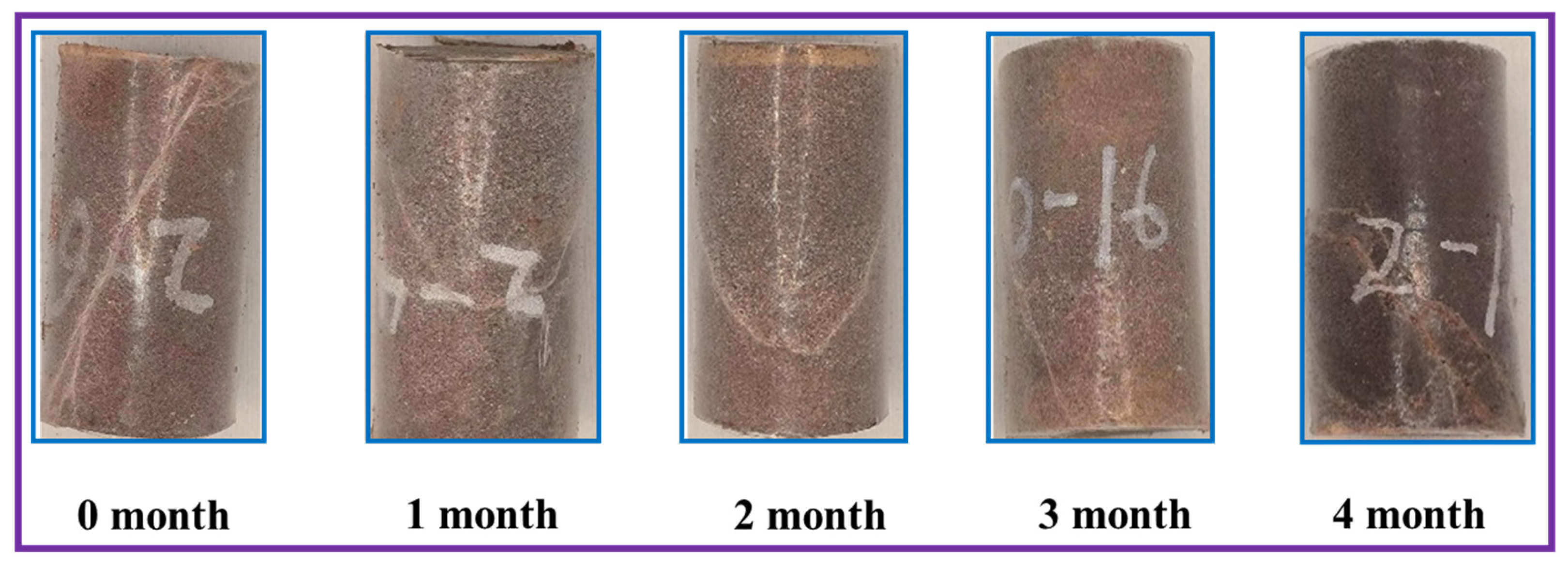
4.1. The Influence of Soaking Time on Cracks in Sandstones
4.2. Soaking Rule of Sandstone
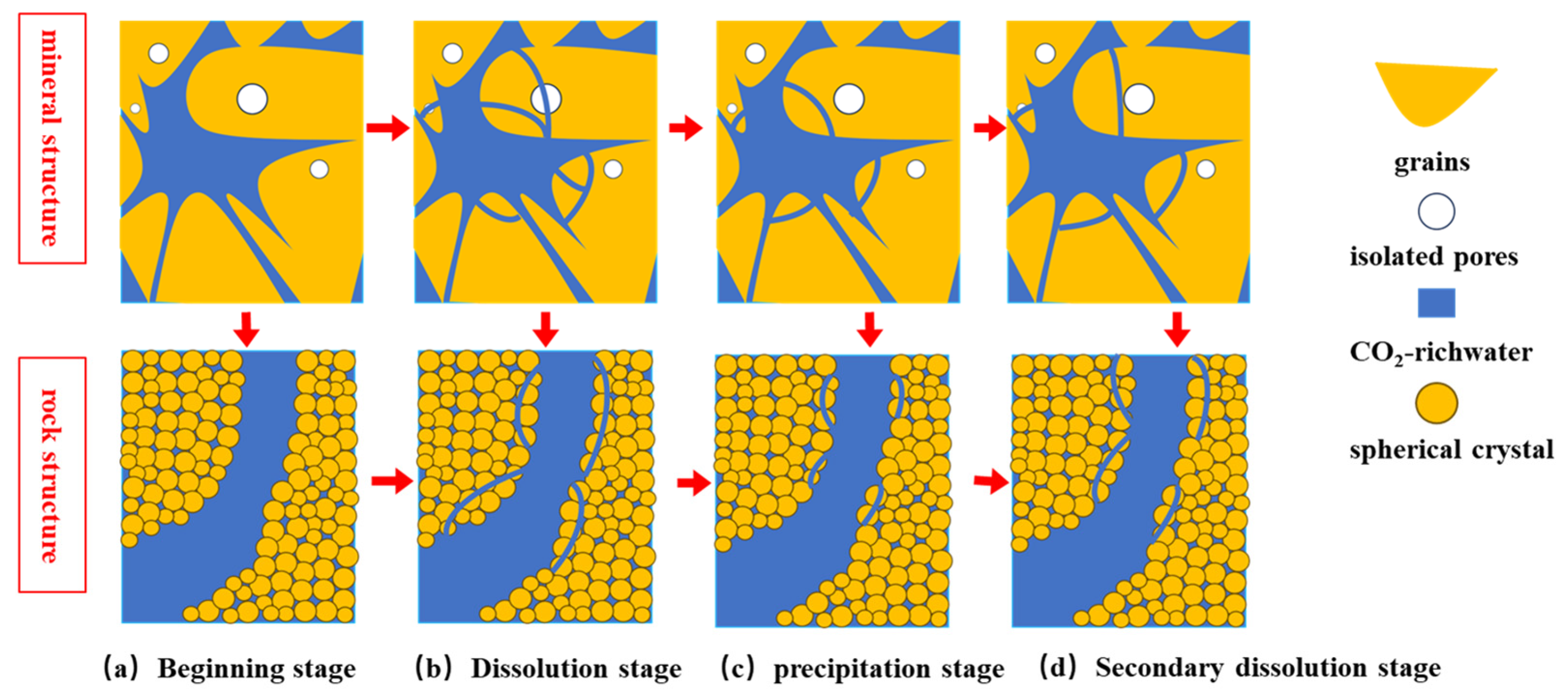
4.3. Model Diagram of the Influence of Soaking Time on Microstructure
5. Conclusions and Prospects
- In sandstone samples soaked in salt water–CO2 for an extended period, a significant decrease in compressive strength was observed; however, this decline did not persist with prolonged soaking time. Specifically, the compressive strength decreased from 152.7 MPa to 121.8 MPa during the initial months, rebounded to 148.9 MPa by the third month, and subsequently declined again by 16.8 MPa in the fourth month. Concurrently, similar trends were noted in the evolution of porosity and fractal dimension. As the immersion time increased, the failure mode progressively transitioned from simple shear failure to a combination of tension and shear failure.
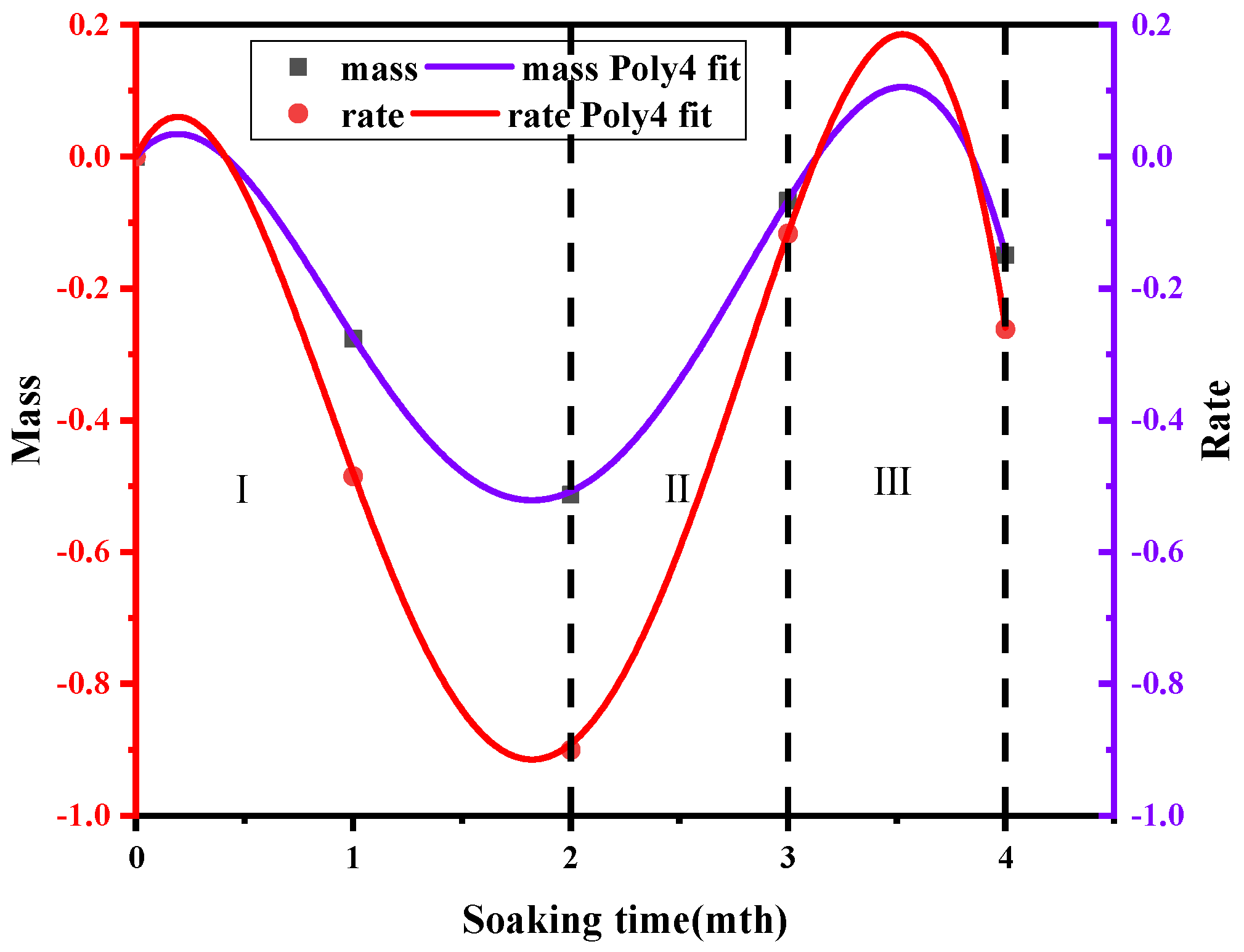
- In addition, based on the mathematical relationship between sandstone mass reduction and soaking time, the researchers established an exponential relationship curve. Through this curve, they defined the reaction rate of Ca2+ and developed a corresponding formula for salinity.
- The change curve of sandstone mass reduction can be categorized into three distinct reaction stages: the dissolution stage, the precipitation stage and the secondary dissolution stage. An analysis of the variations in the proportion of microscopic pore structures reveals that the dissolution of calcium carbonate predominantly occurs during the dissolution and secondary dissolution stages, whereas the precipitation of Ca minerals is primarily concentrated between the dissolution stages. Building on these findings, a microstructure model diagram of rocks in a saltwater–CO2 environment was established.
- Based on the three-dimensional crack expansion shapes of coarse sandstone observed over different corrosion durations, it was found that as the immersion time increased, the complexity of cracks in the reaction samples exhibited a pattern of first increasing, then decreasing, and subsequently increasing again. This variation is primarily concentrated during the dissolution stage, where fracture complexity increases, while a decreasing trend is noted during the precipitation stage.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hao, J.F.; Guo, C.Y.; Sun, W.J.; Liang, B.; Qin, B.; Li, L. Study on the deterioration and damage evolution characteristics of mechanical properties of siltstone after supercritical CO2 treatment. J. CO2 Util. 2024, 89, 2212–9820. [Google Scholar] [CrossRef]
- Feng, G.; Kang, Y.; Sun, Z.D.; Wang, X.-C.; Hu, Y.-Q. Effects of supercritical CO2 adsorption on the mechanical characteristics and failure mechanisms of shale. Energy 2019, 173, 0360–5442. [Google Scholar] [CrossRef]
- Vafaie, A.; Cama, J.; Soler, J.M.; Kivi, I.R.; Vilarrasa, V. Chemo-hydro-mechanical effects of CO2 injection on reservoir and seal rocks: A review on laboratory experiments. Renew. Sustain. Energy Rev. 2023, 178, 113270. [Google Scholar] [CrossRef]
- Bai, B.; Ni, H.J.; Shi, X.; Guo, X.; Ding, L. The experimental investigation of effect of supercritical CO2 immersion on mechanical properties and pore structure of shale. Energy 2021, 228, 0360–5442. [Google Scholar] [CrossRef]
- Ao, X.; Lu, Y.Y.; Tang, J.R.; Chen, Y.; Li, H. Investigation on the physics structure and chemical properties of the shale treated by supercritical CO2. J. CO2 Util. 2017, 20, 2212–9820. [Google Scholar] [CrossRef]
- Zhao, J.S.; Wang, P.F.; Zhang, Y.M.; Ye, L.; Shi, Y. Influence of CO2 injection on the pore size distribution and petrophysical properties of tight sandstone cores using nuclear magnetic resonance. Energy Sci. Eng. 2020, 8, 2286–2296. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.C.; Liu, M.X.; Song, Z.; Sun, Y.; Ni, S.; Fu, S.; Pan, R. Effect of supercritical carbon dioxide on pore structure and methane adsorption of shale with different particle sizes. J. Supercrit. Fluids 2024, 212, 0896–8446. [Google Scholar] [CrossRef]
- Pan, Y.; Hui, D.; Luo, P.Y.; Zhang, Y.; Zhang, L.; Sun, L. Influences of subcritical and supercritical CO2 treatment on the pore structure characteristics of marine and terrestrial shales. J. CO2 Util. 2018, 28, 2212–9820. [Google Scholar] [CrossRef]
- Yu, C.S.; Deng, H.C.; Jiang, Q.; Fan, Z.; Zhou, X.; Huang, S.; Lin, Q.; Peng, L. Mechanism of pore expansion and fracturing effect of high-temperature ScCO2 on shale. Fuel 2024, 363, 0016–0236. [Google Scholar] [CrossRef]
- Jiang, Y.D.; Luo, Y.H.; Lu, Y.Y.; Qin, C.; Liu, H. Effects of supercritical CO2 treatment time, pressure, and temperature on microstructure of shale. Energy 2016, 97, 0360–5442. [Google Scholar] [CrossRef]
- An, Q.Y.; Zhang, Q.S.; Li, X.H.; Yu, H.; Zhang, X. Experimental study on alteration kinetics for predicting rock mechanics damage caused by SC-CO2. Energy 2022, 259, 0360–5442. [Google Scholar] [CrossRef]
- Suto, Y.; Liu, L.H.; Yamasaki, N.; Hashida, T. Initial behavior of granite in response to injection of CO2-saturated fluid. Appl. Geochem. 2007, 22, 0883–2927. [Google Scholar] [CrossRef]
- Kaszuba, J.; Yardley, B.; Andreani, M. Experimental Perspectives of Mineral Dissolution and Precipitation due to Carbon Dioxide-Water-Rock Interactions. Rev. Mineral. Geochem. 2013, 77, 153–188. [Google Scholar] [CrossRef]
- Li, Y.; Ma, H.S.; Li, H.T.; Tang, Z.; Li, K.; Luo, H. Dissolution of supercritical CO2 on carbonate reservoirs. Pet. Reserv. Eval. Dev. 2023, 13, 288–295. [Google Scholar] [CrossRef]
- Liu, N.; Haugen, M.; Benali, B.; Landa-Marbán, D.; Fernø, M.A. Pore-scale kinetics of calcium dissolution and secondary precipitation during geological carbon storage. Chem. Geol. 2023, 641, 121782. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Zhou, J.K.; Li, H.L.; Chen, X.; Tang, J. Different Effect Mechanisms of Supercritical CO2 on the Shale Microscopic Structure. ACS omega 2020, 5, 22568–22577. [Google Scholar] [CrossRef]
- Lahann, R.; Mastalerz, M.; Rupp, J.A.; Drobniak, A. Influence of CO2 on New Albany Shale composition and pore structure. Int. J. Coal Geol. 2013, 108, 0166–5162. [Google Scholar] [CrossRef]
- Major, J.R.; Eichhubl, P.; Dewers, T.A.; Olson, J.E. Effect of CO2–brine–rock interaction on fracture mechanical properties of CO2 reservoirs and seals. Earth Planet. Sci. Lett. 2018, 499, 0012–0821X. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhang, F.S.; Song, X.H.; Wei, J.; Liu, S.; Wang, J. Stimulation of tight basalt reservoirs using supercritical carbon dioxide: Implications for large-scale carbon sequestration. J. Rock Mech. Geotech. Eng. 2024; in press. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, J.X.; Li, B.Y.; Zhu, C. Crack propagation patterns and factors controlling complex crack network formation in coal bodies during tri-axial supercritical carbon dioxide fracturing. Fuel 2021, 286, 0016–2361. [Google Scholar] [CrossRef]
- Ma, D.; Wu, Y.; Ma, X.; Hu, D.; Zhou, H.; Li, D. A Preliminary Experimental and Numerical Study on the Applicability of Liquid CO2 Fracturing in Sparse Sandstone. Rock Mech. Rock Eng. 2023, 56, 7315–7332. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, W.; Wei, C.; Elsworth, D.; Wang, J. Microcrack-based geomechanical modeling of rock-gas interaction during supercritical CO2 fracturing. J. Pet. Sci. Eng. 2018, 164, 91–102. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; He, J.; Mao, T.; Zheng, B.; Li, G. A laboratory investigation of fracture propagation induced by supercritical carbon dioxide fracturing in continental shale with interbeds. J. Pet. Sci. Eng. 2018, 166, 739–746. [Google Scholar] [CrossRef]
- Jiang, X.H.; Li, C.D.; Zhou, J.Q.; Zhang, Z.; Yao, W.; Chen, W.; Liu, H.-B. Salt-induced structure damage and permeability enhancement of Three Gorges Reservoir sandstone under wetting-drying cycles. Int. J. Rock Mech. Min. Sci. 2022, 153, 1365–1609. [Google Scholar] [CrossRef]
- Liang, W.G.; Yang, X.Q.; Gao, H.B.; Zhang, C.; Zhao, Y.; Dusseault, M.B. Experimental study of mechanical properties of gypsum soaked in brine. Int. J. Rock Mech. Min. Sci. 2012, 53, 1365–1609. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Y.L. Combined effects of salt, cyclic wetting and drying cycles on the physical and mechanical properties of sandstone. Eng. Geol. 2019, 248, 0013–7952. [Google Scholar] [CrossRef]
- Menéndez, B.; Petráňová, V. Effect of mixed vs single brine composition on salt weathering in porous carbonate building stones for different environmental conditions. Eng. Geol. 2016, 210, 0013–7952. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Mees, F.; Jacobs, P.; Rodriguez-Navarro, C. The role of saline solution properties on porous limestone salt weathering by magnesium and sodium sulfates. Environ. Geol. Int. J. Geosci. 2007, 52, 269–281. [Google Scholar] [CrossRef]
- Espinosa-Marzal, M.R.; Hamilton, A.; McNall, M.; Whitaker, K.; Scherer, G.W. The chemomechanics of crystallization during rewetting of limestone impregnated with sodium sulfate. J. Mater. Res. 2011, 26, 1472–1481. [Google Scholar] [CrossRef]
- Yuan, W.; Liu, X.; Fu, Y. Chemical thermodynamics and chemical kinetics analysis of sandstone dissolution under the action of dry–wet cycles in acid and alkaline environments. Bull. Eng. Geol. Environ. 2019, 78, 793–801. [Google Scholar] [CrossRef]
- Feng, X.T.; Chen, S.L.; Zhou, H. Real-time computerized tomography (CT) experiments on sandstone damage evolution during triaxial compression with chemical corrosion. Int. J. Rock Mech. Min. Sci. 2004, 41, 1365–1609. [Google Scholar] [CrossRef]
- Ai, T.; Zhang, R.; Zhou, H.; Pei, J. Box-counting methods to directly estimate the fractal dimension of a rock surface. Appl. Surf. Sci. 2014, 314, 610–621. [Google Scholar] [CrossRef]
- Panigrahy, C.; Seal, A.; Mahato, N.K. Quantitative texture measurement of gray-scale images: Fractal dimension using an improved differential box counting method. Measurement 2019, 147, 106859. [Google Scholar] [CrossRef]
- Elsayed, M.; Isah, A.; Hiba, M.; Hassan, A.; Al-Garadi, K.; Mahmoud, M.; El-Husseiny, A.; Radwan, A.E. A review on the applications of nuclear magnetic resonance (NMR) in the oil and gas industry: Laboratory and field-scale measurements. J. Pet. Explor. Prod. Technol. 2022, 12, 2747–2784. [Google Scholar] [CrossRef]
- Slijkerman, W.; Hofman, F.J.; Looyestijn, W.J.; Volokitin, Y. A Practical Approach To Obtain Primary Drainage Capillary Pressure Curves From Nmr Core And Log Data. Petrophysics 2001, 42, SPWLA-2001-v42n4a3. [Google Scholar]
- Zhao, P.; Wang, L.; Xu, C.; Fu, J.; Shi, Y.; Mao, Z.; Xiao, D. Nuclear magnetic resonance surface relaxivity and its advanced application in calculating pore size distributions. Mar. Pet. Geol. 2020, 111, 66–74. [Google Scholar] [CrossRef]
- Xu, Y.L.; Li, X.L.; Wu, X.K.; Zheng, W.; Zhou, B.; Tong, J. Experimental study on pore fluid characteristics of fractured sandstone based on nuclear magnetic resonance technology. J. Pet. Sci. Eng. 2022, 214, 110408. [Google Scholar] [CrossRef]
- Matteson, A.; Tomanic, J.; Herron, M.M.; Allen, D.F.; Kenyon, W.E. NMR Relaxation of Clay/Brine Mixtures. SPE Reserv. Eval. Eng. 2000, 3, 408–413. [Google Scholar] [CrossRef]
- Bi, J.; Ning, L.; Zhao, Y.; Wu, Z.; Wang, C. Analysis of the microscopic evolution of rock damage based on real-time nuclear magnetic resonance. Rock Mech. Rock Eng. 2023, 56, 3399–3411. [Google Scholar] [CrossRef]
- Zheng, S.J.; Yao, Y.B.; Liu, D.M.; Cai, Y.; Liu, Y. Characterizations of full-scale pore size distribution, porosity and permeability of coals: A novel methodology by nuclear magnetic resonance and fractal analysis theory. Int. J. Coal Geol. 2018, 196, 148–158. [Google Scholar] [CrossRef]
- Michael, K.; Golab, A.; Shulakova, V.; Ennis-King, J.; Allinson, G.; Sharma, S.; Aiken, T. Geological storage of CO2 in saline aquifers—A review of the experience from existing storage operations. Int. J. Greenh. Gas Control. 2009, 4, 659–667. [Google Scholar] [CrossRef]
- Vilarrasa, V.; Silva, O.; Carrera, J.; Olivella, S. Liquid CO2 injection for geological storage in deep saline aquifers. Int. J. Greenh. Gas Control. 2013, 14, 1750–5836. [Google Scholar] [CrossRef]
- Luo, A.; Li, Y.M.; Chen, X.; Zhu, Z.; Peng, Y. Review of CO2 sequestration mechanism in saline aquifers. Nat. Gas Ind. B 2022, 9, 2352–8540. [Google Scholar] [CrossRef]
- Zeidouni, M.; Pooladi-Darvish, M.; Keith, D. Analytical solution to evaluate salt precipitation during CO2 injection in saline aquifers. Int. J. Greenh. Gas Control. 2009, 3, 1750–5836. [Google Scholar] [CrossRef]


| Reference Serial Number | 0 | 1–5 | 6–10 | 11–17 | 18–24 | 25 | 26–30 |
|---|---|---|---|---|---|---|---|
| Soaking medium | Brine–CO2 | CO2 | Brine | ||||
| Maximum soaking time | 120 days | 30 days | 2 days | 7 days | Close to 5 days | 80 days | Close to 3 days |
| Influence of mechanical property parameters (compressive strength, etc.) | First decrease, then increase, then decrease | Simple reduction | / | / | / | Simple reduction | / |
| Influence of physical property parameters (total porosity, etc.) | First increase, then decrease, then increase | / | Simple increase | / | / | / | / |
| Influence of mineral composition | Dynamic changes of dissolution, precipitation and secondary dissolution | / | / | It is mainly minerals that are dissolved | / | / | There is an effect, but the mechanism is not completely clear |
| Influence of the fracture propagation mode | The crack deforms from “/” to “V”, then to a local “/” shape and finally to an “X” shape | / | / | / | Simple surface spalling and internal cracking of the rock | / | |
| ID | Diameter/mm | Altitude/mm | Weight/g | Density/g/cm3 | Wave Velocity/m/s |
|---|---|---|---|---|---|
| 0-1 | 25.10 | 50.20 | 56.70 | 2.28 | 3980 |
| 0-2 | 25.15 | 50.08 | 58.98 | 2.37 | 3979 |
| 0-3 | 25.05 | 50.13 | 58.20 | 2.36 | 3979 |
| 1-1 | 25.11 | 50.04 | 58.10 | 2.34 | 4002 |
| 1-2 | 25.14 | 50.17 | 57.60 | 2.31 | 3979 |
| 2-1 | 25.15 | 50.16 | 58.70 | 2.36 | 3994 |
| 2-2 | 25.24 | 50.22 | 58.06 | 2.31 | 3966 |
| 3-1 | 25.21 | 50.14 | 58.30 | 2.33 | 3914 |
| 3-2 | 25.23 | 50.09 | 60.20 | 2.40 | 3932 |
| 4-1 | 25.10 | 50.15 | 58.13 | 2.34 | 3948 |
| 4-2 | 25.09 | 50.27 | 58.95 | 2.37 | 3946 |
| Soaking Time/m | Compressive Strength/MPa | Elasticity Modulus/GPa | Poisson Ratio | Permeability/mD |
|---|---|---|---|---|
| 0 | 152.68 | 11.64 | 0.301 | 1.00 |
| 1 | 134.97 | 9.53 | 0.228 | 1.32 |
| 2 | 121.8 | 8.21 | 0.200 | 2.09 |
| 3 | 148.86 | 11.08 | 0.289 | 1.04 |
| 4 | 138.98 | 10.71 | 0.244 | 1.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, M.; Mao, H.; Zhang, G.; Liu, J.; Zhu, S.; Wang, D.; Xie, H. Saline–CO2 Solution Effects on the Mechanical Properties of Sandstones: An Experimental Study. Appl. Sci. 2025, 15, 607. https://doi.org/10.3390/app15020607
Duan M, Mao H, Zhang G, Liu J, Zhu S, Wang D, Xie H. Saline–CO2 Solution Effects on the Mechanical Properties of Sandstones: An Experimental Study. Applied Sciences. 2025; 15(2):607. https://doi.org/10.3390/app15020607
Chicago/Turabian StyleDuan, Motao, Haijun Mao, Guangquan Zhang, Junxin Liu, Sinan Zhu, Di Wang, and Hao Xie. 2025. "Saline–CO2 Solution Effects on the Mechanical Properties of Sandstones: An Experimental Study" Applied Sciences 15, no. 2: 607. https://doi.org/10.3390/app15020607
APA StyleDuan, M., Mao, H., Zhang, G., Liu, J., Zhu, S., Wang, D., & Xie, H. (2025). Saline–CO2 Solution Effects on the Mechanical Properties of Sandstones: An Experimental Study. Applied Sciences, 15(2), 607. https://doi.org/10.3390/app15020607






