Fermentation of Grapefruit Juice with Lacticaseibacillus rhamnosus and Enzymatic Debittering by Naringinase
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Immobilization of Naringinase
2.2.1. Immobilization and Stabilization of Naringinase on a Polysaccharide Magnetic Carrier
2.2.2. Immobilization and Stabilization of Naringinase on a Chitosan Magnetic Carrier
2.3. Fermentation and Debittering of Grapefruit Juice
2.4. Determination of Naringin Concentration
2.5. Lacticaseibacillus rhamnosus Cell Counts
2.6. Determination of Total Phenol Content (TPC)
2.7. Antioxidant Activity
2.8. Analysis of Organic Acids and Carbohydrates
2.9. Statistical Analysis
3. Results and Discussion
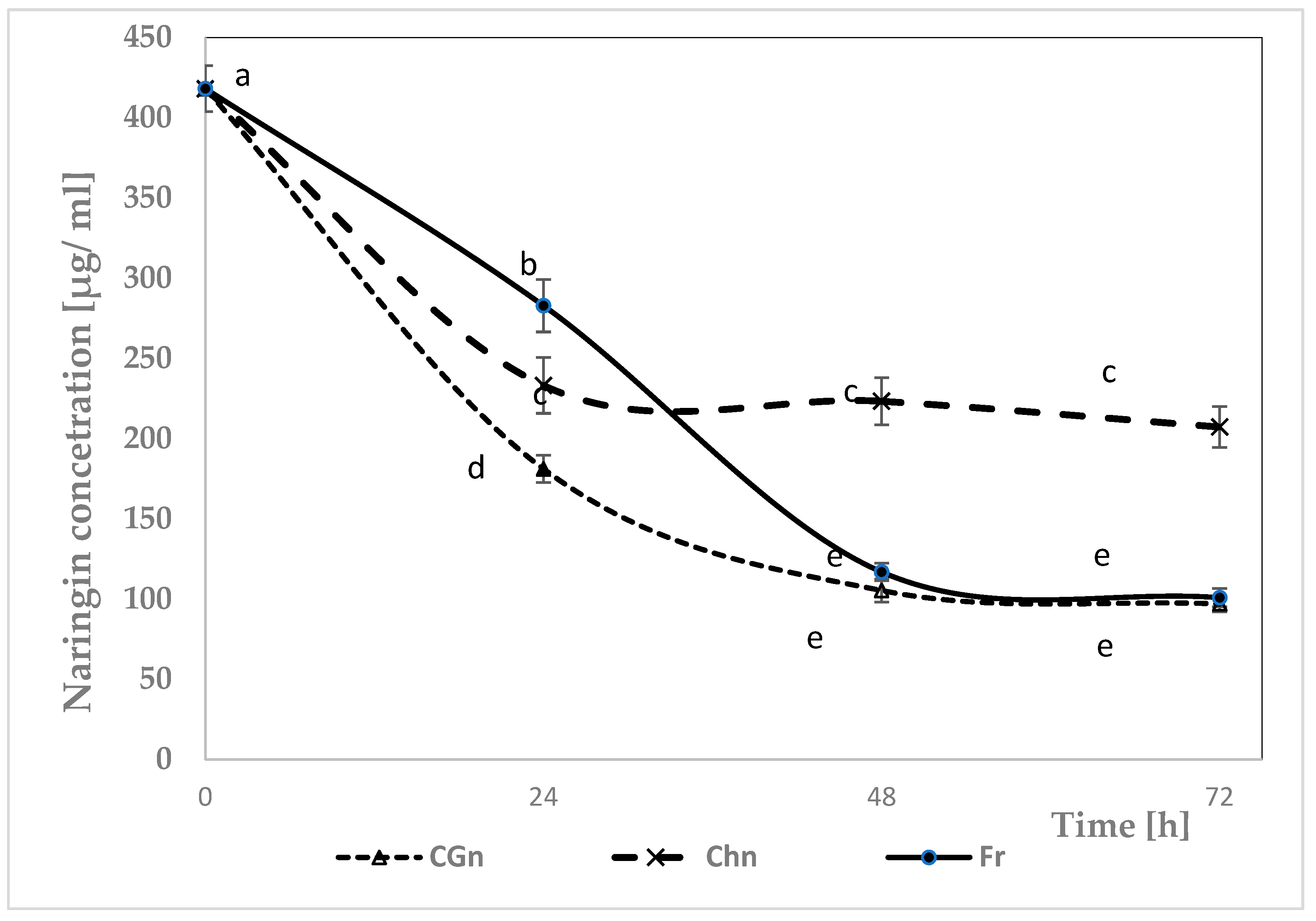
3.1. Concentration of Naringin
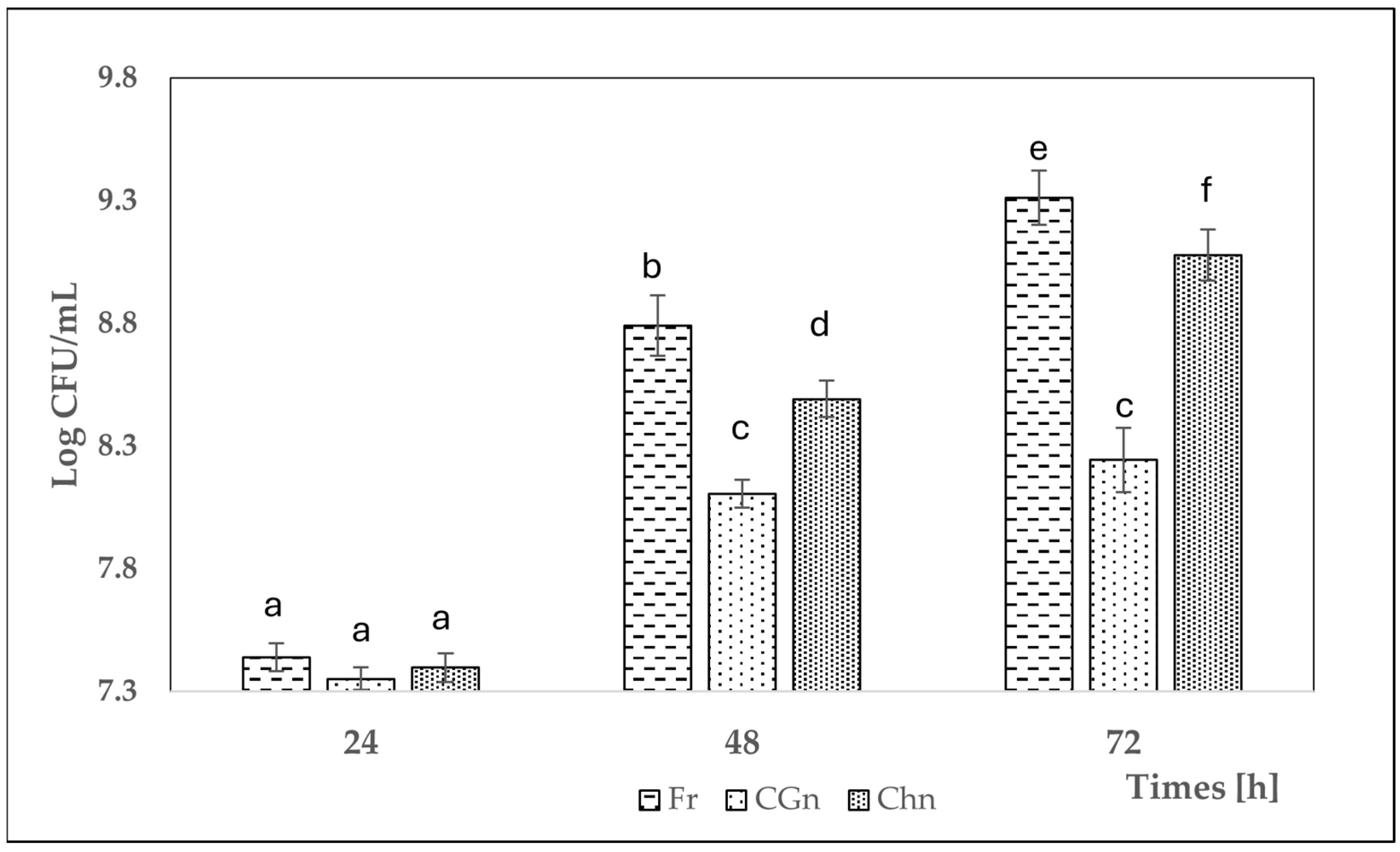
3.2. Changes of Lacticaseibacillus rhamnosus Cell Counts
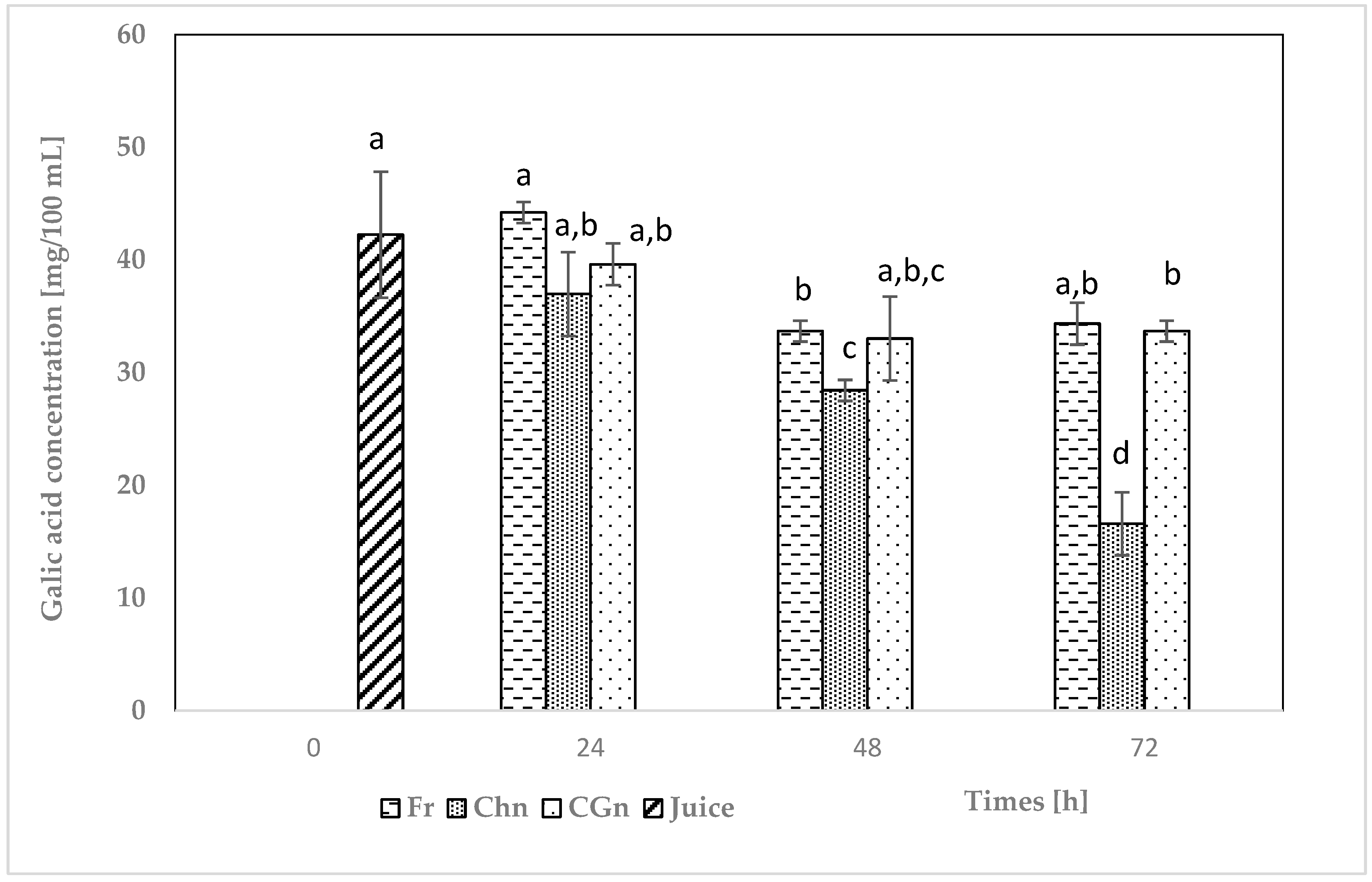
3.3. Total Phenol Content
3.4. Antioxidant Potentials
3.5. Analysis of Carbohydrates and Organic Acids
3.6. Changes in pH of Grapefruit Juice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Fr | grapefruit juice + Lactic. rhamnosus + free naringinase |
| CGn | grapefruit juice + Lactic. rhamnosus + naringinase immobilized on locust bean gum |
| Chn | grapefruit juice + Lactic. rhamnosus + naringinase immobilized on chitosan |
| Fr-1d, Fr-2d, Fr-3d | grapefruit juice + Lactic. rhamnosus + free naringinase after 1, 2, and 3 days of fermentation |
| CGn-1d, CGn-2d, CGn-3d | grapefruit juice + Lactic. rhamnosus + naringinase immobilized on locust bean gum after 1, 2, and 3 days of fermentation |
| Chn-1d, Chn-2d, Chn-3d | grapefruit juice + Lactic. rhamnosus + naringinase immobilized on chitosan after 1, 2, and 3 days of fermentation |
References
- Perricone, M.; Bevilacqua, A.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Challenges for the Production of Probiotic Fruit Juices. Beverages 2015, 1, 95–103. [Google Scholar] [CrossRef]
- Kandylis, P.; Pissaridi, K.; Argyro, B.; Kanellaki, M.; Los Koutinas, A. Dairy and non-dairy probiotic beverages. Curr. Opin. Food Sci. Vol. 2016, 7, 2016. [Google Scholar] [CrossRef]
- de Oliveira Vieira, K.C.; Ferreira, C.D.S.; Bueno, E.B.T.; De Moraes, Y.A.; Toledo, A.C.C.G.; Nakagaki, W.R.; Winkelstroter, L.K. Development and viability of probiotic orange juice supplemented by Pediococcus acidilactici CE51. LWT Food Sci. Technol. 2020, 130, 109637. [Google Scholar] [CrossRef]
- Prieto-Santiago, V.; Aguil, I.; Schorn-García, D.; Ezenarro, J.; Rico, D.; Martín-Diana, A.B.; Anguera, M.; Abadias, M. Effects of Lacticaseibacillus casei fermentation on the physicochemical, nutritional, volatile, and sensory profile of synbiotic peach and grape juice during refrigerated storage. Appl. Food Biotechnol. 2025, 5, 101002. [Google Scholar] [CrossRef]
- Rizzi, F.; Juan, B.; Espadaler-Mazo, J.; Capellas, M.; Huedo, P. Lactiplantibacillus plantarum KABP051: Stability in Fruit Juices and Production of Bioactive Compounds During Their Fermentation. Foods 2024, 13, 3851. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Feng, Y.; Yang, N.; Jiang, T.; Xu, H.; Lei, H. Fermentation of kiwifruit juice from two cultivars by probiotic bacteria: Bioactive phenolics, antioxidant activities and flavor volatiles. Food Chem. 2022, 373, 131455. [Google Scholar] [CrossRef] [PubMed]
- Naseem, Z.; Ahmad, S.; Wani, S.M.; Rouf, M.A.; Bashir, I.; Sc, M. Probiotic-fortified fruit juices: Health benefits, challenges, and future perspective. Nutrition 2023, 115, 112154. [Google Scholar] [CrossRef] [PubMed]
- Imece, A.; Sengul, M.; Cetin, B.; Aktas, H. Effect of probiotic Lactiplantibacillus plantarum strains on some properties of grapefruit juice and naringin. J. Stored Prod. Res. 2024, 108, 102359. [Google Scholar] [CrossRef]
- Nualkaekul, S.; Gurjot, S.; Dimitris, C. Survival of freeze dried Lactobacillus plantarum in instant fruit powders and reconstituted fruit juices. Food Res. Int. 2012, 48, 627–633. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Li, L.; Xu, Q.; Han, R.; Wu, Z.; Zhang, Y. Debittering and Antioxidant Activity of Fermented Grapefruit Juice with Lactiplantibacillus plantarum 1-1-2. Shipin Kexue/Food Sci. 2024, 45, 98–107. [Google Scholar] [CrossRef]
- Tran, A.M.; Nguyen, T.B.; Nguyen, V.D.; Bujna, E.; Dam, M.S.; Nguyen, Q.D. Changes in bitterness, antioxidant activity and total phenolic content of grapefruit juice fermented by Lactobacillus and Bifidobacterium strains. Acta Aliment. 2020, 49, 103–110. [Google Scholar] [CrossRef]
- Irkin, R.; Dogan, S.; Degirmenioglu, N.; Diken, M.E.; Guldas, M. Phenolic content, antioxidant activities and stimulatory roles of citrus fruits on some lactic acid bacteria. Arch. Biol. Sci. 2015, 67, 1313–1321. [Google Scholar] [CrossRef]
- Nualkaekul, S.; Salmeron, I.; Charalampopoulos, D. Investigation of the factors influencing the survival of Bifidobacterium longum in model acidic solutions and fruit juices. Food Chem. 2011, 129, 1037–1044. [Google Scholar] [CrossRef]
- Gous, A.G.S.; Almli, L.; Coetzee, V. Levels of a Grapefruit-Like Model Beverage on the Sensory Properties and Liking of the Consumer. Nutrients 2019, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Bodakowska-Boczniewicz, J.; Garncarek, Z. Use of Naringinase to Modify the Sensory Quality of Foods and Increase the Bioavailability of Flavonoids: A Systematic Review. Molecules 2025, 30, 2376. [Google Scholar] [CrossRef] [PubMed]
- Bodakowska-Boczniewicz, J.; Garncarek, Z. Immobilization of Naringinase from Aspergillus Niger on a Magnetic Polysaccharide Carrier. Molecules 2020, 25, 2731. [Google Scholar] [CrossRef]
- Kimmins, S.D.; Henríquez, A.; Torres, C.; Wilson, L.; Flores, M.; Pio, E.; Jullian, D.; Urbano, B.; Braun-Galleani, S.; Ottone, C.; et al. Immobilization of Naringinase onto Polydopamine-Coated Magnetic Iron Oxide Nanoparticles for Juice Debittering Applications. Polymers 2024, 16, 3279. [Google Scholar] [CrossRef]
- González-Temiño, Y.; Ruíz, M.O.; Ortega, N.; Ramos-Gómez, S.; Busto, M.D. Immobilization of naringinase on asymmetric organic membranes: Application for debittering of grapefruit juice. Innov. Food Sci. Emerg. Technol. 2021, 73, 102790. [Google Scholar] [CrossRef]
- Capurso, L. Thirty Years of Lactobacillus rhamnosus GG A Review. J. Clin. Gastroenterol. 2019, 53, 1–41. [Google Scholar] [CrossRef]
- Verdenelli, M.; Ghelefi, F.; Silvi, S.; Orpianesi, C.; Cecchini, C.; Cresci, A. Probiotic properties of Lactobacillus rhamnosus and Lactobacillus paracasei isolated from human faeces. Eur. J. Nutr. 2009, 48, 355–363. [Google Scholar] [CrossRef]
- Feng, J.; Cen, Q.; Cui, Y.; Hu, X.; Li, M.; Wang, L.; Wei, J.; Sun, N.; Wang, J.; Zhang, A. Lactobacillus rhamnosus: An emerging probiotic with therapeutic potential for depression. Pharmacol. Res. 2025, 211, 107541. [Google Scholar] [CrossRef] [PubMed]
- Nancib, N.; Nancib, A.; Boudjelal, A.; Benslimane, C.; Blanchard, F.; Boudrant, J. The effect of supplementation by different nitrogen sources on the production of lactic acid from date juice by Lactobacillus casei subsp. Rhamnosus. Bioresour. Technol. 2001, 78, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Güney, D.; Güngörmü, M. Development and Comparative Evaluation of a Novel Fermented Juice Mixture with Probiotic Strains of Lactic Acid Bacteria and Bifidobacteria. Probiotics Antimicrob. Proteins 2021, 13, 495–505. [Google Scholar] [CrossRef]
- White, J.; Hekmat, S. Development of Probiotic Fruit Juices Using Lactobacillus rhamnosus GR-1 Fortified with Short Chain and Long Chain Inulin Fiber. Fermentation 2018, 4, 27. [Google Scholar] [CrossRef]
- Bodakowska-Boczniewicz, J.; Garncarek, Z. Naringinase Biosynthesis by Aspergillus niger on an Optimized Medium Containing Red Grapefruit Albedo. Molecules 2022, 27, 8763. [Google Scholar] [CrossRef]
- Rajdeo, K.; Harini, T.; Lavanya, K.; Fadnavis, N.W. Immobilization of pectinase on reusable polymer support for clarification of apple juice. Food Bioprod. Process. 2016, 99, 12–19. [Google Scholar] [CrossRef]
- Bodakowska-Boczniewicz, J.; Garncarek, Z. Immobilization of naringinase from Penicillium decumbens on chitosan microspheres for debittering grapefruit juice. Molecules 2019, 24, 4234. [Google Scholar] [CrossRef]
- Davis, W.B. Determination of Flavanones in Citrus Fruits. Anal. Chem. 1947, 19, 476–478. [Google Scholar] [CrossRef]
- Modrzejewska, K.; Bogusławska-Wąs, E. Comparison of selected microbiological and biochemical parameters of kombucha beverages. Żywność. Nauk. Technol. Jakość 2020, 4, 39–51. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 1999. [Google Scholar]
- Wilczyńska, A. Methods for determining the antioxidant activity of bee honey. Bromatol. I Chem. Toksykol. 2009, 3, 870–874. [Google Scholar]
- Soares, N.F.F.; Hotchkiss, J.H. Bitterness reduction in grapefruit juice through active packaging. Packag. Technol. Sci. 1998, 11, 9–18. [Google Scholar] [CrossRef]
- Urrutia, P.; Arrieta, R.; Torres, C.; Guerrero, C.; Wilson, L. Amination of naringinase to improve citrus juice debittering using a catalyst immobilized on glyoxyl-agarose. Food Chem. 2024, 452, 139600. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; Pintado, C.; Rotger, R.; Goñi, I. Stimulatory role of grape pomace polyphenols on Lactobacillus acidophilus growth. Int. J. Food Microbiol. 2009, 136, 119–122. [Google Scholar] [CrossRef]
- Akarca, G.; Baytal, F. Effects of fermentation on the quality characteristics and biological activities of citrus juices. Process Biochem. 2023, 130, 634–644. [Google Scholar] [CrossRef]
- Oruç, S.Ö.; Çakır, I. A research on production of fermented watermelon juice by probiotic culture. GIDA J. Food 2019, 44, 1030–1041. [Google Scholar] [CrossRef]
- Quan, Q.; Liu, W.; Guo, J.; Ye, M.; Zhang, J. Effect of Six Lactic Acid Bacteria Strains on Physicochemical Characteristics, Antioxidant Activities and Sensory Properties of Fermented Orange Juices. Foods 2022, 11, 1920. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Lai, X.; Nong, J.; Zhao, G.; Xiao, X. One-pot biocatalytic synthesis and antioxidant activities of highly lipophilic naringin derivatives by using bi-functional whole-cells. Food Res. Int. 2020, 136, 109291. [Google Scholar] [CrossRef]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef] [PubMed]
- Forouhandeh, H.; Zunumi Vahed, S.; Hejazi, M.S.; Hahaei, M.R.; Akbari Dibavar, M. Isolation and Phenotypic Characterization of Lactobacillus Species from Various Dairy Products. Curr. Res. Bacteriol. 2010, 3, 84–88. [Google Scholar] [CrossRef]
- Paulo, U.D.S.; Science, L. Effect of inulin on the growth and metabolism of a probiotic strain of Lactobacillus rhamnosus in co-culture with Streptococcus thermophilus. LWT Food Sci. Technol. 2012, 47, 358–363. [Google Scholar] [CrossRef]
- Nancib, A.; Nancib, N.; Meziane-Cherif, D.; Boubendir, A. Joint effect of nitrogen sources and B vitamin supplementation of date juice on lactic acid production by Lactobacillus casei subsp. rhamnosus. Bioresour. Technol. 2005, 96, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Helland, M.H.; Wicklund, T.; Narvhus, J.A. Growth and metabolism of selected strains of probiotic bacteria in maize porridge with added malted barley. Int. J. Food Microbiol. 2004, 91, 305–313. [Google Scholar] [CrossRef] [PubMed]

| Glucose | Fructose | Sucrose | |
|---|---|---|---|
| [mM] | |||
| Grapefruit juice | 160.78 ± 0.66 a | 142.63 ± 4.20 a | 58.90 ± 3.65 a |
| Fr-1d | 89.80 ± 0.59 b | 84.23 ± 4.18 b | 54.65 ± 2.02 ab |
| Fr-2d | 8.77 ± 0.44 c | n.d. | 37.48 ± 1.72 c |
| Fr-3d | 6.56 ± 0.24 d | n.d. | 24.79 ± 2.65 d |
| CGn-1d | 103.58 ± 2.85 e | 94.61 ± 4.61 c | 53.33 ± 1.90 b |
| CGn-2d | 9.91 ± 0.31 e | n.d. | 46.02 ± 2.41 e |
| CGn-3d | 9.41 ± 1.31 e | n.d. | 25.96 ± 2.02 d |
| Chn-1d | 81.95 ± 1.31 f | 75.48 ± 3.91 d | 56.85 ± 4.00 ab |
| Chn-2d | 7.41 ± 0.57 cd | n.d. | 34.72 ± 2.29 c |
| Chn-3d | 4.94 ± 0.20 d | n.d. | 19.77 ± 1.17 f |
| Citric Acid | Malic Acid | Lactic Acid | Acetic Acid | Propionic Acid | |
|---|---|---|---|---|---|
| Concentration [mM] | |||||
| Grapefruit juice | 63.21 ± 2.61 a | 3.07 ± 0.12 a | 0.94 ± 0.12 a | n.d. | 12.00 ± 1.18 ad |
| Fr-1d | 61.24 ± 2.72 ab | 2.35 ± 0.05 b | 7.96 ± 0.45 b | n.d. | 11.53 ± 1.31 ad |
| Fr-2d | 58.31 ± 4.06 ab | 0.29 ± 0.01 c | 7.73 ± 0.59 c | n.d. | 13.40 ± 1.40 a |
| Fri-3d | 57.00 ± 5.00 ab | 0.19 ± 0.01 d | 6.84 ± 0.58 ch | n.d. | 13.11 ± 1.27 a |
| CGn-1d | 59.97 ± 5.27 ab | 2.98 ± 0.02 e | 2.26 ± 0.19 d | n.d. | 12.43 ± 1.40 ad |
| CGn-2d | 59.69 ± 5.57 ab | 0.21 ± 0.01 d | 13.47 ± 0.83 e | 4.14 ± 0.84 a | 16.17 ± 0.90 b |
| CGn-3d | 58.14 ± 5.11 ab | 0.11 ± 0.01 d | 12.26 ± 0.94 f | n.d. | 19.06 ± 0.91 c |
| Chn-1d | 58.94 ± 5.40 ab | 3.15 ± 0.06 f | 4.23 ± 0.42 g | n.d. | 16.14 ± 0.99 b |
| Chn-2d | 52.27 ± 4.41 b | 0.18 ± 0.01 d | 6.51 ± 0.77 h | 5.05 ± 0.95 a | 10.10 ± 0.85 ad |
| Chn-3d | 47.97 ± 5.21 b | 0.17 ± 0.01 d | 6.35 ± 0.56 h | 1.28 ± 0.45 b | 10.72 ± 1.74 ad |
| pH [-] | |
|---|---|
| Grapefruit juice | 3.26 ± 0.03 a |
| Fr-1d | 3.21 ± 0.06 a |
| Fr-2d | 3.12 ± 0.10 b |
| Fr-3d | 3.00 ± 0.06 c |
| CGn-1d | 3.03 ± 0.10 c |
| CGn-2d | 2.92 ± 0.07 d |
| CGn-3d | 2.87 ± 0.20 d |
| Chn-1d | 3.25 ± 0.20 a |
| Chn-2d | 3.12 ± 0.18 b |
| Chn-3d | 3.07 ± 0.12 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górska, K.; Bodakowska-Boczniewicz, J.; Garncarek, Z. Fermentation of Grapefruit Juice with Lacticaseibacillus rhamnosus and Enzymatic Debittering by Naringinase. Appl. Sci. 2025, 15, 10858. https://doi.org/10.3390/app151910858
Górska K, Bodakowska-Boczniewicz J, Garncarek Z. Fermentation of Grapefruit Juice with Lacticaseibacillus rhamnosus and Enzymatic Debittering by Naringinase. Applied Sciences. 2025; 15(19):10858. https://doi.org/10.3390/app151910858
Chicago/Turabian StyleGórska, Katarzyna, Joanna Bodakowska-Boczniewicz, and Zbigniew Garncarek. 2025. "Fermentation of Grapefruit Juice with Lacticaseibacillus rhamnosus and Enzymatic Debittering by Naringinase" Applied Sciences 15, no. 19: 10858. https://doi.org/10.3390/app151910858
APA StyleGórska, K., Bodakowska-Boczniewicz, J., & Garncarek, Z. (2025). Fermentation of Grapefruit Juice with Lacticaseibacillus rhamnosus and Enzymatic Debittering by Naringinase. Applied Sciences, 15(19), 10858. https://doi.org/10.3390/app151910858






