Development and Validation of an HPLC-PDA Method for NMN Quantification in Commercial Pet Foods
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Materials
2.2. Solution Preparation
2.2.1. Standard Stock Solution
2.2.2. Standard Working Solution
2.3. Sample Pretreatment
2.4. LC Conditions
2.5. Plotting of Standard Curves
2.6. Instrument Precision
2.7. Stability
2.8. Repeatability
2.9. LOD and LOQ
2.10. Recovery
2.11. Data Processing
3. Results
3.1. Optimization of Liquid Chromatography Conditions
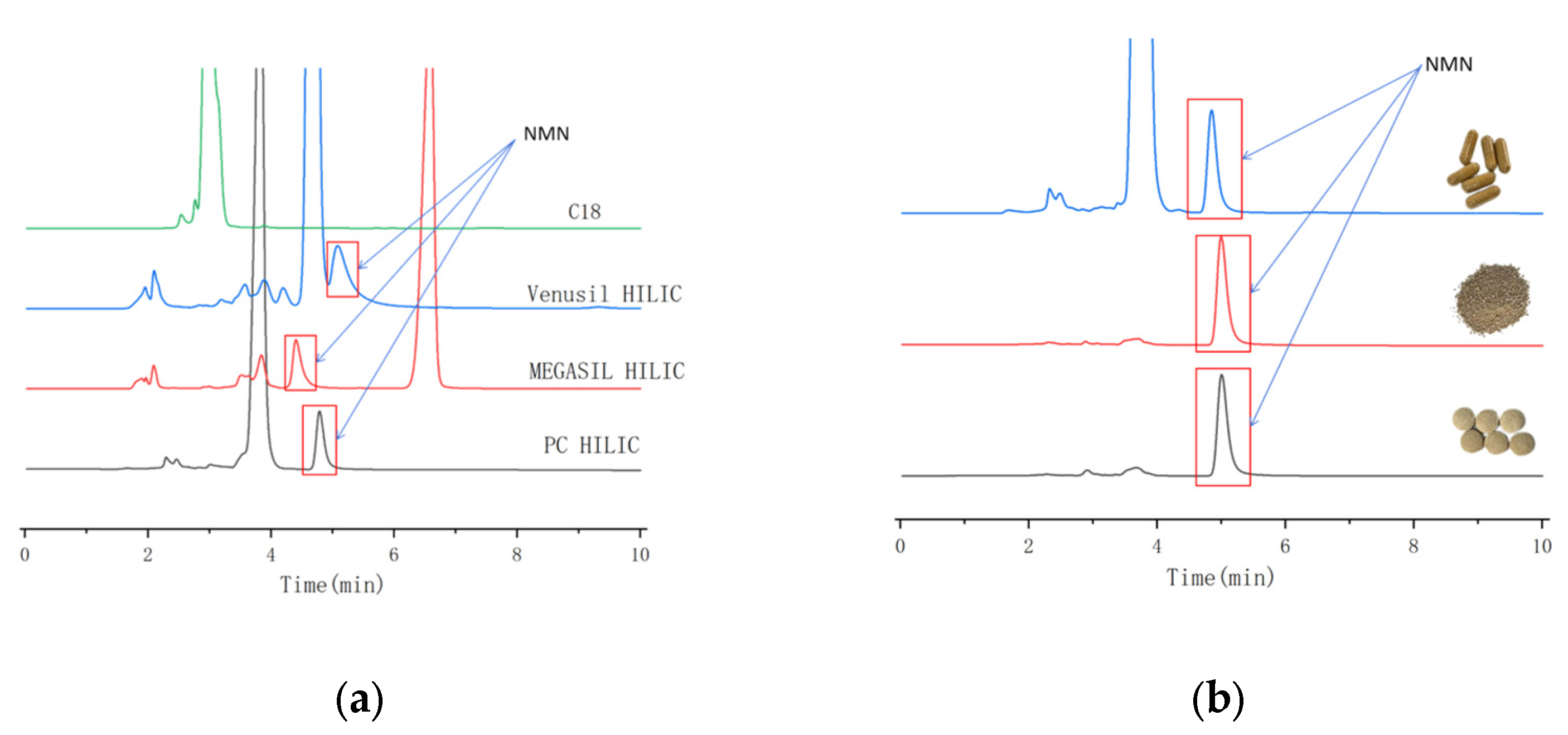
3.1.1. Chromatographic Columns Optimization
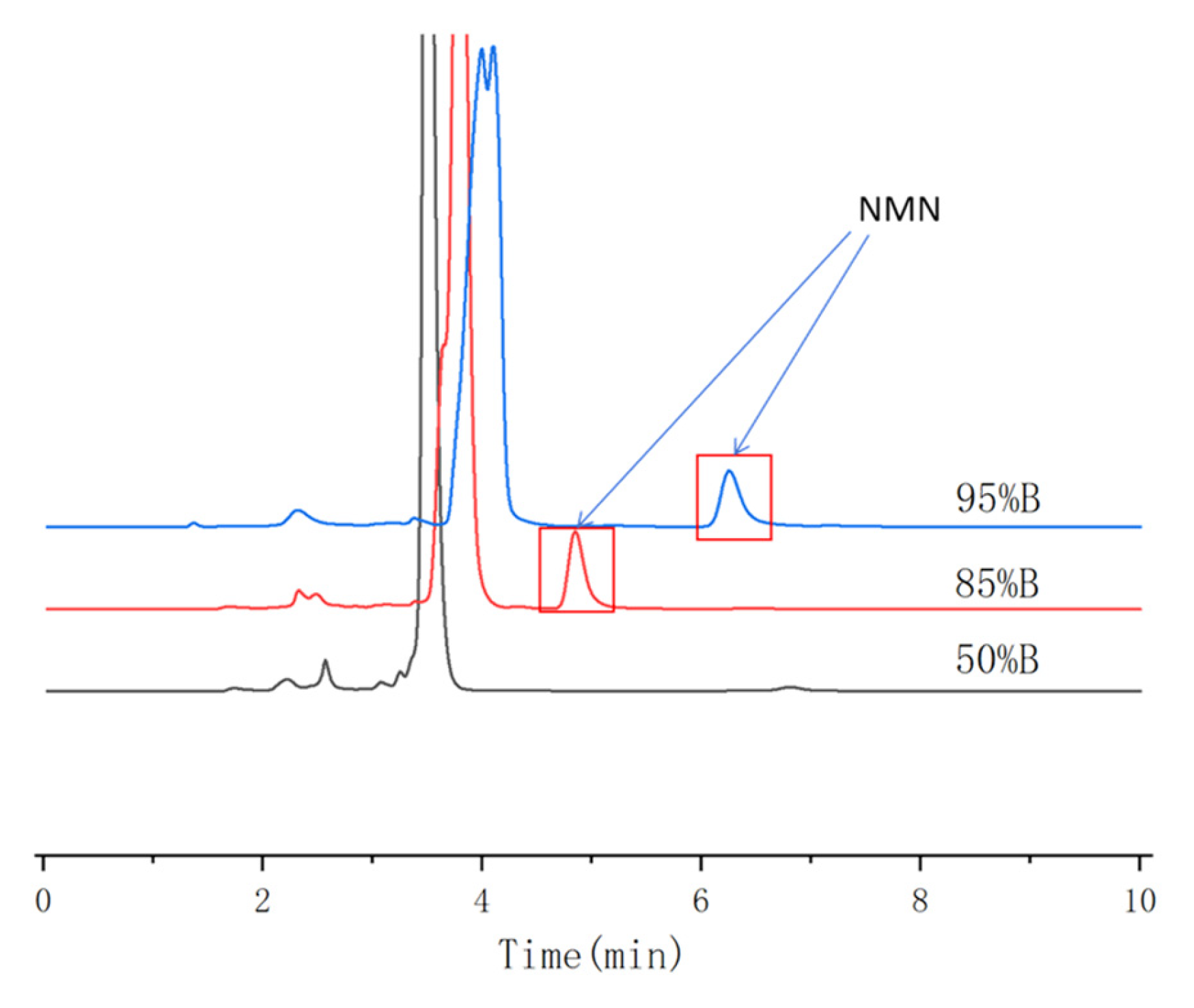
3.1.2. Selection of Mobile Phase
3.2. Optimization of Extraction Method
3.2.1. Extraction Solvent Optimization
3.2.2. Optimization of Ultrasonic Extraction Conditions
3.3. Standard Curve, LOD, and LOQ
3.4. Precision Results
3.5. Stability Results
3.6. Repeatability Results
3.7. Recovery Results
3.8. Detection of Actual Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NMN | Nicotinamide Mononucleotide |
| HPLC | High-Performance Liquid Chromatography |
| PDA | Photodiode Array Detector |
| HILIC | Hydrophilic Interaction Liquid Chromatography |
References
- Ministry of Agriculture and Rural Development of the People’s Republic of China. Measures for the Administration of Pet Feed. 2024. Available online: https://www.gov.cn/ (accessed on 28 January 2025).
- U.S Food and Drug Administration. Federal Food, Drug, and Cosmetic Act (FD&C Act). Available online: https://www.fda.gov/regulatory-information/laws-enforced-fda/federal-food-drug-and-cosmetic-act-fdc-act (accessed on 28 January 2025).
- Compilation Committee of the Compendium of EU Feed Regulations, Compendium of EU Feed Regulations; China Quality Inspection Press: Beijing, China, 2013. Available online: https://www.legislation.gov.uk/eur/2013/68/contents (accessed on 28 January 2025).
- European Pet Food Industry Association. Nutritional Guidelines: For Complete and Complementary Pet Food for Cats and Dogs. 2024. Available online: https://europeanpetfood.org/pet-food-facts/fact-sheets/nutrition/additives/ (accessed on 28 January 2025).
- The Association of American Feed Control Officials. Model Regulations for Pet Food and Special Pet Food. 2023. Available online: https://www.aafco.org (accessed on 28 January 2025).
- Nadeeshani, H.; Li, J.; Ying, T.; Zhang, B.; Lu, J. Nicotinamide mononucleotide (NMN) as an anti-aging health product—Promises and safety concerns. J. Adv. Res. 2021, 37, 267–278. [Google Scholar] [CrossRef]
- Camacho-Pereira, J.; Tarragó, M.G.; Chini, C.C.S.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A.; et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef]
- Ru, M.; Wang, W.; Zhai, Z.; Wang, R.; Li, Y.; Liang, J.; Kothari, D.; Niu, K.; Wu, X. Nicotinamide mononucleotide supplementation protects the intestinal function in aging mice and D-galactose induced senescent cells. Food Funct. 2022, 13, 7507–7519. [Google Scholar] [CrossRef] [PubMed]
- Amazon Official Website. NMN-Containing Pet Supplements. 2025. Available online: https://www.amazon.com/s?k=NMN-containing+pet+supplements&__mk_zh_CN=%E4%BA%9A%E9%A9%AC%E9%80%8A%E7%BD%91%E7%AB%99&ref=nb_sb_noss (accessed on 28 January 2025).
- Han, M.; Hua, J. β-Nicotinamide mononucleotide (NMN) anti-aging research progress. J. Physiol. 2024, 76, 1032–1042. [Google Scholar] [CrossRef]
- Zhou, C.; Peng, H.; Liu, X.; Tao, J. Synthesis and detection of nicotinamide mononucleotide and its application in animal production. Feed. Res. 2023, 46, 131–134. [Google Scholar] [CrossRef]
- Unno, J.; Mills, K.F.; Ogura, T.; Nishimura, M.; Imai, S.-I. Absolute quantification of nicotinamide mononucleotide in biological samples by double isotope- mediated liquid chromatography-tandem mass spectrometry (dimeLC-MS/MS). npj Aging 2024, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yingjuan, H.; Zeng, J.; Bai, W.; Dong, H. Simultaneous determination of nicotinamide mononucleotide α,β isomers and nicotinamide adenine dinucleotide in foods by solid phase extraction-ultra performance liquid chromatography-tandem mass spectrometry. J. Food Saf. Qual. Test. 2023, 14, 206–213. [Google Scholar] [CrossRef]
- Zhang, M. Molecularly Imprinted Solid-Phase Extraction Combined with UHPLC-MS for the Determination of Nicotinamide Mononucleotides in Complex Samples; Shanxi University of Science and Technology: Beijing, China, 2022. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; Wang, C.; Li, X.; Yang, Z.; Leng, K. Determination of nicotinamide mononucleotide in food raw materials by high performance liquid chromatography-tandem mass spectrometry. Food Sci. Technol. 2021, 46, 251–256+262. [Google Scholar] [CrossRef]
- Ma, X.; Wu, J.; Pan, Z.; Qin, Y. Determination of Diflorasone Diacetate in Health Products by Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. Food Saf. Guide 2025, 16, 87-90+94. [Google Scholar] [CrossRef]
- Long, A.N.; Owens, K.; Schlappal, A.E.; Kristian, T.; Fishman, P.S.; Schuh, R.A. Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer’s disease-relevant murine model. BMC Neurol. 2015, 15, 19–2015. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.Y.; Zhao, M.; Guo, Y.Q.; Yang, j.; Xu, L. Determination of whitening ingredients in cosmetics by solvent-based demulsification dispersive liquid-liquid microextraction combined with on-line enrichment capillary electrophoresis. Chin. J. Anal. Lab. 2025, 1–11. [Google Scholar]
- Fan, Y.X. Study on the Determination of Biogenic Amines in Food by Solid-Phase Extraction-Capillary Electrophoresis; Shandong Agricultural University: Tai’an, China, 2024. [Google Scholar] [CrossRef]
- Sun, X.F.; Wang, F.Y. Establishment of a method for determining amoxicillin and ampicillin residues in freshwater fish meat by high-performance capillary electrophoresis. Meat Res. 2023, 37, 29–33. [Google Scholar]
- Wang, X.; Yang, X.Y.; Zhao, H.; Zhou, T.H.; Han, N.N.; Dai, Q. Ji X.; Ye N.S.; Wang X. Discussion on the feasibility of capillary electrophoresis in the quality control of veterinary drugs. Chin. J. Vet. Drug 2023, 57, 73–80. [Google Scholar]
- Zhang, J.L.; Fan, C.L.; Zhang, Z.J. Simultaneous quantitative determination of nicotinamide adenine dinucleotide and its precursor compounds in nutraceuticals by nuclear magnetic resonance spectroscopy. J. Food Saf. Qual. Test. 2023, 14, 246–254. [Google Scholar] [CrossRef]
- Zhou, Q. Optimization of Detection Conditions for Quality Indexes of Vegetable Oils Based on LF-NMR Technology. Henan University of Technology: Beijing, China, 2024. [Google Scholar] [CrossRef]
- Chen, D.W.; Mo, X.Y.; Zhang, H.R.; Hu, X.; Xiao, J.; Zhou, X.; Zhao, Z. Determination of lipid components in wheat germ by NMR technology. Trans. Chin. Soc. Agric. Eng. 2024, 40, 254–263. [Google Scholar]
- Mao, S.C.; Hao, M.; Wang, L.; Zhou, Z.; Bear, G.; Shi, L. Progress in the application of NMR in quality detection of meat products. Mod. Food Sci. Technol. 2023, 39, 354–366. [Google Scholar] [CrossRef]
- Zhao, C.X. Rapid Detection of Non-Protein Nitrogen Adulteration in Feed and Fatty Acids in Milk Based on NMR Technology; Tianjin University of Technology: Beijing, China, 2022. [Google Scholar] [CrossRef]
- He, M.H.; Luo, W.Q.; Chen, Y.Y.; Wu, Z.L.; He, W.X. Determination of β-nicotinamide mononucleotide and its analogs in milk powder. Food Res. Dev. 2024, 45, 166–172. [Google Scholar]
- Zhang, W.Y.; Lan, T.; Zhao, X.; Wu, Q.; Chu, Q.; Yu, C.; Wang, D.; Zhang, W.; Yun, Z. Determination of NMN content in cross-border products with β-nicotinamide mononucleotide. Food Ind. Sci. Technol. 2022, 43, 1–7+22. [Google Scholar] [CrossRef]
- Feng, X.P.; Zhu, Y.H.; Cheng, Q.; Li, C.; Zhang, H. Determination of β-nicotinamide mononucleotide in health food by high performance liquid chromatography. China Food Addit. 2021, 32, 153–157. [Google Scholar] [CrossRef]
- Xie, N.; Zheng, G.J. Determination of nicotinamide mononucleotide and nicotinamide adenine dinucleotide in health food by high performance liquid chromatography. J. Food Saf. Qual. Test. 2025, 16, 224–230. [Google Scholar] [CrossRef]
| Water | 30% Methanol–Water | 50% Methanol–Water | 85% Methanol–Water | |
|---|---|---|---|---|
| recovery rate | 75.6% | 95.3% | 91.4% | 77.6% |
| RSD | 8.5% | 0.9% | 5.7% | 14.0% |
| 20 Min | 20 Min Ice Bath | 30 Min | 30 Min Ice Bath | 40 Min | 40 Min Ice Bath | |
|---|---|---|---|---|---|---|
| recovery rate | 119.4% | 79.1% | 113.4% | 97.8% | 99.8% | 114.5% |
| RSD | 2.2% | 8.7% | 9.4% | 1.5% | 6.7% | 8.3% |
| Rt (Min) | Linear Equation | R | Linear Range (μg/mL) | LOD (mg/kg) | LOQ (mg/kg) | |
|---|---|---|---|---|---|---|
| NMN | 4.907 | y = 8769.3x − 19,035 | 1.000 | 5.0–500.0 | 1.0 | 2.0 |
| Detection Method | Detection Matrix | LOD | LOQ | Document Number |
|---|---|---|---|---|
| HPLC-PDA (Present Method) | Pet Food (Capsules, Tablets, Granules) | 1.0 mg/kg | 2.0 mg/kg | |
| HPLC-DAD | Milk Powder | 0.635 mg/kg | 2.120 mg/kg | [28] |
| LC-MS/MS | Food Raw Materials | 5.0 μg/L | 10 μg/L | [16] |
| CE-UV | Cosmetics | 25 ng/mL | 50 ng/mL | [19] |
| NMR | Dietary Supplements | 0.1 mmol/L | 0.2 mmol/L | [23] |
| 1 | 2 | 3 | 4 | 5 | 6 | RSD | |
|---|---|---|---|---|---|---|---|
| NMN | 51.29 | 50.34 | 52.48 | 53.85 | 52.73 | 52.54 | 2.3% |
| Samples | 1 | 2 | 3 | 4 | 5 | 6 | RSD |
|---|---|---|---|---|---|---|---|
| Capsules (mg/kg) | 25.7 | 25.5 | 25.2 | 25.4 | 25.1 | 25.5 | 0.8% |
| tablets (mg/kg) | 77.8 | 78.3 | 76.9 | 77.5 | 77.4 | 77.1 | 0.6% |
| Granules (mg/kg) | 78.9 | 78.8 | 79.6 | 79.2 | 80.6 | 81.1 | 1.1% |
| Samples | 1 | 2 | 3 | 4 | 5 | 6 | RSD |
|---|---|---|---|---|---|---|---|
| Capsules (mg/kg) | 25.7 | 25.2 | 26.9 | 25.2 | 23.8 | 25.5 | 3.9% |
| tablets (mg/kg) | 77.6 | 78.4 | 80.0 | 78.3 | 78.4 | 79.2 | 0.9% |
| Granules (mg/kg) | 80.1 | 81.6 | 80.8 | 81.7 | 80.0 | 81.4 | 0.9% |
| Samples | Background Value | 5 mg/kg | 10 mg/kg | 50 mg/kg | |||
|---|---|---|---|---|---|---|---|
| Recovery Rate | RSD | Recovery Rate | RSD | Recovery Rate | RSD | ||
| Capsules | 25.4 mg/kg | 97.3% | 5.7% | 103.3% | 0.5% | 107.2% | 0.5% |
| tablets | 79.4 mg/kg | 107% | 6.3% | 103.3% | 3.8% | 108% | 0.5% |
| Granules | 80.2 mg/kg | 101% | 6.0% | 104.6% | 3.9% | 109% | 0.6% |
| Number | Formulations | NMN (Measured Value) | Product Specification |
|---|---|---|---|
| 1 | Capsules 1 | 76.4 mg/kg | unlabeled |
| 2 | Capsules 2 | 76.7 mg/kg | unlabeled |
| 3 | Capsules 3 | 25.5 mg/kg | unlabeled |
| 4 | tablets | 79.4 mg/kg | 80 mg/kg |
| 5 | Granules | 80.1 mg/kg | 80 mg/kg |
| Milk Powder Method [28] | Health Food Method [30] | Cross-Border Food Method [29] | Pet Food Method (This Study) | |
|---|---|---|---|---|
| Chromatographic Column | Venusil HILIC | Welch Xtimate C18 | Venusil HILIC | PC HILIC |
| Pretreatment Purification | C18 solid-phase extraction required | No purification needed | No purification needed | No purification needed |
| Extraction Solvent | Deionized water + acetic acid | 2% acetonitrile–water | 50% methanol–water | 30% methanol–water |
| Ultrasonic Conditions | Room temperature (temperature control not mentioned) | Room temperature, 10 min | Room temperature, 20 min | Ice bath, 30 min |
| Mobile phase ratio | 0–2 min (18% ultrapure water, 57% acetonitrile, 25% 0.1% trifluoroacetic acid aqueous solution) → 2–28 min (gradient to 5% ultrapure water, 25% acetonitrile, 70% 0.1% trifluoroacetic acid aqueous solution) → 28.1–40 min (back to initial proportions). | mobile phase A (50 mmol/L potassium dihydrogen phosphate) and mobile phase B (acetonitrile): 0–10 min (A: 98% → 95%, B: 2% → 5%); 10–15 min (A: 95% → 80%, B: 5% → 20%); 15–16 min (A: 80% → 98%, B: 20% → 2%), then hold for 9 min. | Mobile phase A: Mobile phase B = 0.1% formic acid aqueous solution: 0.1% formic acid methanol solution = 15:85 (v/v) | Mobile phase A: Mobile phase B = 0.1% formic acid aqueous solution: 0.1% formic acid methanol solution = 15:85 (v/v) |
| Column temperature | 25 °C | 30 °C | 35 °C | 35 °C |
| Injection volume | 10 μL | 5 μL | 10 μL | 10 μL |
| flow velocity | 1 mL/min | 0.8 mL/min | 1 mL/min | 1 mL/min |
| Linear Range | 5~30 mg/L | 0.5~2.0 mg/mL | 5~500 μg/mL | 5~500 μg/mL |
| Matrix Adaptability (Pet Food) | Poor (impurity coelution) | Poor (peak tailing) | Fair (insufficient stability) | Excellent (high resolution, good stability) |
| Single Sample Processing Time | >30 min | 9 min | 10 min | 10 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Li, C.; Lan, T.; Wang, L.; Zhang, J. Development and Validation of an HPLC-PDA Method for NMN Quantification in Commercial Pet Foods. Appl. Sci. 2025, 15, 10797. https://doi.org/10.3390/app151910797
Meng Y, Li C, Lan T, Wang L, Zhang J. Development and Validation of an HPLC-PDA Method for NMN Quantification in Commercial Pet Foods. Applied Sciences. 2025; 15(19):10797. https://doi.org/10.3390/app151910797
Chicago/Turabian StyleMeng, Yuxin, Chujun Li, Tao Lan, Lihong Wang, and Jingxuan Zhang. 2025. "Development and Validation of an HPLC-PDA Method for NMN Quantification in Commercial Pet Foods" Applied Sciences 15, no. 19: 10797. https://doi.org/10.3390/app151910797
APA StyleMeng, Y., Li, C., Lan, T., Wang, L., & Zhang, J. (2025). Development and Validation of an HPLC-PDA Method for NMN Quantification in Commercial Pet Foods. Applied Sciences, 15(19), 10797. https://doi.org/10.3390/app151910797









